NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.016

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsAnimal cells and some bacteria produce hyaluronan, a high-molecular-weight, nonsulfated glycosaminoglycan synthesized at the cell surface and extruded into the extracellular environment. This chapter describes the structure and metabolism of hyaluronan, its chemical and physical attributes, and its highly diverse and versatile biological functions.
HISTORICAL AND EVOLUTIONARY PERSPECTIVES
Sulfated glycosaminoglycans were first isolated in the late 1800s, and the isolation of hyaluronic acid (now called hyaluronan) followed in the early 1930s. In their classic paper, Karl Meyer and John Palmer named the “polysaccharide acid of high molecular weight” that they purified from bovine vitreous humor as “hyaluronic acid” (from hyaloid, meaning vitreous), and they showed that it contained “uronic acid (and) an amino sugar.” It took almost 20 years to determine the actual structure of the repeating disaccharide motif (GlcNAcβ4GlcAβ3) of hyaluronan (Figure 16.1). In contrast to the other classes of glycosaminoglycans, hyaluronan is not further modified by sulfation or by epimerization of the glucuronic acid moiety to iduronic acid. Thus, the chemical structure shown in Figure 16.1 is faithfully reproduced by any cell that synthesizes hyaluronan, including animal cells and bacteria.
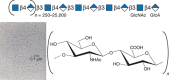
FIGURE 16.1.
Hyaluronan consists of repeating disaccharides composed of N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA). It is the largest polysaccharide found in vertebrates, and it forms hydrated matrices. (Electron micrograph provided by Dr. Richard Mayne (more...)
At first glance, the simplicity of hyaluronan might suggest that it arose early in evolution relative to other glycosaminoglycans. However, this is not the case, because Drosophila melanogaster and Caenorhabditis elegans do not contain the necessary synthases for its assembly. Instead, it appears that hyaluronan arose during the evolution of the notocord shortly before or concurrent with the advent of cartilage and appendicular skeletons apparently as a paralog of the more ancient cell-surface enzymes producing other β-linked polymers like chitin and cellulose. Virtually all cells from vertebrate species can produce hyaluronan, and its expression correlates with tissue expansion and cell motility. As discussed below, hyaluronan has essential roles in development, tissue architecture, cell proliferation, signaling reactions across the plasma membrane, inflammation, and microbial virulence.
STRUCTURE AND BIOPHYSICAL PROPERTIES
Hyaluronan has an indefinite and very high degree of polymerization, typically in the range of 104 disaccharides, with an end-to-end length of ∼10 µm (∼1 nm/disaccharide). Thus, a single molecule of hyaluronan could stretch about halfway around the circumference of a typical mammalian cell. The carboxyl groups on the glucuronic acid residues (pKa 4–5) are negatively charged at physiological pH and ionic strength, making hyaluronan polyanionic. The anionic nature of hyaluronan together with spatial restrictions around the glycosidic bonds confer a relatively stiff, random coil structure to individual hyaluronan molecules in most biological settings. Hyaluronan chains occupy a large hydrodynamic volume such that in a solution containing 3–5 mg/mL hyaluronan, individual molecules occupy essentially all of the solvent. This arrangement creates a size-selective barrier in which small molecules can diffuse freely, whereas larger molecules are partially or completely excluded. Additionally, this solution shows high viscosity with viscoelastic properties, conditions found in the vitreous humor of the human eye and in synovial fluid of joints. Hyaluronan in synovial fluids of articular joints is essential for distributing load during joint motion and for protecting the cartilaginous surfaces. Thus, in both eye and joint tissues, the physical properties of hyaluronan relate directly to tissue function.
BIOSYNTHESIS
Hyaluronan biosynthesis is catalyzed by hyaluronan synthases (HASs) (Figure 16.2). The first bona fide HAS gene (spHas) was cloned from Streptococcus, and the protein expressed in Escherichia coli (E. coli) was shown to synthesize high-molecular-weight hyaluronan from the UDP-sugar substrates. The gene shows homology with a Xenopus gene, DG42 (now known as xlHAS1; Chapter 27). The homology was instrumental in the subsequent identification of the three members of the mammalian Has gene family, Has1–3. These genes code for homologous proteins predicted to contain five to six membrane-spanning segments and a central cytoplasmic domain.
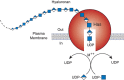
FIGURE 16.2.
Hyaluronan biosynthesis by hyaluronan synthase (Has) occurs by addition of UDP-sugars (UDP-N-acetyl-glucosamine and UDP-glucuronic acid) to the reducing end of the polymer with release of the anchoring UDP. M++ refers to a metal ion cofactor.
As described in Chapter 17, cells synthesize sulfated glycosaminoglycans (heparan sulfate, chondroitin sulfate, and keratan sulfate) on core proteins of proteoglycans as they transit through the Golgi, and elongation of the chains occurs at their nonreducing ends. In contrast, hyaluronan synthesis normally occurs at the inner surface of the plasma membrane in eukaryotic cells and at the cytoplasmic membrane of bacteria that produce hyaluronan capsules. The synthases use the cytosolic substrates UDP-glucuronic acid (UDP-GlcA) and UDP-N-acetylglucosamine (UDP-GlcNAc) and extrude the growing polymer through the membrane to form extracellular matrices (Figure 16.2). According to the model, the reducing end of the growing chain would have a UDP moiety that is displaced when the next nucleotide sugar is added. In keeping with its evolutionary history, it appears HA synthesis is initiated by production of a short chain of chitin-like GlcNAcβ4GlcNAc.
In typical cultured mammalian cells, division under conditions of hyperglycemia (two to three times normal glucose level) results in hyaluronan synthesis in the endoplasmic reticulum (ER), Golgi, and transport vesicles. Under these conditions, the elongating hyaluronan chains are inserted inappropriately into these compartments, inducing abnormalities in cellular functions (e.g., kidney nephropathy and proteinurea). The activity of the HAS enzymes also can be regulated by phosphorylation and the addition of O-GlcNAc (Chapter 19).
Hyaluronan biosynthesis in bacteria involves the expression of multiple enzymes, usually as an operon. For example, in Streptococcus, hasC encodes an enzyme that makes UDP-Glc from UTP and glucose-1-P; hasB encodes the dehydrogenase that converts UDP-Glc to UDP-GlcA; hasD generates UDP-GlcNAc from glucosamine-1-P, acetyl CoA, and UTP; and hasA (spHas) encodes the hyaluronan synthase. The Streptococcus hasA gene encodes a bifunctional protein that contains both transferase activities. Thus, spHas assembles the polysaccharide from the reducing end. The synthase spans the membrane multiple times, presumably forming a pore for hyaluronan extrusion during capsule formation. In contrast, Pasteurella synthesizes hyaluronan by an enzyme that is unrelated to hasA and the mammalian Has gene family. In this case, the enzyme has two separable domains with independent glycosyltransferase activities—one for UDP-GlcNAc and the other for UDP-GlcA, and the elongation is on the nonreducing end.
THE HYALURONIDASES AND HYALURONAN TURNOVER
Animal cells express a set of catabolic enzymes that degrade hyaluronan. The human hyaluronidase gene (HYAL) family is complex, with two sets of three contiguous genes located on two chromosomes, a pattern that suggests two ancient gene duplications followed by a block duplication. In humans, the cluster on chromosome 3p21.3 (HYAL1, 2, and 3) appears to have major roles in somatic tissues. HYAL4 in the cluster on chromosome 7q31.3 codes for a protein that appears to have chondroitinase, but not hyaluronidase, activity; PHYAL1 is a pseudogene; and SPAM1 (sperm adhesion molecule 1, PH-20) is restricted to testes. The role of SPAM1 in fertilization is discussed below.
The turnover of hyaluronan in most tissues is rapid (e.g., a half-life of ∼1 day in epidermal tissues), but its residence time in some tissues can be quite long and dependent on location (e.g., in cartilage). It has been estimated that an adult human contains approximately 15 g of hyaluronan and that about one-third turns over daily. Turnover appears to occur by receptor-mediated endocytosis and lysosomal degradation either locally or after transport by lymph to lymph nodes or by blood to liver. The endothelial cells of the lymph node and liver sinusoids remove hyaluronan via specific receptors, such as LYVE-1 (a homolog of CD44) and HARE (hyaluronan receptor for endocytosis). HARE appears to be the major clearance receptor for hyaluronan delivered systemically by lymph and blood. The current understanding of this catabolic process is that hyaluronidases at the cell surface and in the lysosome cooperate to degrade the chains. Large hyaluronan molecules in the extracellular space interact with cell-surface receptors that internalize fragments produced by membrane-associated hyaluronidases, including HYAL2 and TMEM2. The fragments are transported into a unique vesicular endosomal compartment and eventually enter a pathway to lysosomes for complete degradation to monosaccharides, probably involving HYAL1 and the two exoglycosidases β-glucuronidase and β-N-acetylglucosaminidase. The importance of this process is demonstrated by the facts that Hyal2-null mice show lethality at embryonic day 9.5 and Tmem2-deficient mice accumulate hyaluronan and by the identification of a lysosomal storage disorder in a person with a mutation in HYAL1.
Hyaluronan fragments have been suggested to act as an endogenous signal of injury, or during infection by Group A Streptococcus, which contains a hyaluronan capsule. The signaling activity of HA fragments is mediated through binding of cell-surface receptors, such as CD44, which in turn modulates response through toll-like receptors. Signaling through these and other receptors is affected by the size of the hyaluronan fragments, but the mechanism underlying the dependence of activity on the size of the fragments remains an area of active research.
HYALURONAN FUNCTION IN THE EXTRACELLULAR MATRIX
Hyaluronan has multiple roles in early development, tissue organization, and cell proliferation. The Has2-null mouse shows an embryonic lethal phenotype at the time of heart formation, whereas Has1-, Has3-null, and Has1/3 compound mutant mice show no obvious developmental phenotype. Interestingly, explanted cells from the Has2-null embryonic heart do not synthesize hyaluronan or undergo epithelial–mesenchymal transformation and migration unless small amounts of hyaluronan are added to the culture medium. This finding indicates that the production of hyaluronan at key points may be essential for many tissue morphogenetic transformations—in this case, formation of the tricuspid and mitral valves.
Many of the activities of hyaluronan depend on binding proteins present on the cell surface and/or secreted into the extracellular matrix. A class of proteins that bind selectively to hyaluronan was first discovered in cartilage. This class is now referred to as the link module family of hyaladherins (Figure 16.3). Proteoglycans were efficiently extracted from this tissue with denaturing solvents and were shown to reaggregate when restored to renaturing conditions. An essential protein, referred to as the link protein (HAPLN-1), was shown to be necessary for stabilizing the proteoglycan aggregates, and subsequently, the structure of the aggregate was defined (Figure 16.4). The link protein contains two homologous repeats of a sequence motif, now named the link module. Proteins that have a link module, including link proteins (HAPLN-1 through -4 in humans), several proteoglycans, and other extracellular matrix proteins, can interact specifically with hyaluronan. The major cartilage proteoglycan, now named aggrecan (Chapter 17), also contains a globular domain, the G1 domain, with two homologous link modules that interact with hyaluronan. An additional domain in the link protein (HAPLN-1) cooperatively interacts with a homologous domain in G1, which locks the proteoglycan on the hyaluronan chain. In the absence of the HAPLN-1, aggrecan fails to anchor to hyaluronan. Mice deficient in HAPLN-1 show defects in cartilage development and delayed bone formation (short limbs and craniofacial anomalies). Most mutant mice die shortly after birth as a result of respiratory failure, and the few survivors develop progressive skeletal deformities.
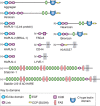
FIGURE 16.3.
Modular organization of the link module superfamily of hyaluronan-binding proteins. These proteins contain one or two link modules that bind to hyaluronan. Like many extracellular matrix proteins, the link module superfamily members contain various subdomains, (more...)
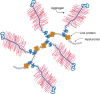
FIGURE 16.4.
The large cartilage chondroitin sulfate (CS) proteoglycan (aggrecan) forms an aggregate with hyaluronan and link protein.
Interestingly, there are four proteoglycan genes with homologous G1 domains that interact with hyaluronan (versican, neurocan, brevican, and aggrecan) (Figure 16.3). Versican is a major component of many soft tissues and is especially important in vascular biology. Neurocan and brevican are expressed predominantly in brain tissue. Versican and aggrecan are anchored to hyaluronan in tissues by similar link protein-dependent mechanisms, and it is likely that neurocan and brevican are organized similarly. In the brain, these complexes form perineuronal nets. Thus, hyaluronan acts as a scaffold on which to build proteoglycan aggregate structures adapted to diverse tissue functions.
An impressive example of the requirement for a hyaluronan-based matrix occurs during the process of cumulus oophorus expansion in the mammalian preovulatory follicle. At the beginning of this process, the oocyte is surrounded by about 1000 cumulus cells tightly compacted and in gap-junction contact with the oocyte. In response to hormonal stimuli, the cumulus cells up-regulate HAS2 and a link module family hyaladherin encoded by tumor necrosis factor–stimulated gene 6 (TSG-6). The expression of these proteins initiates production of hyaluronan and its organization into an expanding matrix around the cumulus cells. Concurrently, the follicle becomes permeable to serum, which introduces an unusual molecule called inter-α-trypsin inhibitor (ITI), composed of the trypsin inhibitor bikunin and two heavy chains all covalently bound to a chondroitin sulfate chain. In a complex process, TSG-6 catalyzes the transfer of heavy chains that are covalently linked to chondroitin sulfate (via an ester bond between the carboxy-terminal aspartate residue of the heavy chains and the C-6 of N-acetylgalactosamine in chondroitin-4-sulfate) onto the newly synthesized hyaluronan by transesterification to C6 of an N-acetylglucosamine residue. In the absence of either TSG-6 or ITI, the matrix does not form, and the phenotype of mice null for either of these molecules is female infertility. At the time of ovulation, hyaluronan synthesis ceases, and ovulation of the expanded cumulus cell–oocyte complex occurs. Before fertilization, individual sperm undergo capacitation enabling them to penetrate and fertilize an ovum. During this process, SPAM1/PH20, a GPI-anchored hyaluronidase, redistributes and accumulates in the sperm head. SPAM1 binds hyaluronan in the cumulus, causing an increase in Ca++ flux and sperm motility. It also helps dissolve the cumulus matrix as the sperm moves through the hyaluronan vestment. A soluble form of SPAM1 is secreted during the acrosome reaction. The release of acrosomal hyaluronidase and proteases renders the sperm capable of fusing with the egg and eventually destroys the entire matrix to allow the fertilized oocyte to implant and develop.
HYALURONAN-BINDING PROTEINS WITH LINK MODULES
There are several hyaluronan-binding proteins with homologous link modules (Figure 16.3). The four homologous link proteins belong to a subfamily called the “hyaluronan and proteoglycan link proteins” (HAPLNs); these are expressed in many tissues. Four cell-surface receptors have extracellular domains with one link module: CD44, LYVE-1 (lymphatic vessel endothelial hyaluronan receptor), HARE/STABILIN-2 (hepatic hyaluronan clearance receptor), and STABILIN-1, which are expressed on discontinuous endothelial cells and some activated macrophages. Other hyaluronan-binding proteins are secreted and include the chondroitin sulfate proteoglycans that comprise the aggrecan superfamily (aggrecan, versican, brevican, and neurocan) and tumor necrosis factor (TNF)-α-stimulated gene 6 (TSG-6), which has one link module.
The three-dimensional structure of the link module fold in TSG-6 has been determined by nuclear magnetic resonance and defines a consensus fold of the two α-helices and two triple-stranded antiparallel β-sheets (Figure 16.5). The fold consists of about 100 amino acids and contains four cysteines disulfide-bonded in the pattern Cys1-Cys4 and Cys2-Cys3. This fold has only been found in vertebrates, consistent with the fact that hyaluronan is a relatively recent evolutionary invention. The link module fold is related to that found in the C-type lectins, but it lacks the Ca++ binding motif (Chapter 34). In the case of TSG-6, the interaction of hyaluronan with the protein involves (1) ionic interactions between positively charged amino acid residues and the carboxyl groups of the uronic acids, and (2) hydrophobic interactions between the acetamido side chains of two N-acetylglucosamine residues and hydrophobic pockets on either side of adjacent tyrosines (Figure 16.5). Many of these features are conserved in other members of the hyaluronan-binding proteins. Subgroups, however, differ in the preferred size and length of hyaluronan for binding (e.g., hexasaccharides to decasaccharides).
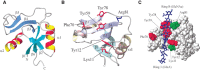
FIGURE 16.5.
Structure of the link module. (A) TSG-6 contains a prototypical link module defined by two α-helices (α1 and α2) and two triple-stranded antiparallel β-sheets (β1,2,6 and β3–5). (Redrawn, with permission, (more...)
Some hyaluronan-binding proteins do not contain a link module (RHAMM, ITI, SPACR, SPACRCAN, CD38, CDC37, HABP1/P-32, Siglec-9, and IHABP4), and most of these are unrelated to one another by primary sequence. Some of these proteins contain clusters of basic amino acids, referred to as BX7B motifs (where B is either lysine or arginine and X can be any amino acid other than acidic residues), but the actual hyaluronan docking site of the chain with this motif has not been established. Thus, the presence of the BX7B motif should not be taken as proof that the protein interacts with hyaluronan.
HYALURONAN AND CELL SIGNALING
Hyaluronan expression has long been implicated in enhanced cell adhesion and locomotion because it is expressed abundantly during morphogenesis and in both physiological and pathological invasive processes. A search for cell-surface receptors revealed two major hyaluronan-binding proteins: CD44 and RHAMM (receptor for hyaluronan-mediated motility). CD44 is a transmembrane receptor expressed by many cell types, and it varies markedly in glycosylation, oligomerization, and protein sequence because of differential messenger RNA (mRNA) splicing. CD44H (the isoform expressed by hematopoietic cells) binds to hyaluronan, and the interaction can mediate leukocyte rolling and extravasation in some tissues. Changes in CD44 expression, notably expression of CD44 variants, are associated with a wide variety of tumors and the metastatic spread of cancer. Many cells also express the receptor RHAMM, which is involved in cell motility and cell transformation. The RHAMM pathway is thought to induce focal adhesions to signal the cytoskeletal changes required for elevated cell motility seen in tumor progression, invasion, and metastasis. Like CD44, RHAMM splice variants exist, some of which may be intracellular.
CD44 contains a cytoplasmic domain, a transmembrane segment, and an ectodomain with a single link module that can bind hyaluronan. When hyaluronan binds to CD44, the cytoplasmic tail can interact with regulatory and adaptor molecules, such as SRC kinases, RHO (ras homolog) GTPases, VAV2 (a human proto-oncogene), GAB1 (a GRB2-associated binding protein), and ankyrin and ezrin (which regulate cytoskeletal assembly/disassembly and cell migration). Hyaluronan binding to RHAMM also transduces signals that influence growth and motility, for example, by activating SRC, FAK (focal adhesion kinase), ERK (extracellular mitogen-regulated protein kinase), and PKC (protein tyrosine kinase C) (Chapter 40).
Interaction of hyaluronan with CD44 can also regulate ERBB-family (epithelial growth factor receptor) signaling, thereby activating the PI3K (phosphatidylinositol 3-kinase)–PKB/AKT (protein kinase B) signaling pathway and phosphorylation of FAK and BAD (BCL2-antagonist of cell death), which promote cell survival. RHAMM can interact with and activate ERK1, which can also phosphorylate BAD. Thus, both CD44 and RHAMM interactions with hyaluronan can influence cell survival. These pathways are relevant to tumor cell survival and invasion; their inhibition by hyaluronan oligomers and soluble hyaluronan-binding proteins suggests novel therapeutic approaches for treating cancer (Chapter 47).
HYALURONAN CAPSULES IN BACTERIA
Some pathogenic bacteria (e.g., certain strains of Streptococcus and Pasteurella) produce hyaluronan and deposit it as an extracellular capsule (Figure 16.6; also see Chapter 21). Capsular hyaluronan, like other capsular polysaccharides, increases virulence by helping to shield the microbe from host defenses. For example, the capsule blocks phagocytosis and protects against complement-mediated killing. Because bacterial hyaluronan is identical in structure to host hyaluronan, the capsule can also prevent the formation of protective antibodies. Thus, the formation of hyaluronan capsules by bacteria is a form of molecular mimicry. The capsule also can aid in bacterial adhesion to host tissue, facilitating colonization (Chapter 37). Finally, the production of hyaluronan by invading bacteria can also induce a number of signaling events through hyaluronan-binding proteins that modulate the host physiology (i.e., cytokine production [Chapter 42]).
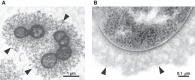
FIGURE 16.6.
Hyaluronan capsule. Cross-sectioned Streptococcus zooepidemicus (S. zooepidemicus) cells surrounded by a hyaluronan capsule and observed at differing magnifications. (A) Scale bar, 1 µm. (Reprinted, with permission, from Chong BF, Blank LM, McLaughlin (more...)
In addition to bacteria, an algal virus (Chlorella) encodes a hyaluronan synthase. The functional significance of viral hyaluronan production is unknown, but could be related to prevention of secondary viral infection, increase in host capacity to produce virus, or viral burst size. The origin of viral HAS is unknown, but based on sequence homology it most likely arose from a vertebrate.
HYALURONAN AS A THERAPEUTIC AGENT
Hyaluronan has been used therapeutically for a number of years. Patients with osteoarthritis obtain short-term relief by direct injection of high-molecular-weight hyaluronan into the synovial space of an affected joint. The mechanism of action is complex and probably involves both the viscoelastic properties of the polymer as well as effects on the growth of synovial cells in the joint capsule. Hyaluronan suppresses cartilage degeneration, acts as a lubricant (thereby protecting the surface of articular cartilage), and reduces pain perception.
The application of hyaluronan in ophthalmology is widespread. During surgery for lens replacement due to cataracts, a high potential for injury of fragile intraocular tissues exists, especially for the endothelial layer of the cornea. High-molecular-weight hyaluronan is injected to maintain operative space and structure and to protect the endothelial layer from physical damage. Hyaluronan also has been approved for cosmetic use (e.g., by subdermal injection to fill wrinkles or pockets under the skin).
The fact that the small organic molecule 4-methylumbelliferone (4-MU) acts as a sink for consumption of UDP-GlcA has been taken advantage of to reduce HA synthesis. A low-dose formulation of 4-MU is already clinically approved for treatment of biliary dyskinesia, presumably by altering the glucuronidation of bile acids. However, depletion of UDP-GlcA could affect many other biochemical pathways, and these high levels of 4-MU could well have other effects.
Low-molecular-weight hyaluronan oligosaccharides (∼103–104 Da) also have potent biological activities by altering selective signaling pathways. In cancer cells, hyaluronan oligosaccharides induce apoptosis and inhibit tumor growth in vivo. Thus, short hyaluronan chains may prove useful for preventing cancer metastasis by boosting certain immune responses or altering new blood vessel growth. Recently, recombinant forms of the Pasteurella synthase (pmHas) have been engineered to produce hyaluronan oligosaccharides of defined size. This strategy has great promise for exploring the relationship of hyaluronan size to function, which may in turn yield new therapeutic agents with selective activities.
ACKNOWLEDGMENTS
The authors appreciate helpful comments and suggestions from Nicholas Keul, Sumit Rai, Kristin Stanford, and Kekoa Taparra.
FURTHER READING
- Simoni RD, Hill RL, Vaughan M, Hascall V. 2002. The discovery of hyaluronan by Karl Meyer. J Biol Chem 277: e27.
- Toole BP. 2004. Hyaluronan: From extracellular glue to pericellular cue. Nat Rev Cancer 4: 528–539. [PubMed: 15229478]
- Jiang D, Liang J, Noble PW. 2007. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23: 435–461. [PubMed: 17506690]
- Weigel PH, Deangelis PL. 2007. Hyaluronan synthases: A decade-plus of novel glycosyltransferases. J Biol Chem 282: 36777–36781. [PubMed: 17981795]
- Zhuo L, Kimata K. 2008. Structure and function of inter-α-trypsin inhibitor heavy chains. Connect Tissue Res 49: 311–320. [PubMed: 18991084]
- Wang A, de la Motte C, Lauer M, Hascall V. 2011. Hyaluronan matrices in pathobiological processes. FEBS J 278: 1412–1418. [PMC free article: PMC4401461] [PubMed: 21362136]
- Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, Vigetti D, Hanson RW, Hart GW. 2014. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol 35: 14–17. [PMC free article: PMC4039572] [PubMed: 24486448]
- Petrey AC, de la Motte CA. 2014. Hyaluronan, a crucial regulator of inflammation. Frontiers Immunol 5: 101. [PMC free article: PMC3949149] [PubMed: 24653726]
- Smith PD, Coulson-Thomas VJ, Foscarin S, Kwok JC, Fawcett JW. 2015. GAG-ing with the neuron: The role of glycosaminoglycan patterning in the central nervous system. Exp Neurol 274: 100–114. [PubMed: 26277685]
- Weigel PH. 2015. Hyaluronan synthase: The mechanism of initiation at the reducing end and a pendulum model for polysaccharide translocation to the cell exterior. Int J Cell Biol 2015: 367579. [PMC free article: PMC4581545] [PubMed: 26472958]
- Liang J, Jiang D, Noble PW. 2017. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev 97: 186–203. [PMC free article: PMC4753080] [PubMed: 26541745]
- Weigel PH, Baggenstoss BA, Washburn JL. 2017. Hyaluronan synthase assembles hyaluronan on a [GlcNAcβ1,4]n-GlcNAcα-UDP primer and hyaluronan retains this residual chitin oligomer as a cap at the nonreducing end. Glycobiology 27: 536–554. [PMC free article: PMC5421502] [PubMed: 28138013]
- Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Oyama C, Yamaguchi Y. 2017. A mammalian homolog of the zebrafish Transmembrane Protein 2 (TMEM2) is the long-sought-after cell surface hyaluronidase. J Biol Chem 292: 7304–7313. [PMC free article: PMC5418033] [PubMed: 28246172]
- HISTORICAL AND EVOLUTIONARY PERSPECTIVES
- STRUCTURE AND BIOPHYSICAL PROPERTIES
- BIOSYNTHESIS
- THE HYALURONIDASES AND HYALURONAN TURNOVER
- HYALURONAN FUNCTION IN THE EXTRACELLULAR MATRIX
- HYALURONAN-BINDING PROTEINS WITH LINK MODULES
- HYALURONAN AND CELL SIGNALING
- HYALURONAN CAPSULES IN BACTERIA
- HYALURONAN AS A THERAPEUTIC AGENT
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Hyaluronan.[Essentials of Glycobiology. 2022]Review Hyaluronan.Simpson M, Schaefer L, Hascall V, Esko JD. Essentials of Glycobiology. 2022
- Review Hyaluronan.[Essentials of Glycobiology. 2009]Review Hyaluronan.Hascall V, Esko JD. Essentials of Glycobiology. 2009
- Review Hyaluronan in the Tumor Microenvironment.[Adv Exp Med Biol. 2020]Review Hyaluronan in the Tumor Microenvironment.Spinelli FM, Vitale DL, Sevic I, Alaniz L. Adv Exp Med Biol. 2020; 1245:67-83.
- Low molecular weight hyaluronan induces malignant mesothelioma cell (MMC) proliferation and haptotaxis: role of CD44 receptor in MMC proliferation and haptotaxis.[Oncol Res. 2002]Low molecular weight hyaluronan induces malignant mesothelioma cell (MMC) proliferation and haptotaxis: role of CD44 receptor in MMC proliferation and haptotaxis.Nasreen N, Mohammed KA, Hardwick J, Van Horn RD, Sanders K, Kathuria H, Loghmani F, Antony VB. Oncol Res. 2002; 13(2):71-8.
- Review [Hyaluronan: structure, metabolism, functions, and role in wound healing].[Postepy Hig Med Dosw (Online)....]Review [Hyaluronan: structure, metabolism, functions, and role in wound healing].Olczyk P, Komosińska-Vassev K, Winsz-Szczotka K, Kuźnik-Trocha K, Olczyk K. Postepy Hig Med Dosw (Online). 2008 Dec 2; 62:651-9. Epub 2008 Dec 2.
- Hyaluronan - Essentials of GlycobiologyHyaluronan - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

