NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.004

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsThis chapter provides an overview of glycosylation from the perspective of a single cell, taking into account the patterns of expression, topology, and other features of the biosynthetic and degradative enzymes that are common to most cell types. The focus is mostly on the organization of glycosylation in eukaryotic cells. Chapters 21 and 22 further address prokaryotic glycosylation mechanisms.
GLYCOSYLATION IS UNIVERSAL IN LIVING ORGANISMS
After more than three billion years of evolution, every free-living cell and every cell type within multicellular organisms remains covered with a dense and complex layer of glycans (Chapter 20). Even enveloped viruses that bud from infected cells carry with them the glycosylation patterns of the host. Additionally, most secreted molecules are glycosylated and extracellular matrices of multicellular organisms are rich in glycans and glycoconjugates. Matrices secreted by unicellular organisms when they congregate (e.g., bacterial biofilms [Chapter 21]) also contain glycans. Thus, evolution has repeatedly and consistently selected glycans as being the most diverse and flexible molecules to position at the interface between cells and the extracellular milieu. Possible reasons include their relative hydrophilicity, flexibility, and mobility in aqueous environments and their extreme diversity, allowing facile short-term and long-term adaptations to changing environments and pathogen regimes.
In bacteria, Archaea, and fungi, glycans serve critical structural roles in the cell wall and in resisting large differences in osmolarity between cytoplasm and environment. In eukaryotes, both secretory proteins and membrane proteins typically pass through an endoplasmic reticulum (ER)–Golgi pathway, the cellular system in which many major glycosylation reactions occur (see below). Most proteins in the blood plasma of animals (with the exception of albumin) are heavily glycosylated, and the glycosylation of these and other secreted proteins may provide solubility, hydrophilicity, and negative charge, thus reducing unwanted intermolecular interactions and protecting against proteolysis. Cell-surface membrane proteins like receptors, adhesion molecules, and channels are typically glycosylated, and this modification can promote their proper folding, ensure their stability, and impact function.
TOPOLOGICAL ISSUES IN GLYCAN BIOSYNTHESIS
The ER–Golgi Pathway of Eukaryotes
The classic work of George Palade indicated that most cell-surface and secreted proteins in eukaryotic cells are cotranslationally translocated into the ER where they are folded, modified, and subjected to quality control mechanisms. They then make their way via an intermediate compartment (IC) through multiple stacks of the Golgi apparatus, finally being distributed to various destinations from the trans-Golgi network (TGN). Secretory pathway proteins can be N-glycosylated, O-glycosylated, and modified with glycosylphosphatidylinositol (GPI) anchors, and some called proteoglycans are modified with glycosaminoglycan chains. The enzymes involved in each of these modification pathways are distinct. N-linked glycans and GPI anchors are preassembled before being transferred to proteins and then further modified in the ER–Golgi pathway. The stepwise assembly of O-linked glycans and glycosaminoglycans, as well as the glycosylation of lipids, involve reactions in both the ER and Golgi (Chapters 9–13 and 17). Figure 4.1 superficially depicts some steps in the synthesis of the major glycan classes in the ER–Golgi pathway of animal cells.
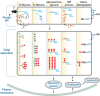
FIGURE 4.1.
Initiation and maturation of the major types of eukaryotic glycoconjugates in relation to subcellular trafficking in the ER–Golgi–plasma membrane pathway. This illustration outlines the different mechanisms and topology for initiation, (more...)
In the ER–Golgi pathway, some glycan chains are made on the cytoplasmic face of intracellular membranes and flipped across to the other side, but most are added to the growing chain on the inside of the ER or the Golgi (Figure 4.1). Regardless, the portion of a molecule that faces the inside of the lumen of the ER or Golgi will ultimately face the outside of the cell or the inside of a secretory granule or lysosome. To date, there are no well-documented exceptions to this topological rule. Of course, these topological considerations are reversed for nuclear and cytoplasmic glycosylation (see below), because the active sites of the relevant glycosyltransferases for these reactions face the cytoplasm. Not surprisingly then, the types of glycans found on the two sides of the cell membrane so far appear to be distinct from each other.
Donors for Glycosylation Reactions
Regardless of location, most glycosylation reactions use activated forms of monosaccharides (often nucleotide sugars and in some cases lipid-phosphate-linked sugars, such as dolichol phosphate mannose) as donors for glycosyltransferases (see Chapter 5 for a listing of some enzymes, their donors and corresponding transporters, and details about their biochemistry). A variety of glycan modifications also occur in nature (Chapter 6). Of these, the most common are generated by sulfotransferases, acetyltransferases, and methyltransferases, which use activated forms of sulfate (3′phosphoadenyl-5′-phosphosulfate; PAPS), acetate (acetyl-CoA), and methyl groups (S-adenosylmethionine; AdoMet), respectively. Almost all donors for glycosylation reactions and glycan modifications are synthesized within the cytoplasmic compartment, from precursors of endogenous origin. In eukaryotes, most of these donors are actively transported across a membrane bilayer by specific multipass transporters, becoming available for reactions within the lumen of the ER–Golgi pathway.
Nuclear and Cytosolic Glycosylation in Eukaryotes
For many years, the nucleus and cytosol (which are topologically semicontinuous via nuclear pores) were assumed to be devoid of glycosylation capacity. It is now established that distinct glycoconjugates are synthesized and reside within these compartments. Indeed, one of them (O-linked GlcNAc; Chapter 19) may well be quantitatively the most common type of glycoconjugate in many cells. Both the O-GlcNAc transferase (OGT) responsible for synthesizing O-linked GlcNAc on nuclear and cytosolic proteins and the O-GlcNAcase (OGA) that removes this monosaccharide are soluble proteins in these compartments. In addition, select cytosolic proteins can be modified with O-Glc, O-Fuc, or O-Man (Chapters 18 and 19).
Glycosylation Reactions at the Plasma Membrane
Because prokaryotic cells do not have an ER–Golgi pathway, they typically generate their cell-surface glycans at the interface of the cytoplasmic membrane and the cytoplasm or in the periplasm (see below). Other glycoconjugates like hyaluronan in vertebrate cells, chitin in invertebrate cells, and cellulose in plant cells are synthesized on the cytoplasmic face of the plasma membrane and simultaneously extruded across the membrane to the outside (Chapters 16, 24, and 26). The enzymes involved in the synthesis of these glycoconjugates appear to mediate this extrusion. Typical eukaryotic Golgi glycosylation enzymes have also been found at the cell surface or as soluble forms in the extracellular space, as described below. Whether these glycosyltransferases would routinely have adequate supplies of nucleotide sugar donors to modify cell-surface glycans is unclear, but at least one example has been documented (see below). On the other hand, there are examples of remodeling of cell-surface glycans in animal cells—for example, the Sulf enzymes that modify heparan sulfate glycosaminoglycan (Chapter 17) and endogenous sialidases that remove cell-surface sialic acids (Chapter 15). Some protozoan parasites, such as trypanosomes, transfer sialic acids from host glycoconjugates to their cell-surface glycans using trans-sialidases.
Glycosylation Pathways in Eubacteria and Archaea
Prokaryotic assembly pathways of polysaccharides and oligosaccharides are very similar to pathways found in the ER and the plasma membrane of eukaryotes. They are assembled in the cytoplasm and then translocated across the plasma membrane. For many biosynthetic pathways, such as O-antigen biosynthesis in Gram-negative bacteria, N-linked protein glycosylation in Archaea, and some Gram-negative bacteria or S-layer biosynthesis in Gram-positive bacteria, oligosaccharides are assembled on lipid carriers at the inner site of the plasma membrane and then flipped to the periplasmic site (Chapters 21 and 22). Synthesis of oligosaccharides can continue in the periplasm, but for these reactions, isoprenoid-linked monosaccharides serve as activated substrates as is the case in cell wall biogenesis of actinobacteria (mycobacteria) (Chapter 21).
GLYCOSYLATION IN THE EUKARYOTIC SECRETORY PATHWAY
Much effort has gone into understanding the mechanisms of glycosylation and glycan modification within the ER and the Golgi apparatus, and it is clear that a variety of interacting and competing factors determine the final outcome of the reactions. The glycosyltransferases and processing glycosidases are well studied (Chapter 6), and their location has helped to define various functional compartments of the ER–Golgi pathway.
Many Golgi Enzymes Share a Similar Topology
Despite lack of sequence homology among different glycosyltransferase families, most Golgi enzymes share some features. Early studies of vertebrate glycosyltransferases found some of these activities in soluble form in secretions and body fluids; others were identified as membrane-bound activities within cells, and some showed both properties. Subsequent molecular cloning defined the sequences of Golgi glycosyltransferases, revealing that they share a common topology and domain structure that can account for these observations.
The majority of Golgi glycosylation enzymes are type II membrane proteins consisting of three parts: an amino-terminal cytoplasmic tail, followed by a transmembrane (TM) region that also acts as an uncleavable signal sequence, and a large carboxy-terminal region containing a membrane proximal, proteolytically sensitive stem region as well as a large catalytic domain. The type II topology of these Golgi enzymes places their catalytic sequences in the Golgi lumen, where they participate in the synthesis of the glycan chains on proteins and lipids during their transit through the secretory pathway (Figure 4.2).
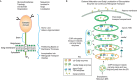
FIGURE 4.2.
Topology and localization of Golgi glycosylation enzymes. Golgi glycosyltransferases and glycosidases are type II membrane proteins with their catalytic sequences facing the lumen of the Golgi. According to the cisternal maturation model of intra-Golgi (more...)
Many Golgi enzymes are secreted by cells, sometimes in large quantities, and can be found in cell culture supernatants and various body fluids. These soluble, secreted enzymes are derived from their membrane-associated forms by one or more proteolytic cleavage events that occur within the enzyme's stem region (Figure 4.2). These cleavage events are catalyzed by proteases in the trans regions of the Golgi and in post-Golgi compartments. The production of soluble enzymes from cell types such as hepatocytes and endothelium can be dramatically up-regulated under inflammatory conditions. Because circulating and cell-surface localized glycosyltransferases are not expected to have access to adequate concentrations of donor nucleotide sugars (primarily located inside cells), it was thought that they should be functionally incapable of performing transfer reactions in extracellular spaces. However, recent evidence suggests that the release of nucleotide sugar donors by activated platelets may allow soluble, secreted sialyltransferase ST6Gal-I to modify glycans on cell surfaces beyond the original source of the enzyme.
Not all glycosylation enzymes in the secretory pathway are type II membrane proteins. For example, the UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase (GlcNAc-phosphotransferase) is a multisubunit complex and the GlcNAc-1-phosphodiester α-N-acetylglucosaminidase is a type I membrane protein with its amino terminus in the lumen of the Golgi. These enzymes are involved in the synthesis of the Man-6-P targeting signal of newly synthesized lysosomal hydrolases (see Chapter 33). Some ER glycosylation enzymes are synthesized as soluble proteins. These include the UDP-glucose glycoprotein glucosyltransferase (UGGT) involved in ER quality control (Chapter 39), and enzymes involved in epidermal growth factor (EGF) repeat or thrombospondin repeat (TSR) glycosylation such as the two protein O-fucosyltransferases, POFUT 1 (EGF) and 2 (TSR), protein O-glucosyltransferase 1 (POGLUT1; EGF), and β1-3-glucosyltransferase (B3GLCT; TSR) (Chapter 13). In addition, one of the sulfotransferases involved in heparan sulfate synthesis, GlcNAc 3-O-sulfotransferase 1, is a soluble enzyme in the Golgi.
Localization of Glycosylation Enzymes in Golgi Compartments
All forms of glycosylation in the secretory pathway are highly ordered and sequential processes, typically involving glycosyltransferase reactions. These enzymes, their glycan substrates (attached to protein or lipid), and the appropriate nucleotide sugar donor, must be located in the same compartment. Biochemical and ultrastructural studies indicate that glycosyltransferases segregate into distinct overlapping compartments within the secretory pathway. Generally speaking, enzymes acting early in the biosynthetic pathway localize to cis and medial Golgi compartments, whereas those acting later in the pathway tend to localize in the trans-Golgi cisternae and the TGN. These observations prompted extensive exploration of mechanisms whereby glycosyltransferases and processing glycosidases achieve this compartmental segregation. Early studies were directed at identifying enzyme sequences required for their retention in the Golgi cisternae per the vesicular transport model of protein trafficking, whereas more recent studies have included the framework of the cisternal maturation model (see below).
The view of how proteins traverse the Golgi stack and how Golgi enzymes “retain” their relative positions in the Golgi cisternae has evolved substantially in the recent past and includes the two primary models mentioned above. These models are not mutually exclusive and may function together in cells. The vesicular transport model posits that the Golgi is a stable compartment, and that cargo proteins are transported in coated vesicles from the ER to an intermediate compartment and between each Golgi cisterna in a vectorial fashion, during which time these proteins are modified by Golgi glycosylation enzymes retained in each cisterna. More recent data support a cisternal maturation model that can explain the intra-Golgi transport of larger cargo molecules, which cannot fit into small transport vesicles (Figure 4.2). In this model, a new Golgi cisterna is formed on the cis face of the stack by the transport of cargo molecules in COPII-coated vesicles from the ER to an intermediate compartment, and the retrograde transport of cis-Golgi enzymes in COPI-coated vesicles from an “older” cis cisterna into the newly formed compartment that then becomes the cis cisterna. The cisterna and its cargo mature as later Golgi glycosylation enzymes are sequentially transported into the “younger” cisternae. The cisternae progress and mature until they effectively dissolve at the TGN stage as membrane and cargo are transported to the plasma membrane for residence or constitutive secretion, to secretory granules for regulated secretion, or to the endosome/lysosome system. The cisternal maturation model is thus distinct from the vesicular transport model in that Golgi enzymes are not retained in stable compartments, but continuously transported in a retrograde fashion to “mature” younger cisternae and their cargo.
The role of the cisternal maturation in Golgi enzyme localization is supported by observations that mutations in conserved oligomeric Golgi (COG) complex proteins involved in retrograde vesicular transport impact Golgi enzyme distribution and overall protein glycosylation. The COG complex is a hetero-oligomer of eight subunits that is believed to function as a cytoplasmic tethering complex that links incoming vesicles to their target compartments before vesicle fusion. This complex is thought to cooperate with COPI subunits in retrograde vesicular transport related to intra-Golgi and Golgi-to-ER trafficking. Mutations in COG subunits lead to the instability and/or mislocalization of several Golgi glycosyltransferases across the stack leading to corresponding glycosylation defects. The COG complex does not directly interact with Golgi enzymes, but it is critical for retrograde vesicular transport in the Golgi system and in this way impacts overall Golgi structure, and thus ensures efficient glycosylation. Notably, several congenital disorders of glycosylation type II (CDG-II) are the result of mutations in COG subunits (Chapter 45).
Studies using mutant and chimeric Golgi enzymes showed that different enzymes have different requirements for their localization. Early work pointed to the TM regions of enzymes such as the GlcNAcT-1 (medial Golgi), GalT-1 (trans-Golgi), and ST6Gal-I (trans-Golgi and TGN), but later studies revealed that for many enzymes, multiple signals and mechanisms are responsible. The role of both homo- and hetero-oligomerization in the localization of some Golgi enzymes has been established. In addition, substantial evidence supports the role of glycosyltransferase cytoplasmic tails in enzyme retrograde transport and Golgi localization (see below).
The length and hydrophobicity of a membrane protein's TM region determine its ability to partition into membrane microdomains and are now appreciated to be involved in membrane protein trafficking and localization throughout the cell. Both the concentration of cholesterol and the width of the membrane increase throughout the secretory pathway with the widest, most cholesterol-rich membranes found at the cell surface. Experiments using cholesterol-containing model membranes showed that shorter TM peptides partition into thinner membranes, whereas longer TM peptides partition into thicker membranes. It is possible that cholesterol's tendency to “straighten” the lipid acyl chains may make it more energetically difficult to partition TM peptides into membranes with mismatched thicknesses. In support of the idea that membrane thickness may contribute to membrane protein localization in the secretory pathway, it has been noted that ER proteins have shorter TM regions than plasma membrane proteins, and that the TM regions of Golgi enzymes are intermediate between those of ER and plasma membrane proteins. However, among the Golgi enzymes there is not a strict increase in TM length as one moves from the cis to the trans face of the organelle. One possibility is that the relative impact of TM region length on cisternal localization depends on what other sequences and mechanisms are involved in the localization of a specific enzyme. Nevertheless, at minimum, the shorter TM regions of Golgi enzymes may prevent these proteins from leaving the Golgi system by reducing their ability to partition to the thicker, cholesterol-rich membranes of carriers destined for post-Golgi compartments like the plasma membrane (Figure 4.2).
Another mechanism contributing to Golgi localization of enzymes is their ability to form oligomeric complexes (Figure 4.2). Nearly all enzymes in the N-linked and O-linked glycosylation pathways form homodimers, and many also form heteromeric complexes. In some cases, heteromeric complex formation is pH dependent. Heteromeric complex formation is observed between enzymes that catalyze sequential reactions in the same pathway and that are localized in the same cisternae. For example, in the N-glycosylation pathway, complexes are formed between two N-acetylglucosaminyltransferases (GlcNAcT-I and GlcNAcT-II) in the medial Golgi, and between GalT-I and ST6Gal-I in the trans-Golgi. Notably, enzymes not in the same pathway (e.g., O-glycosylation and N-glycosylation enzymes), and enzymes in the same pathway, but which catalyze competing or nonsequential events, do not form heteromeric complexes even if they are localized in the same cisterna. Complexes between sequential enzymes in a pathway could increase the efficiency of glycosylation by promoting substrate channeling, wherein one enzyme hands the newly modified substrate off to the next enzyme in the pathway.
Taken together, evidence indicates that glycosylation enzymes use multiple mechanisms to maintain their Golgi localization. The number of signals and mechanisms used by an enzyme could determine how stable its Golgi localization is, whether it is able to move to a later compartment, and whether it is cleaved and secreted into the extracellular space.
GLYCOSYLATION IN UNEXPECTED SUBCELLULAR LOCATIONS
There are scattered reports of glycosylation in unexpected locations, for example, gangliosides in mitochondria and GAGs and N-glycans in the nucleus. Many of these claims are based on incomplete evidence (Chapter 18). One possibility is that there are indeed glycans in these unexpected locations, but that their true structures are novel. Conversely, although structural evidence might be strong, there may be inadequate evidence to be certain about the topology of the claimed structures. Regardless, past experience tells us that the cell biology of glycosylation can hold many surprises, and dogmatic positions about such controversial issues are not warranted.
TURNOVER AND RECYCLING OF GLYCANS
Like all components of living cells, glycans turn over constantly. Some glycoconjugates, such as transmembrane heparan sulfate proteoglycans, turn over by shedding from the cell surface through limited proteolysis. Most glycoconjugate turnover occurs by endocytosis and subsequent degradation in lysosomes (Chapter 44). Endoglycosidases can initially cleave glycans internally, producing substrates for exoglycosidases in the lysosome. Once broken down, individual monosaccharides are then typically exported from the lysosome into the cytoplasm, so that they can be reused (Figure 1.8, Chapter 1). In contrast to the relatively slow turnover of glycans derived from the ER–Golgi pathway, glycans of the nucleus and cytoplasm may be more dynamic and rapidly turned over (Chapters 18 and 19). Glycans in bacterial cells (especially those in the cell wall) also turn over during cell division when the cell wall undergoes cleavage and remodeling.
ACKNOWLEDGMENTS
The authors acknowledge contributions to previous versions of this chapter by Jeffrey D. Esko and appreciate helpful comments and suggestions from Chrissa Dwyer, Simone Kurz, and Daniel Sandoval.
FURTHER READING
- Paulson JC, Colley KJ. 1989. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem 264: 17615–17618. [PubMed: 2681181]
- Calo D, Kaminski L, Eichler J. 2010. Protein glycosylation in Archaea: Sweet and extreme. Glycobiology 20: 1065–1076. [PubMed: 20371512]
- Dell A, Galadari A, Sastre F, Hitchen P. 2010. Similarities and differences in the glycosylation mechanisms in prokaryotes and eukaryotes. Int J Microbiol 2010: 148178. doi: 10.1155/2010/148178. [PMC free article: PMC3068309] [PubMed: 21490701] [CrossRef]
- Nothaft H, Szymanski CM. 2010. Protein glycosylation in bacteria: Sweeter than ever. Nat Rev Microbiol 8: 765–778. [PubMed: 20948550]
- Banfield DK. 2011. Mechanisms of protein retention in the Golgi. Cold Spring Harb Perspect Biol 3: a005264. [PMC free article: PMC3140682] [PubMed: 21525512]
- Glick BS, Luini A. 2011. Models for Golgi traffic: A critical assessment. Cold Spring Harb Perspect Biol 3: a005215. [PMC free article: PMC3220355] [PubMed: 21875986]
- Reynders E, Foulquier F, Annaert W, Matthijs G. 2011. How Golgi glycosylation meets and needs trafficking: The case of the COG complex. Glycobiology 21: 853–863. [PubMed: 21112967]
- Varki A. 2011. Evolutionary forces shaping the Golgi glycosylation machinery: Why cell surface glycans are universal to living cells. Cold Spring Harb Perspect Biol 3: a005462. [PMC free article: PMC3098673] [PubMed: 21525513]
- Moremen KW, Tiemeyer M, Nairn AV. 2012. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat Rev Mol Cell Biol 13: 448–462. [PMC free article: PMC3934011] [PubMed: 22722607]
- Review Cellular Organization of Glycosylation.[Essentials of Glycobiology. 2022]Review Cellular Organization of Glycosylation.Colley KJ, Varki A, Haltiwanger RS, Kinoshita T. Essentials of Glycobiology. 2022
- Review Cellular Organization of Glycosylation.[Essentials of Glycobiology. 2009]Review Cellular Organization of Glycosylation.Varki A, Esko JD, Colley KJ. Essentials of Glycobiology. 2009
- Review Oligosaccharyltransferase structures provide novel insight into the mechanism of asparagine-linked glycosylation in prokaryotic and eukaryotic cells.[Glycobiology. 2019]Review Oligosaccharyltransferase structures provide novel insight into the mechanism of asparagine-linked glycosylation in prokaryotic and eukaryotic cells.Shrimal S, Gilmore R. Glycobiology. 2019 Apr 1; 29(4):288-297.
- Review Introduction to glycosylation and mass spectrometry.[Methods Mol Biol. 2013]Review Introduction to glycosylation and mass spectrometry.Patrie SM, Roth MJ, Kohler JJ. Methods Mol Biol. 2013; 951:1-17.
- Review Complex glycosylation of Skp1 in Dictyostelium: implications for the modification of other eukaryotic cytoplasmic and nuclear proteins.[Glycobiology. 2002]Review Complex glycosylation of Skp1 in Dictyostelium: implications for the modification of other eukaryotic cytoplasmic and nuclear proteins.West CM, van der Wel H, Gaucher EA. Glycobiology. 2002 Feb; 12(2):17R-27R.
- Cellular Organization of Glycosylation - Essentials of GlycobiologyCellular Organization of Glycosylation - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

