NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.045

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsThis chapter discusses inherited human diseases that affect glycan biosynthesis and metabolism. Representative examples of diseases caused by defects in several major glycan families are described. Disorders affecting the degradation of glycans are described in Chapter 44.
INHERITED PATHOLOGICAL MUTATIONS OCCUR IN ALL MAJOR GLYCAN FAMILIES
Nearly all inherited disorders in glycan biosynthesis were discovered in the last 20 years. They are rare, biochemically and clinically heterogeneous, and usually affect multiple organ systems. Some defects strike only a single glycosylation pathway, whereas others impact several. Defects occur in (1) the activation, presentation, and transport of sugar precursors; (2) glycosidases and glycosyltransferases; and (3) proteins that traffic glycosylation machinery or maintain Golgi homeostasis. A few disorders can be treated by the consumption of monosacccharides. The rapid growth in the number of these discovered disorders is shown in Figure 45.1.
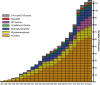
FIGURE 45.1.
Glycosylation-related disorders. The graph shows the cumulative number of human glycosylation disorders in various biosynthetic pathways and the year of their identification. In most cases, the year indicates the definitive proof of gene and specific (more...)
Selected disorders are listed in Table 45.1 and all known disorders in Online Appendix 45A. Disease nomenclature has evolved. Congenital disorders of glycosylation (CDG) were originally defined as genetic defects in N-glycosylation, but now the term is applied to any glycosylation defect, by indicating the mutated gene followed by “-CDG” suffix (e.g., PMM2-CDG). CDGs are rare primarily because embryos with complete defects in a step of glycosylation do not usually survive to be born, documenting the critical biological roles of glycans in humans. CDG patients that survive are usually hypomorphic, retaining at least some activity of the pathways involved.
TABLE 45.1.
Selected genetic defects of glycans in humans
DEFECTS IN N-GLYCAN BIOSYNTHESIS
Clinical and Laboratory Features and Diagnosis
The broad clinical features of disorders in which N-glycan biosynthesis is defective involve many organ systems, but are especially common in the central and peripheral nervous systems, hepatic, visual, and immune systems. The generality and variability of clinical features makes it difficult for physicians to recognize CDG patients. The first were identified in the early 1980s based primarily on deficiencies in multiple plasma glycoproteins. The patients were also delayed in reaching growth and developmental milestones and had low muscle tone, incomplete brain development, visual problems, coagulation defects, and endocrine abnormalities. However, many of these symptoms are seen in patients with other inherited multisystemic metabolic disorders, such as mitochondria-based diseases. CDG patients can be distinguished because they often have abnormal glycosylation of common liver-derived serum proteins containing disialylated, biantennary N-glycans. Serum transferrin is especially convenient because it has two N-glycosylation sites each containing disialylated biantennary N-glycans. Different glycoforms can be resolved by isoelectric focusing (IEF) or ion-exchange chromatography, but better accuracy and sensitivity is achieved by mass spectrometry (MS) of purified transferrin. This simple litmus test alerts physicians to likely CDG patients without knowing the gene or molecular basis of the disease.
CDG defects may be divided into two types based on transferrin glycoforms. Type I (CDG-I) patients lack one or both N-glycans because of defects in the biosynthesis of the lipid-linked oligosaccharide (LLO) and its transfer to proteins. Type II (CDG-II) patients have incomplete protein-bound glycans because of abnormal processing. These differences were used to name the disorders (e.g., CDG-Ia and CDG-IIa), which oriented the search for a defective gene. This system is gradually being replaced by the affected gene name with a CDG suffix, such as PMM2-CDG. The biosynthetic pathways and locations of N-glycan defects are shown in Figure 45.2.
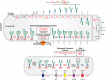
FIGURE 45.2.
Congenital disorders of glycosylation in the N-glycosylation pathway. The figure shows individual steps in lipid-linked oligosaccharide (LLO) biosynthesis, glycan transfer to protein, and N-glycan processing similar to Figure 9.3 and Figure 9.4. The shuttling (more...)
Type I Congenital Disorders of Glycosylation
A complete absence of N-glycans is lethal. Therefore, known mutations generate hypomorphic alleles, not complete knockouts. A deficiency in any of the steps required for the assembly of LLO in the endoplasmic reticulum (ER) (e.g., nucleotide sugar synthesis or sugar addition catalyzed by a glycosyltransferase) (Chapter 9) produces a structurally incomplete LLO. Because the oligosaccharyltransferase prefers full-sized LLO glycans, this results in hypoglycosylation of multiple glycoproteins. This means that some N-glycan sites are not modified. Importantly, many deficiencies in LLO synthesis produce incomplete LLO intermediates. Most of the LLO assembly steps are not easy to assay, but LLO assembly is conserved from yeast to humans, and intermediates that accumulate in CDG patients often correspond to the intermediates seen in mutant Saccharomyces cerevisiae strains with known defects in LLO assembly. Some mutant mammalian cells (e.g., Chinese hamster ovary cells) have been shown to have similar defects (Chapter 49). The close homology between yeast and human genes enables the normal human orthologs to rescue defective glycosylation in mutant yeast strains, whereas mutant orthologs from patients do not. This provides substantial clues to the likely human defect, along with a system in which to perform functional assays.
PMM2-CDG (CDG-Ia) in Table 45.1 is the most common CDG with more than 800 cases identified worldwide. The patients have moderate to severe developmental and motor deficits, hypotonia, dysmorphic features, failure to thrive, liver dysfunction, coagulopathy, and abnormal endocrine functions. More than 100 mutations found in phosphomannomutase 2 (PMM2) impair conversion of Man-6-P to Man-1-P, which is a precursor required for the synthesis of GDP-mannose (GDP-Man) and dolichol-P-mannose (Dol-P-Man). Both donors are substrates for the mannosyltransferases involved in the synthesis of Glc3Man9GlcNAc2-P-P-Dol and its level is decreased in cells from PMM2-CDG patients. Patients have hypomorphic alleles because complete loss of PMM2 function is lethal. Mouse embryos lacking Pmm2 die 2–4 days after fertilization, whereas some of those with hypomorphic alleles survive. There are currently no therapeutic options for PMM2-CDG patients.
MPI-CDG (CDG-Ib) in Table 45.1 is caused by mutations in MPI (mannose-6-phosphate isomerase). This enzyme interconverts fructose-6-P and mannose-6-P (Man-6-P). In contrast to PMM2-CDG, these patients do not have intellectual disability or developmental abnormalities. Instead, they have impaired growth, hypoglycemia, coagulopathy, severe vomiting and diarrhea, protein-losing enteropathy, and hepatic fibrosis. Several patients died of severe bleeding before the basis of this CDG was known. Mannose dietary supplements effectively treat these patients. Man-6-P can be generated directly by hexokinase-catalyzed phosphorylation of mannose (Chapter 5). This pathway is intact in MPI-CDG patients. Human plasma contains ∼50 µm mannose because of export following glycan degradation and processing. Mannose supplements correct coagulopathy, hypoglycemia, protein-losing enteropathy, and intermittent gastrointestinal problems, as well as normalize the glycosylation of plasma transferrin and other serum glycoproteins. Because orally administered mannose is well tolerated, this approach is clearly a satisfyingly effective, although not curative, therapy for this life-threatening condition.
Complete loss of the Mpi gene in mice is lethal at about embryonic day 11.5. N-Glycosylation is normal, but death results from accumulation of intracellular Man-6-P, which depletes ATP and inhibits several glycolytic enzymes. Providing dams with extra mannose during pregnancy only hastens the embryo's demise via the “honeybee effect,” which occurs when bees are given only mannose instead of glucose. The bees continue flying for a short time and then literally drop dead. They have low MPI activity compared with hexokinase and therefore accumulate Man-6-P, which they degrade, and perhaps rephosphorylate, further depleting ATP. Even more serious is that Man-6-P inhibits several glycolytic enzymes. Entry of Man-6-P into glycolysis is very slow and thus the bees become energy-starved and die within a few minutes. MPI-CDG patients have sufficient residual MPI activity and do not accumulate intracellular Man-6-P when given mannose, although the amount is sufficient to correct impaired glycosylation. However, the honeybee effect may be at play again in Mpi-hypomorphic mice because dams given modest amounts of mannose during pregnancy produce pups with no lenses. The effect is quite specific to lens development in which the MPI activity is very low even in normal mice. A hypomorphic mutation and increased substrate load combines so that Man-6-P accumulates.
Other types of CDG-I have a broad range of clinical phenotypes including low LDL, low IgG, kidney failure, genital hypoplasia, and cerebellar hypoplasia. The reasons for these effects are unknown. Patients with mutations in nearly all the remaining steps of LLO biogenesis have been found (Table 45.1; Online Appendix 45A; Figure 45.1) including defects in dolichol biosynthesis (cis-isoprenyl transferase, dolichol-kinase, and polyprenol reductase) and in a putative LLO flippase. Mutations in five of the oligosaccharyltransferase subunits also cause a CDG.
Type II Congenital Disorders of Glycosylation
CDG-II disorders (Figure 45.2) affect N-glycan processing and include defects in glycosyltransferases, nucleotide sugar transporters, vacuolar pH regulators, and multiple cytoplasmic proteins that traffic glycosylation machinery within the cell and maintain Golgi homeostasis.
In B4GALT1-CDG, glycans showed the loss of both galactose (Gal) and sialic acid (Sia) from transferrin because of loss of β1-4 galactosyltransferase I activity. A similar glycan pattern occurs in the X-linked SLC35A2-CDG, because of loss of UDP-Gal transporter activity. Surprisingly, within a few years after birth, abnormal glycosylation becomes normal. This is probably due to somatic mosaicism of cells carrying the mutated SLC35A2 gene and unaffected cells and selection against the affected cells.
Patients with leukocyte adhesion deficiency type II (LAD-II or SLC35C1-CDG) were found to have mutations in SLC35C1, encoding a GDP-Fucose (Fuc) transporter. Here, transferrin sialylation was normal, so this defect was not detected by the usual test, but some serum proteins and O-linked glycans on leukocyte surface proteins were deficient in Fuc. One leukocyte protein carries a selectin ligand glycan, sialyl Lewis x, that mediates leukocyte rolling before extravasation of leukocytes from capillaries into tissues (Chapter 34). This defect greatly elevates circulating leukocytes and decreases leukocyte extravasation so patients have frequent infections. A few patients responded to dietary Fuc therapy. Sialyl Lewis x reappeared on their leukocytes, and circulating neutrophils promptly returned to normal levels. Fuc is converted into Fuc-1-P by fucose kinase and then to GDP-Fuc by GDP-Fuc pyrophosphorylase (Chapter 5). Fuc supplements must increase the amount of GDP-Fuc enough to correct the defect. A mouse model of Fuc deficiency lacks de novo biosynthesis of GDP-Fuc from GDP-Man (Chapter 5). The mice die without Fuc supplements, but providing Fuc in the drinking water rapidly normalizes their elevated neutrophils. The treatment also corrects abnormal hematopoeisis resulting from disrupted O-Fuc-dependent Notch signaling.
CDG-II defects are also caused by mutations in the eight-subunit conserved oligomeric Golgi (COG) complex, which has multiple roles in trafficking within the Golgi. COG7-CDG (Table 45.1) was discovered first. Trafficking of multiple glycosyltransferases and nucleotide sugar transporters were disrupted in COG7-CDG. The mutation affects the synthesis of both N- and O-glycans and glycosaminoglycan (GAG) chains. Mutations have now been found in all COG subunits except COG3. Mammalian cells deficient in COG1, COG2, COG3, and COG5 also show various degrees of altered glycosylation. Various mutations in a vacuolar H+-ATPase subunit also disrupt multiple glycosylation pathways, presumably because of an increase in Golgi pH and concomitant decrease in glycosyltransferase activities.
Other genetic defects that impair N-glycan synthesis include I-cell disease, which results from the lack of Man-6-P on lysosomal enzyme N-glycans (Chapter 33). An unusual disorder called HEMPAS (hereditary erythroblastic multinuclearity with positive acidified-serum test) leads to abnormal red cell shape and instability (hemolysis) due to mutations in SEC23B, another intracellular trafficking protein that produces abnormal red blood cell glycans in several pathways.
In an interesting twist, it is possible to have diseases caused by “excessive” glycosylation. For example, Marfan syndrome results from mutations in fibrillin1 (FBN1) and one of these creates an N-glycosylation site that disrupts multimeric assembly of FBN1. This may not be an isolated case. A survey of nearly 600 known pathological mutations in proteins traveling the ER–Golgi pathway showed that 13% of them create novel glycosylation sites. This is far greater than predicted by random missense mutations and may mean that hyperglycosylation leads to a new class of CDGs.
GALACTOSEMIA
Galactosemia refers to mutations in three genes involved in Gal metabolism. In “classical galactosemia,” Gal-1-P uridyltransferase (GALT; Figure 45.3) is deficient. This results in excess Gal-1-P and decreased synthesis and availability of UDP-Gal. Defects in UDP-Gal-4′-epimerase (GALE; Figure 45.3) or galactokinase (GALK; Figure 45.3) also cause the disease, but they are more rare.
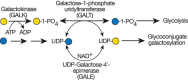
FIGURE 45.3.
UDP-Gal synthesis and galactosemia. The most common form of galactosemia is due to a deficiency of galactose-1-phosphate uridyltransferase (GALT). This enzyme normally uses Gal-1-P derived from dietary Gal. In the absence of GALT, Gal-1-P accumulates, (more...)
GALT-deficient infants fail to thrive and have enlarged liver, jaundice, and cataracts. A lactose-free diet ameliorates most of the acute symptoms by reducing the amount of Gal entering the pathway and the accumulation of Gal and Gal-1-P. Reducing Gal decreases galactitol and galactonate, which are produced via reductive or oxidative metabolism of Gal, respectively. Galactitol is not metabolized further and has osmotic properties that contribute to cataract formation. Unfortunately, a Gal-free diet apparently does not prevent the appearance of cognitive disability, ataxia, growth retardation, and ovarian dysfunction that are characteristic of this disease. The long-term complications in treated GALT-deficient individuals may be due to small amounts of toxic metabolites that accumulate. Alternatively, the complications may reflect dysfunctions that originated during fetal life. GALT deficiency may decrease UDP-Gal and galactosylated glycans. Hypogalactosylation of glycoproteins and glycolipids has been observed in some GALT-deficient individuals. But, in addition to loss of Gal on glycans, some patients who mistakenly receive Gal also synthesize transferrin that is missing both N-glycans. The basis of the combined absence of Gal/Sia and entire N-glycans is not understood, but the pattern returns to normal when the patients are placed on a Gal-free diet.
MUSCULAR DYSTROPHIES
Congenital Muscular Dystrophies
Mutations altering O-Man glycans (Chapter 13), primarily on α-dystroglycan (α-DG) cause at least 16 types of congenital muscular dystrophy (CMD) termed dystroglycanopathies (Figure 45.4). α-DG at neuromuscular junctions links skeletal muscle cell cytoskeleton to laminin in the extracellular matrix. The clinical spectrum of dystroglycanopathies is broad, ranging from very severe, and often lethal, musculo-oculo-encephalopathies, such as Walker–Warburg syndrome (WWS), muscle–eye–brain disease (MEB), and Fukuyama congenital muscular dystrophy (FCMD) to milder forms of limb-girdle muscular dystrophy. Genetic analysis of these disorders has been indispensable for discovering functional glycans and their biosynthesis. The complex pathway is presented in Figure 45.4 and Chapter 13. The pathway is initiated in the ER by the transfer of Man to Ser/Thr via the protein O-mannosyltransferase complex containing POMT1 and POMT2 (Table 45.1). In the Golgi, this pathway generates more than 20 O-Man glycans in mammals. An unusual feature on one subset of O-Man glycans is the existence of a Man-6-P generated by POMK. The Man-6-P containing CoreM3 glycan consists of Man-6-P, GlcNAc (transferred by POMGNT2), and GalNAc (transferred by B3GALNT2). The trisaccharide core is extended by two units of ribitol-5-phosphate and a single repeat of xylose (Xyl) and glucuronic acid (GlcA). This structure is elongated by an alternating disaccharide (β1-3Xylα1-3GlcA). Two ribitol-5-phosphates are sequentially transferred by FKTN (fukutin) and FKRP (fukutin-related protein) from CDP-ribitol, which is generated by ISPD. Xyl and GlcA in the single repeat are transferred by TMEM5 and B4GAT1, respectively. The elongation by the alternating disaccharide is catalyzed by LARGE. The polymeric glycan (matriglycan) is recognized by several diagnostic monoclonal antibodies and is necessary for the binding of laminin and other molecules to α-DG.
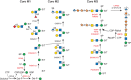
FIGURE 45.4.
O-Man glycan biosynthetic pathway. The biosynthetic pathways of representative O-Man glycans are shown. Genes that cause a disorder are indicated in red. Three main groups are identified: core M1–M3. All O-Man glycans are initiated on either a (more...)
O-Man glycans can also play a traitorous role, because they are receptor molecules for Lassa Virus entry into cells. This feature was cleverly exploited to screen libraries and identify genes required for virus entry. The method correctly identified all previously known WWS-causing genes and predicted new culprits. So far, sixteen genes have been proven to cause a Dystroglycanopathy (POMT1, POMT2, POMGNT1, FKTN, FKRP, ISPD, LARGE, POMGNT2, TMEM5, B3GALNT2, POMK, B4GAT1, GMPPB, DPM1, DPM2, and DPM3) of which three (DPM1–3) are required for N-glycan and glycosylphosphatidylinositol (GPI)-anchor biosynthesis.
Although α-DG is the major carrier of LARGE-modified O-Man glycans, cadherins carry O-Man glycans important for their roles in cell–cell adhesion. Clustered protocadherins that contain O-Man glycans are regulated during brain development and form larger oligomers. The O-Man glycans seem to orient cadherin domains for critical interactions. O-Man in clustered protocadherins may help explain ocular and brain malformations in disorders such as WWS.
Clinical criteria previously defined the Dystroglycanopathies, but now they are defined by the mutated gene as mutations in genes of the pathway fit the clinical criteria. WWS is the most severe CMD. Patients live about one year and have multiple brain abnormalities, and severe muscular dystrophy. About 20% of patients have mutations in POMT1 and a few have mutations in POMT2. Others have defects in FKTN and FKRP, but mutations in both also cause milder forms of muscular dystrophy.
POMGNT1 is mutated in MEB, which is characterized by symptoms similar to, but milder than, WWS. The most severely affected MEB patients die during the first years of life, but the majority of mild cases survive to adulthood. Fukuyama muscular dystrophy (FCMD) is caused by a single 3-kb 3′-retrotransposon insertional event into the FKTN gene, which occurred 2000–2500 years ago. This partially reduces the stability of the mRNA, making it a relatively mild mutation. FCMD is one of the most common types of CMD in Japan with a carrier frequency of 1/188. Fktn-null mice die by E9.5 in embryogenesis and appear to have basement membrane defects. Congenital muscular dystrophy type 1C (MDC1C) is a relatively mild disorder that is caused by mutations in FKRP. Patients with MDC1D, a limb-girdle muscular dystrophy, contain mutations in LARGE, originally described in myodystrophic mice (myd; now called Largemyd). The protein has two glycosyltransferase signatures (DXD) in different domains that account for xylosyl- and glucuronosyl-transferase actitivities respectively (Chapter 13).
GNE Myopathy
Recessive mutations in GNE cause adult-onset GNE myopathy (previously named hereditary inclusion body myopathy type 2 (HIBM2 or Nonaka myopathy) (Table 45.1). It occurs worldwide, but one mutation (p.Met745Thr) is especially common among Persian Jews (1:1500) and occurs in the kinase domain (Chapter 5). GNE mutations occur in various combinations in both GNE enzymatic domains, and variably affect enzyme activity. The mutations moderately reduce enzymatic activity and reduce sialylation in mouse models. Sia is efficiently salvaged from degraded glycoproteins, but there is little information on the cell-type preference or age-dependent contributions of the de novo versus salvage pathways. Gne-null mice die during embryogenesis and most mice homozygous for the knockin p.Met743Thr mutation die a few days after birth because of severe hematuria and proteinuria, not myopathy. Glomerular abnormalities in the podocyte basement membrane result from undersialylated in foot podocytes such as podocalyxin and nephrin. Providing N-acetylmannosamine (ManNAc) to the pups in the neonatal period rescues some of them and increases sialylation of podocalyxin and nephrin. Mutant p.Met743Thr survivors who did not receive ManNAc after the neonatal period develop adult-onset hyposialylation of muscle tissue, which can be rescued by oral ManNAc therapy at adult age. Oral ManNAc is being tested as a therapy for GNE myopathy patients, as well as for patients with primary glomerular diseases (focal segmental glomerulosclerosis, minimal change disease, and membranous nephropathy).
Another mouse model, carrying a transgenic Gne mutation (p.Asp207Val) common in the Japanese population, develops a pathological adult-onset muscle phenotype involving β-amyloid deposition that precedes the accumulation of inclusion bodies. Providing modest amounts of Sia, N-acetylmannosamine or sialyllactose to these mice prevents and even reverses muscle deterioration. Surprisingly, sialylactose supplements are most effective. We still know relatively little about how each of these sugars are imported into various cells or preferentially used in glycan synthesis (Chapter 15).
DEFECTS IN O-GalNAc GLYCANS
A defect in O-GalNAc synthesis by a particular polypeptide GalNAc-transferase (GALNT3) causes familial tumoral calcinosis. This severe autosomal-recessive metabolic disorder shows phosphatemia and massive calcium deposits in the skin and subcutaneous tissues. Mutations in the O-glycosylated fibroblast growth factor 23 (FGF23) also cause phosphatemia, suggesting that GALNT3 modifies FGF23. The rare autoimmune disease, Tn syndrome, is caused by somatic mutations in the X-linked gene C1GALT1C1, which encodes a highly specific chaperone COSMC required for the proper folding and normal activity of the β1-3galactosyltransferase C1GALT1 needed for synthesis of core 1 and 2 O-glycans (Chapters 10 and 46).
DEFECTS IN PROTEOGLYCAN SYNTHESIS
Proteoglycans and their GAG chains are critical components in extracellular matrices. For a discussion of their biosynthesis, core proteins, and function, see Chapter 17.
Ehlers–Danlos Syndrome (Progeroid Type)
Ehlers–Danlos syndrome (progeroid type) is a connective tissue disorder characterized by failure to thrive, loose skin, skeletal abnormalities, hypotonia, and hypermobile joints, together with delayed motor development and delayed speech. The molecular basis of the disorder is reduced synthesis of the core region of GAGs initiated by Xyl. Galactosyltransferase I (B4GALT7) the enzyme that adds Gal to Xyl-Ser is mutated in this disease. The activity of galactosyltransferase II (B3GALT6), the enzyme responsible for adding the second Gal to the core of a GAG, may also be reduced. One possible explanation for the dual effect is that the primary mutation affects the formation or stability of a biosynthetic complex involving several GAG biosynthetic enzymes.
Congenital Exostosis
Defects in the formation of heparan sulfate (HS) cause hereditary multiple exocytosis (HME), an autosomal-dominant disease with a prevalence of about 1:50,000 (Table 45.1). It is caused by mutations in two genes EXT1 and EXT2, which are involved in HS synthesis. HME patients have bony outgrowths, usually at the growth plates of the long bones. Normally, the growth plate contains chondrocytes in various stages of development, which are enmeshed in an ordered matrix composed of collagen and chondroitin sulfate (CS). In HME, however, the outgrowths are often capped by disorganized cartilagenous masses with chrondrocytes in different stages of development. About 1%–2% of patients also develop osteosarcoma. HME mutations occur in EXT1 (60%–70%) and EXT2 (30%–40%). The encoded proteins may form a complex in the Golgi and both are required for polymerizing N-acetylglucosamine (GlcNAc)α1-4 and GlcAβ1-3 into HS. However, the partial loss of one allele of either gene appears sufficient to cause HME. This means that haploinsufficiency decreases the amount of HS and that EXT activity is rate limiting for HS biosynthesis. This is unusual because most glycan biosynthetic enzymes are in substantial excess.
The mechanism of HME pathology is likely rooted in a disruption of the normal distribution of HS-binding growth factors, which include fibroblast growth factor (FGF) and morphogens such as hedgehog, Wnt, and members of the transforming growth factor β (TGF-β) family. The loss of HS disrupts these pathways in Drosophila. Mice that are null for either Ext gene are embryonic lethal and fail to gastrulate; however, Ext heterozygous animals are viable, and do not develop exostoses on the long bones, in contrast to patients with HME. However, mice with chondrocyte-specific somatic mutations in Ext1 cause a loss of heterzygosity and develop exostoses and growth abnormalities in the growth plates of those bones. HS is required to establish and maintain the perichondrium phenotype, and it also restrains prochondrogenic signaling proteins including bone morphogenetic proteins (BMPs) that normally restrict chondrogenesis. Without HS, chondrogenesis increases in localized areas of actively growing cells.
Achondrogenesis, Diastrophic Dystrophy, and Atelosteogenesis
Three autosomal-recessive disorders, diastrophic dystrophy (DTD), atelosteogenesis type II (AOII), and achondrogenesis type IB (ACG-IB) result from defective cartilage proteoglycan sulfation. These forms of osteochondrodysplasia have various outcomes. AOII and ACG-IB are perinatal lethal because of respiratory insufficiency, whereas DTD patients develop symptoms only in cartilage and bone, including cleft palate, clubfeet, and other skeletal abnormalities. Those DTD patients surviving infancy often live a nearly normal life span. All of these disorders result from different mutations in the DTD gene (SLC26A2), which encodes a plasma membrane sulfate transporter. Unlike monosaccharides, sulfate released from degraded macromolecules in the lysosome is not salvaged well. The heavy demand for sulfate in bone and cartilage proteoglycan synthesis probably explains why the symptoms are most evident in these locations. Defects in the UDP-GlcA/UDP-N-acetylgalactosamine (GalNAc) Golgi transporter (SLC35D1) cause Schneckenbecken dysplasia. Patients have bone abnormalities similar to those seen in other chrondrodysplasias, and a mouse model of the disease shows similar features.
Macular Corneal Dystrophy
Keratan sulfate I (KS-I) in the cornea is an N-linked oligosaccharide with poly-N-acetyllactosamine repeats (Galβ1-4GlcNAcβ1-3) variably sulfated at the 6-positions. Macular corneal dystrophy (MCD), an autosomal-recessive disease, causes the cornea to become opaque and corneal lesions to develop. Two types of MCD have been described. MCD I appears to be due to a deficiency in sulfating the repeating units. Both Gal and GlcNAc are sulfated in KS; sulfation of Gal and GalNAc in CS are also affected in MCD patients.
DEFECTS IN GPI-ANCHORED PROTEINS
Complete deletion of the GPI pathway in mice causes embryonic lethality. This is not surprising because more than 150 membrane proteins require a GPI anchor for cell surface expression (Chapter 12). Hypomorphic mutations in multiple genes in the pathway lead to a partial reduction in GPI-anchored proteins. These include PIGA, PIGQ, PIGY, PIGC, PIGP, PIGL, PIGW, PIGM, PIGV, and PIGO in anchor assembly (Table 45.1), and PIGT in the transfer of the glycan to proteins. Defects in side-chain modifications (PIGN and PIGG) and maturation of GPI following attachment to proteins (PGAP1, PGAP2, and PGAP3) also cause inherited GPI deficiency but not embryonic death. GPI deficiency has immense and variable consequences including neurologic symptoms, particularly developmental delay/intellectual disability and seizures, epileptic encephalopathy, progressive cerebral and/or cerebellar atrophy, hypotonia, cortical visual impairment, sensorineural deafness, and Hirschsprung disease. Nonneurologic phenotypes include brachytelephalangy, anorectal anomaly, renal abnormality, cleft palate, heart defect, and characteristic facial features such as hypertelorism, broad nasal bridge, and tented mouth. Other symptoms such as ichthyosis, iron deposition, hepatosplenomegaly, diaphragmatic hernia, and hepatic and/or portal vein thrombosis were reported in small fractions of the affected individuals. It is not easy to causally relate specific symptoms to deficiency of particular GPI-anchored protein except for few instances. Deficiency of tissue-nonspecific alkaline phosphatase (TNALP) accounts for seizures in some of the patients. Death within a year after birth due to aspiration or status epilepticus is not rare among severely affected individuals with GPI deficiency, whereas mildly affected individuals live with GPI deficiency.
DEFECTS IN GLYCOSPHINGOLIPID (GSL) SYNTHESIS
Only three disorders in GSL synthesis are known in humans. Mutations in ST3GAL5 cause autosomal-recessive Amish infantile epilepsy syndrome and also “salt-and-pepper syndrome.” This gene encodes a sialyltransferase required for the synthesis of the ganglioside GM3 (Siaα2-3Galβ1-4Glc-ceramide) from lactosylceramide (Galβ1-4Glc-ceramide). GM3 is also a precursor for some more complex gangliosides. The patients' plasma glycosphingolipids (GSLs) are nonsialylated. In contrast to the human form of the disease, mice that lack GM3 do not have seizures or a shortened life span. However, mouse strains that are null for the sialyltransferase and an N-acetylgalactosaminyltransferase that is required for making other complex gangliosides, do develop seizures, suggesting that it is the absence of these more complex gangliosides that may be the underlying problem (Chapter 11). Mutations in B4GALNT1 (also known as GM2/GD2 synthase) cause hereditary spastic paraplegia subtype 26. These patients have developmental delays and varying cognitive impairments with early-onset progressive spasticity owing to axonal degeneration. Cerebellar ataxia, peripheral neuropathy, cortical atrophy, and white-matter hyperintensities were also consistent across the disorder. A B4galnt1−/− mouse recapitulates several of the neurological characteristics of SPG26, most prominently the progressive gait disorder
ST3GAL3 makes more complex gangliosides as well as N- and O-glycans. It is required for the development of high cognitive functions and is mutated in some individuals with West syndrome. An St3gal3−/− mouse model also exists, but these mice appear to have no overt neurological phenotype. GSL disorders are difficult to identify biochemically because no convenient biomarkers exist. Next-generation sequencing will reveal new candidates.
A Deglycosylation Disorder
Early on, it was assumed that glycosylation disorders would result from defects in glycan biosynthetic enzymes, but that perspective has changed. Discovery of defects in Golgi organization and homeostasis, in ER chaperones such as COSMC or EDEM, and in ER quality control have broadened the perspective. A new defect in the ER-associated degradation (ERAD) continuum (Chapter 39) is caused by defects in NGLY1, an enzyme that cleaves N-glycans from misfolded glycoproteins transported into the cytoplasm before their proteasomal degradation (Table 45.1). The defect does not appear to induce the ERAD pathway, accumulate undegraded glycoproteins in vesicles, or trigger autophagy. It is unclear how the defect causes symptoms such as developmental delay, movement disorder, seizures, and a curious lack of tear production, but their clinical similarity to other CDGs emphasizes that glycosylation defects cannot simply be divided into “synthesis” or “degradation.”
PHENOTYPES, MULTIPLE ALLELES, AND GENETIC BACKGROUND
Phenotypic expression of the same mutation can have highly variable impact, even among affected siblings. Explanations based on residual activity for these “simple Mendelian disorders” are neither simple nor generally satisfying. It is often attributed to “genetic background.” A knockout mutation may be lethal in one highly inbred mouse strain, but not in another because compensatory pathways may exist. Dietary and environmental impacts are substantial as seen in MPI-CDG patients with and without oral mannose therapy. Multiple simultaneous or sequential environmental insults may impinge on borderline genetic insufficiencies to produce overt disease.
ACKNOWLEDGMENTS
The authors acknowledge contribution of Bobby G. Ng and appreciate helpful comments and suggestions from Aime Lopez Aguilar, Kekoa Taparra, Krithika Vaidyanathan, and Shweta Varshney.
FURTHER READING
- Dobson CM, Hempel SJ, Stalnaker SH, Stuart R, Wells L. 2013. O-Mannosylation and human disease. Cell Mol Life Sci 70: 2849–2857. [PMC free article: PMC3984002] [PubMed: 23115008]
- Huegel J, Sgariglia F, Enomoto-Iwamoto M, Koyama E, Dormans JP, Pacifici M. 2013. Heparan sulfate in skeletal development, growth, and pathology: The case of hereditary multiple exostoses. Dev Dyn 242: 1021–1032. [PMC free article: PMC4007065] [PubMed: 23821404]
- Jaeken J. 2013. Congenital disorders of glycosylation. Handb Clin Neurol 113: 1737–1743. [PubMed: 23622397]
- Rosnoblet C, Peanne R, Legrand D, Foulquier F. 2013. Glycosylation disorders of membrane trafficking. Glycoconj J 30: 23–31. [PubMed: 22584409]
- Kinoshita T. 2014. Biosynthesis and deficiencies of glycosylphosphatidylinositol. Proc Jpn Acad Ser B Phys Biol Sci 90: 130–143. [PMC free article: PMC4055706] [PubMed: 24727937]
- Freeze HH, Eklund EA, Ng BG, Patterson MC. 2015. Neurological aspects of human glycosylation disorders. Annu Rev Neurosci 38: 105–125. [PMC free article: PMC4809143] [PubMed: 25840006]
- Hennet T, Cabalzar J. 2015. Congenital disorders of glycosylation: A concise chart of glycocalyx dysfunction. Trends Biochem Sci 40: 377–384. [PubMed: 25840516]
- Maeda N. 2015. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front Neurosci 9: 98. [PMC free article: PMC4369650] [PubMed: 25852466]
- Nishino I, Carrillo-Carrasco N, Argov Z. 2015. GNE myopathy: Current update and future therapy. J Neurol Neurosurg Psychiatry 86: 385–392. [PMC free article: PMC4394625] [PubMed: 25002140]
- INHERITED PATHOLOGICAL MUTATIONS OCCUR IN ALL MAJOR GLYCAN FAMILIES
- DEFECTS IN N-GLYCAN BIOSYNTHESIS
- GALACTOSEMIA
- MUSCULAR DYSTROPHIES
- DEFECTS IN O-GalNAc GLYCANS
- DEFECTS IN PROTEOGLYCAN SYNTHESIS
- DEFECTS IN GPI-ANCHORED PROTEINS
- DEFECTS IN GLYCOSPHINGOLIPID (GSL) SYNTHESIS
- PHENOTYPES, MULTIPLE ALLELES, AND GENETIC BACKGROUND
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Congenital Disorders of Glycosylation.[Essentials of Glycobiology. 2022]Review Congenital Disorders of Glycosylation.Lefeber DJ, Freeze HH, Steet R, Kinoshita T. Essentials of Glycobiology. 2022
- Review Genetic Disorders of Glycosylation.[Essentials of Glycobiology. 2009]Review Genetic Disorders of Glycosylation.Freeze HH, Schachter H. Essentials of Glycobiology. 2009
- Review Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review.[Clin Chem. 2006]Review Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Clin Chem. 2006 Apr; 52(4):574-600. Epub 2006 Feb 23.
- Review Glycans in Acquired Human Diseases.[Essentials of Glycobiology. 2009]Review Glycans in Acquired Human Diseases.Varki A, Freeze HH. Essentials of Glycobiology. 2009
- Review Myo-Glyco disease Biology: Genetic Myopathies Caused by Abnormal Glycan Synthesis and Degradation.[J Neuromuscul Dis. 2019]Review Myo-Glyco disease Biology: Genetic Myopathies Caused by Abnormal Glycan Synthesis and Degradation.Kanagawa M. J Neuromuscul Dis. 2019; 6(2):175-187.
- Genetic Disorders of Glycosylation - Essentials of GlycobiologyGenetic Disorders of Glycosylation - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

