NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.022

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsThis chapter describes current knowledge of archaeal glycobiology. Like in bacteria, glycans are essential parts of the cell wall and the extracellular matrix of the archaeal cell. Recent discoveries shed light on a tremendous structural and functional diversity of carbohydrates in this domain of life. In particular, the pathways of N-linked protein glycosylation, homologous to the eukaryotic N-glycosylation machinery, generate a wide variety of N-linked glycans in different archaeal species.
BACKGROUND
Based on Carl Woese's pioneering use of 16S ribosomal (r)RNA analysis, the Archaea were first recognized as a separate domain of life, distinct from either Bacteria or Eukarya. As the first Archaea identified were isolated from some of the most physically challenging environments on the planet, such as those defined by extremes in salinity, pH, or temperature, it was assumed that all Archaea were extremophiles. However, it has since become clear that Archaea account for a major portion of the microbial population in a variety of “normal” biological niches. In addition to expanding our views on where life can exist, the study of Archaea has also led to many important discoveries.
From the earliest studies on Archaea, glycobiology-based efforts proved important. Most importantly, they helped to break dogma, such as the long-held belief that protein glycosylation was a posttranslational modification restricted to eukaryotes. The discovery that Archaea did not contain peptidoglycan in their cell wall was one of the main arguments used to distinguish this group of microbes from Bacteria. Indeed, at the time, cell wall composition was considered to be “the only useful phylogenetic criterion, other than direct molecular phylogenetic measurement” to distinguish between the two prokaryotic domains. However, some Archaea were shown to include a distinct polymer, termed pseudomurein (or pseudopeptidoglycan), in their cell wall, whereas other archaeal species were found to assemble cell walls based on different sugar-based polymers. Today, as more and more archaeal species are cultivated, it is becoming clear that Archaea present numerous variations in the composition of the cell surface. For instance, although many species seem to mainly rely on a cell envelope in which the cytoplasmic membrane is enclosed by a two-dimensional crystalline proteinaceous layer called the surface (S)-layer, strains surrounded by two membranes have been identified. Figure 22.1 summarizes current knowledge about archaeal cell surfaces.
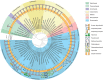
FIGURE 22.1.
Diversity of cell wall structure in the domain of Archaea. The phylogenetic tree, based on 16S RNA sequences, and the makeup of the cell wall of the corresponding species are shown. The different cell wall components are shown on the right. In the inner (more...)
Since Neuberger's discovery of protein glycosylation in the late 1930s (Chapter 1) and his later description of the N-acetylglucosamine (GlcNAc)-β-asparagine link connecting the glycan to the modified protein, it became generally accepted that protein glycosylation was a process limited to eukaryotes. This belief persevered until 1976, when Mescher and Strominger showed that the Halobacterium salinarum S-layer glycoprotein, the sole component of the S-layer in this organism, was subject to both N- and O-glycosylation, thus offering the first example of a noneukaryotic glycoprotein. Available evidence argues for protein glycosylation being an almost universal trait of the Archaea. Accordingly, lessons learned from the study of archaeal glycobiology reveal evolutionary insights into mechanisms present in all domains of life.
The Archaeal Cell Wall
Similar to the bacterial domain, there is no cell wall structure unique to all Archaea. However, like in Bacteria, there are building blocks that are found in different archaeal clades. Some of these cell wall components are very similar in structure to bacterial counterparts, but they seem to be the product of convergent evolution, whereas other cell wall–generating processes seem to be homologous to pathways in the eukaryotic extracellular matrix assembly. The biophysical properties of these cell wall building blocks provide the basis for the ability of many archaeal species to thrive in extreme habitats.
Pseudomurein (Pseudopeptidoglycan)
Although pseudomurein was identified as a component of the cell wall early in the study of Archaea, it subsequently became clear that in terms of distribution, use of this structure was relatively limited. Pseudomurein shares structural similarities with bacterial murein yet presents significant differences (Figure 22.2). Pseudomurein usually consist of N-acetyl-L-talosaminuronic acid linked via a β1-3 linkage to N-acetyl-D-glucosamine, unlike murein, which consists of alternating N–acetylmuramic acids linked via β1-4 linkage to GlcNAc. Moreover, the glycan strands of pseudomurein are cross-linked by peptides composed of L-amino acids (glutamic acid, alanine, and lysine), in contrast to the D-amino acids used in murein. Pseudomurein surrounds cells of all species belonging to the genus Methanopyrus and the order Methanobacteriales, which can, as in the case of Methanothermus fervidus, be bordered by an outer S-layer. As no archaeal homologs of bacterial murein biosynthesis proteins have been identified, a novel biosynthesis pathway for pseudomurein has been proposed. However, notable core sequences consist of alternating β-linked GlcNAc and an acidic sugar, suggesting a shared evolutionary origin.

FIGURE 22.2.
The chemical structure of pseudomurein.
S-Layers
The majority of characterized Archaea rely on a proteinaceous cell wall, the S-layer, comprising a regularly structured two-dimensional array based on a single protein species, the S-layer glycoprotein, or a limited number of proteins. S-layer glycoproteins have proven to be excellent reporters of posttranslational modifications in Archaea, including N-glycosylation as discussed in detail below.
Proteinaceous Sheaths
The rod-shaped cells of Methanospirillum hungatei and Methanosaeta concilii form long filamentous chains in which each cell is surrounded by an S-layer presenting hexagonal symmetry or by a rigid granular layer similar to an S-layer, respectively. The long filamentous chains are further enclosed by a tubular proteinaceous sheath. These sheaths form a paracrystalline structure based on a simple p2 lattice distinct from that of the S-layer. Based on several cross-links involving cysteines, these sheaths are highly stable against proteases and detergents. Depending on the species, the proteinaceous sheaths can be posttranslationally modified by the attachment of various sugars.
Halomucin
The extremely halophilic Haloquadratum walsbyi has a unique square shape with a length of 1.5–11 µm but a thickness of only 0.1–0.5 µm. These cells are surrounded either by an S-layer or by two S-layer sheets, depending on the strain. These cells also secrete halomucin, an extremely large glycoprotein that is highly similar to mammalian mucin, which stays loosely connected to the cell. Halomucin is heavily glycosylated, containing more than 280 potential N-glycosylation sites, with an average possibility of modification every 32 residues. This cell envelope is further enforced by analogs of halomucin, termed Hmu2 and Hmu3, and most likely by a poly-γ-glutamate capsule, because homologs of bacterial genes encoding for CapBCA, involved in the biosynthesis of the protein complex, were found. It is hypothesized that this unique protective cell envelope allows H. walsbyi to cope with salinities in solar salterns that approach the limit of life.
Glutaminylglycan
The cell wall of the highly halophilic and alkaliphilic genus Natronococcus (3.5 M salt and pH 9.5–10) consists of a glutamine polymer. In contrast to poly-γ-D-glutamyl polymers in the bacteria Bacillus, Sporosarcina, or Planococcus, the archaeal polymer is formed from L-glutamines linked via the γ-carboxylic group, yielding a chain of about 60 monomers. Also in contrast to the bacterial polymer, the poly-γ-L-glutamine chain is glycosylated, containing two types of oligosaccharide. The first oligosaccharide consists of a GlcNAc pentasaccharide at the reducing end and multiple GalA residues at the nonreducing end. The second presents a GalNAc disaccharide at the reducing end and two Glc units at the nonreducing end.
Heteropolysaccharides
Halococcus morrhuae (H. morrhuae) is an extreme halophile surrounded by an electron-dense 50–60-nm-thick cell wall composed of a complex, highly sulfated heterosaccharide consisting of glucosamine, galactosamine, gulosaminuronic acid, glucose, galactose, mannose, glucuronic acid, galacturonic acid, N-acetylated amino sugars, and sulfated subunits. Different heteropolysaccharides are thought to be connected via glycine bridges between the amino groups of the glucosamines and the carboxyl groups of the uronic residues. Although the building blocks of heteropolysaccharide have been suggested, biosynthesis of this cell wall structure has yet to be described.
Methanochondroitin
Individual cells of Methanosarcina rely on an S-layer as their cell wall. A cubic aggregate of four cells (Sarcina) is covered by an additional rigid fibrillar polymer called methanochondroitin. Degradation of methanochondroitin results in disaggregation of the cells, underlining that the matrix is responsible for maintenance of the aggregate. Methanochondroitin, which is similar to eukaryotic connective tissue chondroitin, is composed of a repeating trimer of uronic acid and two GalNAc residues. Yet unlike chondroitin, methanochondroitin is not sulfated. A pathway of methanochondroitin biosynthesis has been proposed based on activated precursors in Methanosarcina barkeri (M. barkeri) cell extracts. Methanosarcina species can further modify the methanochondroitin condition largely through the addition of glucose and galactose acids.
Lipoglycan
Members of the thermoacidophilic order Thermoplasmatales (pH 1–2 and ∼60°C), such as Ferroplasma acidophilum and Thermoplasma acidophilum, lack a rigid cell envelope. Such organisms thus display a pleomorphic shape, similar to mycoplasma. Stabilization of the cell is most likely realized by the oligosaccharide portions of lipoglycans and membrane-associated glycoproteins. The outwardly oriented glycan chains form a protective slime coat called the glycocalyx. Studies of the glycan composition of the major membrane glycoprotein revealed that the glycans mainly comprise mannose residues. A more recent study of different cell-surface glycoproteins identified an N-linked branched octosaccharide described below.
N-Glycosylation of Proteins in Archaea
The Diversity of N-Linked Glycans in Archaea
To date, S-layer glycoproteins from Archaea isolated from a wide range of habitats have been studied to various degrees of detail. Possibly reflecting the varied niches occupied by these organisms, their S-layer glycoproteins (and indeed, their other glycoproteins, such as archaellins) bear N-linked glycans that present wider diversity in terms of size, degree of branching, identity of the linking sugar, modification of sugar components by amino acids, sulfate, and methyl groups, and the presence of unique sugars than reported to date in Bacteria or Eukarya. Currently defined archaeal N-linked glycans are depicted in Figure 22.3.

FIGURE 22.3.
The structural diversity of N-linked glycans in Archaea. The structures of N-linked glycans found in the Archaea species shown is given. For a comparison, the eukaryotic lipid-linked oligosaccharide translocated across the membrane (bottom) and transferred (more...)
Delineated Pathways of Archaeal N-Glycosylation
The first archaeal N-glycosylated protein, the H. salinarum S-layer glycoprotein, was reported to be modified by two different N-linked oligosaccharides, a repeating sulfated pentasaccharide linked via N-glycosylamine to Asn-2 and a sulfated glycan linked by a glucose residue to 10 other Asn residues. The latter glycan is also N-linked to archaellins in this haloarchaeon. Efforts undertaken at the time aimed at deciphering the pathways responsible for the synthesis of these glycans solely relied on biochemical approaches because neither suitable genetic tools nor a genome sequence were available. Accordingly, dolichol pyrophosphate (Dol-PP) was determined to be the lipid carrier of the repeating sulfated pentasaccharide GlcNAc-linked to S-layer glycoprotein Asn-2, whereas dolichol phosphate (Dol-P) was shown to bear the glucose-linked sulfated glycan decorating the other N-glycosylated sites of this protein and of archaellin. It was also shown that the sulfated polysaccharide is methylated when Dol-P-linked but not when protein-bound. Finally, the ability of H. salinarum cells to modify sequon-bearing cell-impermeable hexapeptides with sulfated oligosaccharides served to localize oligosaccharyltransferase activity to the external cell surface.
Despite these biochemical advances, delineation of archaeal N-glycosylation pathways had to wait until the genome age and the development of tools for the genetic manipulation of various species. Through the subsequent identification of homologs of eukaryotic and/or bacterial N-glycosylation pathway components, genome scanning for additional components, the generation of deletion strains, and characterization of reporter glycoproteins, agl (archaeal glycosylation) genes comprising archaeal N-glycosylation pathways have been identified in several halophilic, methanogenic, and thermophilic Archaea.
Halophiles
In the last decade, progress in defining pathways of N-glycosylation has relied on Haloferax volcanii as a model organism. In H. volcanii, a series of Agl proteins mediate the assembly and attachment of a pentasaccharide to select Asn residues of the S-layer glycoprotein and archaellin (Figure 22.4). Acting at the cytoplasmic face of the plasma membrane, the glycosyltransferases AglJ, AglG, AglI, and AglE sequentially add the first four pentasaccharide residues (i.e., a glucose, a glucuronic acid, a galacturonic acid, and a methylated glucuronic acid) onto a common Dol-P carrier, whereas AglD adds the final pentasaccharide residue, mannose, to a distinct Dol-P. Assembly of the Dol-P-linked tetrasaccharide also involves AglF, a glucose-1-phosphate uridyltransferase; AglM, a UDP-glucose dehydrogenase; AglP, a methyltransferase; and AglQ, a predicted isomerase. AglF and AglM have been shown to act in a sequential and coordinated manner, transforming glucose-1-phophosphate into UDP-glucuronic acid in vitro. AglB, the archaeal oligosaccharyltransferase, transfers the lipid-linked tetrasaccharide to select Asn residues of target proteins. The final mannose residue is subsequently transferred from its Dol-P carrier to the protein-bound tetrasaccharide in a reaction requiring AglR, a protein that either serves as the Dol-P-mannose flippase or contributes to such activity, and AglS, a Dol-P-mannose mannosyltransferase. Most recently, the N-linked pentasaccharide has been reported to comprise mannose-1,2-[methyl-O-4-]glucuronic acid-β1-4-galacturonic acid-α1-4-glucuronic acid-β1-4-glucose-β-Asn.

FIGURE 22.4.
The pathway of N-glycosylation in Haloferax volcanii. The oligosaccharide is assembled on the lipid carrier dolichol phosphate, translocated across the plasma membrane, and transferred to protein by the AglB oligosaccharyltransferase. The N-linked glycan (more...)
Interestingly, N-glycosylation of the S-layer protein is altered in response to a change of environmental conditions: growing H. volcanii in low-salt medium alters the N-glycan structure in a site-specific manner.
Methanogens
Mass spectrometry efforts had elucidated the glycan N-linked to archaellins of Methanococcus voltae strain PS. GlcNAc, the linking sugar, is connected to a diacetylated glucuronic acid, in turn linked to an acetylated mannuronic acid modified by a threonine at the C-6 position (β-ManpNAcA6Thr-(1-4)-β-Glc-pNAc3NAcA-(1-3)-β-GlcpNAc), although archaellins from other versions of M. voltae strain PS presented an N-glycan bearing an additional mass of either 220 or 260 Da at the reducing end, likely representing an additional sugar. It emerges that an oligosaccharide is assembled on a lipid carrier, translocated across the membrane, and transferred en bloc to asparagine residues of polypeptides. As with H. volcanii, the identification of M. voltae N-glycosylation pathway components initially relied on gene deletion and subsequent analysis of the N-linked glycans generated in the mutant strain. As such, the oligosaccharyltransferase AglB and the glycosyltransferase AglA, responsible for transfer of the third sugar of the glycan, were discovered. The same strategy was later used to identify AglC and AglK, glycosyltransferases proposed to be involved in the biosynthesis or transfer of the second sugar. A genetics approach also assigned AglH responsibility for adding the linking sugar GlcNAc to the lipid carrier on which the N-linked glycan is assembled. Although aglH could not be deleted in M. voltae, it was able to complement a conditional lethal mutation in the alg7 gene of S. cerevisiae. Alg7, sharing 25% identity with M. voltae AglH, catalyzes the conversion of UDP-GlcNAc and Dol-P to UMP and Dol-PP-GlcNAc in the eukaryotic N-glycosylation process.
Additional insight into M. voltae N-glycosylation has come from in vitro studies. Such efforts revealed the transfer of Dol-P-linked glycans to model peptides by heterologously expressed and purified M. voltae AglB. In contrast to the earlier genetics-based studies showing AglH to be the first glycosyltransferase of the pathway, a bacterially expressed version of the enzyme was not able to add GlcNAc to Dol-P. On the other hand, purified AglK catalyzed the formation of Dol-P-GlcNAc from Dol-P and UDP-GlcNAc. The seeming disagreement between the genetics and biochemical results concerning AglH and AglK functions remains to be solved.
Methanococcus maripaludis has become an important model for genetic and structural research on N-glycosylation in the methanogens. In M. maripaludis, archaellins are modified by an N-linked tetrasaccharide similar to its M. voltae counterpart. In the M. maripaludis glycan, the linking sugar is GalNAc and not the GlcNAc used by M. voltae. The second sugar in the M. maripaludis glycan is a diacetylated glucuronic acid, as in M. voltae. Although the third sugar is a modified mannuronic acid with a threonine attached at the C-6 position in both organisms, there is an additional acetamidino group added at position C-3 of the M. maripaludis glycan. The fourth and terminal sugar of the M. maripaludis glycan is a novel sugar, (5S)-2-acetamido-2,4-dideoxy-5-O-methyl-α-L-erythro-hexos-5-ulo-1,5-pyranose. It was later reported that the major M. maripaludis pilin is modified by the same N-linked tetrasaccharide bearing an extra hexose branching from the linking GalNAc subunit. The pathway used for N-glycosylation of M. mariplaudis for archaellin has been largely delineated. The process seemingly starts with the addition of UDP-GalNAc to Dol-P by an unidentified glycosyltransferase. Like H. volcanii Dol-P, M. mariplaudis Dol-P includes two saturated isoprenes, likely at the α- and ω-positions. The AglO, AglA, and AglL glycosyltransferases add the next three nucleotide-activated sugars, respectively. AglU adds the threonine moiety to sugar three, apparently only following addition of the fourth sugar by the glycosyltransferase AglL. AglV then methylates sugar four. The Dol-P-bound tetrasaccharide is then flipped across the membrane by an unidentified flippase, when AglB transfers the lipid-linked glycan to target Asn residues.
Thermophiles
Studies on the N-glycosylation process in thermophilic Archaea have thus far focused on Sulfolobus acidocaldarius, a thermoacidophilic archaeon that grows optimally at 80°C and pH 2. In S. acidocaldarius, the S-layer glycoprotein and cytochrome b558/566 are modified by a N-linked hexasaccharide comprising a glucose, two mannoses, two GlcNAc residues, and 6-sulfoquinovose (6-deoxy-6-sulfoglucose). This glycan is unusual in that it is tribranched, in contrast to the linear or dibranched glycans N-linked glycans described in halophiles and methanogens, and in that it contains the typical eukaryal N-acetylated chitobiose core and 6-sulfoquinovose, a sugar generally found only in photosynthetic membranes of plants and phototrophic bacteria. Biosynthesis of the N-linked glycan is thought to begin with GlcNAc, derived from a nucleotide-activated precursor, being transferred onto the unusually short and highly saturated Dol-PP lipid carrier by AglH, a predicted UDP-GlcNAc-1-P: Dol-P-GlcNAc-1-P transferase. Information concerning addition of the second and third sugars is lacking. However, Agl3 converts UDP-glucose and sodium sulphite into UDP-sulfoquinovose, which is subsequently added to Dol-PP-bound trisaccharide by an unknown glycosyltransferase. In the final steps of N-linked glycan assembly, the terminal mannose and glucose moieties are added, with Agl16, a soluble glycosyltransferase, adding the final glucose. A so far unidentified flippase translocates the Dol-PP-bound hexasaccharide across the membrane, where AglB transfers the glycan to target protein Asn residues. In contrast to what occurs in H. volcanii, M. voltae and M. maripaludis, namely, species in which N-glycosylation pathways have been described, aglB is essential in S. acidocaldarius. This could be a reflection of S. acidocaldarius belonging to the Crenarchaeota rather than the Euryarchaeota, as do the other strains. As the two best studied archaeal phyla, Crenarchaeota and Euryarchaeota differ significantly with respect to several basic cellular processes, such as replication and cell division.
Physiological Roles of Archaeal N-Glycosylation
A survey of 168 archaeal genomes revealed that all but two encode AglB, the archaeal oligosaccharyltransferase. As such, it would appear that N-glycosylation is a common posttranslational modification in Archaea. N-glycosylation has also been considered as assisting Archaea to cope with the challenges of the extreme environments they often occupy. For instance, enhanced surface charge in the face of hypersaline conditions and hence increased solubility was offered as an explanation for the high sulfated sugar content of N-linked glycans decorating the H. salinarum S-layer glycoprotein relative to its H. volcanii counterpart, given the higher salinity of the locale in which the former lives. In other instances, it is not clear how a given N-glycosylation profile contributes to life in harsh surroundings. In H. volcanii, however, N-glycosylation may provide cells with the ability to respond to changes in the surrounding salinity. As noted above, the N-glycosylation profile of the S-layer glycoprotein differs in cells grown in 3.4 or 1.75 m NaCl-containing medium. Modified glycosylation in response to environmental conditions has also been reported in the case of Methanospirillum hungatei, in which archaellins are only modified in low phosphate-containing medium.
Although N-glycosylation is apparently important for archaeal protein stability and maintenance of cell integrity, it may be more so in the face of high temperatures. This could also explain why N-glycosylation is essential in S. acidocaldarius, in contrast to other Archaea studied to date. Accordingly, the S-layer glycoproteins of the hyperthermophilic methanogens Methanocaldococcus jannaschii and Methanotorris igneus present higher numbers of potential N-glycosylation sites, relative to their counterparts in mesophilic methanogens.
ACKNOWLEDGMENTS
The authors acknowledge contributions to previous versions of this chapter by Jeffrey D. Esko, Tamara L. Doering, and the late Christian R.H. Raetz, thank Benjamin H. Meyer for the preparation of the original figures, and appreciate helpful comments and suggestions from M. Osman Sheikh, Corinna Landig, and Robert Townley.
FURTHER READING
- Sumper M. 1987. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta 906: 69–79. [PubMed: 2882779]
- Lechner J, Wieland F. 1989. Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem 58: 173–194. [PubMed: 2673008]
- Kandler O, Konig H. 1998. Cell wall polymers in Archaea (Archaebacteria). Cell Mol Life Sci 54: 305–308. [PubMed: 9614965]
- Schaffer C, Messner P. 2001. Glycobiology of surface layer proteins. Biochimie 83: 591–599. [PubMed: 11522387]
- Albers SV, Meyer BH. 2011. The archaeal cell envelope. Nat Rev Microbiol 9: 414–426. [PubMed: 21572458]
- Visweswaran GR, Dijkstra BW, Kok J. 2011. Murein and pseudomurein cell wall binding domains of bacteria and archaea—A comparative view. Appl Microbiol Biotechnol 92: 921–928. [PMC free article: PMC3210951] [PubMed: 22012341]
- Eichler J. 2013. Extreme sweetness: Protein glycosylation in Archaea. Nat Rev Microbiol 11: 151–156. [PubMed: 23353769]
- Larkin A, Chang MM, Whitworth GE, Imperiali B. 2013. Biochemical evidence for an alternate pathway in N-linked glycoprotein biosynthesis. Nat Chem Biol 9: 367–373. [PMC free article: PMC3661703] [PubMed: 23624439]
- Jarrell KF, Ding Y, Meyer BH, Albers SV, Kaminski L, Eichler J. 2014. N-linked glycosylation in Archaea: A structural, functional, and genetic analysis. Microbiol Mol Biol Rev 78: 304–341. [PMC free article: PMC4054257] [PubMed: 24847024]
- Klingl A. 2014. S-layer and cytoplasmic membrane—Exceptions from the typical archaeal cell wall with a focus on double membranes. Front Microbiol 5: 624. [PMC free article: PMC4243693] [PubMed: 25505452]
- Review Archaea.[Essentials of Glycobiology. 2022]Review Archaea.Meyer BH, Albers SV, Eichler J, Aebi M. Essentials of Glycobiology. 2022
- Review Protein glycosylation in Archaea: sweet and extreme.[Glycobiology. 2010]Review Protein glycosylation in Archaea: sweet and extreme.Calo D, Kaminski L, Eichler J. Glycobiology. 2010 Sep; 20(9):1065-76. Epub 2010 Apr 5.
- Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea.[Mol Microbiol. 2006]Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea.Chaban B, Voisin S, Kelly J, Logan SM, Jarrell KF. Mol Microbiol. 2006 Jul; 61(1):259-68.
- Review Lipid sugar carriers at the extremes: The phosphodolichols Archaea use in N-glycosylation.[Biochim Biophys Acta Mol Cell ...]Review Lipid sugar carriers at the extremes: The phosphodolichols Archaea use in N-glycosylation.Eichler J, Guan Z. Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Jun; 1862(6):589-599. Epub 2017 Mar 19.
- Review Hot and sweet: protein glycosylation in Crenarchaeota.[Biochem Soc Trans. 2013]Review Hot and sweet: protein glycosylation in Crenarchaeota.Meyer BH, Albers SV. Biochem Soc Trans. 2013 Feb 1; 41(1):384-92.
- Archaea - Essentials of GlycobiologyArchaea - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

