NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings R, Esko J, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1999.

Essentials of Glycobiology.
Show detailsPrimary contributions to this chapter were made by A. Varki (University of California at San Diego).
THIS CHAPTER PROVIDES HISTORICAL BACKGROUND to the emergence of the field of glycobiology, as well as an overview of this book. General terms and definitions found throughout the volume are also considered. The common monosaccharide units of glycoconjugates are mentioned and a uniform symbol nomenclature used for structural depictions throughout the book is presented. The general oligosaccharide classes to be discussed in the book are mentioned, and an overview of the general pathways for their biosynthesis is provided. Topological issues relevant to biosynthesis and function are also considered.
What Is Glycobiology? (1–4)
The central paradigm of modern molecular biology is that biological information flows from DNA to RNA to protein. The power of this concept lies not only in its template-driven precision, but also in the ability to manipulate any one class of molecules based on knowledge of another, and in the patterns of sequence homology and relatedness that predict function and reveal evolutionary relationships. With the upcoming completion of the genomic sequences of humans and several other commonly studied model organisms, even more spectacular gains in the understanding of biological systems are anticipated. However, there is often a tendency to assume the following extension of the central paradigm:
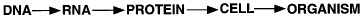
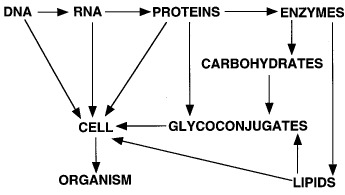
In actual fact, creating a cell requires two other major classes of molecules: lipids and carbohydrates. These molecules can serve as intermediates in generating energy, as signaling molecules, or as structural components. The structural roles of carbohydrates become particularly important in constructing complex multicellular organs and organisms, which requires interactions of cells with one another and with the surrounding matrix. Indeed, all cells and many macromolecules in nature carry a dense and complex array of covalently attached sugar chains (called oligosaccharides or glycans). In some instances, these glycans can also be free-standing entities. Since most glycans are on the outer surface of cellular and secreted macromolecules, they are in a position to modulate or mediate a wide variety of events in cell-cell and cell-matrix interactions crucial to the development and function of a complex multicellular organism. They are also in a position to mediate interactions between organisms (e.g., between host and parasite). In addition, simple, highly dynamic protein-bound glycans are abundant in the nucleus and cytoplasm, where they appear to serve as regulatory switches. An extended paradigm of molecular biology can thus be rendered as follows:
In the first part of this century, the chemistry, biochemistry, and biology of carbohydrates were very prominent matters of interest. However, during the initial phase of the modern revolution in molecular biology, studies of glycans lagged far behind those of other major classes of molecules. This was in large part due to their inherent structural complexity, the difficulty in easily determining their sequence, and the fact that their biosynthesis could not be directly predicted from the DNA template. The development of a variety of new technologies for exploring the structures of these sugar chains has opened up a new frontier of molecular biology which has been called glycobiology. This word was first coined in 1988 by Rademacher, Parekh, and Dwek to recognize the coming together of the traditional disciplines of carbohydrate chemistry and biochemistry with modern understanding of the cellular and molecular biology of glycans. The term glycobiology has gained wide acceptance, with a major biomedical journal, a growing scientific society, and a Gordon Research Conference now bearing this name.
Defined in the broadest sense, glycobiology is then the study of the structure, biosynthesis, and biology of saccharides (sugar chains or glycans) that are widely distributed in nature. It is one of the more rapidly growing fields in the biomedical sciences, with relevance to basic research, biomedicine, and biotechnology. Indeed, several biotechnology, pharmaceutical, and laboratory supply companies have invested heavily in the area. The field ranges from the chemistry of carbohydrates and the enzymology of glycan-modifying proteins to the functions of glycans in complex biological systems, and their manipulation by a variety of techniques. Research in glycobiology requires a foundation not only in the nomenclature, biosynthesis, structure, chemical synthesis, and functions of complex glycans, but also in the general disciplines of molecular genetics, cellular biology, physiology, and protein chemistry. This volume provides an overview of the field of glycobiology, with a particular emphasis on the glycans of higher animal systems, about which the greatest amount is currently known. It is assumed that the reader has a basic background in graduate-level chemistry, biochemistry, and cell biology.
Monosaccharides Are the Basic Structural Units of Glycans (5)
Carbohydrates are defined as polyhydroxyaldehydes or polyhydroxyketones, or larger compounds that can be hydrolyzed into such units (for examples, see below and for more details, see Chapter 2). A monosaccharide is a carbohydrate that cannot be hydrolyzed into a simpler unit. It has a potential carbonyl group at the end of the carbon chain (an aldehyde group) or at an inner carbon (a ketone group). These two types of monosaccharides are therefore named aldoses and ketoses. Free monosaccharides can exist in open chain or ring forms (Figure 1.1).

Figure 1.1
Open chain and ring forms of galactose. Changes in the orientation of hydroxyl groups around specific carbon atoms result in new molecules that have a distinct biology and biochemistry (e.g., glucose is the 4-epimer of galactose).
Ring forms of the monosaccharides are the rule in oligosaccharides, which are branched or linear chains of monosaccharides attached to one another via glycosidic linkages (the term polysaccharide is typically reserved for large glycans that are composed of repeating oligosaccharide motifs). The ring form of a monosaccharide generates a chiral (anomeric) center (at C-1 for aldo sugars or at C-2 for keto sugars) (for details, see Chapter 2). A glycosidic linkage involves the attachment of a monosaccharide to another residue, typically via the hydroxyl group of this anomeric center, which can be α linkages or β linkages depending on the relationship of the oxygen to the anomeric carbon (see Chapter 2). It is important to realize that these two types of linkages confer very different structural properties and biological functions upon sequences that are otherwise identical in composition. A glycoconjugate is a compound in which one or more monosaccharide or oligosaccharide units (the glycone) are covalently linked to a noncarbohydrate moiety (the aglycone). An oligosaccharide that is unattached to an aglycone usually retains the potential reducing power of the aldehyde or ketone in its terminal monosaccharide component. This end of a sugar chain is therefore often called the reducing terminus or reducing end (this term tends to be used even when the sugar chain is attached to an aglycone and thus has actually lost its reducing power). Correspondingly, the outer end of the chain tends to be called the nonreducing end (note the analogy to the 5′ and 3′ ends of nucleotide chains or the amino and carboxyl termini of polypeptides).
Glycans Can Constitute a Major Portion of a Glycoconjugate (2)
In naturally occurring glycoconjugates, the portion of the molecule comprising the glycans can vary greatly, from being very minor in amount to being the dominant component. Indeed, it is striking that sugar chains make up a substantial portion of the mass of most glycoconjugates (for a typical example, see Figure 1.2). For this reason, the surfaces of most types of cells (which are heavily decorated with different kinds of glycoconjugates) are effectively covered with a dense coating of sugars, giving rise to the so-called glycocalyx. This cell surface structure was first observed by electron microscopists many years ago as an anionic layer external to the plasmalemma, which could be decorated with polycationic reagents like cationized ferritin (for an example, see Figure 1.3).

Figure 1.2
Schematic representation of the Thy-1 glycoprotein including the three N-glycans and a glycophospholipid (GPI-glycan) anchor whose acyl chains would normally be embedded in the membrane bilayer. Note that the polypeptide represents only a relatively small (more...)
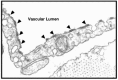
Figure 1.3
Electron micrograph of endothelial cells from a blood capillary in the diaphragm muscle of a rat, showing the lumenal plasmalemma of the cells (facing the blood) decorated with particles of (pI 8.4) cationized ferritin (see arrowheads). These particles (more...)
Monosaccharides Generate More Linkage Variation Than Amino Acids or Nucleotides (1,6)
Nucleotides and proteins are linear polymers that can each have only one basic type of linkage. In contrast, each monosaccharide can theoretically generate an α or a β linkage to any one of several positions on another monosaccharide in a chain or to another type of molecule. Thus, it has been pointed out that although three nucleotide bases or amino acids can only generate six variations, three hexoses could produce (depending on which factors are considered) anywhere from 1,056 to 27,648 unique trisaccharides. As the number of units in the polymer increases, this difference in complexity becomes even greater. For example, a hexasaccharide with six hexoses could have more than 1 trillion possible combinations. Thus, an almost unimaginable number of possible saccharide units could be theoretically present in biological systems. Fortunately, for the student of glycobiology, naturally occurring biological macromolecules contain relatively few of the possible monosaccharide units in a limited number of combinations.
Common Monosaccharide Units of Animal Glycoconjugates (5,7)
The common monosaccharides found in higher animal oligosaccharides are listed below, along with their standard abbreviations (for more details regarding their structures, see Chapter 2).
- Sialic Acids: Family of nine-carbon acidic sugars (generic abbreviation is Sia), of which the most common is N-acetyl neuraminic acid (Neu5Ac, also sometimes called NeuNAc, NeuAc, or NANA) (for more details, see Chapter 15).
- Hexoses: Six-carbon neutral sugars, including glucose (Glc), galactose (Gal), mannose (Man).
- Hexosamines: Hexose with an amino group at the 2-position, which can be either free or, more commonly, N-acetylated: N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc).
- Deoxyhexoses: Six-carbon neutral sugar without the hydroxyl group at the 6-position, fucose (Fuc).
- Pentoses: Five-carbon sugar, xylose (Xyl).
- Uronic Acids: Hexose with a negatively charged carboxylate at the 6-position, glucuronic acid (GlcA) and iduronic acid (IdA).This limited set of monosaccharides seems to dominate the glycobiology of higher animals, but several others can be found in lower animals (e.g., tyvelose; see Chapter 36), bacteria (e.g., keto-deoxyoctulosonic acid, rhamnose, heptose, and muramic acid; see Chapter 21), or plants (e.g., apiose and galacturonic acid; see Chapter 20).A variety of modifications of oligosaccharides enhance the diversity of oligosaccharides in nature and frequently serve to mediate specific biological functions. The hydroxyl groups of different monosaccharides can be subject to phosphorylation, sulfation, methylation, O-acetylation, or fatty acylation. Amino groups can remain free or be N-acetylated or N-sulfated. Carboxyl groups are occasionally subject to lactonization to nearby hydroxyl groups.Details regarding the structural depiction of monosaccharides, linkages, and oligosaccharides are discussed in Chapter 2. Many figures in this book utilize a simplified style of depiction of sugar chains (as outlined in Figure 1.4). This figure (reproduced on the inside front cover) also indicates a uniform system of symbols that is used in several figures through this book.

Figure 1.4
Recommended symbols and conventions for drawing glycan structures. The example used is a typical branched “biantennary” N-glycan with two types of outer termini. This symbolic system for representing monosaccharides is used throughout (more...)
Major Classes of Glycoconjugates and Oligosaccharides (8–12)
The common classes of oligosaccharides found on eukaryotic cells are primarily defined according to the nature of the linkage (core) regions to the aglycone (protein or lipid) (see Figure 1.5). An N-glycan (N-linked oligosaccharide, N-(Asn)-linked oligosaccharide) is a sugar chain covalently linked to an asparagine residue of a polypeptide chain within the consensus peptide sequence: Asn-X-Ser/Thr. N-glycans share a common pentasaccharide core region and can be generally divided into three main classes: high-mannose-type, complex-type, and hybrid-type (see Chapter 7). An O-glycan (O-linked oligosaccharide, O-(Ser/Thr)-linked oligosaccharide) is typically linked to the polypeptide via N-acetylgalactosamine (GalNAc) to a serine or threonine residue and can be extended into a variety of different structural core classes (see Chapter 8). Other types of “O-linked oligosaccharides” do exist (e.g., O-linked mannose). However, since the O-GalNAc linkage is the best known, it is often described by the generic term O-glycan. A glycophospholipid anchor is a glycan bridge between phosphatidylinositol and a phosphoethanolamine in amide linkage to the carboxyl terminus of a protein. This structure typically constitutes the only anchor to the lipid bilayer membrane for such proteins (see Chapter 9). A glycoprotein is a glycoconjugate in which a protein carries one or more oligosaccharide chains covalently attached to a polypeptide backbone, usually via N- or O-linkages (see above). A proteoglycan is a glycoconjugate having one or more covalently attached glycosaminoglycan chains (see definition below). The distinction from a glycoprotein is otherwise arbitrary, since some polypeptides can carry both glycosaminoglycan chains and N- or O-linked chains (see Chapter 10). A mucin is a large glycoprotein that carries many O-glycans that are often closely spaced (clustered). A glycosphingolipid (often called glycolipid) is an oligosaccharide usually attached via glucose or galactose to the terminal primary hydroxyl group of the lipid moiety ceramide, which is itself composed of a long chain base (i.e., sphingosine) and a fatty acid (see Chapter 6). Glycolipids can be neutral or anionic. A ganglioside is an anionic glycolipid containing one or more residues of sialic acid. It should be emphasized that these represent only the most common classes of glycans reported in eukaryotic cells. There are several other less common types found on both sides of the cell membrane in animal cells (see Chapters 12 and 13).
Figure 1.5
Basic core structures of the common classes of animal glycans. (For the monosaccharide symbol code used for the depictions, see Figure 1.4.)
Topological Issues Relevant to the Biosynthesis of Glycans (10,12–22)
Most well-characterized pathways for the biosynthesis of different classes of glycans occur within the ER-Golgi-plasmalemma pathway and its other ramifications. Thus, for example, newly synthesized proteins or lipids originating from the ER are either cotranslationally or posttranslationally modified with sugar chains at various stages in their itinerary toward their final destinations. Most glycosylation reactions utilize activated forms of monosaccharides (sugar nucleotides) as donors for reactions that are catalyzed by enzymes called glycosyltransferases (for details about their biochemistry, molecular genetics, and cell biology, see Chapter 17). These nucleotide donors are synthesized within the cytosolic compartment from monosaccharide precursors of endogenous or exogenous origin (see Chapter 6). To be available to carry out glycosylation reactions within the lumen of the ER-Golgi pathway, these donors must be actively transported across a membrane bilayer. Much effort has therefore gone into understanding the mechanisms of glycosylation within the ER and the Golgi apparatus, and it is clear that a variety of factors determine the final outcome of glycosylation reactions.
Some bulky sugar chains are made on the cytoplasmic face of these intracellular membranes and flipped across to the other side, but most are added to the growing chain on the inside of the ER or the Golgi. Regardless, whatever portion of a molecule faces the inside of the ER or Golgi will ultimately face the inside of a secretory granule or lysosome, but it is topologically considered to be outside of the cell. The biosynthetic enzymes (mostly glycosyltransferases) responsible for these reactions are well studied (see Chapter 17), and their location has helped to define various functional compartments of the ER-Golgi pathway. The commonly held model envisions these enzymes as being physically lined up along this pathway in the precise sequence in which they actually work. This appears to be an oversimplified view, since there is considerable overlap among these enzymes, and the actual distribution of a given enzyme probably depends on the cell type. The low-molecular-weight sugar nucleotides that act as donors for most of the biosynthetic steps are made in the cytosol (see Chapter 6) and specifically transported into the lumen of the organelles. Another consequence of this topological asymmetry is that many classes of glycans are designed to be involved in cell-cell and cell-matrix interactions. Of course, these topological considerations do not apply to nuclear and cytoplasmic glycosylation (see below), since the active sites of the relevant glycosyltransferases face the cytosol.
Nuclear and Cytoplasmic Glycosylation Is Common (10)
Until the mid 1980s, a commonly stated dogma was that glycoconjugates, such as glycoprotein and glycolipids, occur exclusively on the outer surface of cells, on the internal (luminal) surface of intracellular organelles, and on secreted molecules. As discussed above, this fit well with knowledge of the topology of the biosynthesis of the classes of glycans known at the time, which took place within the lumen of the Golgi-ER pathway. Thus, despite several clues to the contrary, the cytosol and nucleus (which are topologically semicontinuous because of the existence of nuclear pores) were assumed to be devoid of glycosylation capacity. However, in the last two decades, it has become clear that certain types of glycoconjugates are synthesized and reside within the cytosol and nucleus. Indeed, one of them (O-linked GlcNAc; see Chapter 14) may well be numerically the most common type of glycoconjugate in many cells. The fact that this major form of glycosylation was missed by so many investigators for so long emphasizes the relatively unexplored state of the whole field of glycobiology.
Outer Structures Are Often Shared among Classes of Glycans (14,16)
In contrast to the unique core regions of different classes of glycans, certain outer structural sequences are often shared among different classes of glycans. For example, N- and O-linked glycans and GSLs often carry the subterminal disaccharide Galβ1-4(3)GlcNAcβ1 (lactosamine or LacNAc units) which can sometimes be repeated (polylactosamines, polylactosaminoglycans, or poly-N-acetyllactosamines), or less commonly, GalNAcβ1-4GlcNAcβ1-(LacdiNAc) units. These chains may be modified by fucosylation or branching and are typically capped by sialic acids, fucose, α-Gal, β-GalNAc, or β-GlcA units (see Chapter 16). A glycosaminoglycan is a linear copolymer of acidic disaccharide repeating units, each containing a hexosamine and a hexose (Gal) or a hexuronic acid (GlcA or IdoA). These are the chains whose presence defines a proteoglycan (see Chapter 11). The type of disaccharide unit defines the glycosaminoglycans as chondroitin or dermatan sulfate (GalNAcβ1-4GlcA/IdoA), heparin or heparan sulfate (GlcNAcα1-4GlcA/IdoA), or keratan sulfate (Galβ1-4GlcNAc). Keratan sulfate is actually a 6-O-sulfated form of a polylactosamine and is therefore attached to a N- or O-glycan core rather than a typical proteoglycan core region. One type of glycosaminoglycan, hyaluronan (GlcNAcβ1-4GlcA)n, appears to exist primarily as a free sugar chain, unattached to any aglycone. The glycosaminoglycans (except for hyaluronan) also typically have sulfate esters substituting either hydroxyl or amino groups (N- or O-sulfate groups). Polysialic acid is a homopolymer of sialic acid selectively expressed on a few mammalian proteins and on the capsular polysaccharides of certain pathogenic bacteria.
Microheterogeneity: A Common Feature of Protein Glycosylation (2,4,8,23)
One of the most fascinating and yet frustrating aspects of protein glycosylation is the phenomenon of microheterogeneity. This term indicates that at any given glycosylation site on a given protein synthesized by a particular cell type, a range of variations can be found in the precise structure of the glycan. Even the extent of this heterogeneity can vary considerably from glycosylation site to glycosylation site, from protein to protein, and from cell type to cell type. Thus, a given glycoprotein can exist in numerous glycoforms, each effectively being a distinct molecular species. Mechanistically, this heterogeneity might be explained by the rapidity with which multiple, sequential, partially competitive glycosylation reactions must take place in the Golgi apparatus through which the newly synthesized glycoprotein is passing. An alternate possibility is that each individual cell is in fact exquisitely specific in the details of glycosylation which its Golgi apparatus produces, but intercellular variations result in the observed heterogeneity of samples from natural multicellular sources.
From the practical point of view, microheterogeneity explains the anomalous behavior of glycoproteins in various forms of chromatography (such as the diffuse bands observed on SDS-PAGE gels) and makes the complete structural analysis of most glycoproteins a difficult task. From a functional point of view, the meaning of this heterogeneity remains unclear. It is possible that this is a type of “diversity generator” intended for either diversifying endogenous recognition functions and/or for evading microbes and parasites that can bind with high specificity to certain glycan structures.
Turnover and Degradation of Glycans (24,25)
Like all components of living cells, glycans are constantly being created and degraded (see Chapter 18). The latter reactions are mediated by enzymes that cleave sugar chains either at the outer (nonreducing) terminal end (exoglycosidases) or internally (endoglycosidases). Some outer units can also be removed and then reattached without degradation of the underlying chain. The final complete degradation of most glycans is generally carried out by a series of glycosidases in the lysosome. Once broken down, their individual unit monosaccharides are then typically exported from the lysosome into the cytosol so that they can be reutilized again (see Figure 1.6). In contrast to the relatively slow turnover of glycans derived from the ER-Golgi pathway, glycans of the nucleus and cytoplasm may be more dynamic and rapidly turned over (see Chapters 13–14).
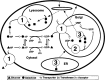
Figure 1.6
Biosynthesis, utilization, and turnover of a common monosaccharide. This schematic shows the biosynthesis, fate, and turnover of one common monosaccharide constituent of animal glycans, galactose. Although small amounts of galactose can be taken up from (more...)
Tools Used to Study Glycosylation (26–36)
Unlike oligonucleotides and proteins, glycan chains are rarely expressed in a linear, unbranched fashion, and even when they are, such chains are often subject to various modifications. Thus, the complete sequencing of oligosaccharides is difficult to accomplish by a single method and therefore requires iterative combinations of physical and chemical approaches that eventually yield the details of the structure under study (for a discussion of the various forms of low- and high-resolution separation and analysis, including mass spectrometery and NMR, see Chapter 38). Likewise, glycosylation can be perturbed in a variety of ways to explore the biology of glycans. Other tools and methods used to study glycosylation include enzymes (endoglycosidases and exoglycosidases), lectins (carbohydrate-binding proteins) (see Chapter 30), chemical modification or cleavage, metabolic radioactive labeling, glycosylation inhibitors and primers (Chapter 40), antibodies, molecular cloning of glycosyltransferases (Chapter 17), and the genetic manipulation of glycosylation in intact cells and organisms (Chapters 31 and 32). The directed in vitro synthesis of glycans using chemical and enzymatic methods has also taken great strides forward in recent years, providing many new tools for exploring glycobiology (Chapter 39). The generation of complex oligosaccharide libraries by a variety of routes has further enhanced this interface of chemistry and biology (Chapter 39).
Genetic Glycosylation Defects in Cultured Cells and Intact Animals (12,37–40)
A variety of specific genetic defects have been defined in mutant variants of cultured cell lines that express specific defects in glycan biosynthesis. Although there are some exceptions, defects have been obtained at many steps of almost all of the pathways of glycan biosynthesis in cultured animal cells. This has been of great value in elucidating the details of glycan biosynthetic pathways (see Chapter 31). However, it also implies that the specific details of biosynthesis of many types of glycans are not crucial to the housekeeping activities of single cells living in the environment of the tissue culture dish. Rather, they may be more important in mediating cell-cell and cell-matrix interactions in intact multicellular organisms and/or in mediating interactions between organisms. In keeping with this, genetic defects of glycosylation in intact animals have been found with relative rarity (see Chapter 32). On the other hand, such naturally occurring mutants may simply have complex or unexpected phenotypes that may not have lent themselves to easy elucidation with the limited diagnostic tools currently available. Regardless, there is clearly much to be learned by generating genetic defects in intact animals and examining the consequences. In recent years, this has become an important new frontier in glycobiology (see Chapter 33).
Biological Roles of Glycans Appear to Be Diverse (4,7,41–50)
A major theme of this volume is the exploration of the biological roles of glycans. As with any biological system, the best approach carefully considers the relationship of the structure and biosynthesis of glycans to their actual functions. As might be imagined from their ubiquitous and complex nature, the biological roles of glycans are quite varied. Thus, all of the proposed theories regarding glycan function appear to be partly correct, but exceptions to each can be found. As might be expected for such a diverse group of molecules, the biological roles of glycans span the spectrum from those that are trivial to those that are crucial for the development, growth, function, or survival of an organism (for further discussion, see Chapter 5).
The diverse biological functions ascribed to glycans can be more simply grouped into two general classes: (1) structural and modulatory functions involving the glycans themselves or their modulation of molecules to which they are attached and (2) specific recognition of glycans by lectins (carbohydrate-binding proteins). Such lectins can be either endogenous to the organism that synthesized the glycans (e.g., see Chapters 22–27 concerning animal lectins) or exogenous (Chapter 28 concerns microbial lectins that bind to specific glycans on host cells). In several instances, the detailed kinetics and the atomic details of these carbohydrate-protein interactions have been elucidated (see Chapter 4). The following is a common emerging theme: Monovalent carbohydrate-lectin binding tends to be of relatively low affinity, and such systems typically achieve their specificity and function by creating multivalent arrays of carbohydrate and lectin to enhance avidity (see Chapters 30 and 40).
Glycosylation Changes in Development, Differentiation, Malignancy, and Phylogeny (12,51–57)
Whenever a new probe (e.g., antibody or lectin) specific for a particular glycan is developed and used to study its expression in intact organisms, it is typical to find temporal and spatial patterns of expression in relation to cellular activation, embryonic development, organogenesis, and differentiation (see Chapter 33). Changes in expression of glycans are also often found in the setting of transformation and progression to malignancy (see Chapter 35). These spatially and temporally controlled patterns of glycan expression imply the mechanistic involvement of the glycans in many processes.
Remarkably little is known about the evolution of glycosylation. There are clearly shared and unique features of glycosylation in different kingdoms and taxa, and among animals, an increasing complexity is often seen in higher forms. Intra- and interspecies variations in glycosylation are also relatively common. It has been suggested that the more specific biological roles of oligosaccharides are often mediated by unusual glycan structures, unusual presentations of common structures, or further modifications of the saccharides themselves. Such structures likely result from the unique expression patterns of the relevant glycosyltransferases. However, such glycans are also more likely to be targets for recognition by pathogenic toxins and microorganisms. Thus, at least a portion of the diversity in oligosaccharide expression must be related to the evolutionary selection pressures generated by interspecies interactions (e.g., host-pathogen or host-symbiont interactions). In other words, the two different classes of glycan recognition mentioned above are in constant competition with each other with regard to any specific glycan-receptor interaction. Of course, the specialized glycans expressed by parasites and microbes (see Chapters 36 and 37) are themselves presumably subject to evolutionary selection pressures and are of great interest from the biomedical point of view. These evolutionary issues are considered further in Chapter 3, which also considers how various glycan biosynthetic pathways appear to have evolved and diverged in different life forms.
Glycobiology in Biotechnology and Medicine (23,58–61)
Many natural bioactive molecules are glycoconjugates, and the attached glycans can have dramatic effects on the biosynthesis, stability, action, and turnover of these molecules in intact organisms. For this reason alone, glycobiology and carbohydrate chemistry have become of increasing importance in modern biotechnology. In addition, many important biological interactions and functions mediated by glycans are potentially amenable to manipulation in vivo. Furthermore, several human disease states are characterized by changes in glycan biosynthesis that can be of diagnostic and/or therapeutic significance. The emerging importance of glycobiology in biotechnology and medicine is further considered in Chapters 37 and 41.
References
- 1.
- Lis H, Sharon N. Protein glycosylation—Structural and functional aspects. Eur. J. Biochem. 1993;218:1–27. [PubMed: 8243456]
- 2.
- Rademacher T W, Parekh R B, Dwek R A. Glycobiology. Annu. Rev. Biochem. 1988;57:785–838. [PubMed: 3052290]
- 3.
- Varki A. and Freeze H.H. 1994. The major glycosylation pathways of mammalian membranes: A summary. In Subcellular biochemistry (ed. Maddy A.H. and Harris J.R.), pp. 71–100. Plenum Press, New York. [PubMed: 8146888]
- 4.
- Hart G. Glycosylation. Curr. Opin. Cell Biol. 1992;4:1017–1023. [PubMed: 1485955]
- 5.
- Varki A., Manzi A.E., and Freeze H.H. 1996. Introduction: Preparation and analysis of glycoconjugates. In Current protocols in molecular biology (ed. Ausubel F.M. et al.), Unit 17.0. Wiley, New York.
- 6.
- Laine R A. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: The Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology. 1994;4:759–767. [PubMed: 7734838]
- 7.
- Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3:97–130. [PMC free article: PMC7108619] [PubMed: 8490246]
- 8.
- Furukawa K, Kobata A. Protein glycosylation. Curr. Opin. Biotechnol. 1992;3:554–559. [PubMed: 1368939]
- 9.
- Hascall V C, Calabro A, Midura R J, Yanagishita M. Isolation and characterization of proteoglycans. Methods Enzymol. 1994;230:390–417. [PubMed: 8139509]
- 10.
- Hart G W. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu. Rev. Biochem. 1997;66:315–335. [PubMed: 9242909]
- 11.
- Ferguson M A J. Lipid anchors on membrane proteins. Curr. Opin. Struct. Biol. 1992;1:522–529.
- 12.
- Varki A, Marth J. Oligosaccharides in vertebrate development. Semin. Dev. Biol. 1995;6:127–138.
- 13.
- Driouich A, Faye L, Staehelin L A. The plant Golgi apparatus: A factory for complex polysaccharides and glycoproteins. Trends Biochem. Sci. 1993;18:210–214. [PubMed: 8346556]
- 14.
- Van den Eijnden D H, Joziasse D H. Enzymes associated with glycosylation. Curr. Opin. Struct. Biol. 1993;3:711–721.
- 15.
- Moremen K W, Trimble R B, Herscovics A. Glycosidases of the asparagine-linked oligosaccharide processing pathway. Glycobiology. 1994;4:113–126. [PubMed: 8054711]
- 16.
- Natsuka S, Lowe J B. Enzymes involved in mammalian oligosaccharide biosynthesis. Curr. Opin. Struct. Biol. 1994;4:683–691.
- 17.
- Abeijon C, Mandon E C, Hirschberg C B. Transporters of nucleotide sugars, nucleotide sulfate and ATP in the Golgi apparatus. Trends Biochem. Sci. 1997;22:203–207. [PubMed: 9204706]
- 18.
- Colley K J. Golgi localization of glycosyltransferases: More questions than answers. Glycobiology. 1997;7:1–13. [PMC free article: PMC7108620] [PubMed: 9061359]
- 19.
- Esko J D, Zhang L J. Influence of core protein sequence on glycosaminoglycan assembly. Curr. Opin. Struct. Biol. 1996;6:663–670. [PubMed: 8913690]
- 20.
- Traub L M, Kornfeld S. The trans-Golgi network: A late secretory sorting station. Curr. Opin. Cell Biol. 1997;9:527–533. [PubMed: 9261049]
- 21.
- Farquhar M G, Palade G E. The Golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 1998;8:2–10. [PMC free article: PMC7135405] [PubMed: 9695800]
- 22.
- Varki A. Factors controlling the glycosylation potential of the Golgi apparatus. Trends Cell Biol. 1998;8:34–40. [PubMed: 9695806]
- 23.
- Parekh R B, Patel T P. Comparing the glycosylation patterns of recombinant glycoproteins. Trends Biotechnol. 1992;10:276–280. [PubMed: 1368380]
- 24.
- Neufeld E F. Lysosomal storage diseases. Annu. Rev. Biochem. 1991;60:257–280. [PubMed: 1883197]
- 25.
- Sandhoff K, Kolter T. Topology of glycosphingolipid degradation. Trends Cell Biol. 1996;6:98–103. [PubMed: 15157485]
- 26.
- Cummings R D. Use of lectins in analysis of glycoconjugates. Methods Enzymol. 1994;230:66–86. [PubMed: 8139516]
- 27.
- Dell A, Reason A J, Khoo K, Panico M, McDowell R A, Morris H R. Mass spectrometry of carbohydrate-containing bipolymers. Methods Enzymol. 1994;230:108–132. [PubMed: 8139492]
- 28.
- Geyer R, Geyer H. Saccharide linkage analysis using methylation and other techniques. Methods Enzymol. 1994;230:86–108. [PubMed: 8139517]
- 29.
- Hardy M R, Townsend R R. High-pH anion-exchange chromatography of glycoprotein-derived carbohydrates. Methods Enzymol. 1994;230:208–225. [PubMed: 8139497]
- 30.
- Kaushal G P, Elbein A D. Glycosidase inhibitors in study of glycoconjugates. Methods Enzymol. 1994;230:316–329. [PubMed: 8139504]
- 31.
- Mellors A, Sutherland D R. Tools to cleave glycoproteins. Trends Biotechnol. 1994;12:15–18. [PubMed: 7764554]
- 32.
- van Halbeek H. 1H nuclear magnetic resonance spectroscopy of carbohydrate chains of glycoproteins. Methods Enzymol. 1994;230:132–168. [PubMed: 8139493]
- 33.
- Varki A. Metabolic radiolabeling of glycoconjugates. Methods Enzymol. 1994;230:16–32. [PubMed: 8139494]
- 34.
- Palcic M M, Pierce M, Hindsgaul O. Synthetic neoglycoconjugates in glycosyltransferase assay and purification. Methods Enzymol. 1994;247:215–227. [PubMed: 7898354]
- 35.
- Burlingame A L. Characterization of protein glycosylation by mass spectrometry. Curr. Opin. Biotechnol. 1996;7:4–10. [PubMed: 8742373]
- 36.
- Reinhold V N, Reinhold B B, Chan S. Carbohydrate sequence analysis by electrospray ionization mass spectrometry. Methods Enzymol. 1996;271:377–402. [PubMed: 8782562]
- 37.
- Esko J. Animal cell mutants defective in heparan sulfate polymerization. Adv. Exp. Med. Biol. 1992;313:97–106. [PubMed: 1442273]
- 38.
- Jaeken J, Carchon H, Stibler H. The carbohydrate-deficient glycoprotein syndromes: Pre-Golgi and Golgi disorders? Glycobiology. 1993;3:423–428. [PubMed: 8286854]
- 39.
- Marth J D. Will the transgenic mouse serve as a Rosetta Stone to glycoconjugate function? Glycoconj. J. 1994;11:3–8. [PubMed: 8193551]
- 40.
- Stanley P, Ioffe E. Glycosyltransferase mutants: Key to new insights in glycobiology. FASEB J. 1995;9:1436–1444. [PubMed: 7589985]
- 41.
- Paulson J C. Glycoproteins: What are the sugar chains for? Trends Biochem. Sci. 1989;14:272–276. [PubMed: 2672447]
- 42.
- Chrispeels M J, Raikhel N V. Lectins, lectin genes, and their role in plant defense. Plant Cell. 1991;3:1–9. [PMC free article: PMC159974] [PubMed: 1824332]
- 43.
- Sharon N. Lectin-carbohydrate complexes of plants and animals: An atomic view. Trends Biochem. Sci. 1993;18:221–226. [PubMed: 8346557]
- 44.
- Karlsson K A. Microbial recognition of target-cell glycoconjugates. Curr. Opin. Struct. Biol. 1995;5:622–635. [PubMed: 8574698]
- 45.
- Nelson R M, Venot A, Bevilacqua M P, Linhardt R J, Stamenkovic I. Carbohydrate-protein interactions in vascular biology. Annu. Rev. Cell Dev. Biol. 1995;11:601–631. [PubMed: 8689570]
- 46.
- Crocker P R, Feizi T. Carbohydrate recognition systems: Functional triads in cell-cell interactions. Curr. Opin. Struct. Biol. 1996;6:679–691. [PubMed: 8913692]
- 47.
- Dénarié J, Debellé F, Promé J C. Rhizobium lipo-chitooligosaccharide nodulation factors: Signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 1996;65:503–535. [PubMed: 8811188]
- 48.
- Gahmberg C G, Tolvanen M. Why mammalian cell surface proteins are glycoproteins. Trends Biochem. Sci. 1996;21:308–311. [PubMed: 8772385]
- 49.
- Sharon N, Weis W. Carbohydrates and glycoconjugates—From cellulose and polysialic acids to the control of intracellular protein trafficking: New insights into carbohydrate structure and function. Curr. Opin. Struct. Biol. 1998;8:545–547.
- 50.
- Drickamer K, Taylor M E. Evolving views of protein glycosylation. Trends Biochem. Sci. 1998;23:321–324. [PubMed: 9787635]
- 51.
- Muramatsu T. Carbohydrate signals in metastasis and prognosis of human carcinomas. Glycobiology. 1993;3:291–296. [PubMed: 8400544]
- 52.
- Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed: 8625291]
- 53.
- Kim Y J, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 1997;14:569–576. [PubMed: 9298689]
- 54.
- Galili U, Shohet S B, Kobrin E, Stults C L, Macher B A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988;263:17755–17762. [PubMed: 2460463]
- 55.
- Manzella S M, Dharmesh S M, Beranek M C, Swanson P, Baenziger J U. Evolutionary conservation of the sulfated oligosaccharides on vertebrate glycoprotein hormones that control circulatory half-life. J. Biol. Chem. 1995;270:21665–21671. [PubMed: 7545167]
- 56.
- Dairaku K, Spiro R G. Phylogenetic survey of endomannosidase indicates late evolutionary appearance of this N-linked oligosaccharide processing enzyme. Glycobiology. 1997;7:579–586. [PubMed: 9184840]
- 57.
- Costache M, Apoil P A, Cailleau A, Elmgren A, Larson G, Henry S, Blancher A, Iordachescu D, Oriol R, Mollicone R. Evolution of fucosyltransferase genes in vertebrates. J. Biol. Chem. 1997;272:29721–29728. [PubMed: 9368041]
- 58.
- Warren C E. Glycosylation. Curr. Opin. Biotechnol. 1993;4:596–602. [PubMed: 7764212]
- 59.
- Ichikawa Y, Wang R, Wong C. Regeneration of sugar nucleotide for enzymatic oligosaccharide synthesis. Methods Enzymol. 1994;247:107–127. [PubMed: 7898347]
- 60.
- Ding Y, Kanie O, Labbe J, Palcic M M, Ernst B, Hindsgaul O. Synthesis and biological activity of oligosaccharide libraries. Adv. Exp. Med. Biol. 1995;376:261–269. [PubMed: 8597257]
- 61.
- Wright A, Morrison S L. Effect of glycosylation on antibody function: Implications for genetic engineering. Trends Biotechnol. 1997;15:26–32. [PubMed: 9032990]
- What Is Glycobiology?
- Monosaccharides Are the Basic Structural Units of Glycans
- Glycans Can Constitute a Major Portion of a Glycoconjugate
- Monosaccharides Generate More Linkage Variation Than Amino Acids or Nucleotides
- Common Monosaccharide Units of Animal Glycoconjugates
- Major Classes of Glycoconjugates and Oligosaccharides
- Topological Issues Relevant to the Biosynthesis of Glycans
- Nuclear and Cytoplasmic Glycosylation Is Common
- Outer Structures Are Often Shared among Classes of Glycans
- Microheterogeneity: A Common Feature of Protein Glycosylation
- Turnover and Degradation of Glycans
- Tools Used to Study Glycosylation
- Genetic Glycosylation Defects in Cultured Cells and Intact Animals
- Biological Roles of Glycans Appear to Be Diverse
- Glycosylation Changes in Development, Differentiation, Malignancy, and Phylogeny
- Glycobiology in Biotechnology and Medicine
- References
- Historical Background and Overview - Essentials of GlycobiologyHistorical Background and Overview - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
