NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009.

Essentials of Glycobiology. 2nd edition.
Show detailsThis chapter provides an overview of the biological roles of glycans and attempts to synthesize some general principles for understanding and exploring these roles. For details, see the reviews cited and the other chapters in this book.
GENERAL PRINCIPLES
As with other major classes of macromolecules, the biological roles of glycans span the spectrum from those that appear to be relatively subtle, to those that are crucial for the development, growth, functioning, or survival of the organism that synthesizes them. Many glycans have not yet been assigned a function, because efforts to study them have not been made or a function is not yet evident. Over the years, many theories have been advanced regarding the biological roles of glycans. Although there is evidence to support all of these theories, exceptions to each can also be easily found. This should not be surprising, given the enormous diversity of glycans in nature. Added complexities arise from the fact that glycans are frequently targets for the binding of microbes and microbial toxins, that is, they can be detrimental to the organism that synthesizes them.
The biological roles of glycans can be divided into two broad categories: (1) the structural and modulatory properties of glycans and (2) the specific recognition of glycans by other molecules—most commonly, glycan-binding proteins (GBPs) (Figure 6.1). The GBPs can be subdivided into two major groups: (1) intrinsic GBPs, which recognize glycans from the same organism and (2) extrinsic GBPs, which recognize glycans from a different organism. Intrinsic GBPs typically mediate cell–cell interactions or recognize extracellular molecules, but they can also recognize glycans on the same cell. Extrinsic GBPs consist mostly of pathogenic microbial adhesins, agglutinins, or toxins, but some also mediate symbiotic relationships. As discussed in Chapter 19, these two types of glycan recognition likely act as opposing selective forces driving evolutionary change, at least partly accounting for the enormous diversity of glycan structure found in nature. Further complexity arises from the fact that some microbial pathogens engage in “molecular mimicry,” evading immune reactions by decorating themselves with glycans typical of their hosts. Finally, some microbes are themselves targets of their own pathogens (e.g., bacteriophages that invade bacteria), and glycan recognition is a common feature of these interactions as well.
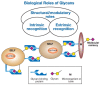
FIGURE 6.1
General classification of the biological roles of glycans. A simplified and broad classification is presented, emphasizing the roles of organism-intrinsic and -extrinsic glycan-binding proteins in recognizing glycans. There is some overlap between the (more...)
Other general principles emerge after reviewing the extant literature on this subject. The biological consequences of altering glycosylation in various systems seem to be highly variable and unpredictable. A given glycan can have different roles in different tissues or at different times in development (organism-intrinsic functions) or in different environmental contexts (organism-extrinsic functions). As a broad generalization, it can be stated that terminal sequences, unusual structures, and modifications of glycans probably mediate the more specific biological roles within the organism. However, such glycans or modifications are also more likely to be targets for pathogens and toxins. Perhaps as a consequence, intraspecies and interspecies variations in glycosylation are relatively common, and at least some of the diversity of glycans in nature may represent the signatures of past or current host-pathogen interactions (for discussion see Chapter 19). Finally, genetic defects in glycosylation are easily obtained in cultured cells, but often have limited biological consequences. In contrast, the same defects in intact organisms can have major and even catastrophic consequences. This indicates that many major functions of glycans are operative only within an intact organism. Each of these principles is briefly discussed below.
BIOLOGICAL CONSEQUENCES OF ALTERING GLYCOSYLATION ARE VARIABLE
Approaches taken to understand the biological roles of glycans include the prevention of initial glycosylation, prevention of glycan chain elongation, alteration of glycan processing, enzymatic or chemical deglycosylation of completed chains, genetic elimination of glycosylation sites, and the study of naturally occurring genetic variants and mutants in glycosylation (see further discussion below). The consequences of such manipulations range from being essentially undetectable to the complete loss of particular functions or even loss of the entire glycoconjugate bearing the altered glycan. Even within a particular class of molecules, for example cell-surface receptors, the effects of altering glycosylation are variable and unpredictable. Moreover, the same glycosylation change can have markedly different effects in different cell types, or when studied in vivo or in vitro. The answer obtained may depend on the structure of the glycan, the biological context (intrinsic or extrinsic interaction), and the specific biological question being asked. Given all of the above considerations, it is difficult to predict a priori the functions that a given glycan on a given glycoconjugate might mediate and its relative importance to the organism.
STRUCTURAL AND MODULATORY ROLES OF GLYCANS
There is little doubt that glycans have many protective, stabilizing, organizational, and barrier functions. As discussed in Chapter 1, the glycocalyx that covers all eukaryotic cells and the polysaccharide coats of various prokaryotes can represent a substantial physical barrier. Glycans attached to matrix molecules, such as proteoglycans, are important for the maintenance of tissue structure, porosity, and integrity. Such molecules can also contain binding sites for other specific types of glycans that in turn aid the overall organization of the matrix. The external location of glycans on most glycoproteins can provide a general shield, protecting the underlying polypeptide from recognition by proteases or antibodies. Glycans are also involved in the proper folding of newly synthesized polypeptides in the endoplasmic reticulum (ER) and/or in the subsequent maintenance of protein solubility and conformation. Indeed, if some proteins are incorrectly glycosylated, they fail to fold properly and/or to exit the ER, being consigned instead to degradation in proteasomes. Conversely, there are also examples of glycoproteins whose synthesis, folding, trafficking, sensitivity to proteolysis, or immune recognition seem quite unaffected by altering their glycosylation. Moreover, inhibitors (see Chapter 50) or genetic mutations (see Table 6.1 and Chapter 42) that only affect the later steps of glycan processing often do not interfere with basic structural functions. Although the structural functions of glycans are obviously of great importance to the intact organism, they do not explain why such a diverse range of complex glycan molecules has evolved.
TABLE 6.1
A few examples of mouse and human genetic defects in glycosylation
Glycosylation can also modulate the interaction of proteins with one another. Some growth factor receptors seem to acquire their binding abilities in a glycosylation-dependent manner while they are in transit through the Golgi apparatus. This may limit unwanted early interactions of a newly synthesized receptor with a growth factor that is synthesized in the same cell. Glycosylation of a polypeptide can also mediate an on-off or switching effect. For example, when the hormone beta-human chorionic gonadotrophin is deglycosylated, it is still able to bind to its receptor with similar affinity, but it fails to stimulate adenylate cyclase. In most instances, the effects of glycosylation are incomplete, that is, glycosylation appears to be “tuning” a primary function of the protein rather than turning it on or off. For example, the activity of some glycosylated growth factors and hormones can be modulated over a wide range by the extent and type of their glycosylation. This becomes particularly evident when recombinant forms of such molecules are produced in biotechnology, bearing different types and extents of glycosylation. A striking example is the role of polysialic acid chains attached to the neural cell adhesion molecule (NCAM). This adhesion receptor normally mediates homophilic binding between neuronal cells. In the embryonic state, or in other states of neural “plasticity,” these anionic polysialic chains tend to be very long, thereby interfering with homophilic binding (see Chapter 14). There are also instances wherein protein functions can be tuned by glycans attached to other neighboring structures. For example, the polysialic acids of embryonic NCAM can interfere with the interactions of other unrelated receptor–ligand pairs, simply by physically separating the cells. Also, the tyrosine phosphorylation activity of the epidermal growth factor (EGF) receptor and the insulin receptor can be modulated by endogenous cell-surface gangliosides, possibly by organizing them into membrane microdomains (see Chapter 10). Although the precise mechanisms of these latter effects are uncertain, specificity is implied by the requirement for a defined glycan sequence in the ganglioside. Because most such tuning effects of glycans are partial, their overall importance might be questioned. However, the sum total of several such partial effects can be a dramatic effect on the final biological outcome. Thus, glycosylation appears to be a mechanism for generating important functional diversity from the limited set of basic receptor–ligand interactions that are possible, when using the gene products derived from the typical genome. Of course, as with most other functions of glycans, exceptions to these concepts can be found. There are many receptors whose ligand binding is not acquired in a glycosylation-dependent manner and many peptide ligands whose binding and action are not obviously affected by glycosylation.
Another structural/modulatory function of glycans appears to be to act as a protective storage depot for biologically important molecules. For example, many heparin-binding growth factors (see Chapters 16 and 35) are found attached to the glycosaminoglycan (GAG) chains of the extracellular matrix, adjacent to cells that need to be stimulated, for example, in the basement membrane underlying epithelial and endothelial cells. This prevents diffusion of the factors away from the site (sometimes generating morphogenic gradients), protects them from nonspecific proteolysis, prolongs their active lives, and allows them to be released under specific conditions. Likewise, the GAG chains found in secretory granules seem to bind and protect the protein contents of the granule and modulate their functions. There are several other instances in which glycans act as sinks or depots for biologically important molecules, such as water, ions, and immune regulatory proteins.
GLYCANS AS SPECIFIC LIGANDS FOR CELL–CELL INTERACTIONS (INTRINSIC RECOGNITION)
The first intrinsic glycan receptors to be identified were those that mediate clearance, turnover, and intracellular trafficking of soluble blood-plasma glycoproteins (for examples, see Chapter 31). Most of these receptors specifically recognize certain terminal or subterminal glycans on the soluble glycoprotein. However, even the most elegantly precise examples, such as the role of mannose-6-phosphate (Man-6-P) in the trafficking of lysosomal enzymes to lysosomes (see Chapter 30), feature some exceptions. Thus, Man-6-phosphorylation is not absolutely required for the trafficking of lysosomal enzymes in certain cell types nor is it operative at all in some lower eukaryotes. There are also endocytic receptors, whose functions have yet to be assigned, that recognize specific glycan sequences. Several instances exist wherein free glycans can have hormonal actions that induce specific responses in a highly structure-specific manner. Examples include the interaction of small glycans from bacterial symbionts with plant roots (see Chapter 37) and the bioactive properties of fragments of hyaluronan in mammalian systems (see Chapter 15), both of which can induce biological responses in a size- and structure-dependent manner. Like wise, free heparan or dermatan sulfate fragments released by certain cell types can have major biological effects in complex situations such as wound healing. In many of these instances, the putative receptors for these molecules and their precise mechanisms of action are still being defined.
It is now clear that glycans have many specific biological roles in cell–cell recognition and cell–matrix interactions. One of the best characterized examples concerns the selectin family of adhesion molecules, which recognize glycan structures on their ligands and thereby mediate critical interactions between blood cells and vascular cells in a wide variety of normal and pathological situations (see Chapter 31). As indicated above, GBPs and glycans present on cell surfaces can interact specifically with molecules in the matrix or even with glycans on the same cell surface. In some such instances, the specific biological significance of recognition has yet to be conclusively demonstrated in the intact animal. Also, it is becoming clear that some critical recognition sites are actually combinations of glycans and protein. For example, P-selectin recognizes the generic selectin ligand sialyl Lewisx with high affinity only in the context of the amino-terminal 13 amino acids of P-selectin glycoprotein ligand-1 (PSGL-1), which include certain required sulfated tyrosine residues (see Chapter 31). More recently, a different form of intrinsic recognition has been described, in which glycan-binding sites of cell-surface receptors are masked by cognate glycans on the same cell surface, making them unavailable for recognition by external ligands (see Chapter 32).
Carbohydrate–carbohydrate interactions may also have a specific role in cell–cell interactions and adhesion. A dramatic example is the species-specific interaction between marine sponges, which is mediated via homotypic binding of the glycans on a large cell-surface glycoprotein. Another example is the compaction of the mouse embryo at the morula stage, which seems to be facilitated by a Lewisx–Lewisx interaction. The single-site affinities of such interactions are not very strong and are sometimes difficult to measure. However, if the molecules in question are present in very high copy numbers on the cell surface, a large number of relatively low-affinity interactions can collaborate to produce a high-avidity “Velcro” effect that is sufficient to mediate biologically relevant interactions.
GLYCANS AS SPECIFIC LIGANDS FOR CELL–MICROBE INTERACTIONS (EXTRINSIC RECOGNITION)
As discussed in Chapter 34, certain glycans act as specific binding sites for a variety of viruses, bacteria, and parasites, and as recognition targets for many plant and bacterial toxins. In such situations, there is typically excellent recognition specificity for the sequence of the glycan involved. For example, the hemagglutinins of many viruses specifically recognize the type of host sialic acid, its modifications, and its linkage to the underlying sugar chain. Likewise, various toxins bind with great specificity to certain gangliosides but not to related structures (see Chapters 10 and 34). There is little doubt about the importance of structural specificity with respect to these functions of glycans. Indeed, many of the microbial binding proteins involved have been harnessed as specific tools for studying the expression of the cognate sugar chains. However, providing signposts to aid the success of pathogenic microorganisms has little obvious value to the organism that synthesized such glycans. Perhaps to counter such deleterious consequences, some organisms may have also evolved the ability to mask or modify glycans recognized by microorganisms or toxins. Conversely, glycan sequences on soluble glycoconjugates, such as secreted mucins, can also act as decoys for microorganisms and parasites. Thus, a pathogenic organism or toxin seeking to bind to mucosal cell membranes may first encounter the specific glycan ligand attached to a soluble mucin, which can then be washed away, removing the potential danger to the cells underneath. In contrast, instances occur in which symbiosis is mediated by specific glycan recognition, such as some commensal bacteria in the gut lumen of animals and the bacteria involved in forming plant root nodules (see Chapter 37).
MOLECULAR MIMICRY OF HOST GLYCANS BY PATHOGENS
Pathogens that invade multicellular animals sometimes decorate themselves with glycan structures that appear to be identical or nearly identical to those found on their host cell surfaces (see Chapters 39 and 40). These glycans form a thick coating on the surface of the microbe and therefore represent a very successful strategy for evading host immune responses. Perhaps not surprisingly, pathogens appear to have evolved to achieve this state of molecular mimicry by making use of “every possible trick in the book,” for example, direct or indirect appropriation of host glycans, convergent evolution toward similar biosynthetic pathways, and even lateral gene transfer. In some instances, the impact of the pathogen is aggravated by autoimmune reactions, resulting from host reactions to these host-like antigens.
THE SAME GLYCAN CAN HAVE DIFFERENT ROLES WITHIN AN ORGANISM
The expression of certain types of glycans on different glycoconjugates in different tissues at different times of development implies that these structures have diverse roles within the same organism. For example, Man-6-P-containing glycans were first found on lysosomal enzymes and are involved in lysosomal trafficking (see Chapter 30). However, Man-6-P-containing glycans are now known to occur on a variety of apparently unrelated proteins, including proliferin, thyroglobulin, the EGF receptor, and the transforming growth factor-β (TGF-β) precursor, and they have certain functional roles in the biology of these proteins or their role remains to be discovered. Likewise, the sialylated fucosylated lactosamines critical for selectin recognition (see Chapter 31) are found in a variety of unrelated cell types in mammals, and the polysialic acid chains that play such an important part in embryonic NCAM function (Chapter 14) are found on fish-egg-jelly coat proteins and on a sodium channel protein. Given that glycans are added posttranslationally, these observations should not be surprising. Once a new glycan or modification has been expressed in an organism, several distinct functions could evolve independently in different tissues and at different times in development. If any of these situations mediated a function valuable to the survival of the organism, the genetic mechanisms responsible for expression of the glycan and its expression pattern would remain conserved in evolution.
INTRASPECIES AND INTERSPECIES VARIATIONS IN GLYCOSYLATION
The underlying core structures of the major classes of glycans tend to be conserved across many species, for example, the core structure of N-glycans is conserved across all eukaryotes and at least some of the Archaea (see Chapter 8). However, as outlined in Chapters 19, 20, 21, 22, 23, 24, and 25, there can be considerable diversity in outer-chain glycosylation, even among relatively similar species. Such interspecies variations in glycan structure indicate that some glycan sequences do not have fundamental and universal roles in all tissues and cell types in which they are expressed. Of course, such diversity could be involved in generating differences in morphology and function observed between species. Such variations could also reflect differing selection pressures resulting from exposure to different pathogen regimes. Furthermore, significant intraspecies polymorphism in glycan structure can exist without obvious functional value. The potential role of such polymorphisms in the interplay between parasites and host populations is discussed in Chapter 19. Extensive interspecies variability in primary sequence also occurs in conventional genes and proteins, without any obvious consequences to essential functions. For example, some yeast proteins are functional when transfected into mammalian cells and vice versa, despite relatively limited sequence homology.
IMPORTANCE OF TERMINAL SEQUENCES, MODIFICATIONS, AND UNUSUAL STRUCTURES
Given all of the above, it is challenging to predict which glycan structures are likely to mediate the more specific or crucial biological roles within an organism. As mentioned above, terminal sugar sequences, unusual structures, or modifications of the glycans are more likely to be involved in such specific roles. The predictive value of this observation is reduced by the fact that such terminal sequences, unusual glycans, or modifications are also more likely to be involved in interactions with microorganisms and other noxious agents, because the balance between the organism-intrinsic and -extrinsic functions of glycans, discussed above, tends to involve such structures. A further complexity arises from “micro-heterogeneity” in glycan structure (see discussion in Chapter 19), wherein the same glycosylation site on the same protein in the same species can carry a variety of related glycan structures. The challenge then is to predict and sort out which of these two distinct roles is to be assigned to a given glycan structure in a given cell type in a given organism.
ARE THERE “JUNK” GLYCANS?
Because microorganisms and parasites that bind glycans evolve in parallel with their multicellular hosts, they must adapt their glycan-binding “repertoire” to any change in glycan structure presented by the host. In response, the host population may select for new modifications of the target structure, especially if the latter had meanwhile evolved a vital function elsewhere within the organism. Thus, there would be no choice but to preserve the underlying scaffolding upon which the latest modification was placed, while adding yet another layer of complexity to its glycans. Such cycles of evolutionary interaction between microbes and hosts might explain some of the complex and extended sugar chains found in multicellular organisms, especially in areas of frequent microbial contact, such as on mucosal surfaces and secreted mucins. In this manner, “junk” glycans could accumulate, akin to “junk” DNA. Although such structures may still function as structural scaffolding, they may have no other specific role in that particular cell type, organism, or at that particular time in evolution. They would, of course, provide fodder for future evolutionary selection, either for new organism-intrinsic functions or for population-based selective responses to a new pathogen. Additionally, neutral unselected drift (which is now acknowledged as a major process in evolution) can also explain some of the “junk” glycans.
APPROACHES TO ELUCIDATING SPECIFIC BIOLOGICAL ROLES OF GLYCANS
Some functions of glycans are discovered serendipitously. In other instances, the investigator who has elucidated complete details of the structure and biosynthesis of a specific glycan is left without knowing its functions. It is necessary to design experiments that can differentiate between the trivial and crucial functions mediated by each glycan. Various approaches that can be considered are discussed below, and we emphasize the pros and cons of each. These approaches are also presented in schematic form in Figure 6.2.
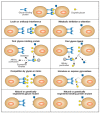
FIGURE 6.2
Approaches for elucidating the biological roles of glycans. The figure assumes that a specific biological role is being mediated by recognition of a certain glycan structure by a specific glycan-binding protein. Clues to this biological role could be (more...)
Localization of or Interference with Specific Glycans Using Glycan-binding Proteins or Antibodies
Most current approaches to understanding glycan diversity (see Chapter 48) involve the extraction and identification of the entire complement of glycans found in a given organ or tissue, without regard to the fact that individual cell types within that organ or tissue can have widely varying patterns of glycan expression. However, the cell-type-specific localization of glycans can be explored using the numerous highly specific GBPs and antibodies now available (see Chapter 45). Once a specific glycan has been localized in an interesting biological context, it is natural to consider introducing the cognate GBP or antibody into the intact system, hoping that it will interfere with a specific function and generate an interpretable phenotype. A similar approach with antibodies can be very successful when investigating the function of a protein, but with rare exceptions, this strategy is likely to give confusing results with regard to glycan function. Most antibodies against glycans are of the IgM variety and hence tend to have weak affinity and show cross-reactivity between species. Although high-affinity IgG antibodies are preferred, they are hard to obtain, because glycans tend to be T-independent antigens, and often do not generate high-titer immune responses. Likewise, although some plant lectins seem to be very specific for animal glycans, they originate from organisms that typically do not contain the same ligand. Thus, their apparent specificity may not be as reliable when introducing them into complex animal biological systems where unknown cross-reacting glycan structures are potentially present. Finally, both antibodies and GBPs are multivalent, and their cognate ligands (the glycans) tend to be present in multiple copies on multiple glycoconjugates. Thus, introduction of a GBP or antibody into a complex biological system is likely to cause nonspecific aggregation of various molecules and cell types, and the effects seen may have nothing to do with the biological functions of the glycan in question. It would seem more worthwhile to develop recombinant monovalent GBP modules that are derived from the same system being investigated. Providing they are of high enough affinity, the effects of introducing such monovalent GBPs into a complex system as competitors of the native function may yield more interpretable clues.
Metabolic Inhibition or Alteration of Glycosylation
As outlined in Chapter 50, many pharmacological agents can metabolically inhibit or alter glycosylation in intact cells and animals. Although metabolic inhibitors are powerful tools to elucidate biosynthetic pathways, they can sometimes yield confusing results in complex systems. One concern is that the inhibitor may have effects on other unrelated pathways. For example, the inhibitor tunicamycin that blocks N-linked glycosylation can also inhibit UDP-Gal uptake into the Golgi. The second concern is that the inhibitor may cause such massive changes in glycan synthesis that the physical properties of the glycoconjugates and/or membranes are altered, making it difficult to interpret the results. Somewhat more useful results can be obtained by introducing low-molecular-weight primers of terminal glycosylation (see Chapter 50), which can act as alternate substrates for Golgi enzymes, diverting synthesis away from the endogenous glycoproteins. However, this approach can simultaneously generate incomplete glycans on the endogenous glycoconjugates, as well as produce secreted glycan chains, each of which could have its own biological effects.
Finding Natural Glycan Ligands for Specific Receptors
Because specific “carbohydrate-recognition domains” can be identified within a primary amino acid sequence (see Chapters 26 and 27), it is now possible to predict whether a newly cloned protein can bind glycans. If a potential GBP can be produced in sufficient quantities, techniques such as hemagglutination, flow cytometry, surface plasmon resonance, and affinity chromatography (see Chapter 27) can then be used to search for specific ligands. However, the monovalent affinity of the putative GBP for its ligand may not be high. Thus, high densities and/or multivalent arrays may be needed to avoid missing a biologically relevant interaction. The question also arises as to where exactly to look for the biologically relevant ligands in a complex multicellular system. Furthermore, because many glycan structures can be expressed in different tissues at different times in development and growth, a recombinant GBP may detect a cognate structure in a location and at a time that it is not actually of major biological relevance. Careful consideration of the natural occurrence and expression profile of the GBPs should lead to a rational decision as to where to look for its biologically relevant glycan ligands.
Finding Receptors that Recognize Specific Glycans
The converse situation arises when an unusual glycan is found to be expressed in an interesting context and is hypothesized to be a ligand for a specific receptor. It is possible to search for such a receptor by techniques similar to those mentioned above, such as hemagglutination, flow cytometry, and affinity chromatography (see Chapter 27). To facilitate the search, it is necessary to have reasonable quantities of the pure defined glycan in question, as well as a variety of closely related structures that can act as negative controls. Because many biologically relevant lectin-like interactions are of low affinity, it is probably advisable to use a multivalent form of the glycan as the probe. Finally, it may not be obvious where to look for the glycan-binding protein. For example, the receptor that specifically recognizes the unusual sulfated N-glycans of pituitary glycoprotein hormones was eventually found not in the pituitary itself nor in any of the target tissues for these hormones, but in the endothelial cells of the liver, where it serves to regulate the circulating half-life of the hormones (see Chapter 28). Indeed, the most biologically relevant receptor for a particular glycan might even be found in another organism (a pathogen or a symbiont).
Interference by Soluble Glycans or Structural Mimics
The addition of soluble glycans or their structural mimics into the system can cause interference with the interaction between an endogenous GBP and a specific glycan (see Chapter 27). If a sufficient concentration of the specific inhibitor can be achieved, the resulting phenotypic changes can be instructive. When studying in vitro systems, even monosaccharides can be used to advantage in such experiments, as exemplified by the exploration of the Man-6-P receptor pathway (Chapter 30). However, it is often necessary to use competing glycans in somewhat large quantities to block the relatively low-affinity interactions between a GBP and its specific ligand. Effective blockade may also require multivalency of the cognate glycan. Finally, especially when studying complex multicellular systems, the glycans introduced could be cross-recognized by other as-yet-unknown binding proteins, giving a confusing phenotypic readout.
Eliminating Specific Glycan Structures by Glycosidases
A powerful approach to understanding the biological roles of glycans is to use degradative enzymes known to be highly specific for a particular glycan sequence. Many such specific enzymes can be obtained from microbial pathogens. The advantage of this approach is that one is not interfering with the basic biosynthetic cellular machinery, but simply eliminating certain structures selectively after normal synthesis has been completed. Thus, for example, sialidase treatment abolished lymphocyte binding to the high endothelial venules of lymph nodes and provided the first prediction of the nature of endogenous ligands for L-selectin (Chapter 31); injection of endoneuraminidase into the developing retina suggested specific roles for polysialic acids (Chapter 14), and injection of heparanase into developing embryos resulted in a randomization of left–right axis formation (Chapter 16). In all such studies, the purity of the enzyme used is critical and appropriate controls are necessary (including, if possible, a specific inhibitor of the enzyme or a catalytically inactive version of the enzyme). If the enzyme is of bacterial origin, trace amounts of potent contaminants such as endotoxin are also of concern. A genetic approach can be used to avoid problems of contamination by expressing a cDNA for the glycan-modifying enzyme in the intact cell or animal. For example, transgenic expression in mice of an influenza sialic-acid-specific 9-O-acetylesterase gave either early or late abnormalities in development, depending on the promoter used. Unfortunately, many such glycosidases may not function well or at all in the context of an intact animal, which can limit the spectrum of glycan structures that may be probed for function with this approach.
Studying Natural or Genetically Engineered Glycan Mutants
This is intuitively a powerful approach for understanding glycan function. Technically, it is easiest to study glycosylation mutants in cultured cell lines (see Chapter 46). However, although genetic or acquired defects in glycosylation are obtained relatively easily in cultured cell lines, these defects may have limited or not easily discernible biological consequences. This may be because of the lack of other factors or cell types that would be present in the intact organism. For example, the cognate receptor for the glycan may not be present in the same cell type. Of course, such mutants can still be used to analyze basic structural functions of the glycans and their relevance to the physiology of a single cell. Furthermore, one can add back external factors or other cell types thought to interact with the modified glycan. Some mutants can also be reintroduced into intact organisms, for example, to study tumorigenicity or metastatic behavior of malignant cells.
Although much useful information can be gained by such approaches, many of the more specific roles of glycans need to be uncovered by studying mutations in the intact multicellular organism. Genetic defects in glycosylation in intact organisms were initially thought to be relatively uncommon. Looking back on the many glycosylation mutants that have been recently discovered in flies, worms, mice, and humans (see Table 6.1 and Chapter 42), it is clear that glycan changes often affect multiple systems and that the phenotypes are unpredictable and highly variable. In retrospect, the apparent rarity of naturally occurring mutations can now be explained in several ways. In some cases, they appear to have limited biological consequences in the otherwise healthy animal because of redundant pathways. For example, our understanding of the role of GalNAc-initiated O-glycans in animals has been hampered by the remarkable multiplicity of polypeptide GalNAc transferase loci and the consequent inability to easily “delete” O-glycan synthesis using gene “knockout” approaches in mice. In other cases, specific challenges are needed to elucidate the phenotype, and the nature of such challenges is not always initially apparent. Alternatively, many mutations cause lethal aberrations that prevent completion of embryogenesis. This has become apparent, in part, by comparing the genotype-phenotype relationships in naturally occuring human disorders of glycosylation and in experimentally induced glycosylation disorders in mice. In humans, naturally occurring disease-associated mutations in glycosylation pathways almost always correspond to missense mutations that leave some residual enzymatic function intact, whereas deletion of the corresponding enzymatic locus in mice often leads to a lethal phenotype during embryogenesis. Another possibility is that such genetic abnormalities remain undetected because their consequences are pleiotropic. Indeed, it has only been recently discovered that several pediatric developmental disorders are caused by genetic abnormalities in glycan biosynthesis (see Chapter 42). Regardless, the value of constructing glycosylation mutants in intact animals is evident. Indeed, it can now be stated that complete elimination of most of the major glycan classes of vertebrate cells has been genetically accomplished in the mouse, and every instance has lead to embryonic lethality. Given the complex phenotypes and the potential for early developmental lethality, the ability to disrupt glycosylation-related genes in a temporally controlled and cell-type-specific manner can be particularly valuable.
Studying Natural or Genetically Engineered Glycan Receptor Mutants
Eliminating a specific glycan receptor can yield a phenotype that may be very instructive with regard to the functions of the glycan. As with genetic modification of the glycan, the results are more likely to be useful if studied in the intact organism. However, the receptor protein may have other functions unrelated to glycan recognition. Conversely, the glycan in question may have other functions not mediated by the receptor. Thus, for example, the genetic elimination of the CD22/Siglec-2 receptor and the ST6Gal I enzyme that generates its ligand gave complementary, but not identical, phenotypes (see Chapter 32). However, breeding the two mutations into the same mouse indicated that there were indeed epistatic interactions. Similar results were obtained by mating mice deficient in making polysialic acid and in synthesizing the protein carrier of polysialic acids, NCAM.
FURTHER READING
- Roseman S. The synthesis of carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970;5:270–297. [PubMed: 5476326]
- Montreuil J. Primary structure of glycoprotein glycans: Basis for the molecular biology of glycoproteins. Adv. Carbohydr. Chem. Biochem. 1980;37:157–223. [PubMed: 6996449]
- Berger EG, Buddecke E, Kamerling JP, Kobata A, Paulson JC, Vliegenthart JFG. Structure, biosynthesis and functions of glycoprotein glycans. Experientia. 1982;38:1129–1162. [PubMed: 6754417]
- Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu. Rev. Biochem. 1988;57:785–838. [PubMed: 3052290]
- Hart GW. Glycosylation. Curr. Opin. Cell. Biol. 1992;4:1017–1023. [PubMed: 1485955]
- Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur. J. Biochem. 1992;209:483–501. [PubMed: 1358608]
- Lis H, Sharon N. Protein glycosylation—Structural and functional aspects. Eur. J. Biochem. 1993;218:1–27. [PubMed: 8243456]
- Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3:97–130. [PMC free article: PMC7108619] [PubMed: 8490246]
- Stanley P, Ioffe E. Glycosyltransferase mutants: Key to new insights in glycobiology. FASEB J. 1995;9:1436–1444. [PubMed: 7589985]
- Gahmberg CG, Tolvanen M. Why mammalian cell surface proteins are glycoproteins. Trends Biochem. Sci. 1996;21:308–311. [PubMed: 8772385]
- Hooper LV, Manzella SM, Baenziger JU. From legumes to leukocytes: Biological roles for sulfated carbohydrates. FASEB J. 1996;10:1137–1146. [PubMed: 8751716]
- Salmivirta M, Lidholt K, Lindahl U. Heparan sulfate: A piece of information. FASEB J. 1996;10:1270–1279. [PubMed: 8836040]
- Spillmann D, Burger MM. Carbohydrate–carbohydrate interactions in adhesion. J. Cell. Biochem. 1996;61:562–568. [PubMed: 8806079]
- Drickamer K, Taylor ME. Evolving views of protein glycosylation. Trends Biochem. Sci. 1998;23:321–324. [PubMed: 9787635]
- Ferguson MAJ. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell. Sci. 1999;112:2799–2809. [PubMed: 10444375]
- Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. [PubMed: 10406840]
- Angata T, Varki A. Chemical diversity in the sialic acids and related α-keto acids: An evolutionary perspective. Chem. Rev. 2002;102:439–470. [PubMed: 11841250]
- Esko JD, Selleck SB. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002;71:435–471. [PubMed: 12045103]
- Freeze HH. Human disorders in N-glycosylation and animal models. Biochim. Biophys. Acta. 2002;1573:388–393. [PubMed: 12417423]
- Hakomori SI. The glycosynapse. Proc. Natl. Acad. Sci. 2002;99:225–232. [PMC free article: PMC117543] [PubMed: 11773621]
- Spiro RG. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. [PubMed: 12042244]
- Lowe JB, Marth JD. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 2003;72:643–691. [PubMed: 12676797]
- Wells L, Hart GW. O-GlcNAc turns twenty: Functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–158. [PubMed: 12829252]
- Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu. Rev. Biochem. 2004;73:491–537. [PubMed: 15189151]
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. [PubMed: 16959566]
- Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. [PubMed: 16959563]
- Varki NM, Varki A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Invest. 2007;87:851–857. [PMC free article: PMC7100186] [PubMed: 17632542]
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. [PubMed: 17460664]
- GENERAL PRINCIPLES
- BIOLOGICAL CONSEQUENCES OF ALTERING GLYCOSYLATION ARE VARIABLE
- STRUCTURAL AND MODULATORY ROLES OF GLYCANS
- GLYCANS AS SPECIFIC LIGANDS FOR CELL–CELL INTERACTIONS (INTRINSIC RECOGNITION)
- GLYCANS AS SPECIFIC LIGANDS FOR CELL–MICROBE INTERACTIONS (EXTRINSIC RECOGNITION)
- MOLECULAR MIMICRY OF HOST GLYCANS BY PATHOGENS
- THE SAME GLYCAN CAN HAVE DIFFERENT ROLES WITHIN AN ORGANISM
- INTRASPECIES AND INTERSPECIES VARIATIONS IN GLYCOSYLATION
- IMPORTANCE OF TERMINAL SEQUENCES, MODIFICATIONS, AND UNUSUAL STRUCTURES
- ARE THERE “JUNK” GLYCANS?
- APPROACHES TO ELUCIDATING SPECIFIC BIOLOGICAL ROLES OF GLYCANS
- FURTHER READING
- Review Biological Functions of Glycans.[Essentials of Glycobiology. 2015]Review Biological Functions of Glycans.Varki A, Gagneux P. Essentials of Glycobiology. 2015
- Review Biological Functions of Glycans.[Essentials of Glycobiology. 2022]Review Biological Functions of Glycans.Gagneux P, Hennet T, Varki A. Essentials of Glycobiology. 2022
- Review Glycans in Development and Systemic Physiology.[Essentials of Glycobiology. 2009]Review Glycans in Development and Systemic Physiology.Varki A, Freeze HH, Vacquier VD. Essentials of Glycobiology. 2009
- Review Discovery and Classification of Glycan-Binding Proteins.[Essentials of Glycobiology. 2009]Review Discovery and Classification of Glycan-Binding Proteins.Varki A, Etzler ME, Cummings RD, Esko JD. Essentials of Glycobiology. 2009
- Review Glycans in Acquired Human Diseases.[Essentials of Glycobiology. 2009]Review Glycans in Acquired Human Diseases.Varki A, Freeze HH. Essentials of Glycobiology. 2009
- Biological Roles of Glycans - Essentials of GlycobiologyBiological Roles of Glycans - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

