NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.040

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsThere has been a growing recognition that free glycans are used as signals for the initiation of a wide variety of biological processes. Such signaling events are found in development, in defense responses of plants and animals, and in interactions of organisms with one another. This chapter covers the current information on this field of study.
NATURE AND SCOPE OF GLYCAN SIGNALING SYSTEMS
Glycan signaling systems are diverse. Sugars (glucose, fructose, and sucrose) may be used by sensing systems, which are typically linked with the metabolism of the sugar, and form complex webs of signaling events linked to hormones. Glycan-signaling systems also involve various glycoconjugates. The addition of O-linked N-acetylglucosamine (GlcNAc) to cytoplasmic and nuclear proteins results in changes in the cytoskeleton, gene transcription, and enzyme activation (Chapter 19). Glycosphingolipids may form lipid rafts, which act as a platform for sequestering signaling receptors, or can associate with receptor tyrosine kinases and modulate their activity (Chapter 11). Membrane proteoglycans containing sulfated glycosaminoglycans, including syndecans, glypicans, and phosphacan may act as signaling molecules by interacting with kinases or phosphatidylinositol-4,5-bisphosphate (Chapters 17 and 38). Most plasma membrane signaling receptors, including receptor tyrosine kinases and G-protein-coupled receptors, contain N-glycans and O-glycans that modulate their stability and activity (Chapters 9 and 10). Binding of galectins to these glycans (Chapter 36) or removal of sialic acids by cell-surface sialidases (Chapter 15) is also thought to modulate signaling. These and other signaling processes are described elsewhere in this book and are not discussed further here.
There is increasing evidence that low concentrations of specific free glycans are signals that initiate numerous biological processes. The first evidence for such signals was obtained during studies of defense responses in plants. Subsequently, glycan perception and signaling have been shown to be important in plant and animal development, in innate immunity, and in the initiation of the nitrogen-fixing Rhizobium–legume symbiosis. The glycans that function as signals have been identified in many of these processes. In contrast, only a few of the receptors and the mechanisms of signal transduction have been identified and characterized.
GLYCAN SIGNALS TRIGGER THE INITIATION OF THE PLANT DEFENSE RESPONSE
The plant defense response includes the recognition of the pathogen, changes in ion flux across the plasma membrane, the formation of reactive oxygen species, the activation of genes that lead to changes in the plant cell wall, the production of glycanases that fragment the pathogens cell wall, and the production of compounds (phytoalexins), which kill the pathogen (Figure 40.1). This defense response often leads to localized cell death in the plant tissue, which is visible as necrotic spots at the site of infection, and limits the pathogens spread.
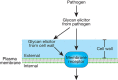
FIGURE 40.1.
Plant defense response. This response is initiated by a glycan elicitor produced by the pathogen or generated following the interaction of the pathogen with the plant cell wall. The elicitor interacts with a membrane receptor to initiate numerous cellular (more...)
Early studies showed that oligosaccharides, referred to as “oligosaccharins,” derived from cell wall glycans of the plant or the pathogen elicit numerous plant defense responses. Oligogalacturonides composed of 1-4-linked α-GalA residues are one example of oligosaccharins released from plant cell wall pectin by endopolygalacturonases (EPGs) secreted by the pathogen. Oligoglucosides composed of 1-3- and 1-6-linked β-Glc are an example of oligosaccharins released from the pathogens cell wall by plant endo-glucanases. There is evidence that the plant defense system and the innate immune system in animals have similarities, most notably where specific pattern recognition occurs (Chapter 42).
Several structurally well-defined oligosaccharides generated from glycans present in plant and fungal cell walls elicit defense responses at nanomolar concentrations (Figure 40.2A). A single active hepta-glucoside generated from the cell wall of the soybean pathogen Phytophthora megasperma was isolated from a mixture of approximately 300 inactive structural isomers of this oligosaccharide. Both the size and location of the β1-3 branches are important for elicitor activity (Figure 40.2B). Elongating the oligosaccharide at its reducing end had no discernible effect on bioactivity. Most activity was also retained by removal of a single glucose from the reducing end. However, the hexa-glucoside was the minimal structure that has appreciable elicitor activity.

FIGURE 40.2.
(A) Oligosaccharide elicitors of the plant defense response. (B) Relative biological activities of synthetic oligosaccharide isomers and derivatives of hepta-glucoside. (Adapted, with permission, from Darvill A, et al. 1992. Glycobiology 2: 181–198, (more...)
Other glycan elicitors that have been identified are linear homo-oligomers and include oligogalacturonides, chitosan, and chitin oligosaccharides (Figure 40.2A). Initial studies focused primarily on the degree of polymerization (dp) required for biological activity. Oligogalacturonides with a dp of 10–14 are biologically active, whereas dps of >7 and >4 are necessary for the oligochitosans and oligochitins, respectively. Plants produce polygalacturonase-inhibiting proteins (PGIP) that inhibit fungal endopolygalacturonases (PGs). This inhibition likely results in the accumulation in the plant apoplast of biologically active oligogalacturonides, which elicit numerous defense responses.
The low quantities and different types of glycan signal molecules that elicit a defense response suggest that these glycans are recognized by specific plasma membrane-localized receptors. A high-affinity binding protein for chitin elicitors has been identified in rice cell plasma membranes and proposed to be involved in elicitor perception and signal transduction.
Specific cell-surface or membrane-binding sites that are saturable and have binding specificities similar to those required for biological activity have been identified in plant cells and isolated plasma membranes. A 75-kDa plasma membrane protein was identified as a chitin oligosaccharide elicitor-binding protein (CEBiP) in rice cells. Reducing expression of the CEBiP gene by RNA interference (RNAi) resulted in suppression of the defense response. Although CEBiP is a membrane protein, no appreciable portion of the protein is located on the cytoplasmic side of the membrane. A plasma membrane-localized receptor-like kinase (CERK1), which is required for chitin elicitor signaling in Arabidopsis, has been identified. Thus, several proteins may be required to form an elicitor–receptor complex.
Nod FACTORS ARE SIGNALS FOR THE INITIATION OF THE NITROGEN-FIXING RHIZOBIUM–LEGUME SYMBIOSIS
The interaction between Rhizobium and the roots of legumes is an agriculturally and economically important symbiotic relationship because it enables the plant to fix atmospheric nitrogen. An early step in this process is the plants recognition of lipooligosaccharide signals (Nod factors), which are produced by the bacteria (Figure 40.3). Nod factors have a chitin oligosaccharide backbone containing from three to five GlcNAc residues. However, the types of modifications of this backbone, which include methylation, acylation (typically with a C16 or C18 fatty acid), acetylation, carbamylation, sulfation, glycosylation, and the addition of glycerol, differ among Rhizobium strains.
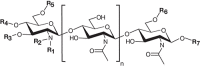
FIGURE 40.3.
Generic structure of a Nod factor. Sites on the molecule where species-specific modifications can occur are designated by R1–R7. R1 = H, methyl; R2 = C16:2, C16:3, C18:1, C18:3, C18:4, C20:3, C20:4; R3 = H, carbamate; R4 = H, carbamate; R5 = H, (more...)
Nod factors are effective at subnanomolar amounts, are host-specific, and stimulate numerous changes in the plants root hairs and roots that allow the bacteria to enter the root cortex and induce the formation of nodules where nitrogen fixation occurs. The initiation of nodule formation and Rhizobium entry into the root are host strain–specific; this specificity is determined by the structure of the Nod factor produced by a particular Rhizobium strain and the ability of a leguminous species to recognize that signal.
Genetic and biochemical approaches have been used to identify potential plant root Nod factor receptors and proteins involved in the signaling events. The putative receptors are transmembrane proteins with a serine/threonine receptor kinase motif on the cytoplasmic side of the membrane and lysozyme motif (LysM) domains that may recognize Nod factors on the exterior of the membrane. Two receptors (NFR5 and NFR1) have been reported to bind Nod factor directly at high-affinity binding sites, although only limited carbohydrate-binding studies were conducted. A lectin nucleotide phosphohydrolase (LNP) has been identified in legume roots and reported to bind Nod factors from Rhizobium symbionts of the plant species from which it was obtained. LNP is a peripheral membrane protein that may function in a receptor complex with one or more LysM-type proteins or act downstream of the Nod factor receptors.
Rhizobium exopolysaccharides (EPS) also have important roles in the development of nitrogen-fixing root nodules in the legume–rhizobium symbiosis. A root receptor-like kinase (EPR3) has been identified and shown to have a role in the recognition of the bacterial EPS. Thus, receptor-mediated recognition of Nod factors and EPS signals may be involved in plant-bacterial compatibility and bacterial access to legume roots.
OLIGOSACCHARIDE SIGNALS IN EARLY PLANT AND ANIMAL DEVELOPMENT
Several glycans have been shown to affect plant growth and plant organogenesis. Nanomolar concentrations of oligogalacturonides (Figure 40.2) with a dp between 12 and 14 induce flower formation but inhibit root formation. Oligogalacturonides also enhance cell expansion and thereby affect plant growth and development. Many of these effects may result from the ability of oligogalacturonides to alter the plants responses to the hormone auxin. Auxin-induced elongation of pea stem segments is inhibited by nanomolar concentrations of a nonasaccharide-rich fragment of xyloglucan (Figure 40.4).
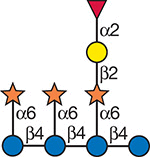
FIGURE 40.4.
A nonasaccharide from xyloglucan that shows signaling properties.
A family of plant proteins known as wall-associated kinases (WAKs) have been identified and reported to bind to cell wall pectin and thereby affect plant cell expansion. WAKs also bind pectin fragments, which may provide the plant with a mechanism to respond to pathogens and mechanical stresses. Plants may also use endogenous Nod-factor-like signals to regulate their growth and development.
Chitin oligosaccharides may have a role in animal embryogenesis. The Xenopus gene DG42 encodes a protein with chitin synthase activity and is transiently expressed in endoderm cells during the mid-late gastrulation stage (Chapter 25). Homologs of DG42 have also been identified in zebrafish and mice. The DG42 protein has sequence homology with the Rhizobium NodC chitin synthase. Transgenic expression of DG42 results in the formation of glycans that are fragmented by chitinase. DG42 is also homologous to a gene encoding a hyaluronan synthase, and studies suggest that the DG42 protein synthesizes chitin and hyaluronan, with the former perhaps as an initiation primer for a single chain (Chapter 16). Injection of chitinases or expression of NodZ (which encodes a fucosyltransferase that can modify chitin) in animal cells has profound effects on development. Thus, chitin oligosaccharides are examples of free glycans that act as intracellular signaling molecules.
The presence of abnormal glycans or the accumulation of glycans in the wrong place may negatively impact signaling pathways in animal cells. Three prime repair exonuclease 1 (TREX1) is an ER-associated negative regulator of innate immunity. Mutations that affect TREX1 function are associated with numerous autoimmune and autoinflammatory diseases (Chapter 45). The ER-localized carboxyl terminus of TREX1 has been proposed to interact with, and stabilize, the catalytic activity of the ER oligosaccharyltransferase (OST) complex and thereby suppress immune activation. The OST complex becomes dysfunctional in the presence of carboxy-terminal truncated TREX1. This leads to the release of free glycans from dolichol-linked oligosaccharides, which has been hypothesized to lead to the activation of genes with immune system-related functions and the production of autoantibodies. Thus, TREX1 may safeguard the cell against free glycan buildup in the ER and thereby prevent glycan and glycosylation defects that can lead to immune disorders.
N-linked glycans have a role in the correct folding of glycoproteins in the ER. Misfolded glycoproteins are targeted for degradation by an ER-associated degradation (ERAD) process in which they are retrotranslocated into the cytosol (Chapter 39). The glycans are then released from the glycoprotein by the N-glycanase NGLY1. The protein is degraded by the proteasome, whereas the released glycans are likely partially de-mannosylated in the cytosol and then transported to the lysosomes by an as yet unidentified oligosaccharide transporter. It is not known if these free glycans have any signaling functions in the cytosolic/nuclear compartment. In the lysosome, glycosidases hydrolyze the glycans into monomeric sugars that can then be reused by the cell. Mutations that disrupt NGLY1 function may cause severe health problems in humans. Studies with Ngly1 mutant mice cells suggest that in the absence of NGLY1, ERAD becomes dysfunctional because a cytosolic endo-β-N-acetylglucosaminidase generates proteins that contain only a single Asn-linked GlcNAc instead of completely deglycosylated proteins. The accumulation of these GlcNAc-proteins may result in the formation of aggregates that are harmful to the cell or they may interfere with intracellular signaling processes.
GLYCOSAMINOGLYCANS AND CELL SIGNALING
Glycosaminoglycans (GAGs) are signaling glycans because they interact with receptor tyrosine kinases and/or their ligands and facilitate changes in cell behavior (Chapters 15, 16, and 35). Hyaluronan oligosaccharides bind to specific membrane proteins, including CD44. In some cells, this binding leads to clustering of CD44, which activates kinases such as c-Src and focal adhesion kinase (FAK). Phosphorylation alters the interaction of the cytoplasmic tail of CD44 with regulatory and adaptor molecules that modulate cytoskeletal assembly/disassembly and cell survival and proliferation (Figure 40.5). Signaling by hyaluronan oligosaccharides depends on the dp of the glycans. Low-molecular-weight glycans are more active in triggering danger responses via binding to Toll-like receptor's (TLR's) responses.
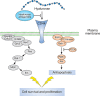
FIGURE 40.5.
Schematic diagram of signaling pathways activated by binding of hyaluronan to CD44. In tumor cells, activation results in proliferation and invasion. In embryonic stem cells, it can result in epithelial to mesenchymal transition.
In contrast to hyaluronan-dependent signal transduction, signaling via sulfated GAGs (heparan sulfate [HS] and chondroitin/dermatan sulfate) occurs by an indirect mechanism. Indeed, few membrane receptors have been described in which sulfated GAGs binding causes a specific downstream response, such as phosphorylation of the receptor or activation of a kinase. Instead, sulfated GAGs bind to many ligand/receptor pairs, thereby lowering the effective concentration of ligand required to engage the receptor or increasing the duration of the response. An example of this is the ability of exogenous heparin or endogenous HS proteoglycans to activate fibroblast growth factor (FGF) receptors by FGF (Chapter 38). No substantial conformational change in the ligand occurs on binding to sulfated GAG, consistent with the idea that the glycan primarily aids in the juxtaposition of components of the signal transduction pathway. Free HS oligosaccharides can be released by the action of secreted heparanase. These glycans may facilitate signaling through the mechanism described above or by the release of growth factors from stored depots in the extracellular matrix. Sulfated GAGs also facilitate the formation of morphogen gradients in tissues during early development. Because the gradient determines cell specification during development, the glycan indirectly affects signaling responses in receptive cells. These examples do not exclude the possibility that sulfated glycosaminoglycans may act as ligands and induce signaling directly (e.g., by ligating receptors).
GLYCANS AS MODULATORS OF INNATE IMMUNITY
In addition to mucins (Chapter 10), the innate immune system developed early in eukaryote evolution is a first line of defense against infection by microorganisms. A key feature of this system is its ability to distinguish self from infectious nonself. In more advanced eukaryotes, this is accomplished by receptors that recognize conserved molecular patterns specific to the pathogens. Many of these pathogen-associated molecular patterns (PAMPs) are glycans located on the surfaces of the microorganism. The glycans include the lipopolysaccharides (LPS) of Gram-negative bacteria, the peptidoglycans and techoic acids of Gram-positive bacteria (Chapters 21 and 22) and the mannans of fungi. The cognate receptors on the host cells are referred to as pattern-recognition receptors (PRRs). Numerous PRRs are present in mammals that recognize diverse PAMPs and induce host-defense pathways, including TLRs and mannan-binding lectin (Chapter 42). Binding of PAMPs to TLRs activates various signaling pathways that induce inflammation and antimicrobial effector responses. Some TLRs are present on antigen-presenting cells and help to activate the adaptive immune response. TLRs also respond to tissue injury via binding of released HA fragments as damage-associated molecular patterns (DAMPs).
One of the best-studied models of innate immunity involves the LPS of Gram-negative bacteria, which has a role in causing septic shock (see Chapter 42). Lipid A (endotoxin) is the glucosamine-based phospholipid anchor of LPS responsible for activating the innate immune system. Lipid A is an excellent PAMP as its structure is highly conserved among Gram-negative bacteria. Picomolar amounts of Lipid A are detected by TLR-4 (Figure 40.5). The LPS is first opsonized and complexed with another host cell-surface protein, CD14. The binding of LPS leads to recruitment of the adaptor proteins MyD88 and IRAK. This complex initiates a signaling cascade of phosphorylation events that ultimately lead to the transcription of proinflammatory genes.
In contrast to PAMPs and DAMPs, inhibitory Siglec receptors (Chapter 35) on innate immune cells recognize endogenous sialylated glyconjugates as self-associated molecular patterns (SAMPs), and dampen unwanted reactions against the host. Details of the sialoglycan specificity involved require further investigation, but pathogens take advantage of the system via molecular mimicry (Chapters 7 and 42).
The examples given in this chapter indicate the diversity of glycan structures that can function as signaling molecules. It is likely that further examples of glycan signals in both plant and animal cells, as well as in their interactions with microbes, will become apparent in the future.
ACKNOWLEDGMENTS
The authors appreciate the helpful comments and suggestions from Ryan Porell, Chengcheng Huang, Nickita Mehta, and Daniela Janevska Carroll.
FURTHER READING
- Darvill A, Augur C, Bergmann C, Carlson RW, Cheong J-J, Eberhard S, Hahn M, Ló V-M, Marfa V, Meyer B. 1992. Oligosaccharins—Oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology 2: 181–198. [PubMed: 1498416]
- Cullimore JV, Ranjeva R, Bono J-J. 2001. Perception of lipo-chitooligosaccharidic Nod factors in legumes. Trends Plant Sci 6: 24–30. [PubMed: 11164374]
- Ronald PC, Beutler B. 2010. Plant and animal sensors of conserved microbial signatures. Science 330: 1061–1064. [PubMed: 21097929]
- Smeekens S, Ma J, Hanson J, Rolland F. 2010. Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13: 273–278. [PubMed: 20056477]
- Broghammer A, Krusell L, Blaise M, Sauer J, Sullivan J, Maolanon N, Vinther M, Lorentzen A, Madsen EB, Jensen KJ. 2012. Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. Proc Nat Acad Sci USA 109: 13859–13864. [PMC free article: PMC3427107] [PubMed: 22859506]
- Ferrari S, Savatin D, Sicilia F, Gramegna G, Cervone F, Lorenzo GD. 2013. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci 4: 10.3389. [PMC free article: PMC3595604] [PubMed: 23493833]
- Liang Y, Cao Y, Tanaka K, Thibivilliers S, Wan J, Choi J, ho Kang C, Qiu J, Stacey G. 2013. Nonlegumes respond to rhizobial Nod factors by suppressing the innate immune response. Science 341: 1384–1387. [PubMed: 24009356]
- Roberts NJ, Morieri G, Kalsi G, Rose A, Stiller J, Edwards A, Xie F, Gresshoff P, Oldroyd GE, Downie JA. 2013. Rhizobial and mycorrhizal symbioses in Lotus japonicus require lectin nucleotide phosphohydrolase, which acts upstream of calcium signaling. Plant Physiol 161: 556–567. [PMC free article: PMC3532285] [PubMed: 23136382]
- Kawaharada Y, Kelly S, Nielsen MW, Hjuler CT, Gysel K, Muszyński A, Carlson R, Thygesen MB, Sandal N, Asmussen M. 2015. Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523: 308–312. [PubMed: 26153863]
- Kohorn BD. 2017. Cell wall–associated kinases and pectin perception. J Exp Bot 67: 489–494. [PubMed: 26507892]
- NATURE AND SCOPE OF GLYCAN SIGNALING SYSTEMS
- GLYCAN SIGNALS TRIGGER THE INITIATION OF THE PLANT DEFENSE RESPONSE
- Nod FACTORS ARE SIGNALS FOR THE INITIATION OF THE NITROGEN-FIXING RHIZOBIUM–LEGUME SYMBIOSIS
- OLIGOSACCHARIDE SIGNALS IN EARLY PLANT AND ANIMAL DEVELOPMENT
- GLYCOSAMINOGLYCANS AND CELL SIGNALING
- GLYCANS AS MODULATORS OF INNATE IMMUNITY
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Free Glycans as Signaling Molecules.[Essentials of Glycobiology. 2009]Review Free Glycans as Signaling Molecules.Etzler ME, Esko JD. Essentials of Glycobiology. 2009
- Review Free Glycans as Bioactive Molecules.[Essentials of Glycobiology. 2022]Review Free Glycans as Bioactive Molecules.Molina A, O'Neill MA, Darvill AG, Etzler ME, Mohnen D, Hahn MG, Esko JD. Essentials of Glycobiology. 2022
- Review In Situ Cellular Glycan Analysis.[Acc Chem Res. 2018]Review In Situ Cellular Glycan Analysis.Chen Y, Ding L, Ju H. Acc Chem Res. 2018 Apr 17; 51(4):890-899. Epub 2018 Mar 29.
- Review Engineering Aspects of Olfaction.[Neuromorphic Olfaction. 2013]Review Engineering Aspects of Olfaction.Persaud KC. Neuromorphic Olfaction. 2013
- Review Viridiplantae.[Essentials of Glycobiology. 2009]Review Viridiplantae.Etzler ME, Mohnen D. Essentials of Glycobiology. 2009
- Free Glycans as Signaling Molecules - Essentials of GlycobiologyFree Glycans as Signaling Molecules - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

