NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.018

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsAlthough glycoconjugates predominate at the cell surface, in cellular secretions, and in the extracellular matrix, within the secretory pathway and lysosomes, there are also stable residential glycoproteins in the cytoplasm, nucleus, and plastids of eukaryotes. The prevalence of β-linked O-GlcNAc is so great in eukaryotes that an entire chapter is devoted to it (Chapter 19). This review discusses all other forms of cytoplasmic glycosylation, both established and speculative. The analysis begins with other well-documented forms of monoglycosylation in prokaryotes, expands to complex forms in protists, and provides examples of monoglycosylation exploited by bacterial toxins to co-opt processes vital to eukaryotes. In addition, conventional N-glycans are reported on a subset of mitochondrial and chloroplast proteins. Finally, the potential for a more extensive than currently appreciated occurrence of cytoplasmic glycosylation, as suggested by many examples of nuclear and cytoplasmic glycoproteins and glycosaminoglycans, which are yet to be confirmed by modern methods, is discussed. The numerous types of cytoplasmic and nuclear lectins that have been described are also reviewed.
DOCUMENTED CASES OF CYTOPLASMIC O-GLYCOSYLATION
The hydroxyl side chains of Thr, Ser, Tyr, hydroxyproline, and hydroxylysine constitute potential sites of attachment of O-linked glycans, and Asn and Arg provide attachment sites for N-glycans. Thr, Ser, and Asn are the predominant linkage residues in the secretory pathway but, interestingly, the best characterized examples of nucleocytoplasmic O-glycosylation (other than O-β-GlcNAc; Chapter 19) occur on Tyr, Hyp (4R,2S-hydroxyproline), and Arg. Also, unlike secretory pathway glycosylation, the responsible glycosyltransferases often target single proteins. The following sections review documented examples of nucleocytoplasmic glycosylation in which the glycosylation reaction occurs in the cytoplasm or nucleus, and the glycosylated target remains and functions within these compartments. Some of these examples reside in the cytoplasm alone, whereas others may also occur within the nucleus. This section also includes examples in which the cytoplasmic glycosyltransferases derived from a bacterial pathogen are injected into the eukaryotic host cytoplasm and concludes with a description of glycosyltransferases that modify DNA. These examples are summarized in Table 18.1.
TABLE 18.1.
Examples of nuclear or cytoplasmic glycosylation events
Glycogenin
Glycogen is a large branched homopolysaccharide used as a short-term storage form of glucose in bacteria, yeast, and animals. Glucose is added to and removed from the nonreducing termini based on availability of the sugar donor UDP-Glc and the nutritional needs of the cell. Glycogen stores in the liver are also important in maintaining glucose homeostasis in the blood. Glycogen is assembled initially from a linear oligoglucoside that is extended by glycogen synthase and branched by a so-called branching enzyme. Based on the pioneering work of Whelan and Cohen, the initial oligoglucose is assembled by another enzyme, glycogenin, which attaches the first sugar to itself in an uncommon Glcα1-Tyr linkage, at Tyr-195 in human glycogenin-1. Thus, every glycogen molecule, which can contain up to 105 Glc residues and 12 generations of branch points, is thought to contain a single glycogenin protein molecule at its nonreducing end. Thus, glycogen is a glycoprotein whose glycosylation is initiated by glycogenin, extended by glycogen synthase, and modified by branching enzyme, debranching enzyme, and glycogen phosphorylase.
The biochemical mechanism of glycogenin glycosylation is interesting because it naturally assembles as a homodimer under cellular conditions. Evidence supports the importance of intersubunit-dependent αGlc transfer to the acceptor hydroxyl on Tyr, and both inter- and intrasubunit extension of additional αGlc residues to the 4-position of underlying αGlc residues up to a chain length of ∼10. Production of the glycogenin primer, especially in muscle cells, can be the rate-limiting step in glycogen formation. Thus, levels of glycogenin could be capable of overriding the better understood hormonally controlled mechanisms that involve enzyme phosphorylation/dephosphorylation and regulate glycogen elongation. There is also crystallographic and mutational evidence for formation of a complex between glycogenin and glycogen synthase that is important for glycogen synthase activity in vitro and in vivo. The physiological impact of this interaction, which is difficult to reconcile with the idea that there is only one glycogenin per glycogen but potentially a glycogen synthase for every nonreducing terminus (1012), remains to be resolved.
Glycogenin-like proteins are found in a wide range of plants, animals, and free-living single-celled eukaryotes. The Escherichia coli (E. coli) homolog is incapable of autoglucosylation. Glycogenin is a GT-A superfamily member from CAZy family GT8 (Carbohydrate-Active Enzymes sequence database) (Chapter 52), which includes bacterial lipopolysaccharide (LPS) glucosyl and galactosyl transferases and galactinol synthases—all cytoplasmic as for glycogenin—as well as other glycosyltransferases in the eukaryotic Golgi. Interestingly, a novel Glc-Arg linkage has been described in a plant protein potentially associated with the synthesis of starch, which is related to glycogen, but this protein has no apparent sequence similarity to glycogenin. The function of this and the glycogenin-like proteins deserve further investigation for their potential to mediate other cytoplasmic glycosylation events.
Hydroxyproline-Linked Skp1 Glycans
West and colleagues identified a small fucosylated protein, Skp1, in the cytoplasm of a free-living soil amoeba, the cellular slime mold Dictyostelium. Skp1 is an adaptor in SCF (Skp1/Cullin1/F-box protein)-type E3-ubiquitin ligase complexes that function in the cytoplasm and nucleus of all eukaryotes. E3SCF-ubiqutin ligases that mediate the polyubiquitination and eventual proteasomal degradation of hundreds of proteins involved in cell cycle regulation, transcription, and signal transduction. Skp1 is subject to O2-dependent hydroxylation at Pro143, which generates the substrate for five glycosyltransferase reactions. Based on mass spectrometric, sequential exoglycosidase treatments and nuclear magnetic resonance (NMR) studies (Chapter 50), the glycan is comprised of a pentasaccharide consisting of a core trisaccharide equivalent to the type 1 blood group H structure, Fucα1-2Galβ1-3GlcNAc1α-, substituted at the 3-position of Fuc by a Galα1,3Galα- disaccharide (Figure 18.1A). In an unrelated protist, the apicomplexan Toxoplasma gondii, a similar core trisaccharide is capped by a Galα1-Glcα1- disaccharide (Figure 18.1B), whose linkages remain to be determined. The linkage of this glycan to protein, its localization in the cytoplasm, and the structures themselves are all novel. Because Pro143 is conserved in the Skp1 genes of plants, invertebrates, and unicellular eukaryotes, and gene sequences related to those of known Skp1 modification enzymes (see below) are present in the genomes of many protists, this modification may be widespread, at least in unicellular eukaryotes.
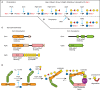
FIGURE 18.1.
Mechanism of glycosylation of Skp1 in the cytoplasm of protists. Glycosylation is enabled by prior hydroxylation of a single Pro-residue by the action of a cytoplasmic, O2-dependent prolyl 4-hydroxylase that is homologous to the HIFα prolyl hydroxylases (more...)
The Dictyostelium glycosyltransferases that glycosylate Skp1 have each been purified based on their enzymatic activities from cytosolic extracts, sequenced and cloned, and the enzyme genes were disrupted in Dictyostelium and expressed in E. coli. The evidence shows the existence of sequential linear pathway NDP-sugar-dependent glycosyltransferases that are Golgi-like except that they are soluble rather than type 2 membrane proteins (Figures 18.1A,C; Table 18.1). The Skp1 GlcNAc-transferase (EC 2.4.1.229), which adds the first sugar, is related to the Thr/Ser polypeptide αGalNAc-transferases that initiate mucin-type O-glycosylation in the Golgi of animals, and therefore forms an anomeric linkage opposite to that of the well-known O-βGlcNAc transferase (Chapter 19). However, unlike known polypeptide αGalNAc-transferases (Chapters 6 and 10), the Skp1 GlcNAc-transferase lacks an amino-terminal signal anchor, consistent with its biochemical fractionation as a soluble cytoplasmic protein (Figure 18.1C). Thus, the mechanism of initiation of Skp1 glycosylation is similar to that of mucin-type domains of secretory proteins, except that a different N-acetylhexosamine (GlcNAc vs. GalNAc) is attached to a distinct hydroxyamino acid (Hyp vs. Thr or Ser) in the cytoplasm versus the Golgi lumen.
The cloning of the gene for the fucosyltransferase, which adds the third sugar, led to the discovery that both the second (βGal) and the third (α-L-Fuc) sugars are added by separate domains of the same soluble protein, PgtA (Figure 18.1C). The β3-galactosyltransferase catalytic domain is most similar to bacterial lipopolysaccharide and capsular glycosyltransferases, which are also cytoplasmic, suggesting that cytoplasmic glycosylation in Dictyostelium has its evolutionary origins in bacterial glycolipid synthesis. The β-galactosyltransferase catalytic domain belongs to the inverting CAZy GT2 family, which is diagnostic for glycosyltransferases with their catalytic domains exposed to the cytoplasm. However, in bacteria and eukaryotes, these enzyme family members are usually membrane-associated and their products, including lipopolysaccharides, Dol-P-sugars, and polysaccharides such as hyaluronic acid, cellulose, and chitin, are translocated to the lumen or cell exterior. In Dictyostelium, the fourth and fifth sugars are added by the two-domain glycosyltransferase AgtA, in which the α3-galactosyltransferase domain is fused to a β-propeller-like domain, which has a second function that may involve constitutive Skp1 sequestration activity. The catalytic domain is related to a large number of plant Golgi glycosyltransferases implicated in pectin biosynthesis and here catalyzes two successive additions. Notably, two glycosyltransferases with independent evolutionary origins catalyze the completion of Skp1 glycan in Toxoplasma.
Dictyostelium undergoes a developmental program when starved that is exquisitely sensitive to extracellular conditions including the level of O2, which is thought to help the cells determine whether they are below or above ground in their native soil environment. Considerable biochemical, genetic, and physiological evidence supports the model that at least a component of O2-dependent development is mediated via the modification of Skp1. Whereas the rate of Skp1 hydroxylation is O2-limited in vitro and in vivo, glycosylation is important for maximal interaction with several different F-box protein subunits of the SCF complex (Figure 18.1D). Currently, it is unclear whether increased association leads to increased turnover of substrates recognized by the F-box proteins, or reduced levels of F-box proteins via autoubiquitination. Further studies are needed to determine whether the glycan functions by reorganizing Skp1 to promote its interaction with F-box proteins, or contributes directly to binding by a lectin-like mechanism.
Prokaryotic Monoglycosylation
Protein glycosylation is becoming increasingly recognized as a prevalent modification in bacteria (Chapter 21). In comparison to eukaryotes, protein glycosylation appears to vary more extremely among bacterial species. A recent proteomics search for glycoproteins in the Gram-positive intestinal bacterium Lactobacillus plantarum, biased only because potential glycopeptides were affinity enriched with the lectin wheat germ agglutinin (WGA), identified several glycoproteins with cytoplasmic localization and functions: the molecular chaperone DnaK, the PdhC E2 subunit of the pyruvate dehydrogenase complex, the DNA translocase FtsK1, the signal recognition particle receptor FtsY, FtsZ involved in cell division, and others of unknown function. Peptides from these proteins were modified with single HexNAc residues attached to Ser residues. Some of the sites were variably modified suggesting that they may be regulatory. Nothing is known about the mechanism of this glycosylation, nor of its function. Interestingly, in L. plantarum several proteins are glycosylated in the cytoplasm and then exported to the cell wall. It can be anticipated from this seminal study that there is much remaining to be discovered regarding glycosylation in prokaryotic cytoplasms.
An example of a specific function for monoglycosylation comes from the EF-P protein, the bacterial homolog of eukaryotic elongation initiation factor 5a. EF-P normally suppresses translational stalling by a mechanism that involves oxidation of a critical lysyl residue. A very recent phylogenetic analysis of EF-P sequences revealed a subset of enzymes with an Arg in place of Lys, and a coevolving gene that was subsequently identified by biochemistry and mass spectrometry as an argininyl rhamnosyltransferase. Assembly of the Rha-Arg linkage activates EF-P and is required for pathogenicity of Pseudomonas, a Gram-negative, opportunistic human pathogen, and a number of other bacteria. This linkage is, like those described in glycogenin and Skp1, highly novel. The uniqueness of these linkages perhaps reflects a long evolutionary separation from the more familiar glycosylation pathways associated with cell surface and extracellular glycoproteins, and suggests that more novel mechanisms of glycosylation are yet to be discovered.
Bacterial Glycosyltransferase Toxins
Small cytoplasmic G-proteins (GTP-binding proteins) of the Rho family are involved in regulating the cytoskeleton. Certain toxins from anaerobic bacteria were found by Aktories and colleagues to contain retaining glycosyltransferase activities that inhibit G-proteins by attaching a glycosyl moiety to a threonine residue (Thr-37) in their GTP-binding sites. These secreted toxins show the remarkable ability of translocating across the surface membrane into the cytoplasm of mammalian target cells. The enterotoxins from Clostridium difficile (ToxA) and Clostridium sordellii are α-glucosyltransferases from CAZy family GT44 that use UDP-Glc as the donor. In contrast, a similar toxin from Clostridium novyi is an O-αGlcNAc transferase that uses UDP-GlcNAc as the donor. The C. novyi toxin has no primary sequence relationship to the endogenous inverting animal O-βGlcNAc (Chapter 19) or the retaining Skp1 αGlcNAc transferases. A distantly related toxin from Legionella pneumophila, also delivered by a type IV secretion system, installs an αGlc residue on elongation factor 1A in a broad range of eukaryotic host cells. The target residue, Ser53, is located in the G domain near the switch-1 region of the GTPase, and glucosylation inhibits its activity in vitro and in vivo. Modification appears to depend on a ternary complex of eEF1A, GTP, and an aminoacyl-tRNA. Interestingly, Legionella encodes yet another toxin with an unknown specificity that controls vesicular trafficking, so even more remains to be discovered.
Recently, an unrelated toxin from Yersinia, which enters host cells via a phage tail-derived translocation system, was found to be an αGlcNAc-transferase that modifies Tyr-34 of RhoA. Finally, another recent study described the formation of a GlcNAcα-Arg linkage on death domains in TRADD, FADD, RIPK1, and TNFR1. This novel reaction is catalyzed by NleB-related glycosyltransferases from various pathogenic bacteria, apparently promoting host cell longevity during infection. In contrast to what is known about the other glycosyltransferase toxins, NleB appears to modify multiple proteins including glyceraldehyde 3-phosphate dehydrogenase. Although these examples involve monoglycosylation mediated by exogenous glycosyltransferases (see Table 18.1), their existence reinforces the potential impact of glycosylation as a regulatory mechanism, and the diversity of glycosylation events that probably remain to be discovered.
Glycosylation of DNA
Although the focus of this chapter is on nuclear and cytoplasmic glycoproteins, it should be noted that specific bases in DNA have long been known to be a target of bacteriophage-encoded glycosyltransferases. Modification of T4-phage DNA hydroxymethylcytosine residues by βGlcTs or αGlcTs (Table 18.1) render them resistant to host restriction enzyme digestion and may have additional functions as well. Some T-even phages assemble a Glc disaccharide and others attach different sugars to different bases, and the full diversity of sugar modifications remains to be described. A related modification also occurs in a protist, Trypanosoma brucei, in the form of base J. By a mechanism that resembles the modification of the Skp1 glycoprotein, DNA thymidine residues are initially hydroxylated by an O2-dependent non-heme dioxygenase, which generates hydroxymethyl moieties suitable for glycosylation by a novel, very recently discovered nuclear β-glucosyltransferase. The enzyme, JGT, is also a CAZy GT2 glycosyltransferase (Table 18.1). Base J influences Pol II transcription and termination in these unicellular eukaryotes. Thus, sugar-based epigenetic modifications occur on DNA, as well as histones, as seen for O-GlcNAc (Chapter 19).
HOW WIDESPREAD IS NUCLEOCYTOPLASMIC GLYCOSYLATION?
The Possibility of “Conventional” Secretory-Type Glycans on Cytoplasmic Glycoproteins
The above examples show that nucleocytoplasmic glucosyltransferases (GTs) can assemble nucleocytoplasmic glycoproteins (or DNA) in both prokaryotes and eukaryotes, as illustrated in Figure 18.2, but they raise the important question of prevalence of cytoplasmic glycosylation. The known examples diverge from traditional glycosylation dogma by their focus on single protein targets, in contrast to Golgi glycosylation pathways that tend to modify multiple proteins. Thus, by definition, there will be fewer glycosylation events from target-restricted pathways.
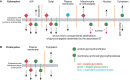
FIGURE 18.2.
Topology of glycosylation reactions and the destinations of their product glycoconjugates. Glycosyltransferases (GTs) are represented as spirals and membrane anchoring sequences as sticks crossing the membrane. GTs that modify lipid carriers are in red (more...)
Yet the literature is replete with many reports suggesting the more widespread occurrence of cytoplasmic glycoconjugates in animal cells. Many of these studies have used plant lectins (Chapter 28) and anticarbohydrate antibodies as tools to detect saccharide-bearing molecules within the nucleoplasmic or cytoplasmic compartments using immunocytochemical techniques. These binding studies have largely been ignored by mainstream biochemists for several reasons.
- Most glycosyltransferases involved in complex glycan biosynthesis are type 2 membrane proteins with their active sites within the lumen of the endoplasmic reticulum or Golgi. Thus, the “enzymatic machinery” is on the “wrong” side of the membrane for the biosynthesis of complex nuclear or cytoplasmic glycoconjugates.
- Most of the lectin-binding studies that make claims of cytosolic or nuclear glycosylation present no supporting structural data to firmly establish the nature of the putative cytoplasmic or nuclear glycoconjugates.
- Plant lectins, such as Concanavalin A, for example, can, under some circumstances, bind to molecules by nonspecific hydrophobic binding that is suppressed by the competing saccharide ligand.
Therefore, although lectins are important tools in glycobiology, conclusions based on their use—particularly those not compatible with known pathways and established concepts of biochemistry and cell biology—must be followed up by rigorous structural analyses. In a recent analysis of yeast extracts, these criticisms were circumvented by mass spectrometric demonstration of O-Man glycans on numerous nucleocytoplasmic proteins, which are also abundant on secretory glycoproteins. In these same types of analyses, O-Man monosaccharides were not found on mammalian nucleocytoplasmic proteins. However, confirmation of cytoplasmic and nuclear O-Man in yeast must await demonstration of the mechanism of the glycosylation reaction and exclusion of glycosylation reactions occurring during extract preparation. Nevertheless, these studies suggest that GlcNAc-, Man-, and Fuc-containing glycans are potentially abundant within the nucleus and cytoplasm, indicating that further chemical analyses are warranted.
Some studies have examined individual glycoprotein candidates directly. For example, the α-subunit of the dog kidney sodium pump (Na+, K+-ATPase), a transmembrane protein, was reported to contain traditional N-linked glycans (like those formed in the rough endoplasmic reticulum [rER]) with terminal GlcNAc residues in its cytosolic domain. This conclusion was based on the enzymatic attachment of radioactive Gal to GlcNAc residues by galactosyltransferase labeling of permeabilized right-side-out membrane vesicles. Peptide-N-glycosidase sensitivity of the radiolabeled products suggested that the acceptors are N-linked glycans (Chapters 9 and 50). This provocative claim has remained unresolved, because of the lack of site mapping of the putative cytosolic glycans and the absence of direct structural data.
Several studies have also indicated that the so-called high mobility group (HMG) proteins, which are important structural components of chromatin, are bona fide “classical-type” glycoproteins. Purified preparations of HMGs 14 and 17 were found to contain GlcNAc, mannose, galactose, glucose, fucose, and possibly xylose, and isolated nucleosomes were recognized by a fucose-specific lectin. Alkali resistance of the saccharides on the HMG proteins is consistent with attachment of the glycan via an N-linkage. In addition, the presence of sugars was validated by metabolic radiolabeling with tritiated Fuc, Gal, Man, or GlcNH2. Later studies suggested that the glycans on HMG 14 and 17 were required for their binding to nuclear matrix, where the HMG proteins play a role in modulating chromatin structure at active sites of gene transcription. Even though these HMG glycosylation studies are widely cited as evidence for nuclear N-glycans, they lack support from orthogonal structural studies such as mass spectrometry or NMR. In fact, more recent studies, using modern methods (Chapter 50), were unable to verify the presence of complex glycans on the HMG proteins but did report the presence of O-βGlcNAc (Chapter 19).
Sialic acid–containing glycoproteins (Chapter 15) have been reported on the cytoplasmic face of the nuclear envelope. The sialic acid–specific lectin, Sambucus nigra (S. nigra) agglutinin (SNA), was shown to bind to several proteins, previously found to also contain O-βGlcNAc. Two of these proteins were identified as major nucleoporins: p62 and p180. Prior sialidase treatment blocked binding of SNA to these nuclear pore proteins. Based on peptide-N-glycosidase sensitivity, the sialic acids on the p180 appeared to be on N-linked glycans. The authors also showed that SNA blocked nuclear protein import in neuroblastoma cells, suggesting that the sialic acids might have functional importance on the nuclear pore proteins. In plants, a novel O-linked glycan has also been reported on a nuclear pore–associated protein. This protein is recognized by the lectin WGA and could be labeled using tritiated UDP-Gal and β4-galactosyltransferase, which is specific for nonreducing terminal GlcNAc. The glycan, with an approximate size of five sugars, was released by mild alkaline degradation consistent with β-elimination from a Thr or Ser residue. Other notable reports of nuclear glycoproteins are a heat-shock-like nuclear chaperone protein with GlcNAc-binding activity, CBP70, which has been suggested to be N-glycosylated. A subpopulation of prion protein may be N-glycosylated and found in the nucleus possibly in association with the GlcNAc-binding lectin. As for the examples above, however, confirmation by structural and linkage analyses are needed to firmly establish the existence of these modifications.
Such cytoplasmic complex glycoconjugates may derive from the secretory pathway itself. A sizable fraction of nascent proteins fail quality control of folding and assembly in the rER (Chapter 39), and are retrotranslocated to the cytoplasm as a part of ERAD (ER-associated degradation), as depicted in Figure 18.2A (rER). Most of these are N-glycosylated and are normally processed by a cytoplasmic N-glycanase to remove the N-glycan before degradation by the 26S-proteasome or an autophagic vacuole, or are recognized by N-glycan-dependent E3 ubiquitin ligases. If these processes do not operate on a given protein, because the N-glycan is modified in such a way that it is not recognized, or is sequestered in a protein complex or the nucleus, then it may accumulate in the cytoplasm and potentially execute a novel function that might depend on its ER-derived glycan. In addition, there is evidence that endocytosed proteins, such as cholera toxin, can gain access to the cytoplasm via this or a related pathway. Furthermore, there is a growing body of literature that supports the concept that cytokines, growth factors, and even their transmembrane receptors, many of which are glycosylated, can translocate to the nucleus to exert direct effects on transcription. Although the existence of these transport pathways is controversial, they would, if they occur, provide a source of cytoplasmic and nuclear glycoproteins with conventional glycan modifications. Finally, occasional reports of cytoplasmic glycosylation in pathological tissues raise the possibility that compartmental breakdowns result in the exposure of cytoplasmic proteins to latent Golgi glycosyltransferases.
The Possibility of Other Novel Glycans on Nuclear or Cytoplasmic Glycoproteins
In 1982, Marchase and colleagues showed the transfer of αGlc-1-phosphate from UDP-Glc to O-linked mannosyl residues onto a single 62-kDa protein. The 62-kDa cytosolic protein bearing O-mannose residues was subsequently identified as parafusin, a protein that, in response to a secretagogue, reversibly dissociates from cellular membranes and coordinates with release of Glc-PO4. There is considerable biochemical evidence in support of the occurrence of Glcα1-PO4 and PO4-Man linkages, likely to be conjoined in a single phosphodiester moiety, in organisms as diverse as Paramecium, birds, and mammals. Parafusin was subsequently identified as a member of the phosphoglucomutase (PGM) protein family, named after the enzyme that catalyzes the interconversion of Glc-1-PO4 and Glc-6-PO4. Although parafusin itself appears not to show PGM activity, there is evidence that the active PGM protein from yeast and rat liver is similarly modified in extracts. Latency studies established that both the novel glucose phosphotransferase acting on parafusin and a Glc-1-phosphate phosphodiesterase that cleaves Glc-PO4 from parafusin have their active sites within the cytoplasm. Cochromatography studies of hydrolyzed parafusin from cells metabolically labeled with radioactive sugar precursors suggest that PGM-like proteins are similarly modified in vivo. The modification of PGM-like proteins is highly responsive to calcium levels, and appears to regulate the association of parafusin with the membrane. Unfortunately, nothing is known about the enzymes that catalyze these modifications or the specific structures themselves. Nonetheless, these findings suggest that both terminal mannose and dynamic Glc-1-phosphorylation of PGM-like proteins may be important regulatory forms of cytoplasmic glycosylation.
Another potential example of a cytoplasmic glycan comes from a study on purified mammalian cytokeratin, which was reported to contain GalNAc and bind lectins that recognize α1-3-linked GalNAc. Lectin binding was sensitive to pretreatment with N-acetylgalactosaminidase, but this does not rule out the possibility of a contaminant protein. Additional potential examples are oxygen-regulated protein 150, a putative cytoplasmic glycoprotein whose nuclear uptake is inhibited by a plant lectin thought to be internalized by epithelial cells, and a cytoplasmic isoform of carbonic anhydrase secreted from seminal gland epithelial cells by ectocytosis. Again, the significance of these provocative findings must await structural proof of the nature of the putative glycans.
Evidence for a cytoplasmic or nuclear function of a glycan would strengthen the case for its normal existence in that compartment. One such function in which a role for sugars has been implicated is nuclear transport. Molecules larger than ∼40 kDa do not diffuse freely through nuclear pores and must be specifically and actively transported into and out of the nucleus. Generally, nuclear localization sequences (NLSs) consist of one or two stretches of positive amino acids. However, Monsigny and colleagues have presented evidence that sugars may also serve as nuclear localization signals. The synthetically generated neoglycoproteins BSA-Glc, BSA-Fuc, and BSA-Man are rapidly transported into the nucleus of permeabilized or microinjected living HeLa cells, whereas bovine serum albumin (BSA) itself is not. Like the classical basic peptide-mediated NLS pathway, the sugar-mediated nuclear transport requires energy and is blocked by the lectin WGA. However, unlike the basic peptide system, the sugar-mediated pathway does not require cytosolic factors, and is not blocked by sulfhydryl-reactive chemicals. Additional evidence shows that BSA substituted with β-di-N-acetylchitobiosides (GlcNAcβ1-4GlcNAc) is rapidly localized to purified nuclei in vitro by a pathway distinct from the classically defined NLS systems. Validation of these fascinating results awaits characterization of the components involved, and identification of natural counterparts to the neoglycoproteins.
The Possibility of Novel Cytoplasmic and Nuclear Glycosyltransferases
There are several possible origins of complex nucleocytoplasmic glycans suggested by cytological and fractionation studies in animals and plants. As exemplified by the above protist Skp1 and DNA glycosyltransferases, and by the bacterially derived glycosyltransferases, nonconventional soluble enzyme proteins may simply reside in the cytoplasm or nucleus and directly modify proteins there. For example, several reports describe glycosyltransferase activities in highly purified preparations of rat liver nuclei judged to be approximately >99% pure by marker enzyme analysis. These studies document the transfer of GlcNAc from UDP-GlcNAc to endogenous acceptors and show that at least 80% of the activity is blocked by low concentrations of the antibiotic tunicamycin (an inhibitor of formation of the N-glycan precursor), suggesting the involvement of N-linked biosynthetic intermediates, such as N-acetylglucosaminyl-pyrophosphoryl-dolichol (GlcNAc-PP-dolichol). Later studies showed the direct transfer of chitobiose (GlcNAcβ1-4GlcNAc) from chitobiosyldolichol to endogenous nuclear acceptors by these mammalian nuclear preparations, suggesting a novel pathway of N-glycosylation. The products of these in vitro reactions were found to be N-linked chitobiosyl moieties, based on their sensitivity to peptide N-glycosidase F and hydrazinolysis but insensitivity to alkali-induced β-elimination (Chapter 50). Similar studies have documented the presence of nuclear mannosyltransferases. The finding that CMP (cytidine 5′-monophosphate)-sialic acid is synthesized in the mammalian nucleus offers further support for the concept of nuclear glycosyltransferases. Although these studies are provocative, they must also be interpreted with caution. The ER, which is the widely accepted site of N-glycosylation (Chapter 9), is functionally contiguous with the outer nuclear envelope, which also can fold into the interior. Even a minor contamination of nuclear envelope could lead to misinterpretation of these findings. Also, it is very difficult to purify nuclei such that other cellular components do not nonspecifically adhere to the otherwise “pure” nuclei during their preparation. Given these potential problems, widespread acceptance of the existence of these nuclear glycosyltransferases must await independent confirmation by alternative methods.
Recently, a remarkable N-linked glycan was characterized on a capsid protein of a virus, PBCV-1. This capsid protein, VP54, is assembled, posttranslationally modified, and incorporated into viral structures within the cytoplasm of Chlorella algae. X-ray diffraction analysis shows that VP54 possesses four sites for unconventional N-glycosylation and two sites of Ser-glycosylation. Van Etten and colleagues have shown that one glycoform consists of a branched decasaccharide containing seven different sugars including D-Glc, D-Gal, D-Man, D-Xyl, L-Fuc, L-Ara, D-Rha, and L-Rha, and one L-Rha is dimethylated. Glycosylation of VP54 is at least partially virally encoded, and indeed the genome of PBCV-1 contains six sequences predicted to encode cytoplasmically localized glycosyltransferases that lack targeting sequences for the secretory pathway. Ten glycosyltransferases are predicted to account for the distinct linkages in the decasaccharides, so it is likely that new classes of glycosyltransferase genes will be discovered in this pathway. N-Glycosylation of VP54 is unusual in that the Asn-attachment site is not associated with the canonical N-glycosylation sequon used for N-glycosylation in the eukaryotic secretory pathway and the linkage sugar is βGlc. This activity is reminiscent of NGT, the prokaryotic cytoplasmic sugar nucleotide-dependent glycosyltransferase that modifies adhesins that are exported to the cell surface (Table 18.1). One or two α6-linked Glc residues extend the βGlc residue on HMW1C adhesin. Because PBCV-1 eventually lyses its host, the product glycoprotein presumably also functions outside of the alga. Thus, there is evidence for complex glycosylation in the cytoplasm of both prokaryotes and eukaryotes, yet these glycoprotein products appear to function at the cell (or viral) surface, converging with the conventional locale expected of protein-linked glycans (Figure 18.2A, cytoplasm).
Finally, the biosynthesis of several classes of glycolipids and polysaccharides is initiated by membrane-associated glycosyltransferases whose catalytic domains are situated at the cytoplasm-facing surface. This includes early steps in the synthesis of glucoceramides, GPI anchors, and dolichyl-linked N-glycosylation precursors. However, these precursors are ultimately “flipped” to the other side of the membrane where they are extended and, in the case of the GPI-anchor and N-glycan precursors, are transferred to proteins within the lumen of the ER in the secretory pathway (Chapter 9). Cytosolic-oriented glycosyltransferases also include transmembrane proteins that polymerize hyaluronic acid (Chapter 16), cellulose, chitin, and lipopolysaccharides, in which the products are directly translocated across the plasma membrane. The glycan products of these membrane-associated glycosyltransferases normally exit the cytoplasmic space but, if this did not occur, they could serve as the origin of novel, cytoplasmic, non-protein-linked glycoconjugates.
Nucleocytoplasmic Glycosaminoglycans?
A special class of nucleocytoplasmic glycans is represented by the glycosaminoglycans (or GAGs) (Chapter 17). As early as 1964, Yamashina and colleagues proposed the presence of glycosaminoglycans in purified nuclei. In a fascinating series of subsequent studies, Kinoshita and colleagues proposed the occurrence of nuclear heparan sulfates (HSs) in developing sea urchin embryos. They found that heparin stimulated messenger RNA (mRNA) synthesis in pregastrula but not in postgastrula embryos. Other glycosaminoglycans, such as chondroitin sulfates and hyaluronan (Chapter 16) were without effect. Pulse-chase studies suggested that the nuclear GAGs appeared first in the cytoplasm and were transported to the nucleus. β-Xylosides, which are primers of chondroitin sulfate and HS biosynthesis (Chapters 17 and 55), blocked development of sea urchins, and addition of postgastrula proteoglycans prevented this block. Pregastrula proteoglycans did not prevent the β-xyloside-induced block to development. Microinjection of proteoglycans into embryos blocked development at the stage from which they were isolated. Clearly, given recent advances in the tools to study proteoglycans, these potentially exciting observations warrant a critical reexamination.
The first structural data proposing nuclear GAGs were published in 1989 by Fedarko and Conrad. These workers radiolabeled rat hepatocytes with 35SO4, isolated subcellular fractions, purified HSs, cleaved the HSs with nitrous acid, and determined the structures of the fragments. Nuclear fractions were found to contain 11% of the total cellular HSs, but were greatly enriched with a unique molecular species with a high content of a novel structure: β-D-glucuronosyl(2-SO4)αD-glucosamine-N,O-(SO4) disaccharides (GlcUA-2-SO4). Given the unique nature of these structures, it is hard to conceive how they could be derived by contamination of nuclear preparations with cell surface HS molecules, which lack such structures. Subsequent pulse-chase studies suggested that cell surface HS is taken into the nucleus and modified to these unique nuclear molecular species. In support of the uptake model, when unlabeled HS proteoglycan, that contains no GlcUA-2-SO4, was incubated with cells, about 10% ended up within the nucleus, and resulted in free HS chains containing the unusual disaccharide. Given the ability of HSs to influence gene transcription in vitro, these studies could eventually prove highly significant. In contrast, Hascall and colleagues used a new nuclear isolation method to prepare nuclei from rat ovarian granulosa cells and found dermatan sulfates, but not HSs, associated with the nucleus. These authors emphasized the difficulties in isolating pure nuclei, and in proving the existence of GAGs in native nuclei. Furthermore, all of the known glycosyltransferases involved in the biosynthesis of glycosaminoglycans have their active sites in luminal compartments (Chapter 17), and pathways for nuclear uptake of such large and negatively charged molecules have not been described.
Using another approach, immunocytochemical studies probing for a GPI-anchored HS proteoglycan (PG), glypican, have provided strong evidence for the nuclear accumulation of this PG in addition to its traditionally accepted presence at the cell surface. Although the mechanism of nuclear compartmentalization is not known, there is evidence that the glypican polypeptide harbors a nuclear localization sequence that is functional when grafted onto a neutral carrier expressed in the cytoplasm. In a separate study, overexpression of a chondroitin sulfate proteoglycan core protein in cultured cells resulted in accumulation of the core polypeptide in the cytoplasm and nucleus in addition to the secretory pathway, indicating, as has been suggested for other proteins, the possibility of dual compartmentalization. However, although these studies provide an explanation for how proteoglycan core proteins might accumulate in the nucleus, it is difficult to imagine how they have been modified by the glycosaminoglycan synthases residing in the Golgi lumen.
Cytochemical studies using naturally occurring proteins with high affinity binding to hyaluronic acid have been used to document accumulation of hyaluronic acid in the nuclei of cultured cells. Intracellular hyaluronan might represent a pool of polysaccharide that failed to translocate following biosynthesis, as the catalytic domain of hyaluronic acid synthases is oriented at the cytoplasmic surface of the plasma membrane (Figure 18.2A, plasma membrane). Some hyaluronic acid–binding proteins naturally occur within the nucleus raising the possibility of a physiological role for nuclear hyaluronan via interaction with these hyaluronic acid–binding proteins. However, it is not certain that hyaluronic acid is the natural ligand for these binding proteins. An alternative possibility is that they interact with nuclear proteins that have been modified by clusters of O-βGlcNAc (Chapter 19).
GLYCOSYLATION WITHIN ORGANELLES: MITOCHONDRIA AND CHLOROPLASTS
Mitochondria have been suggested to contain complex glycoconjugates based on lectin-binding studies. Although most mitochondrial proteins are synthesized within the cytoplasm under the direction of nuclear genes, two mitochondrial glycoproteins appear to be conventionally N-glycosylated in the rER based on pulse-chase labeling studies and susceptibility to N-glycanase. These glycoproteins might transit to mitochondria via a novel vesicle transport pathway. Subsequently, several plant chloroplast proteins, including carbonic anhydrase-1, α-amylase, and pyrophosphatase/phosphodiesterase in “higher” plants, and Glx, Syn, MutS in diatoms, were also reported to be transported from the rER as N-glycosylated proteins (Table 18.1). The presence of N-glycans highlights their interesting biosynthetic origin and implicates a novel vesicular transport pathway, but further studies are needed to assess their roles in plastid functions.
In plants, mono- and digalactolipids (MGDG: 1,2-diacyl-3-O-[β-D-Gal]-sn-glycerol and DGDG: 1,2-diacyl-3-O-[α-D-Gal-(1,6)-O-β-D-Gal]-sn-glycerol) are prominent lipids in thylakoid membranes and MGDG is essential for photosystem function (Chapter 24). Their synthesis is controlled by UDP-Gal-dependent galactosyltransferases residing in the inner and outer layer membranes of the chloroplast. Under conditions of phosphate deprivation, the synthesis of these glycolipids is dramatically increased, becoming up to 70 mol% of glycerolipids. The glycolipids replace conventional phospholipids such as phosphatidylcholine in a variety of other cellular membranes including vacuoles, mitochondria, and the plasma membrane (Table 18.1). DGDG almost exclusively resides in the cytoplasmic leaflet of these membranes and is thus oriented toward the cytoplasm (Figure 18.2A, chloroplasts), which is opposite of the conventional orientation of glycolipids as known from animal and yeast cells. Variants containing sulfoquinovose and GlcUA, which conserve the negative charge, are also produced. Little is known about the trafficking of these glycolipids. Given the functional importance of DGDG for cells based on genetic studies, it will be interesting to understand how the asymmetric distribution contributes to cytoplasmic functionality of the cellular membranes.
NUCLEAR AND CYTOPLASMIC LECTINS
The occurrence of cytoplasmic or nuclear glycoproteins or glycolipids (oriented toward the cytoplasm) would suggest the potential parallel existence of carbohydrate-binding proteins (or lectins) whose binding might contribute to the function of the glycans. Many lines of evidence document the existence of lectins that reside in the cytoplasm and/or nucleus. For example, Monsigny, Hubert, and colleagues have shown that BSA-based neoglycoproteins derivatized with L-Rha, D-GlcNAc, D-Glc, lactose, Man-6-P, and L-Fuc all bind to nuclei at threefold higher affinity than underivatized BSA. In 1980, Feizi and colleagues showed the specific binding of BSA-lactose to cryostat sections of nuclei. However, little is known about the molecular nature of these putative carbohydrate-binding proteins except for galectins-1 and -3 (see below) and the heat-shock chaperone protein CBP70 (described above). CBP70 has GlcNAc-binding activity that might recognize O-βGlcNAc-modified proteins.
The galectin family of lectins in animals, and the discoidin family of cytoplasmic lectins in the amoebazoan Dictyostelium, are high abundance proteins that have carbohydrate-binding specificity generally directed toward β-linked Gal and/or GalNAc (Chapter 36). Large pools of these proteins are soluble in the cytoplasm, but cell biological studies have shown that these proteins also reside at the cell surface and in the pericellular matrix. These proteins lack typical amino-terminal signal peptides and exit the cytoplasm by a poorly characterized, posttranslational, unconventional, secretory mechanism that may avoid premature association with secreted glycoproteins. Biochemical and genetic studies have uncovered primarily extracellular functions for these lectins (Chapter 36), suggesting that the cytoplasmic pools may be precursors of functional extracellular pools. Nevertheless, Wang and colleagues have shown that CBP35 (galectin-3) and possibly galectin-1 are present in the nucleus as part of the HnRnP complex, where they appear to be required for normal mRNA splicing in extracts. Interestingly, a neoglycoprotein containing blood group A tetrasaccharide was found to specifically inhibit mRNA splicing in vitro. However, at present there is only limited evidence for a functional role of the carbohydrate-binding activity of galectin 3 in mRNA splicing. In addition, a cytoplasmic function in regulating apoptosis has been implicated for galectin-1, although a role for the carbohydrate-binding activity has not been established. These findings certainly leave open the possibility that galectins have important untested functions via unknown cytoplasmic glycoconjugates.
Numerous other soluble cytoplasmic proteins have been assigned sugar-binding activity that point to the potential significance of cytoplasmic glycoconjugates. A Golgi-associated actin-binding protein in Dictyostelium—comitin—has a Man-binding activity. In plants, cytoplasmic mannose- and GlcNAc-binding lectins are induced by various kinds of biotic and abiotic effectors. Further studies are warranted to evaluate the functional significance of the glycan-binding activities of these interesting proteins.
Several filamentous fungi express cytoplasmic lectins with glycan-binding specificities that do not seem to be expressed in the fungi themselves (Chapter 23). Aebi, Kunzler, and colleagues have provided compelling evidence that these lectins are toxic to predators like roundworms, mosquitoes, and amoebae, and represent a form of innate immunity. It is interesting to speculate that the cytoplasm represents a “safe” depot to stow carbohydrate reactive proteins as a defense for other cells in the community should damage cause their release from individual members. Thus, the occurrence of a lectin does not necessarily imply the existence of a cognate glycan in the same compartment.
CONCLUSION
Overall, there are many tantalizing clues for the existence and importance of glycoconjugates with simple or complex glycans within the nucleus and cytoplasm, in addition to the ubiquitous O-βGlcNAc (Chapter 19). Well-characterized examples involve novel linkages to the protein via tyrosine in animal and yeast glycogenin, via hydroxyproline in protist Skp1, and via arginine in bacteria (Table 18.1). The known glycosyltransferases that mediate these modifications are traditional cytoplasmically localized proteins that evolved from the same evolutionary lineages that generated the enzymes of the secretory pathway. At present, these modifications appear to be confined to specific protein targets.
Cytoplasmic glycosylation is also a strategy for pathogens to control host cell responses and these mechanisms also frequently involve novel linkages and target single proteins. These examples clearly establish the importance of cytoplasmic and nuclear glycosylation in protein-specific regulation, which contrasts with the relatively broad distribution and heterogeneity of glycans on cell surface, extracellular matrix, and blood proteins. However, much remains to be explored to establish the generality of this concept. Significantly, there exists substantial indirect evidence for much more extensive complex cytoplasmic glycosylation in animal cells, and cell biologists continue to illustrate novel mechanisms of protein compartmentalization that could allow a protein that is glycosylated in one place to be transferred to another, as more commonly occurs in prokaryotes (Figure 18.2B). Nevertheless, proof of the implied prevalence of familiar or unfamiliar glycans will require detailed structural evidence in conjunction with supporting biosynthetic, cell biological and functional studies. Given that much of what is known about cytoplasmic glycosylation has only recently emerged, it is indeed likely that much remains to be discovered in this realm, and that these pathways are much more common in both eukaryotes and prokaryotes than is currently appreciated. This promises to be an exciting and important area of research in the future.
ACKNOWLEDGMENTS
The authors appreciate helpful comments and suggestions from Jenny H.L. Chik, Nicholas Keul, Sarah Baas Robinson, and Krithika Vaidyanathan.
FURTHER READING
- Marchase RB, Hiller AM. 1986. Glucose phosphotransferase and intracellular trafficking. Mol Cell Biochem 72: 101–107. [PubMed: 3029558]
- Hart GW, Haltiwanger RS, Holt GD, Kelly WG. 1989. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem 58: 841–874. [PubMed: 2673024]
- Chandra NC, Spiro MJ, Spiro RG. 1998. Identification of a glycoprotein from rat liver mitochondrial inner membrane and demonstration of its origin in the endoplasmic reticulum. J Biol Chem 273: 19715–19721. [PubMed: 9677401]
- Lee JY, Spicer AP. 2000. Hyaluronan: A multifunctional, megadalton, stealth molecule. Curr Opin Cell Biol 12: 581–586. [PubMed: 10978893]
- Lomako J, Lomako WM, Whelan WJ. 2004. Glycogenin: The primer for mammalian and yeast glycogen synthesis. Biochim Biophys Acta 1673: 45–55. [PubMed: 15238248]
- Monsigny M, Rondanino C, Duverger E, Fajac I, Roche AC. 2004. Glyco-dependent nuclear import of glycoproteins, glycoplexes and glycosylated plasmids. Biochim Biophys Acta 1673: 94–103. [PubMed: 15238252]
- Haudek KC, Spronk KJ, Voss PG, Patterson RJ, Wang JL, Arnoys EJ. 2010. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim Biophys Acta 1800: 181–189. [PMC free article: PMC2815258] [PubMed: 19616076]
- Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. 2012. Glycogen and its metabolism: Some new developments and old themes. Biochem J 441: 763–787. [PMC free article: PMC4945249] [PubMed: 22248338]
- Belyi Y, Jank T, Aktories K. 2013. Cytotoxic glucosyltransferases of Legionella pneumophila. Curr Top Microbiol Immunol 376: 211–226. [PubMed: 23900830]
- De Castro C, Molinaro A, Piacente F, Gurnon JR, Sturiale L, Palmigiano A, Lanzetta R, Parrilli M, Garozzo D, Tonetti MG, Van Etten JL. 2013. Structure of N-linked oligosaccharides attached to chlorovirus PBCV-1 major capsid protein reveals unusual class of complex N-glycans. Proc Natl Acad Sci 110: 13956–13960. [PMC free article: PMC3752267] [PubMed: 23918378]
- Fredriksen L, Moen A, Adzhubei AA, Mathiesen G, Eijsink VG, Egge-Jacobsen W. 2013. Lactobacillus plantarum WCFS1 O-linked protein glycosylation: An extended spectrum of target proteins and modification sites detected by mass spectrometry. Glycobiology 23: 1439–1451. [PubMed: 24000282]
- Li S, Zhang L, Yao Q, Li L, Dong N, Rong J, Gao W, Ding X, Sun L, Chen X, Chen S, Shao F. 2013. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature 501: 242–246. [PubMed: 23955153]
- Peschke M, Hempel F. 2013. Glycoprotein import: A common feature of complex plastids? Plant Signal Behav 8: e26050. doi: 10.4161/psb.26050. [PMC free article: PMC4091080] [PubMed: 24220152] [CrossRef]
- Boudière L, Michaud M, Petroutsos D, Rébeillé F, Falconet D, Bastien O, Roy S, Finazzi G, Rolland N, Jouhet J, Block MA, Maréchal E. 2014. Glycerolipids in photosynthesis: Composition, synthesis and trafficking. Biochim Biophys Acta 1837: 470–480. [PubMed: 24051056]
- Bullard W, Lopes da Rosa-Spiegler J, Liu S, Wang Y, Sabatini R. 2014. Identification of the glucosyltransferase that converts hydroxymethyluracil to base J in the trypanosomatid genome. J Biol Chem 289: 20273–20282. [PMC free article: PMC4106341] [PubMed: 24891501]
- Stewart MD, Sanderson RD. 2014. Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biol 35: 56–59. [PMC free article: PMC4364407] [PubMed: 24309018]
- Lassak J, Keilhauer EC, Fürst M, Wuichet K, Gödeke J, Starosta AL, Chen JM, Søgaard-Andersen L, Rohr J, Wilson DN, Häussler S, Mann M, Jung K. 2015. Arginine-rhamnosylation as new strategy to activate translation elongation factor P. Nat Chem Biol 11: 266–270. [PMC free article: PMC4451828] [PubMed: 25686373]
- West CM, Blader IJ. 2015. Oxygen sensing by protozoans: How they catch their breath. Curr Opin Microbiol 26: 41–47. [PMC free article: PMC4623824] [PubMed: 25988702]
- Review Nucleocytoplasmic Glycosylation.[Essentials of Glycobiology. 2022]Review Nucleocytoplasmic Glycosylation.West CM, Slawson C, Zachara NE, Hart GW. Essentials of Glycobiology. 2022
- Review Nucleocytoplasmic Glycosylation.[Essentials of Glycobiology. 2009]Review Nucleocytoplasmic Glycosylation.Hart GW, West CM. Essentials of Glycobiology. 2009
- Review Nucleocytoplasmic O-glycosylation in protists.[Curr Opin Struct Biol. 2019]Review Nucleocytoplasmic O-glycosylation in protists.West CM, Kim HW. Curr Opin Struct Biol. 2019 Jun; 56:204-212. Epub 2019 May 22.
- Review Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc.[Science. 2001]Review Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc.Wells L, Vosseller K, Hart GW. Science. 2001 Mar 23; 291(5512):2376-8.
- Review Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription.[Prog Nucleic Acid Res Mol Biol...]Review Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription.Kamemura K, Hart GW. Prog Nucleic Acid Res Mol Biol. 2003; 73:107-36.
- Nucleocytoplasmic Glycosylation - Essentials of GlycobiologyNucleocytoplasmic Glycosylation - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

