NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.014

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsThis chapter describes the variable components of N-glycans, O-glycans, and glycolipids attached to the core of each glycan class and presented in Chapters 9, 10, and 11. The glycan extensions of these cores form the mature glycan and may include human blood group determinants. The terminal sugars of the mature glycan often regulate the function(s) or recognition properties of a glycoconjugate. Also discussed are milk oligosaccharides, that carry many of the same extensions on a lactose core.
REGULATED GLYCOSYLATION OF GLYCAN EXTENSIONS
Many glycan extensions are regulated during embryogenesis and in the postnatal period as part of the normal developmental program (Chapter 41). Changes in terminal glycan structure are also often associated with malignant transformation in cancer (Chapter 47). Tissue- and/or lineage-specific regulation of glycan extension biosynthesis is largely due to the regulated expression of the relevant glycosyltransferases. Biological consequences of such changes in glycan extensions are discussed throughout this volume. However, the majority of regulated terminal glycosylations observed likely have many different functions, but they are not well understood.
Type-2 Glycan Units (LacNAc)
The core structures in Figure 14.1 have a terminal GlcNAc and may receive β1-4Gal to generate a Type-2 unit composed of Galβ1-4GlcNAc, also called N-acetyllactosamine (LacNAc) (Figure 14.2). The terminal Gal so generated can receive a β1-3GlcNAc, which in turn can receive a β1-4Gal, thus forming two LacNAc units. These reactions may recur to form poly-N-acetyllactosamine [-3Galβ1-4GlcNAcβ1-]n (poly-LacNAc). Poly-LacNAc chains occur in glycans from most cell types. An alternative is a chain composed of LacdiNAc glycan units [GalNAcβ1-4GlcNAc]n generated by the action of a β1-4GalNAc-transferase. LacdiNAc (GalNAcβ1-4GlcNAc) termini occur on N-glycans in bovine milk, rat prolactin, and kidney epithelial cells, as well as in invertebrates such as snails and worms (Chapter 25). These residues are frequently α2-6-sialylated in vertebrates.
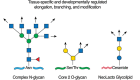
FIGURE 14.1.
N-Glycan synthesis (Chapter 9) leads to complex N-glycans with branching GlcNAc residues that are generally extended (arrows) in glycosylation reactions that may be tissue-specific, developmentally regulated, or even protein-specific. The GlcNAc linked (more...)
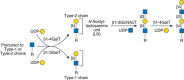
FIGURE 14.2.
Terminal GlcNAc residues are usually galactosylated. Modification by β1-4Gal (top) occurs in all mammalian tissues. This reaction is catalyzed by β1-4 galactosyltransferases (B4GALT1 to B4GALT6) and yields the Galβ1-4GlcNAc (N- (more...)
Type-1 Glycan Units
Terminal GlcNAc residues in N-glycans, O-glycans, and glycolipids may alternatively be modified by β1-3Gal (Figure 14.2) to generate a Type-1 unit composed of Galβ1-3GlcNAc. In humans, expression of Type-1 units is relatively high in O-glycans of glycoproteins and glycolipids in the epithelia of the gastrointestinal or reproductive tracts.
Type-1 and Type-2 units may be further modified by glycosyltransferases that transfer sugars to terminal Gal or subterminal GlcNAc, generating sialylated, fucosylated, or sulfated structures or blood group determinants.
Poly-N-Acetyllactosamines and i and I Human Blood Groups
Some glycoproteins and glycolipids preferentially carry poly-LacNAc. This implies that the glycosyltransferases responsible can discriminate between glycan acceptors with terminal GlcNAc or Gal. For example, poly-LacNAc extensions preferentially occur on multiantennary N-glycans, particularly on the β1-6GlcNAc branch synthesized by N-acetylglucosaminyltransferase V (GlcNAc-TV, MGAT5) (Chapter 9). Similarly, poly-LacNAc extensions on O-GalNAc glycans associated with mucin glycoproteins often preferentially occur on the β1-6GlcNAc transferred by a core 2 β1-6GlcNAcT (CGNT1 to CGNT4; Chapter 10). N-Glycans generally have longer poly-LacNAc extensions than O-glycans, and both may receive sialic acid or Fuc residues or sulfate. Thus, poly-LacNAc chains may serve as linear, extended scaffolds for the presentation of specific terminal glycans, whose functions require them to be presented at a certain distance from the plasma membrane. Poly-LacNAc chains are also recognized with high affinity by galectins that recognize internal as well as terminal Gal residues (Chapter 36). Interestingly, poly-LacNAc is also the backbone of keratan sulfate (KS, see below), in which the Gal and GlcNAc residues are 6-O-sulfated, and such sulfated poly-LacNAc is known not to be recognized by galectins.
Poly-LacNAc chains may also become branched by the addition of β1-6GlcNAc to internal Gal residues. Branched and nonbranched poly-LacNAc chains correspond to the “I” and “i” blood group antigens, respectively (Figure 14.3). These antigens were originally discovered during the analysis of a cold-dependent agglutinating antibody (cold agglutinin) in a patient with acquired hemolytic anemia (Chapter 46). Cold agglutinin antibodies interact with red blood cells (erythrocytes) that express the I blood group (the “I” antigen). Nonreactive donors are classified as having the i blood group. Distinct β1-6GlcNAcTs yield different types of β1-6-branched glycans (Figure 14.3). The i antigen is abundantly expressed on the surface of embryonic erythrocytes and on erythrocytes during times of altered erythropoiesis. Such cells are relatively deficient in the expression of the I antigen. However, during the first 18 months of life, I antigen reactivity on erythrocytes reaches adult levels, and i antigen reactivity declines to very low levels. This developmental regulation is presumed to be due to regulated expression of CGNT1 to CGNT4 genes that encode β1-6GlcNAcTs. Rare individuals never express the I antigen on erythrocytes and maintain embryonic levels of erythrocyte i antigen expression as adults. Individuals with this i phenotype are potentially homozygous for inactive alleles at CGNT loci. There is no obvious pathophysiology associated as yet with the absence of the I blood group in humans.
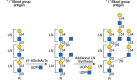
FIGURE 14.3.
Blood group i and I antigen synthesis. Linear poly-N-acetyllactosamine chains (i antigen) synthesized on N- and O-glycans or glycolipids (R) may be modified by β1-6 N-acetylglucosaminyltransferases (GCNT1 to GCNT4). These enzymes transfer GlcNAc (more...)
THE A, B, AND H HUMAN BLOOD GROUPS
The ABO blood group antigens were discovered early in the 20th century by Karl Landsteiner and colleagues. They showed that humans could be divided into different groups according to the presence or absence of serum factors that would agglutinate red blood cells isolated from other humans. We now know that these serum factors are antibodies and that the corresponding antigens are glycan epitopes determined by the inheritance of genes that, for the most part, encode glycosyltransferases.
The A, B, and H blood group antigens are glycans presented on Type-1 or Type-2 LacNAc (Figure 14.2), on O-GalNAc glycans (Type-3), or on glycolipids (Type-4) (Figure 14.4). The blood group antigens are formed by the sequential action of glycosyltransferases encoded by the ABO, H, and Se genes, now termed the ABO, FUT1, and FUT2 loci (Figure 14.5). Blood group antigen synthesis begins with modification of Type-1 or Type-2 LacNAc by the transfer of α1-2Fuc to Gal to form the blood group H determinant. The H allele encodes an α1-2FucT (FUT1) expressed in erythrocyte precursors and transfers Fuc to Type-2 and Type-4 glycan units to form the H antigen on erythrocytes (Figure 14.4). The Se allele encodes another α1-2FucT (FUT2) expressed in epithelial cells and uses Type-1 and Type-3 LacNAc to form the H antigen in epithelia lining the lumen of the gastrointestinal, respiratory, and reproductive tracts and in salivary glands (Figure 14.4), as well as modifying milk oligosaccharides to generate the H antigen.
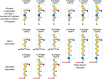
FIGURE 14.4.
Type-1, -2, and -3 H, A, and B antigens that form the O (H), A, and B blood group determinants on N- and O-glycans. Type-4 H, A, and B antigens form the O (H), A, and B blood group determinants on glycolipids.
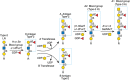
FIGURE 14.5.
Synthesis of H (O), A, and B blood group determinants. For details, see text. LN designates N-acetyllactosamine unit.
A or B blood group determinants are subsequently formed from H Type-1, -2, -3, or -4 determinants by glycosyltransferases encoded by the ABO locus. The A allele encodes the α1-3GalNAcT that generates the A glycan epitope (A3GALNT), forming the A blood group (Figure 14.5). The B allele of the ABO locus encodes the α1-3GalT (A3GALT1) that forms the B glycan determinant and generates the B blood group (Figure 14.5). O alleles at the ABO locus encode a functionally inactive A/B glycosyltransferase. Individuals who synthesize exclusively A determinants are blood group A and have the genotype AA or AO, blood group B individuals are BB or BO, and individuals that express one A and one B allele have the genotype AB. Blood group O individuals expressing inactive A/B glycosyltransferase have the genotype OO. They express only the H antigen. Blood type designations are the same as the blood group genotypes above. In terms of nomenclature, the O blood group includes the H antigen and occasionally the term ABO(H) is used.
The ABO antigens are expressed on membrane glycoproteins and glycolipids on the surface of erythrocytes and many epithelial or endothelial cells in tissues. Some tissues also synthesize soluble forms on secreted glycoproteins, glycolipids, and free glycans. As discussed below, the ability to secrete soluble molecules carrying ABO(H) blood group antigens is a genetically determined function of alleles at the Se (FUT2) locus. On each human red blood cell, ∼80% of the one to two million ABO(H) determinants are attached to the anion transport protein Band 3, and ∼15% are carried by the erythrocyte glucose transport protein Band 4.5. Both of these integral membrane proteins carry ABO(H) antigens on a single branched N-glycan with poly-LacNAc. Each erythrocyte has other glycoproteins and approximately half a million glycolipids with ABO(H) determinants. Many of these glycolipids have A, B, and H determinants on poly-LacNAc chains and have been termed polyglycosylceramides or macroglycolipids. A, B, and H determinants based on Type-4 chains (Figure 14.4) are also present in human erythrocyte glycolipids.
A, B, and H determinants of the epidermis are primarily on Type-2 units, whereas mucins of the gastric mucosa and in ovarian cyst fluid carry A, B, and H antigens on Type-3 units (Figure 14.4). Epithelial cells lining the digestive, respiratory, urinary, and reproductive tracts and epithelia of some salivary and exocrine glands synthesize soluble forms of the ABO(H) determinants, largely carried on Type-1 units (Figure 14.4). Expression of the A, B, and H determinants in secretory tissues is a function of the α1-2FucT encoded by the Se gene (FUT2), because the H gene is not expressed there. Humans with an inactive FUT2 gene do not express soluble forms of the A, B, or H determinants in saliva or milk oligosaccharides or in other tissues and are termed “nonsecretors.”
Serology is used to characterize erythrocytes for transfusion and has identified variants of the A and B blood group determinants that typically yield weak reactivity with blood typing reagents. Interestingly, plant lectins were used historically to aid in typing blood. For example, the lectin from Dolichos biflorus agglutinates erythrocytes from most blood group A individuals (termed A1 individuals), but it does not agglutinate erythrocytes from individuals of the A2 subgroup. The A1 and A2 subgroup antigens are distinct (Figure 14.5). These structural differences reflect the different catalytic activities of the A transferases encoded by the A1 versus A2 allele in the ABO locus.
Heritable erythrocyte antigenic polymorphisms determined by the ABO locus have important medical implications. Early in the postnatal period, the immune system generates IgM antibodies against ABO antigen(s), even though they are absent from erythrocytes. This is because glycan antigens similar or identical to the A and B blood group determinants are carried by colonizing bacteria and fungi. Thus, type-O individuals do not synthesize A or B determinants but show relatively high titers of circulating IgM antibodies (termed isoagglutinins) against A and B blood group determinants. Similarly, blood group B individuals show circulating IgM anti-A isoagglutinins, but they do not make isoagglutinins against the blood group B determinant, a “self” antigen. Conversely, serum from blood group A individuals contains anti-B but not anti-A antibodies. Finally, people with the AB blood group do not make either anti-A or anti-B IgM isoagglutinins, because both are “self” antigens. Anti-H(O) antibodies are not made in most people because a substantial fraction are converted to A or B determinants or they are a “self” antigen.
IgM isoagglutinins efficiently trigger the complement cascade and circulate in human plasma at titers sufficient to cause complement-dependent lysis of transfused erythrocytes that display the corresponding blood group antigens. Such rapid erythrocyte lysis causes an immediate, acute transfusion reaction, which can lead to hypotension, shock, acute renal failure, and death from circulatory collapse. This problem is avoided by ensuring that the ABO type of transfused erythrocytes is compatible with the recipient's ABO type. Thus, an A recipient may receive erythrocytes from A or O persons but not from a person of type B or AB. Blood banks perform typing and cross-matching assays. First, units of erythrocyte products typed for the A and B antigens are chosen to match the patient's ABO type. To ensure that these are truly “compatible,” the patient's serum is cross-matched by mixing with a small aliquot of each prospective erythrocyte unit. Erythrocytes of compatible units do not agglutinate (form an erythrocyte clump), whereas incompatibility is indicated by agglutinated erythrocytes formed by antibodies in the patient's serum. Blood typing is used to ensure compatibility not only for red blood cell transfusions but also for transfusion with plasma. Similar ABO compatibility concerns are important in heart, kidney, liver, and bone marrow transplantation procedures. The “type and cross” procedures have virtually eliminated ABO blood group transfusion reactions in the developed world. Attempts are being made to enzymatically modify A or B erythrocytes to convert them to the “O” type, which is the “universal donor” type. The few individuals with AB type are “universal acceptors.”
Cross-matching procedures helped to identify a rare ABO blood group phenotype termed the Bombay phenotype, so named because the first identified individual lived in that city (now Mumbai). Affected persons have erythrocytes and tissue cells lacking A, B, and H determinants because they have inactive FUT1 and FUT2 genes, and therefore no α1-2FucT enzyme. Bombay sera contain IgM antibodies that react with erythrocytes from virtually all donors, including O erythrocytes (H antigen-positive, A and B antigen-negative). They show robust titers of anti-H, anti-A, and anti-B IgM antibodies and cannot receive erythrocytes from any donor except those of the same Bombay blood type. A related phenotype, termed para-Bombay, occurs in people with an inactive FUT1 gene, but at least one functional Se (FUT2) allele (secretor-positive). The fact that Bombay individuals appear generally healthy implies that developmental or physiological functions for the A, B, and H antigens, if they ever existed, are no longer relevant. However, a variety of associations have been made between the ABO blood group phenotype and relative risk for infection by some pathogens and the acquisition of a spectrum of diseases. For example, people with blood group O who are also Lewis-antigen positive (see below) are the most susceptible to infection by Helicobacter pylori. This is because H. pylori binds well to glycans with terminal Fuc, such as the H and Lewis antigens. The AB blood group is associated with infection by Brucella (Brucellosis) and noroviruses that cause gastroenteritis. Blood group status has also been associated with risk of stomach and pancreatic cancers. ABO status may also be protective. Thus, enveloped viruses carry the ABO(H) glycans of their hosts and are susceptible to lysis following infection of another individual with an ABO-incompatible type. Finally, differences in susceptibility to severe complications of malaria appear to be affected by ABO blood groups. Protective mechanisms relevant to these roles for blood group determinants may explain why the ABO system has survived more than 50 million years of primate evolution, including likely reinventions.
LEWIS BLOOD GROUPS
The Lewis blood group antigens are a related set of glycans that carry α1-3/α1-4 Fuc residues (Figure 14.6). The term “Lewis” derives from a family who suffered from a red blood cell incompatibility. The Lewis a antigen (Lea) is synthesized by an α1-3/α1-4FucT (FUT3) encoded by the Lewis (LE or FUT3) blood group locus. The Lewis b antigen (Leb) is synthesized by the concerted actions of FUT3 and FUT2. Secretor-positive individuals express FUT2 and convert Type-1 units to Type-1 H determinants that may be acted on by FUT3 to form the Leb determinant (Figure 14.6). Nonsecretors who do not synthesize Type-1 H determinants in secretory epithelia express the Lea determinant via FUT3 (Figure 14.6). Individuals with an inactive FUT3 locus (∼10%–20% of the population), are termed Lewis-negative. Lewis-negative secretors express Type-I H determinants that cannot be converted to Lea or Leb determinants. Lewis-negative nonsecretors express Type-1 units that are devoid of Fuc.
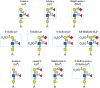
FIGURE 14.6.
Type-1 and -2 Lewis determinants. Type-1 and -2 units differ in the linkage of the outermost galactose (β1-3 or β1-4, respectively) and thus in the linkage of fucose to the internal GlcNAc (α1-4 or α1-3, respectively). (more...)
Expression of Lea and Leb glycans and FUT3 is largely restricted to the same epithelia that express FUT2. Thus, soluble forms of these antigens are released into secretions and body fluids. Lea and Leb antigens are also detectable on erythrocytes. However, the precursors of erythrocytes do not synthesize these determinants. Instead, Lewis antigens are acquired by the erythrocyte membrane through passive adsorption of Lewis-positive glycolipids that circulate in plasma in lipoprotein complexes and aqueous dispersions. Antibodies against the Lea antigens have been implicated in occasional transfusion reactions.
Because of structural similarities, the term “Lewis antigen” was applied to other glycan sequences and includes the Lewis x (Lex) and Lewis y (Ley) determinants and forms of the Lea and Lex determinants that are sialylated and/or sulfated (Figure 14.6). These glycan epitopes are formed through the actions of FUT4, FUT5, FUT6, and/or FUT7. Some Lewis blood group antigens have important functions in selectin-dependent leukocyte extravasation and tumor-cell metastasis. Most strongly implicated are the sialylated and/or sulfated determinants represented by sialyl Lex and its sulfated variants (Figure 14.6), which function as selectin ligands on glycoproteins and glycolipids of leukocytes and tumor cells (Chapters 34 and 47). The Lewis blood group antigens have also been proposed to function in the pathogenesis of H. pylori, the causative agent in chronic active gastritis associated with hypertrophic gastropathy, duodenal ulcer, gastric adenocarcinoma, and gastrointestinal lymphoma (Chapter 37). Also, Lewis antigens may be expressed on glycoproteins in plants (Chapter 24).
P BLOOD GROUPS
The P1PK blood group includes the P1 and Pk antigens. Their synthesis involves two pathways, each beginning with lactosylceramide (Figure 14.7). The Pk antigen is synthesized by an α1-4GalT (Pk transferase; A4GALT) and may be modified by a β1-3GalNAcT (P transferase; B3GALNT1) to form the P antigen. In the second pathway, P1-antigen biosynthesis begins with two reactions leading to paragloboside, which is acted on by A4GALT to form the P1 antigen, the most common P blood group. Individuals with this blood group possess both pathways, and their erythrocytes express P and P1 antigens and small amounts of Pk not converted into P determinants. Individuals with an inactive P1 transferase are quite common and express the P2 blood group. Their erythrocytes show normal levels of P and Pk antigens but are deficient in P1 determinants. Antibodies against P, P1, and Pk determinants have been implicated in transfusion reactions. Complement-fixing, cold-reactive anti-P antibodies known as “Donath–Landsteiner” antibodies cause intravascular hemolysis observed in a syndrome called paroxysmal cold hemoglobinuria (see Chapter 46).
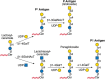
FIGURE 14.7.
Biosynthesis of antigens of the P1PK blood group system: Pk, P, and P1.
Various uropathogenic strains of Escherichia coli express adhesins that bind to the terminal Galα1-4Gal moiety of the Pk and P1 antigens (Chapter 37). The P1 determinant is expressed on the urothelium and probably facilitates infection by mediating attachment of bacteria. P1 individuals have a higher relative risk for urinary tract infections and pyelonephritis. The adhesion of a pyelonephritic strain of E. coli to renal tissue is mediated by a bacterial adhesin specific for the Galα1-4Gal epitope, and deficiency of the adhesin severely attenuates the pyelonephritic activity of the organism. The P blood group antigens may also have a role as receptors for human parvovirus B19. This virus causes erythema infectiosum and leads to congenital anemia and hydrops fetalis following infection in utero. It is also associated with transient aplastic crisis in patients with hemolytic anemia and with cases of pure erythrocyte aplasia and chronic anemia in immunocompromised individuals. Parvovirus B19 replication is restricted to erythroid progenitor cells. An adhesive interaction between the virion and glycolipids with P antigen is involved in viral infection of erythroid progenitors.
MILK OLIGOSACCHARIDES
Mammals make a series of oligosaccharides with a core of lactose (Galβ1-4Glc) and secrete them into the milk. Human milk comprises many hundreds of different glycans with small amounts of glycans containing greater than fifteen sugars. The distribution of structures and amount of each oligosaccharide vary between individuals and during the lactation cycle. There is a relatively high concentration of complex, sialylated, and fucosylated oligosaccharides in milk, and these glycans appear to protect infants against enteric pathogens. Interestingly, in individuals that are “nonsecretors” and Lewis-negative, fucosylated glycans are not present beyond 2’-fucosyllactose, and the overall repertoire of oligosaccharides is reduced. Most other mammals synthesize lactose but also express species-specific repertoires of glycans that largely differ from humans—often being much simpler—but may have similar functions. Beside lactose, which is nutritionally important, the larger human milk oligosaccharides are thought to be important in infant immunoprotection and to have prebiotic activity by contributing to the development of healthy microbiota. Surprisingly, there is essentially no information on the hormone-regulated biosynthesis of these abundant glycans. Lactose is generated by a β1-4 galactosyltransferase (acting as lactose synthase) only in the lactating mammary gland, because of the lactation-specific expression of the modifier protein α-lactalbumin, which causes the enzyme to transfer Gal from UDP-Gal to Glc rather than to GlcNAc. Even though this process has been demonstrated to occur in intact Golgi, the precise mechanisms of how lactose is modified by addition of other sugars by specific glycosyltransferases during lactation are unknown. It is assumed that the same enzymes involved in making termini of other glycan classes are responsible.
THE Galα1-3Gal TERMINUS
The Galα1-3Gal epitope (often called “alpha-Gal”) is synthesized on Type-2 units on glycolipids and glycoproteins by a specific α1-3GalT (Figure 14.8). This epitope and the α1-3GalT that synthesizes it are expressed by New World primates and many nonprimate mammals, but the α1-3GalT gene is inactivated in humans and Old World primates. Mice engineered to lack the α1-3GalT develop cataracts. Species that do not express the Galα1-3Gal epitope, including humans, carry anti-Galα1-3Gal antibodies, likely because of immunization through exposure to the Galα1-3Gal epitope on microbes and food. Anti-Galα1-3Gal antibodies present a major barrier to the use of porcine and other nonprimate organs for xenotransplantation in humans, because they bind to Galα1-3Gal epitopes on the vascular endothelium of xenotransplants and cause hyperacute graft rejection through complement-mediated endothelial cell cytotoxicity. Efforts are in progress to overcome this barrier by using animal organ donors that have been genetically modified. Approaches include transgenic expression of enzymes, such as FUT1, that diminishes Galα1-3Gal expression by diverting Type-2 units toward H antigen synthesis. Unfortunately, pig tissues lacking Galα1-3Gal elicit a graft rejection reaction to other pig antigens. Anti-Galα1-3Gal antibodies have also been shown to significantly diminish the infective efficiency of recombinant retroviruses. The problem has been solved through the generation of packaging cell lines that are deficient in α1-3GalT. Recombinant glycoproteins for therapeutic use in humans must also be prepared in cells that do not express α1-3GalT. Severe allergies to red meat consumption can occur when high titer IgE antibodies against this epitope appear in adult humans, claimed to be the consequence of a prior bite by the Lone Star tick, which may be expressing the same epitope in its saliva.
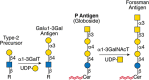
FIGURE 14.8.
Structure and synthesis of the Galα1-3Gal antigen. The α1-3GalT uses unsubstituted Type-2 units on glycoproteins or glycolipids (R) to form the Galα1-3Gal terminal epitope. The glycolipid globoside serves as the substrate for the (more...)
THE FORSSMAN ANTIGEN
The Forssman antigen (also known as globopentosylceramide) is a glycolipid that contains terminal α1-3GalNAc linked to the terminal GlcNAc of globoside transferred by the α1-3 GalNAcT (GBGT1), which is related in sequence to ABO transferases (Figure 14.8). The Forssman antigen, first discovered in sheep erythrocytes by John Frederick Forssman, is expressed during embryonic and adult stages in many mammals. Humans have a mutated GBGT1 and cannot synthesize the Forssman antigen but carry anti-Forssman antibodies in their serum. Rare individuals have a reversion mutation that restores activity to GBGT1, and synthesis of the Forssman antigen. Anti-Forssman antibodies may contribute to the pathogenesis of Guillain–Barré syndrome by binding to cross-reactive glycolipid components of peripheral nerve myelin. It is interesting that anti-Forssman antibodies can disrupt tight junction formation, apical–basal polarization, and cell adhesion.
SULFATED GalNAc: PITUITARY GLYCOPROTEIN HORMONES
Glycans with sulfated terminal β-linked GalNAc are found on the pituitary glycoprotein hormones lutropin (LH) and thyrotropin (TSH) but not on follicle-stimulating hormone (FSH), although it is made in the same cells. These heterodimeric glycoproteins contain a common α subunit and a unique β subunit, each with biantennary N-glycans. The N-glycans of TSH and LH have an unusual 4-O–sulfated GalNAc attached to GlcNAc residues (Figure 14.9). This contrasts with the N-glycans on FSH (and most N-glycans), in which GlcNAc residues are substituted with β1-4Gal, often extended by α2-3 or α2-6 sialic acid residues (Chapter 9). A free α subunit common to LH, TSH, and FSH is present in pituitary cells and it also carries this determinant, as do other glycoproteins synthesized by the pituitary and elsewhere, (e.g., on the O-glycans of proopiomelanocortin). Synthesis of the sulfated GalNAc determinant is controlled by a β1-4GalNAcT, either B4GALNT3 or B4GALNT4 (Figure 14.9). The terminal β1-4GalNAc is then sulfated by a sulfotransferase (CHST8 or CHST11) also expressed in pituitary cells. In some tissues, including the pituitary, the β1-4GalNAc is substituted by an α2-6 sialic acid residue. Both β1-4GalNAcT and β1-4GalT enzymes are expressed in pituitary cells, but the N-glycans on LH and TSH carry the uncommon β1-4GalNAc, whereas the N-glycans on FSH carry the common β1-4Gal residue. This protein-specific glycosylation is a consequence of interactions between B4GALNT3 or B4GALNT4 and a specific peptide motif present on the combined αβ subunits of LH and TSH. This interaction causes an increase in the catalytic efficiency of the β1-4GalNAcT that modifies biantennary N-glycans on LH and TSH at the expense of the competing β1-4GalT. Importantly, the peptide motif recognized by the β1-4GalNAcT is not present in the β subunit of FSH, and the recognition motif on the α subunit of FSH is not accessible to the enzyme. Consequently, the biantennary N-glycans on FSH are modified exclusively by a β1-4GalT.
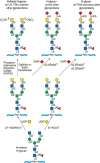
FIGURE 14.9.
Structure and synthesis of N-glycans bearing terminal GalNAc, including those with sulfated-GalNAc found on the pituitary hormones lutropin (LH) and thyrotropin (TSH), but not on follicle-stimulating hormone (FSH).
These differential glycosylation events have profound consequences for the ovulatory cycle in vertebrates. Circulating LH levels increase and decrease in a highly pulsatile manner. This assures maximal stimulation of the ovarian LH receptor at the preovulatory surge, because sustained high LH levels would lead to LH receptor desensitization. The increase and decrease in LH levels is due, in part, to pulsatile release of the hormone by the pituitary. However, the peaks and troughs are accentuated markedly by the rapid clearance of LH from the circulation mediated via recognition of its terminal sulfated-GalNAcβ1-4GlcNAc determinant(s). The “mannose receptor” MRC1 is expressed in the liver by hepatic endothelial cells and by Kupffer cells. LH binding is followed by internalization and lysosomal degradation. MRC1 is also expressed in macrophages. In liver, MRC1 recognizes sulfated-GalNAc via an R-type lectin domain, whereas in macrophages, the same receptor recognizes mannose via an L-type lectin domain (Chapters 31 and 32).
TERMINAL β-LINKED GalNAc: THE Sda BLOOD GROUP
The addition of GalNAc to Gal already substituted with α2-3Sia may also occur on glycoproteins and glycolipids (Figure 14.10). On glycoproteins, this structure forms the human Sda blood group, which is expressed by most individuals. In mice, the Sda antigen was first described on cytotoxic T lymphocytes (CTLs) and was termed the CT antigen. On glycolipids, the same trisaccharide terminus is shared by the ganglioside GM2 (Chapter 11). A related structure to the Sda antigen called the Cad antigen [GalNAcβ1-4(Neu5Acα2-3)Galβ1-3(Neu5Acα2-6)] is found in O-glycans and glycolipids. The human Sda antigen was first sequenced in N-glycans of Tamm–Horsfall glycoprotein from human urine. Both human and mouse β1-4GalNAcTs (B4GALNT1 and B4GALNT2) transfer GalNAc to N- and O-glycans on glycoproteins, but not to the glycolipid GM3 (Siaα2-3Galβ1-4Glc-Cer), even though both can efficiently use 3-sialyllactose (Siaα2-3Galβ1-4Glc) as a substrate in vitro. In the mouse, Sda antigens are recognized by IgM monoclonal antibodies termed CT1 and CT2, which were isolated for their ability to block lysis of cellular targets by a murine CTL clone. Rare humans lack the determinant and form naturally occurring antibodies against it, but they show no apparent pathophysiology. Mice with a dominantly inherited form of von Willebrand's disease express B4GALNT1 aberrantly in vascular endothelium. The presence of the β1-4GalNAcT in this abnormal location generates von Willebrand factor (VWF) carrying the Sda determinant. This VWF glycoform is rapidly cleared from the circulation by the asialoglycoprotein receptor in liver, leading to VWF deficiency and hemorrhagic disease.
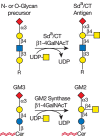
FIGURE 14.10.
Synthesis of the human Sda or mouse CT antigen and the glycolipid GM2.
The glycolipid equivalent of the Sda determinant, termed GM2, which is synthesized by GM2 synthase (B4GALNT1) (Figure 14.10), is widely expressed in the central and peripheral nervous systems and in the adrenal gland. Mice homozygous for a null mutation in B4galnt1 show modest conduction defects in the peripheral nervous system and male sterility (Chapter 41).
α2-3-SIALYLATED GLYCANS
Sialic acids in α2-3 linkage are found on N-glycans, O-glycans, and glycolipids generated by six different α2-3 sialyltransferases (ST3GAL1 to ST3GAL6). ST3GAL3 and ST3GAL4 are broadly expressed in mammals. In mouse, St3gal1 transcripts are most abundant in the spleen, liver, bone marrow, thymus, and salivary glands. St3gal2 expression is most abundant in brain, where α2-3-sialylated glycolipids are common. St3gal5 is expressed well in brain, skeletal muscle, adrenals, and liver, and St3gal6 is most expressed in testes. In vertebrates, α2-3 sialic acid residues are found on terminal Gal residues. The addition of α2-3 sialic acid to Gal inhibits the action of other enzymes, including α1-2FucTs, α1-3GalT, GlcNAcTs, and GalNAcTs, which compete with terminal α2-3 sialyltransferases. Although most α2-3 sialic acid on glycoproteins is found on complex N-glycans (Chapter 9) and O-GalNAc glycans (Chapter 10), sialylation also occurs on O-fucose and O-mannose glycans found on a limited subset of glycoproteins (Chapter 13). As discussed above and in Chapter 34, selectin ligands are α2-3-sialylated glycans.
Glycans bearing α2-3 sialic acid contribute to the circulating half-life of plasma glycoproteins by “masking” terminal Gal residues that contribute to the removal of glycoproteins from serum by the asialoglycoprotein receptor (Chapter 34). ST3GAL1 generates Siaα2-3Galβ1-3GalNAcα-Ser/Thr that is important for the viability of peripheral CD8+ T cells. Mice lacking ST3GAL1 show decreased cytotoxic T-cell responses with an increase in the apoptotic death of naïve CD8+ T cells (Chapter 36).
Sialic acid recognition is important for binding by viruses and bacteria. Binding to sialic acid and subsequent release by neuraminidase are important for infection by influenza virus. Sialic acid residues in α2-3 linkage are recognized by the hemagglutinin (HA) in the envelope of influenza viruses from birds and pigs. Human influenza viruses bind more commonly to sialic acid residues in α2-6 linkage. Mutations in the HA gene of influenza viruses from birds may lead to a human influenza pandemic partly due to their enhanced ability to infect human cells through HA recognition of α2-6Neu5Ac (Chapter 42). Glycans bearing α2-3 sialic acid residues have also been implicated in bacterial pathogenesis. Glycans terminating in α2-3 sialic acid residues support the adhesion of H. pylori, which causes gastritis, gastric ulcers, and stomach cancer. The ganglioside GM1 (Galβ1-3GlcNAcβ1-4[Siaα2-3]Galβ1-4GlcβCer) is a receptor for cholera toxin produced by Vibrio cholerae and the heat-labile enterotoxin (LT-1) produced by enterotoxigenic E. coli (Chapter 42). Glycan-based inhibitors are currently under evaluation in humans for their ability to diminish the symptoms and progression of cholera. A variety of other pathogens and toxins bind to sialylated termini bearing one of many possible modified sialic acids (Chapter 15).
α2-6-SIALYLATED GLYCANS
Sialic acid in α2-6 linkage is found on N-glycans, O-glycans, and glycolipids. Two α2-6 sialyltransferases, ST6GAL1 and ST6GAL2, transfer to Gal, whereas ST6GALNAC1 through ST6GALNAC6 transfer to GalNAc (Figure 14.11). In vertebrates, α2-6 sialic acid is found on terminal Gal, on terminal or subterminal GlcNAc, or, in the case of reactions catalyzed by ST6GALNAC3, on an internal GalNAc. α2-6 Sialic acid is less common than α2-3 sialic acid. Glycans with terminal α2-6 sialic acid are generally not modified further. In mouse, St6gal1 is expressed at a relatively high level in hepatocytes and lymphocytes and is responsible for α2-6 sialylation of serum glycoproteins and glycoproteins of the antigen receptor complex in lymphocytes. St6gal2 expression is mainly restricted to the embryonic and adult brain, and its functions are currently unknown.
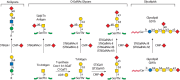
FIGURE 14.11.
Synthesis of α2-6 and α2-3 sialic acid on O-glycans and glycolipids (see Chapters 9 and 10) by the ST3Gal and ST6GalNAc families of sialyltransferases. Enzymes in parentheses contribute at relatively low levels in vitro to the reactions (more...)
The α2-6–sialylated glycans from ST6GALNAC1 and ST6GALNAC2 are restricted to O-glycans. ST6GALNAC3 is responsible for transferring α2-6 sialic acid to the GalNAcα-Ser/Thr core of O-GalNAc glycans and to GalNAc in glycolipids. ST6GALNAC4, ST6GALNAC5, and ST6GALNAC6 appear to use glycolipids as preferred acceptors. Many strains of influenza infectious for humans bind terminal α2-6 sialic acid residues (Chapter 37), and glycoproteins bearing α2-6 sialylation can be cleared from the circulation by the asialoglycoprotein receptor (Chapter 31).
Mice lacking ST6GAL1 show diminished antibody responses to T-lymphocyte-dependent and -independent antigens, reduced B-lymphocyte proliferation in response, reduced B cell surface IgM and CD22 levels, ∼65% reduction in serum IgM levels, and reduced B cell receptor (BCR) signaling (Chapter 35). The extracellular domain of CD22 on B lymphocytes specifically recognizes Siaα2-6Galβ1-4GlcNAc-. In the absence of α2-6 sialic acid on glycans, CD22 shows increased clustering with the BCR, and BCR signaling is down-regulated.
α2-8-SIALYLATED GLYCANS
Glycans modified by α2-8 sialylation occur in vertebrates mainly in developing brain and are carried primarily on the neural cell adhesion molecule NCAM. α2-8-sialylated glycans are also expressed on a few glycoproteins in noneuronal cells and on tumor cells. There are six α2-8 sialyltransferases, ST8SIA1 through ST8SIA6, that transfer sialic acid in α2-8 linkage to a terminal α2-3 or α2-6-linked sialic acid, generally on an N-glycan (Figure 14.12). ST8SIA2 (also called STX) and ST8SIA4 (also called PST) catalyze the synthesis of linear polymers of up to 400 α2-8 sialic acid residues to give polysialic acid (PolySia or PSA) on NCAM. Both ST8SIA2 and ST8SIA4 are autocatalytic and synthesize PolySia on their own N-glycans, although polysialylation is not a prerequisite for their sialyltransferase activity. Thus, some cultured cells that do not express a known substrate of these sialytransferases may express surface PolySia when transfected with ST8SIA2 or ST8SIA4 due to their autosialylation. Glycoproteins with N-glycans carrying only one α2-8 sialic acid or two (disialic acid) to seven α2-8 sialic acids (oligosialic acid) have been described and may be synthesized by ST8SIA3. However, functional studies have focused on PolySia on NCAM (Chapter 15).
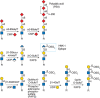
FIGURE 14.12.
Structure and synthesis of glycans with α2-8 sialic acids including PolySia on N-glycans by ST8SIA1 to ST8SIA6. Synthesis and structure of the HNK-1 epitope. GlucuronosylT, glucuronosyltransferase; SulfoT, sulfotransferase.
PolySia is highly negatively charged, highly hydrated, and contributes up to one third of the molecular mass of NCAM. The embryonic form of NCAM is extensively modified by PolySia, which exerts an antiadhesive effect and reduces homotypic interactions. PolySia can also diminish interactions promoted by other adhesion molecules, including L1-dependent attachment to laminin or collagen and also binds extracellular signaling molecules like BDNF and FGF-2. Mice lacking ST8SIA4 show reduced PSA in certain brain regions and have altered neuronal responses in the hippocampal CA1 region. Mice lacking ST8SIA2 have a distinct neuronal phenotype because of misguided migration of a subset of hippocampal neurons and ectopic synapses. When both ST8SIA2 and ST8SIA4 are inactivated, mice have severe neuronal and other problems and die precociously. However, this phenotype is rescued by also removing NCAM in a triple-knockout strain. This shows that the presence of NCAM lacking PSA is a major cause of the severe defects in double-knockout mice (Chapter 41).
Certain glycolipids also carry α2-8 sialic acid linkages, which are constructed by three α2-8 sialyltransferases termed ST8SIA1 (also known as GD3 synthase), ST8SIA3, and ST8SIA5 (Chapter 11). They generate single or oligomeric α2-8 sialic acid resiudes but not polymeric PSA. The three enzymes are generally thought to act primarily on glycolipid substrates, but in vitro studies suggest that ST8SIA3 can also use N-glycans to generate oligosialic acids. These α2-8 sialyltransferases are expressed in the brain, where each shows a distinct developmentally regulated expression pattern. ST8SIA1 is also present in kidney and thymus. In vitro experiments imply that certain α2-8-sialylated glycolipids may participate in signal transduction processes in neuronal cell types. Inactivation of the ST8SIA1 gene in the mouse causes alterations in sensory neuron responses to pain.
SULFATED AND PHOSPHORYLATED GLYCANS
In principle, any free hydroxyl group on a monosaccharide could be modified by sulfation or phosphorylation. However, in vertebrates, glycan sulfation is restricted to Gal, GlcNAc, GlcA, and GalNAc at internal or terminal positions, and phosphorylation has been observed only on Man and Xyl to date. The internally sulfated glycans in heparin, heparan sulfate (HS), and chondroitin sulfate (CS) proteoglycans and the sulfated N-glycans on keratan sulfate are discussed in Chapter 17. This chapter describes sulfated glycans recognized by L-selectin, the HNK-1 epitope, and the pituitary glycoprotein hormones mentioned above. Chapter 17 also mentions transient phosphorylation of the Xyl that initiates the proteoglycan core. Mannose phosphorylation is described in Chapters 9, 13, and 33. Parasites, fungi, and bacteria have a wide variety of phosphorylated glycans (Chapters 21, 22, 23, and 43). However, glycan sulfation is remarkably rare in prokaryotes.
In vertebrates, L-selectin on lymphocytes binds to the high endothelial venules (HEVs) in lymph nodes through recognition of L-selectin ligands present on O-GalNAc glycans of HEV glycoproteins. Sulfated forms of the sialyl Lewis x determinant (Figure 14.6) provide an essential contribution to L-selectin recognition of these glycoproteins. Sulfation occurs at C-6 of Gal by CHST1 and C-6 of GlcNAc by CHST2, both of which contribute to L-selectin ligand activity. Mice lacking the two sulfotransferases show almost no homing of lymphocytes to HEV. The biosynthesis of sulfated L-selectin ligands and the enzymes that participate in this process are discussed in Chapter 34. Sulfated forms of the sialyl Lewis x determinant are also thought to contribute to Siglec recognition, as mentioned in Chapter 35.
The HNK-1 antigen is a terminally sulfated glycan that was first described on human natural killer cells, and is also called CD57. The HNK-1 epitope is expressed in the vertebrate nervous system, and expression patterns change during neural development (Chapter 41). The HNK-1 determinant comprises 3-O-sulfated GlcA attached in α1-3 linkage to a terminal Gal (Figure 14.12) of N-glycans, O-glycans, proteoglycans, and glycolipids. Two different GlcA-transferases participate in HNK-1 GlcA addition: GlcAT-P (B3GAT1) and GlcAT-S (B3GAT2). They have very different activities for glycoprotein or glycolipid substrates in vitro and may generate functionally different HNK-1 epitopes in vivo. Glucuronylation is followed by 3-O sulfation of the GlcA by one or more sulfotransferases. The HNK-1 epitope is present on a variety of neuronal cell glycoproteins, including NCAM, contactin, myelin-associated glycoprotein, telencephalin, L1, and P0 (the major glycoprotein of peripheral nerve myelin). There is evidence that HNK-1 can function as a ligand for laminin, L-selectin, P-selectin, and a cerebellar adhesion protein termed amphoterin. HNK-1 has also been shown to mediate homotypic adhesive interactions involving P0. HNK-1-dependent adhesive interactions have been implicated in cell migration processes involving cell–cell and cell–matrix interactions and are proposed to participate in reinnervation of muscles by motor neurons.
Phosphorylation of sugars is also important in recognition events. In mammals, the phosphorylation of Man on oligomannose N-glycans at the C-6 position of lysosomal hyrolases occurs by a phospho-GlcNAc transferase to created a GlcNAc-phospho-6-Mannose diester (Chapters 9 and 33). Subsequent removal of the GlcNAc exposes monophosphoester Man-6-P for recognition of lysosomal hydrolases by the Man-6-P receptors. Interestingly, mannose-1-6-phosphate-mannose is a common modification in yeast mannans on their cell walls. Phosphorylation of the C-2 of the Xyl that initiates proteoglycan core linker synthesis is mediated by a Golgi kinase Fam20B and is essential for addition of the second Gal in the core linker GlcAβ1-3Galβ1-3Galβ1-4Xylβ1-Ser/Thr core. The phosphate must be removed by a phosphatase, PXYLP1, before GlcA can be added to the core linker, generating the substrate for HS, CS, or DS addition. Interestingly, a GlcNAc may be added to the phosphorylated trisaccharide to block extension of the core linker glycan. This regulation by Xyl phosphorylation is essential for physiological homeostasis of proteoglycans (Chapters 17 and 41). In another example of regulation by phosphorylation, the Golgi kinase POMK phosphorylates the C-6 position of the O-Man that initiates O-mannose glycans on α-dystroglycan, and this has been shown to be essential for the subsequent action of the glycosyltransferase LARGE, which adds a GlcA β1-3Xyl polymer to the O-Man core (Chapters 13 and 45).
ACKNOWLEDGMENTS
The authors appreciate helpful comments and suggestions from Jason W. Labonte.
FURTHER READING
- Yamamoto F. 2004. Review: ABO blood group system—ABH oligosaccharide antigens, anti-A and anti-B, A and B glycosyltransferases, and ABO genes. Immunohematology 20: 3–22. [PubMed: 15373665]
- Audry M, Jeanneau C, Imberty A, Harduin-Lepers A, Delannoy P, Breton C. 2011. Current trends in the structure–activity relationships of sialyltransferases. Glycobiology 21: 716–726. [PubMed: 21098518]
- Fiete D, Beranek M, Baenziger JU. 2012. Molecular basis for protein-specific transfer of N-acetylgalactosamine to N-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J Biol Chem 287: 29194–29203. [PMC free article: PMC3436590] [PubMed: 22722937]
- Patnaik SK, Helmberg W, Blumenfeld OO. 2014. BGMUT database of allelic variants of genes encoding human blood group antigens. Transfus Med Hemother 41: 346–351. [PMC free article: PMC4264482] [PubMed: 25538536]
- Yamamoto F, Cid E, Yamamoto M, Saitou N, Bertranpetit J, Blancher A. 2014. An integrative evolution theory of histo-blood group ABO and related genes. Sci Rep 4: 6601. [PMC free article: PMC5377540] [PubMed: 25307962]
- Bode L. 2015. The functional biology of human milk oligosaccharides. Early Hum Dev 91: 619–622. [PubMed: 26375354]
- Jost T, Lacroix C, Braegger C, Chassard C. 2015. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr Rev 73: 426–437. [PubMed: 26081453]
- Quraishy N, Sapatnekar S. 2017. Advances in blood typing. Adv Clin Chem 77: 221–269. [PubMed: 27717418]
- REGULATED GLYCOSYLATION OF GLYCAN EXTENSIONS
- THE A, B, AND H HUMAN BLOOD GROUPS
- LEWIS BLOOD GROUPS
- P BLOOD GROUPS
- MILK OLIGOSACCHARIDES
- THE Galα1-3Gal TERMINUS
- THE FORSSMAN ANTIGEN
- SULFATED GalNAc: PITUITARY GLYCOPROTEIN HORMONES
- TERMINAL β-LINKED GalNAc: THE Sda BLOOD GROUP
- α2-3-SIALYLATED GLYCANS
- α2-6-SIALYLATED GLYCANS
- α2-8-SIALYLATED GLYCANS
- SULFATED AND PHOSPHORYLATED GLYCANS
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Structures Common to Different Glycans.[Essentials of Glycobiology. 2022]Review Structures Common to Different Glycans.Stanley P, Wuhrer M, Lauc G, Stowell SR, Cummings RD. Essentials of Glycobiology. 2022
- Review Structures Common to Different Glycans.[Essentials of Glycobiology. 2009]Review Structures Common to Different Glycans.Stanley P, Cummings RD. Essentials of Glycobiology. 2009
- Review N-Glycans.[Essentials of Glycobiology. 2015]Review N-Glycans.Stanley P, Taniguchi N, Aebi M. Essentials of Glycobiology. 2015
- Tandem mass spectra of glycan substructures enable the multistage mass spectrometric identification of determinants on oligosaccharides.[Rapid Commun Mass Spectrom. 2013]Tandem mass spectra of glycan substructures enable the multistage mass spectrometric identification of determinants on oligosaccharides.Everest-Dass AV, Kolarich D, Campbell MP, Packer NH. Rapid Commun Mass Spectrom. 2013 May 15; 27(9):931-9.
- Structural mapping of the glycans from the egg glycoproteins of Schistosoma mansoni and Schistosoma japonicum: identification of novel core structures and terminal sequences.[Glycobiology. 1997]Structural mapping of the glycans from the egg glycoproteins of Schistosoma mansoni and Schistosoma japonicum: identification of novel core structures and terminal sequences.Khoo KH, Chatterjee D, Caulfield JP, Morris HR, Dell A. Glycobiology. 1997 Jul; 7(5):663-77.
- Structures Common to Different Glycans - Essentials of GlycobiologyStructures Common to Different Glycans - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

