NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings R, Esko J, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1999.

Essentials of Glycobiology.
Show detailsPrimary contributions to this chapter were made by A. Varki (University of California at San Diego).
THIS CHAPTER PROVIDES AN OVERVIEW concerning the biological roles of the major classes of glycans in eukaryotic cells and makes an attempt to synthesize some general principles for understanding and exploring these roles. For more details regarding many of the specific biological roles mentioned, see the the reviews cited, the other chapters in this book, and the original literature cited therein.
General Principles and Conclusions about Biological Roles of Glycans (1–42)
The commonly heard question—what is the function of glycosylation?—is not actually a reasonable one. As with most other classes of macromolecules, the biological roles of glycans span the complete spectrum, from those that are relatively unimportant to those that appear crucial for the development, growth, functioning, or survival of the organism. Over the years, many theories have been advanced regarding the biological roles of glycans. Although the available evidence provides support for essentially all of these theories, exceptions to each can be easily found. Given the enormous diversity of glycans in nature, this should perhaps not be surprising. An added level of complexity arises from the fact that glycans are very frequently targets for the binding of microbes and pathogens. The biological roles of glycans can be broadly divided into two groups (Figure 5.1). One group relies on the structural and modulatory properties of glycans and the other relies on specific recognition of glycan structures by other molecules (generally receptor proteins or lectins). The latter category can be further subdivided into two major groups: those involving recognition by endogenous receptors within the same organism and those resulting from recognition by exogenous agents. The last group consists mostly of pathogen receptors and toxins, but it can also include symbiotic agents. As discussed in Chapter 3, these two types of recognition are likely to represent opposing selective forces during evolution.
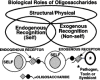
Figure 5.1
General classification of the biological roles of glycans. A simplified and broad classification is presented, emphasizing the roles of endogenous and exogenous lectins in recognizing glycans. There is some overlap between the groups, e.g., some structural (more...)
Other general principles should also be mentioned. The biological consequences of altering glycosylation in various systems seem to be highly variable and unpredictable (see discussion below). A given glycan can also have different roles in different tissues or at different times in development. As a broad generalization, it can be stated that terminal sequences, unusual structures, and modifications of glycans are more likely to be found to mediate specific biological roles within the organism. However, such unusual glycans or modifications are also more likely to be targets for pathogens and toxins. Perhaps as a consequence (for discussion, see Chapter 3), intraspecies and interspecies variations in glycosylation are relatively common, and at least some of the diversity of glycans seen in nature may represent evolutionary “junk.” Finally, since genetic defects in glycosylation are easily obtained in cultured cells, but often have limited consequences, many major functions of glycans may be operative only within an intact organism. Each of these principles is discussed briefly below.
Biological Consequences of Altering Glycosylation Are Variable
Approaches taken to understand the biological roles of glycans include the prevention of initial glycosylation, alteration of oligosaccharide processing, enzymatic or chemical deglycosylation of completed chains, genetic elimination of glycosylation sites, and the study of naturally occurring variants and genetic mutants in glycosylation. In reviewing many such studies, the consequences of altering glycosylation range from being essentially undetectable to the complete loss of particular functions, or even loss of the entire glycoprotein itself. Even within a particular class of proteins (e.g., cell surface receptors), the effects of altering glycosylation are highly variable and unpredictable. Moreover, the same glycosylation change can have markedly different effects when studied in vivo or in vitro. The answer obtained may depend on the structure of the glycan, the biological context, and the specific biological question being asked. Overall, it is difficult to predict a priori the functions that a given oligosaccharide on a given glycoconjugate might mediate or its relative importance to the organism.
Structural and Modulatory Roles of Glycans
There is little doubt that glycans have many protective, stabilizing, organizational, and barrier functions. As discussed in Chapter 1, the glycocalyx that covers most cells can represent a substantial physical barrier. The glycans attached to matrix molecules such as collagens and proteoglycans are important for the maintenance of tissue structure, porosity, and integrity. Such molecules also contain binding sites for specific types of glycans, which in turn help with the overall organization of the matrix. The external location of glycans on most glycoproteins can provide a general shield, protecting the underlying polypeptide from recognition by proteases or antibodies. Glycans are also involved in the proper folding of newly synthesized polypeptides in the ER and/or in the subsequent maintenance of protein solubility and conformation. Indeed, if some proteins are incorrectly glycosylated, they may fail to fold properly and/or fail to exit the ER. Conversely, there are also examples of glycoproteins whose synthesis, folding, trafficking, sensitivity to proteolysis, or immune recognition seem quite unaffected by altering their glycosylation. Overall, these types of functions are obviously of importance to the intact organism. However, they cannot explain the need to evolve the enormous structural complexities found in glycans in nature. In keeping with this view, inhibitors that affect only the later steps of glycan processing usually do not interfere with such basic structural functions.
There are many examples wherein glycosylation can modulate the interaction of proteins with one another. Some growth factor receptors seem to acquire their binding abilities in a glycosylation-dependent manner. This may limit unwanted early interactions of a newly synthesized receptor with a growth factor that is synthesized in the same cell. Glycosylation of a polypeptide can also mediate an on-off or switching effect. For example, when the hormone β-human chorionic gonadotrophin (β-HCG) is deglycosylated, it is still able to bind to its receptor with similar affinity. However, it fails to stimulate adenylate cyclase. The mechanism by which this agonist:antagonist conversion occurs remains unknown. In most instances, such effects of glycosylation are incomplete, i.e., glycosylation appears to be tuning the primary function of the protein. For example, the activity of some glycosylated growth factors and hormones can be modulated over a wide range by the extent and type of their glycosylation. This becomes particularly evident when recombinant forms of such molecules are produced, bearing different types and extents of glycosylation. A striking example of this “tuning” function is demonstrated by polysialic acid chains attached to the N-CAM. This adhesion receptor normally mediates homophilic binding between neuronal cells. In the embryonic state, or in other states of neural “plasticity,” these anionic chains tend to be very long, thereby interfering with homophilic binding (see Chapter 15). There are also instances where protein functions can be tuned by glycans attached to other neighboring structures. Thus, the polysialic acids of embryonic N-CAM mentioned above can interfere with the interactions of other unrelated receptor-ligand pairs, simply by physically separating the cells. Another example is the tyrosine phosphorylation activity of the EGF receptor and the insulin receptor, which can be modulated by endogenous gangliosides (see Chapter 9). Although the precise mechanism of these effects is uncertain, some specificity is implied by the requirement for a defined oligosaccharide sequence in the ganglioside. Perhaps the most dramatic example of a tuning function of a glycan is the effect of heparin/heparan sulfate chains on the action of the natural anticoagulant antithrombin III (see Chapter 11). A specific pentasaccharide sequence within the polysaccharides converts this weak antithrombin into a potent anticoagulant.
Since most such tuning effects of specific glycan sequences are partial, their overall importance might be questioned. However, it should be realized that the sum total of several such partial effects can be a dramatic effect upon the final biological outcome. Thus, glycosylation may be a mechanism for generating important functional diversity while utilizing a limited set of basic receptor-ligand interactions dictated by the size of the genome. Of course, as with most other functions of glycans, exceptions to these concepts can be easily found. There are many receptors whose ligand binding is not acquired in a glycosylation-dependent manner, and there are many peptide ligands whose binding and action are not obviously affected by glycosylation.
Another structural/modulatory function of glycans is to act as a protective storage depot for biologically important molecules. A particularly interesting case is that of the heparin-binding growth factors (see Chapter 11) that are bound to the GAG chains of the extracellular matrix adjacent to cells that need to be stimulated (e.g., the basement membrane underlying endothelial cells). This prevents diffusion of the factors away from the site, protects them from nonspecific proteolysis, prolongs their active lives, and allows them to be released under specific conditions. Likewise, the GAG chains found in secretory granules seem to bind and protect protein contents of the granule and to modulate their functions. There are several other such examples wherein glycans act as sinks or depots for biologically important molecules, ranging from water, to ions, to complement regulatory proteins.
Glycans as Specific Ligands for Exogenous Receptors
As discussed in Chapter 28, certain glycans act as specific receptors for a variety of viruses, bacteria, and parasites. They are also receptors for many plant and bacterial toxins and can serve as antigens for autoimmune and alloimmune reactions. In most instances, there is excellent specificity for the sequence of the oligosaccharide involved. Thus, for example, influenza virus hemagglutinins specifically recognize the type of sialic acid, its modifications, and its linkage to the underlying sugar chain, whereas various toxins bind with great specificity to certain gangliosides and not to related structures (see Chapter 28). Some incompletely synthesized oligosaccharides such as the Tn antigen (GalNAc-O-Ser/Thr on mucins) can also act as autoantigens. There is little doubt about the structural specificity of this group of functions of glycans. Indeed, the relevant binding proteins have even been used as specific tools for studying the expression of the sugar chains. However, as far as the organism that synthesized such glycans is concerned, there is no clear value resulting from providing such traitorous signposts, which can aid the access of pathogenic microorganisms or permit damaging autoimmune reactions. Perhaps to counter these deleterious consequences, the addition of specific monosaccharides or modifications can mask sequences recognized by microorganisms, toxins, or antibodies. Glycan sequences on soluble glycoconjugates, such as the secreted mucins, can also act as decoys for microorganisms and parasites. Thus, a pathogenic organism or toxin seeking to bind to mucosal cell membranes might first encounter the specific oligosaccharide ligand attached to a soluble mucin, which can then be washed away, removing the potential danger to the cells below. In contrast, there are instances in which symbiosis between species is mediated by specific glycan recognition. Examples of these include some commensal bacteria in the gut lumen of animals and bacteria involved in forming plant root nodules.
Glycans as Specific Ligands for Endogenous Receptors
The earliest endogenous receptors for glycans to be recognized were those mediating clearance, turnover, and intracellular trafficking (for examples, see Chapters 23 and 25). However, even the most elegantly precise examples, such as the role of Man-6-P in the trafficking of lysosomal enzymes, include some exceptions. Thus, Man-6-phosphorylation is not absolutely required for the trafficking of lysosomal enzymes in certain cell types, nor is it operative at all in some lower eukaryotes. There are also endocytic receptors recognizing specific glycan sequences whose functions have yet to be assigned. There are several examples wherein free oligosaccharides can have hormonal actions inducing specific responses in a highly structure-specific manner. Examples include the action of oligosaccharins in plants (see Chapter 20) and the bioactive properties of fragments of hyaluronan in mammalian systems, both of which can induce biological responses in a size- and structure-dependent manner. Likewise, free heparan sulfate fragments released by certain cell types have effects in complex situations such as wound healing. In many of these instances, the putative receptors for these molecules and their precise mechanisms of action have yet to be defined.
Many examples are now available of specific biological roles of oligosaccharides in cell- cell recognition and cell-matrix interactions. Perhaps the best-documented case is that of the selectin family of adhesion molecules that mediate critical interactions among blood cells in a wide variety of normal and pathological situations (see Chapter 26). There is also now substantial evidence that sperm:egg binding involves the O-glycans on the egg zona pellucida glycoprotein ZP3 (see Chapter 34). Other clear examples include CD44 recognition of hyaluronan (Chapter 29) and the interactions of bone marrow cells with sialoadhesin on macrophages (Chapter 24). As indicated above, there are also specific interactions between lectins and carbohydrates present on cell surfaces, with molecules in the matrix. In many such instances, the specific biological significance of recognition has yet to be conclusively demonstrated in the intact animal.
Carbohydrate-carbohydrate interactions may also play a specific role in cell-cell interactions and adhesion. The most dramatic example is the species-specific interactions of marine sponges, which are mediated via homotypic binding of the glycans on a large cell-surface proteoglycan. Another example is the compaction of the mouse embryo at the morula stage, which seems to be due to an Lex-Lex interaction. The single-site affinities of such interactions are not very strong and are difficult to measure. However, if the molecules in question are present in very high copy numbers on the cell surface, a large number of relatively low-affinity interactions can collaborate to produce a high-avidity “velcro” effect, which may be clearly sufficient to mediate biologically relevant interactions.
The Same Glycan Can Have Different Roles within An Organism
The expression of specific types of glycosylation on different glyconjugates in different tissues at different times of development implies that these structures have diverse roles within the same organism. For example, Man-6-P-containing oligosaccharides were first found on lysosomal enzymes and are involved in lysosomal trafficking (see Chapter 23). However, Man-6-P-containing glycans are found on a variety of apparently unrelated proteins, including proliferin, thyroglobulin, the EGF receptor, and the TGF-β precursor. Likewise, the sialylated fucosylated lactosamines critical for selectin recognition (see Chapter 26) are found in a variety of unrelated cell types, and the polysialic acid chains that play such an important part in embryonic N-CAM (Chapter 15) are found on egg jelly coat proteins and a sodium channel protein. Since oligosaccharides are added posttranslationally, these observations should not be surprising. Once a new oligosaccharide or modification has been expressed in an organism, several distinct usages could evolve independently in different tissues and at different times in development. If any of these situations provide a function vital to the survival of the organism, then the gene responsible for expression of the glycan would remain conserved in evolution.
Are There “Junk” Glycans?
Since microorganisms and parasites that bind glycans evolve in parallel with their multicellular hosts (see Chapter 3), they have to adapt to bind to any new “masking” glycan structure presented by the host. In response, the host may find it easiest to generate new masking structures, especially if the primary structure had meanwhile evolved a vital function elsewhere within the organism. Thus, there would be no choice but to preserve the underlying scaffolding upon which the latest “mask” was placed, adding yet another layer of complexity to its glycans. Such cycles of interaction between microbes and hosts could perhaps explain some of the complex and extended sugar chains found on mucosal surfaces and secreted mucins. In this manner, “junk” glycans could develop, akin to “junk” DNA. Although such structures may still function as a scaffolding, they may have no other specific role in the organism.
Intraspecies and Interspecies Variations in Glycosylation
Species-specific variations in glycan structure also indicate that some glycans do not have fundamental and universal roles (see Chapter 3). Such diversity in glycosylation could be involved in generating the obvious differences in morphology and function observed between species. These variations could also simply reflect differing selection pressures resulting from exposure to different pathogens. Furthermore, substantial intra-species polymorphism in oligosaccharide structure can exist without obvious functional value. The potential role of such polymorphisms in the interplay between parasites and host populations is discussed in Chapter 3. Of course, it should be noted that extensive interspecies variability in primary sequence also occurs in proteins, without any obvious consequences to essential functions (e.g., some yeast proteins are functional when transfected in mammalian cells, and vice versa, despite relatively limited sequence homology).
Importance of Terminal Sequences, Modifications, and Unusual Structures
Given all of the above, it is a challenge to predict which glycan structures are likely to mediate the more specific or crucial biological roles within an organism. The available data suggest that terminal sugar sequences, unusual structures, or modifications of the glycans are more likely to be involved in such specific roles. The predictive value of this observation is reduced by the fact that such terminal sequences, unusual glycans, or modifications are also more likely to be involved in interactions with microorganisms and other noxious agents, i.e., the balance between the traitorous and masking functions of glycans discussed above tends to involve such structures. The challenge then is to predict and sort out which of these two distinct roles is to be assigned to a given glycan structure.
Approaches to Uncovering Specific Biological Roles of Glycans
Some functions of glycans are discovered serendipitously. In most cases, the investigator who has elucidated complete details of the structure and biosynthesis of a specific glycan is still left without knowing its functions. It is necessary to design experiments that can differentiate between the trivial and crucial functions mediated by each glycan. Various approaches that can be considered are discussed below, emphasizing the pros and cons of each. These approaches are also presented in schematic form in Figure 5.2 (the figure assumes that the investigator is exploring a glycan function mediated by a specific receptor:ligand pair).

Figure 5.2
Approaches toward uncovering biological roles of glycans. The figure assumes that a specific biological role is being mediated by recognition of a certain glycan structure by a specific receptor lectin. Clues to this biological role could be obtained (more...)
Localization of or Interference with Specific Glycans Using Lectins or Antibodies
The availability of numerous lectins and antibodies that are apparently specific for certain glycans has permitted the exploration of the regional and cell-type-specific localization of these structures. Once a specific glycan has been localized in a interesting context, it is natural to consider introducing the same lectin or antibody into the intact system, hoping that it will interfere with a specific function, generating an interpretable phenotype. This approach has proven to be highly successful when investigating the function of proteins by introduction of specific antibodies. However, with some possible exceptions, the same approach is likely to give confusing results with regard to glycan function. Most antibodies against glycans are of the IgM variety and hence tend to have weak affinity and show cross-reactivity between species (although high-affinity IgG antibodies are preferred, they are hard to obtain, because glycans tend to be T-independent antigens and often do not generate high-titer immune responses). Likewise, although some plant lectins seem to be very specific for animal glycans, it must be remembered that the lectins originated from organisms that typically do not contain the cognate ligand. Thus, their apparent specificity may not be as reliable when introducing them into complex animal biological systems where unknown cross-reacting glycan structures are potentially present. Finally, both antibodies and lectins are multivalent and their cognate ligands (the glycans) tend to be present in multiple copies on multiple glycoconjugates. Thus, introduction of a lectin or antibody into a complex biological system is likely to cause nonspecific aggregation of various molecules and cell types, and the effects seen may have nothing to do with the biological functions of the glycan in question. In the long run, it would seem more worthwhile to develop recombinant monovalent high-affinity lectin modules that are derived from the same system being investigated. The effects of introducing such monovalent lectins into a complex system may yield more interpretable clues.
Metabolic Inhibition or Alteration of Glycosylation
As outlined in Chapter 40, a large number of pharmacological agents can metabolically inhibit or alter glycosylation in intact cells and animals. Although metabolic inhibitors are powerful tools to elucidate biosynthetic pathways, they can sometimes yield confusing results in complex systems. One concern is that the inhibitor may have effects on other unrelated pathways (e.g., the inhibitor tunicamycin that blocks N-linked glycosylation can also inhibit UDP-Gal uptake into the Golgi). The second is that the inhibitor may result in such massive changes in glycan synthesis that the physical properties of the glycoconjugates and/or membranes are altered, making it difficult to interpret the results. Somewhat more useful results can be obtained by introducing low-molecular-weight primers of glycosylation, which can act as alternate substrates for Golgi enzymes, diverting synthesis away from the endogenous glycoproteins. However, this approach runs the risk of simultaneously generating incomplete glycans on the endogenous glycoconjugates, as well as producing secreted glycan chains that could have their own biological effects.
Finding Natural Glycan Ligands for Specific Receptors
With the ability to recognize different types of lectin motifs within a primary amino acid sequence (see Chapter 22), many newly cloned proteins may be predicted to bind glycans. If the potential lectin protein can be produced in large enough quantities, techniques such as red cell agglutination, flow cytometry, surface plasmon resonance, and affinity chromatography can then be used to look for the presence of specific ligands. It should be kept in mind that the monovalent affinity of the putative lectin for its ligand may not be high, and high densities and/or multivalent arrays may be needed, to avoid missing a biologically relevant interaction. The question also arises as to where to look for the biologically relevant ligands. Furthermore, since many glycan structures can be expressed in different tissues at different times in development and growth, the recombinant lectin may detect a cognate structure in a location and time where it is not actually of biologically relevance. Careful consideration of the natural occurrence and expression profile of the lectins should lead to a rational decision as to where to look for the glycan ligands.
Finding Receptors Recognizing Specific Glycans
The converse situation arises when an unusual glycan is found to be expressed in an interesting context and is hypothesized to be a ligand for a specific receptor. It is possible to search for such a receptor by techniques similar to those mentioned above, such as hemagglutination, flow cytometry, and affinity chromatography. Of course, to facilitate the search, it is necessary to have reasonable quantities of the pure defined glycan in question, as well as a variety of closely related structures that can act as negative controls. Since many biologically relevant lectin-like interactions can be of low affinity, it is probably also advisable to use a multivalent form of the glycan as the probe. Finally, it may not necessarily be obvious where to look for receptor. For example, the receptor that specifically recognizes the sulfated glycans of pituitary glycoprotein hormones was eventually found not in the pituitary itself, nor in any of the target tissues for the hormones, but in the endothelial cells of the liver, where it regulates the circulating half-life of the hormones. Indeed, the most biologically relevant receptor for a particular glycan might even be found in another organism (a pathogen or a symbiont).
Interference by Soluble Glycans or Structural Mimics
The addition of soluble glycans or their structural mimics into the system can cause interference with the interaction between an endogenous lectin and a specific glycan. If a sufficient concentration of the specific inhibitor can be achieved, the resulting phenotypic changes can be instructive. When studying in vitro systems, even monosaccharides can be used to advantage in such studies (e.g., the exploration of the Man-6-P receptor pathway, Chapter 23). However, is often necessary to use pure glycans in somewhat large quantities to block the relatively low-affinity interactions. Effective blockade may also require multivalency of the cognate glycan. Finally, especially when studying complex multicellular systems, the glycans introduced could cross-react with other as yet unknown receptors, giving a phenotypic readout that is confusing.
Eliminating Specific Glycan Structures by Glycosidases
A powerful approach to understanding the biological roles of glycans is to make use of the many known degradative enzymes that are highly specific for certain glycan sequences. The advantage of this approach is that one is not interfering with the basic biosynthetic cellular machinery, but simply eliminating the structures selectively after normal synthesis has been completed. Thus, for example, sialidase treatment abolished lymphocyte binding to the high endothelial venules of lymph nodes and provided the first prediction of the ligands for l-selectin: Injection of endoneuraminidase into the developing retina suggested specific roles for polysialic acids, and injection of heparanase into developing embryo resulted in a randomization of left-right axis formation. In all such studies, the purity of the enzyme being used is obviously critical, and appropriate controls are necessary. A genetic approach can be used to avoid problems of contamination, by expressing a cDNA for the glycan-modifying enzyme in the intact cell or animal. For example, transgenic expression in mice of an influenza sialic-acid-specific C 9-O-acetylesterase gave either early or late abnormalities in development, depending on the promoter used.
Studying Natural or Genetically Engineered Glycan Mutants
This is intuitively a powerful approach to understanding glycan function. Technically, it is easiest to study glycosylation mutants in cultured cell lines (see Chapter 31). However, although genetic or acquired defects in glycosylation are easily obtained in cultured cell lines, these defects may have limited or not easily discernible consequences. This may be because of the lack of other factors or cell types that would be present in the intact organism; e.g., the cognate receptor for the glycan may not be present in the same cell type. Of course, such mutants can still be used to analyze basic structural functions of the glycans and their relevance to the physiology of a single cell. Furthermore, one can add back external factors or other cell types thought to interact with the modified glycan. Some mutants can also be reintroduced into intact organisms (e.g., to study tumorigenicity or metastatic behavior). Overall, much useful information can be gained by this approach, but many of the more specific roles of glycans need to be uncovered by studying mutations in the intact multicellular organism.
Genetic defects in glycosylation in intact organisms seem to be relatively uncommon and have highly variable consequences. Looking back on the mutants that have been discovered to date in mice and humans (see Chapters 32 and 33), it is clear that glycan changes often affect multiple systems and that the phenotypes are unpredictable and highly variable. The relative rarity of naturally occurring mutations can be explained in several ways. Perhaps they do occur frequently but have little detectable biological consequence. Alternatively, many may cause lethal aberrations that prevent completion of embryogenesis. Another possibility is that genetic glycosylation abnormalities remain undetected because of redundant or fail-safe pathways. Regardless of the reasons, the value of constructing glycosylation mutants in intact animals is evident. Given the complex phenotypes that can result and the potential for early developmental lethality, the ability to disrupt genes in a temporally controlled and cell-type-specific manner can be particularly valuable (for details and examples, see Chapter 32).
Studying Natural or Genetically Engineered Glycan Receptor Mutants
Eliminating a specific glycan receptor can yield a phenotype that may be very instructive with regard to the functions of the glycan. As with genetic modification of the glycan, the results are more likely to be useful if studied in the intact organism. However, the receptor protein may have other functions that are unrelated to glycan recognition. Conversely, the glycan in question may have other functions that are not mediated by the receptor. Thus, for example, the genetic elimination of the CD22 receptor and the ST6Gal I enzyme that generates its ligand gave complementary, but not identical, phenotypes.
Future Directions
The future now appears to be very bright for elucidating many new biological roles of glycans. These roles are likely to become more obvious in the upcoming “post-genomic era,” when more and more attention is going to be focused upon the molecular basis of the development and physiology of whole organs or organisms. The various approaches described in this chapter must be combined as needed to elucidate these biological roles.
References
- 1.
- Roseman S. The synthesis of carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem. Phys. Lipids. 1970;5:270–297. [PubMed: 5476326]
- 2.
- Montreuil J. Primary structure of glycoprotein glycans: Basis for the molecular biology of glycoproteins. Adv. Carbohydr. Chem. Biochem. 1980;37:157–223. [PubMed: 6996449]
- 3.
- Aplin J D, Hughes R C. Complex carbohydrates of the extracellular matrix structures, interactions and biological roles. Biochim. Biophys. Acta. 1982;694:375–418. [PubMed: 6760897]
- 4.
- Berger E G, Buddecke E, Kamerling J P, Kobata A, Paulson J C, Vliegenthart J F G. Structure, biosynthesis and functions of glycoprotein glycans. Experientia. 1982;38:1129–1162. [PubMed: 6754417]
- 5.
- Sharon N, Lis H. Glycoproteins: Research booming on long-ignored ubiquitous compounds. Mol. Cell. Biochem. 1982;42:167–187. [PubMed: 7062912]
- 6.
- Schauer R. Sialic acids and their role as biological masks. Trends Biochem. Sci. 1985;10:357–360.
- 7.
- Barondes S H. Bifunctional properties of lectins: Lectins redefined. Trends Biochem. Sci. 1988;13:480–482. [PubMed: 2855286]
- 8.
- Rademacher T W, Parekh R B, Dwek R A. Glycobiology. Annu. Rev. Biochem. 1988;57:785–838. [PubMed: 3052290]
- 9.
- Paulson J C. Glycoproteins: What are the sugar chains for. Trends Biochem. Sci. 1989;14:272–276. [PubMed: 2672447]
- 10.
- Fenderson B A, Eddy E M, Hakomori S. Glycoconjugate expression during embryogenesis and its biological significance. BioEssays. 1990;12:173–179. [PubMed: 1970725]
- 11.
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem. Sci. 1990;15:291–294. [PubMed: 2204153]
- 12.
- Cumming D A. Glycosylation of recombinant protein therapeutics: Control and functional implications. Glycobiology. 1991;1:115–130. [PubMed: 1823155]
- 13.
- Elbein A D. The role of N-linked oligosaccharides in glycoprotein function. Trends Biotechnol. 1991;9:346–352. [PubMed: 1367760]
- 14.
- Esko J D. Genetic analysis of proteoglycan structure, function and metabolism. Curr. Opin. Cell Biol. 1991;3:805–816. [PubMed: 1931081]
- 15.
- Darvill A, Augur C, Bergmann C, Carlson R W, Cheong J -J, Eberhard S, Hahn M G, Ló V -M, Marfà V, Meyer B, Mohnen D, O'Neill M A, Spiro M D, van Halbeek H, York W S, Albersheim P. Oligosaccharins—Oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology. 1992;2:181–198. [PubMed: 1498416]
- 16.
- Drickamer K, Carver J. Upwardly mobile sugars gain status as information-bearing macromolecules. Curr. Opin. Struct. Biol. 1992;2:653–654.
- 17.
- Hart G W. Glycosylation. Curr. Opin. Cell Biol. 1992;4:1017–1023. [PubMed: 1485955]
- 18.
- Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur. J. Biochem. 1992;209:483–501. [PubMed: 1358608]
- 19.
- Rasmussen J R. Effect of glycosylation on protein function. Curr. Opin. Struct. Biol. 1992;2:682–686.
- 20.
- Zanetta J -P, Kuchler S, Lehmann S, Badache A, Maschke S, Thomas D, Dufourcq P, Vincendon G. Glycoproteins and lectins in cell adhesion and cell recognition processes. Histochem. J. 1992;24:791–804. [PubMed: 1478888]
- 21.
- Zeller C B, Marchase R B. Gangliosides as modulators of cell function. Am. J. Physiol. 1992;262:C1341–C1355. [PubMed: 1616002]
- 22.
- Knudson C B, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed: 7691670]
- 23.
- Lis H, Sharon N. Protein glycosylation—Structural and functional aspects. Eur. J. Biochem. 1993;218:1–27. [PubMed: 8243456]
- 24.
- Opdenakker G, Rudd P M, Ponting C P, Dwek R A. Concepts and principles of glycobiology. FASEB J. 1993;7:1330–1337. [PubMed: 8224606]
- 25.
- Van Echten G, Sandhoff K. Ganglioside metabolism. Enzymology, topology, and regulation. J. Biol. Chem. 1993;268:5341–5344. [PubMed: 8449895]
- 26.
- Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3:97–130. [PMC free article: PMC7108619] [PubMed: 8490246]
- 27.
- Bevilacqua M P, Nelson R M, Mannori G, Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu. Rev. Med. 1994;45:361–378. [PubMed: 7515220]
- 28.
- Marth J D. Will the transgenic mouse serve as a Rosetta Stone to glycoconjugate function? Glycoconj. J. 1994;11:3–8. [PubMed: 8193551]
- 29.
- Dennis R P. A review of the biological significance of carbohydrates on glycoproteins and methods for their analysis. Adv. Exp. Med. Biol. 1995;376:1–11. [PubMed: 8597235]
- 30.
- Stanley P, Ioffe E. Glycosyltransferase mutants: Key to new insights in glycobiology. FASEB J. 1995;9:1436–1444. [PubMed: 7589985]
- 31.
- Wong S Y C. Neoglycoconjugates and their applications in glycobiology. Curr. Opin. Struct. Biol. 1995;5:599–604. [PubMed: 8574694]
- 32.
- Crocker P R, Feizi T. Carbohydrate recognition systems: Functional triads in cell-cell interactions. Curr. Opin. Struct. Biol. 1996;6:679–691. [PubMed: 8913692]
- 33.
- Gahmberg C G, Tolvanen M. Why mammalian cell surface proteins are glycoproteins. Trends Biochem. Sci. 1996;21:308–311. [PubMed: 8772385]
- 34.
- Kansas G S. Selectins and their ligands: Current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed: 8896391]
- 35.
- Kasai K, Hirabayashi J. Galectins: A family of animal lectins that decipher glycocodes. J. Biochem. 1996;119:1–8. [PubMed: 8907168]
- 36.
- Salmivirta M, Lidholt K, Lindahl U. Heparan sulfate: A piece of information. FASEB J. 1996;10:1270–1279. [PubMed: 8836040]
- 37.
- Spillmann D, Burger M M. Carbohydrate-carbohydrate interactions in adhesion. J. Cell. Biochem. 1996;61:562–568. [PubMed: 8806079]
- 38.
- Baenziger J U. Glycosylation: To what end for the glycoprotein hormones? Endocrinology. 1996;137:1520–1522. [PubMed: 8612480]
- 39.
- Gabius H J. Animal lectins. Eur. J. Biochem. 1997;243:543–576. [PubMed: 9057819]
- 40.
- Kim Y J, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 1997;14:569–576. [PubMed: 9298689]
- 41.
- Kinoshita T, Ohishi K, Takeda J. GPI-anchor synthesis in mammalian cells: Genes, their products, and a deficiency. J. Biochem. 1997;122:251–257. [PubMed: 9378699]
- 42.
- Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. [PubMed: 10406840]
- General Principles and Conclusions about Biological Roles of Glycans
- Biological Consequences of Altering Glycosylation Are Variable
- Structural and Modulatory Roles of Glycans
- Glycans as Specific Ligands for Exogenous Receptors
- Glycans as Specific Ligands for Endogenous Receptors
- The Same Glycan Can Have Different Roles within An Organism
- Are There “Junk” Glycans?
- Intraspecies and Interspecies Variations in Glycosylation
- Importance of Terminal Sequences, Modifications, and Unusual Structures
- Approaches to Uncovering Specific Biological Roles of Glycans
- Future Directions
- References
- Exploring the Biological Roles of Glycans - Essentials of GlycobiologyExploring the Biological Roles of Glycans - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
