NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings R, Esko J, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1999.

Essentials of Glycobiology.
Show detailsPrimary contributions to this chapter were made by J.C. Paulson (Ths Scripps Research Institute, La Jolla, California), A. Varki (University of California at San Diego), and J.D. Esko (University of California at San Diego).
THIS CHAPTER PROVIDES AN OVERVIEW of the increasing importance of glycobiology and carbohydrate chemistry in modern biotechnology and the pharmaceutical industry. The carbohydrates of therapeutic recombinant glycoproteins have important roles in determining their pharmacokinetic properties. Important biological interactions and biological functions mediated by glycans are also being targeted for therapeutic manipulation in vivo. Examples of carbohydrate-based therapeutics in development include inhibitors of microbial pathogens and their toxins, cancer vaccines, and drugs designed to suppress the immune system for treatment of inflammation and transplant rejection.
Therapeutic Glycoproteins (1–9)
The most important class of biotechnology products to date are therapeutic glycoproteins. These include erythropoietin, granulocyte macrophage-colony-stimulating factor, and tissue plasminogen activator, which together generate sales of 3–5 billion dollars worldwide. In addition, approximately 60 recombinant glycoproteins are currently in development as therapeutic agents. Therapeutic glycoproteins are typically produced as recombinant products in cell culture systems or in transgenic animals. Control of glycosylation takes on major importance during the development of these drugs, since the glycan chains have dramatic effects on stability, action, and pharmacodynamics in intact organisms. In most cases, glycosylation must be optimized to ensure prolonged circulatory half-life in the blood. Manipulation of glycans to promote targeting to specific tissues and cell types has also been an essential element of drug design for several successful therapeutic glycoproteins.
Optimizing Glycans of Therapeutic Glycoproteins for Prolonged Serum Half-life (1–7)
A well-studied example is that of erythropoietin, perhaps the single most successful biotechnology product to date. Erythropoietin interacts with a membrane-signal-transducing receptor, inducing proliferation and differentiation of erythroid progenitors, and thus, it has great value in treating anemias caused by bone marrow suppression, e.g., after chemotherapy. Natural and recombinant forms of erythropoietin carry four sialylated complex-type N-glycans. Although the in vitro activity of deglycosylated erythropoietin is equal to fully glycosylated erythropoietin, the in vivo activity of the deglycosylated form is less than 10% of the glycosylated erythropoietin, because poorly glycosylated forms of erythropoietin are rapidly cleared by filtration in the kidney. Furthermore, undersialylated erythropoietin is rapidly cleared by Gal/GlcNAc/Man receptors in hepatocytes and macrophages. Both processes can be reduced by having fully sialylated chains and by increasing the number of tetra-antennary chains. Decreasing the rate of clearance of erythropoietin, in turn, increases its in vivo activity nearly tenfold. Erythropoietin is perhaps unusual, since it is small enough to be cleared by the kidney if underglycosylated. For most glycoprotein therapeutics, minimizing clearance by the Gal/GlcNAc/Man receptors by having fully sialylated chains is the most important consideration (see Chapter 25).
Because the glycans can have dramatic effects on the properties of these drugs, it is important to ensure that glycosylation is controlled during their production to satisfy regulatory requirements for batch-to-batch product consistency. Changes in culture pH, the availability of precursors and nutrients, and the presence or absence of various cytokines and hormones can each affect the extent of glycosylation, the degree of branching, and the completeness of sialylation and sulfation. The presence of sialidases and other glycosidases either secreted or released by dead cells can cause degradation of the previously intact product. Thus, during the development of each glycoprotein drug, considerable effort is devoted to defining appropriate conditions for reproducibly producing homogeneous preparations of recombinant glycoproteins.
Targeting of Lysosomal Enzymes in Enzyme Replacement Therapy (10–13)
Unlike most therapeutic glycoproteins that interact with target receptors on the surface of cells, lysosomal enzymes developed for enzyme replacement therapy must be delivered intracellularly to lysosomes, their site of action. These drugs are intended to treat genetic defects for deficiencies in individual lysosomal enzymes leading to pathological accumulation of their substrates in inclusion bodies inside the cells (see lysosomal storage disorders, Chapter 18). During the normal biosynthesis of lysosomal enzymes, the N-linked glycans become modified with Man-6-P residues that target them to lysosomes through the Man-6-P receptor (see Chapter 23). The challenge for enzyme replacement therapy is to get the enzymes targeted properly to lysosomes where they can degrade accumulated substrate.
A special case is enzyme replacement therapy for Gaucher's disease, a glucocerebrosidase deficiency. Exogenous enzyme has been targeted to lysosomes of macrophages through the cell surface mannose receptor (see Chapter 25). This approach required that the recombinant enzyme be produced with N-glycans containing terminal mannose residues. This was accomplished by cleaving the N-glycans of the enzymes produced in mammalian cells with glycosidases (sialidase, galactosidase, and hexosaminidase). Alternatively, the enzyme can be produced in baculovirus-infected insect cells, which elaborate N-glycans with terminal mannose residues. The commercial success of glucocerebrosidase has stimulated the development of lysosomal enzymes for treatment of other lysosomal storage diseases.
Complex Carbohydrate-based Therapeutics (14–20)
An increasing number of therapeutics aimed at modulating processes mediated by carbohydrate groups of glycoproteins and glycolipids are currently in development. Advances in the enzymatic production of carbohydrates are available to facilitate the production and commercialization of such compounds.
Inhibitors of Pathogenic Microbes and Toxins
As discussed in Chapter 28, many microbes and toxins bind to mammalian tissues by recognizing specific carbohydrate ligands. Thus, small soluble glycans or glycan mimics can be used to block the initial attachment of microbes and toxins to cell surfaces (or block their release), and thus prevent or suppress infection. Because many of these organisms naturally gain access through the airways or gut, the carbohydrate-based drugs can be delivered orally or bronchially without the requirement of being distributed systemically. Examples of such applications currently under study are listed in Table 41.1 and Table 41.2. Notable for their advanced stage of development are inhibitors of influenza virus sialidase (Table 41.3). The sialidase is required for the release of newly produced virus from sialic acid receptors on the cell surface. Therefore, inhibition of the sialidase stops further replication of the virus and appears to reduce the severity and duration of the disease.
Table 41.1
Possible carbohydrate antagonists of microbial adhesion.
Table 41.2
Examples of glycan therapeutics being developed to enhance the immune system.
Table 41.3
Examples of enzyme-based inhibitors as potential therapeutics.
Human breast milk oligosaccharides are believed to be natural antagonists of intestinal infection in infants and to promote the growth of beneficial gut flora. Some companies have begun to market nontherapeutic nutritional products that are “fortified” with oligosaccharides to impart the putative beneficial properties provided by these natural oligosaccharides. More defined therapeutic applications are also being evaluated (Table 41.1).
Bacterial Vaccines
Conjugate vaccines with oligosaccharides coupled to carrier proteins are proving to be highly effective. For example, Haemophilus influenzae type b causes an acute lower respiratory infection among young children. As recently as a decade ago, approximately 20,000 children in the United States suffered infections. In the early 1990s, a conjugated form of a Hib-derived oligosaccharide coupled to a protein carrier was shown to provide an effective vaccine.
The Hib vaccine is now routinely given to infants in developed countries and has resulted in a more than 95% decrease in incidence of Hib infections in vaccinated populations. In addition, new vaccines are being developed against conjugated components of the capsular polysaccharides of Neisseria meningitidis and Streptococcus pneumoniae and the preliminary studies look promising.
Cancer Vaccines
Several carbohydrate-based vaccines are under development to treat cancer. Some of these vaccines are based on ganglioside immunogens present on certain types of cancer cells (e.g., gangliosides GM2 and GD2 on melanomas and breast cancer). These vaccines are composed of carbohydrate haptens conjugated to a protein carrier (Table 41.2). In one case, a breast cancer antigen, sialylTn (Sialylα2–6GalNAcα-) is synthesized chemically and conjugated to a protein carrier (keyhole limpet hemocyanin). SialylTn is found on cancer mucins, and its expression correlates with progression to metastatic breast cancer (see Chapter 35).
Xenotransplantation: Acute Transplant Rejection Mediated by Carbohydrates (21–23)
As discussed in Chapter 16, a variety of glycans, including the classical A and B blood group determinants, can act as barriers to blood transfusion and transplantation of organs. Rejection of mismatched blood or organs occurs because hosts have a high titer of preexisting antibodies against the carbohydrate epitopes, presumably as a prior reaction to related structures found on bacteria or other microbes. In the case of the ABO blood groups, incompatibility is routinely managed by blood and tissue typing and finding an appropriate donor for the recipient. However, one approach to circumvent blood group incompatibility is to use immobilized A and B blood groups, which under some circumstances could allow mismatched blood to be used for transfusions. In practice, such circumstances are rare.
A related problem is found in xenotransplantation, transplantation of organs between species, which is actively being pursued as a solution for the shortage of organs for human recipients. The animal donors of preference are pigs, since many porcine organs resemble those of humans in size, physiology, and structure. Unlike humans and certain other primates, pigs and most other mammals produce a terminal Galα1,3Gal linear epitope on glycoproteins and glycolipids. Humans have naturally occurring high-titer antibodies in their blood directed toward the Galα1–3Gal epitope, resulting in hyperacute rejection of porcine organ transplants, which is due to reaction of the antibodies with this antigen on enodothelial cells of blood vessels. Attempts to prevent this reaction include blood filtration over glycan affinity columns to remove xenoreactive antibodies and blockade of the interaction by infusing soluble competing αGal oligosaccharides. Ultimately, producing transgenic pigs lacking the reactive Gal α1–3 transferase may solve this problem. Of course, there are other carbohydrate differences between humans and pigs (e.g., absence of Neu5Gc in humans, see Chapter 15) that could also be of concern.
Inhibition of Selectin-mediated Leukocyte Trafficking (24–26)
If specific glycan-protein interactions in vivo are responsible for selective cell-cell interactions and a resulting pathology, then infusion of small-molecule analogs or mimics of the natural ligand could be theoretically useful as a therapeutic. The best studied example is the selectin-mediated recruitment of neutrophils (and other leukocytes) into sites of inflammation or ischemia/reperfusion injury, which involves specific selectin-glycan interactions occurring in the vascular system (see Chapter 26). Approaches taken include the use of sialyl Lewis X derivatives or small-molecule selectin antagonists. Simple monovalent compounds have already proven effective in various animal inflammatory models. This finding is somewhat surprising since the binding constant for the monovalent ligands is much poorer than that for the physiological ligand. Nevertheless, preliminary studies in animals indicate that sialyl Lewis X derivatives are probably effective at submillimolar concentrations, suggesting that other low-affinity ligands for cellular lectins might prove to be effective antagonists as well.
An alternate approach for interfering with selectin-carbohydrate interactions consists of finding specific inhibitors of the enzymes involved in forming endogenous sialyl Lewis X determinants in vivo. Thus, considerable effort is under way to design inhibitors of the FucT VII enzyme responsible for generating most natural selectin ligands (also see Chapter 40). Other therapeutics under development based on modulating protein-carbohydrate interactions include small-molecule inhibitors of heparin-growth factor interactions in cancer, inflammation, and angiogenesis. A particularly appealing aspect of this work is that antagonists discovered in this way can be used to treat both acute and chronic disorders.
Large-scale Chemoenzymatic Synthesis of Complex Glycans (27)
A limiting factor in developing carbohydrate-based compounds for clinical application is the high cost and complexity of producing glycans in adequate quantities. Advances in cost-effective technology for enzymatic synthesis appear to be promising solutions to this problem (for the generic principles involved, see Figure 41.1). This approach uses recombinant glycosyltransferases, relatively inexpensive precursors, and recycling reactions to regenerate the most costly reagents (nucleotide sugars). The reactions tend to be efficient and specific, giving a single carbohydrate product with very high yields, thus allowing efficient recovery of product from reaction mixtures. By using selected glycosyltransferases in sequence, the approach is suitable for the synthesis of most complex carbohydrates (e.g., see Figure 41.2). At least four compounds have been produced at the 1–10-kg scale using this approach.
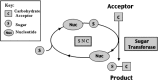
Figure 41.1
Generic principle for sugar nucleotide cycles (SNC).
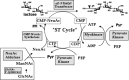
Figure 41.2
Example of a synthesis of a simple sialyloligosaccharide using in situ regeneration of CMP-NeuAc.
Another application of the enzymatic synthesis technology is to produce analogs of lead compounds. Some glycosyltransferases, kinases, and phosphorylases will utilize alternate substrates with low efficiency. However, the reactions can be carried out for long periods of time, resulting in the generation of specific oligosaccharides containing one or more functional group modifications. For example, the β1–4 galactosyltransferases will use a number of UDP-Gal analogs lacking hydroxyl groups or containing amino groups. Although the overall yields are low, the generation of analogs in sufficient quantity for testing purposes is feasible and much less laborious than standard orthogonal blocking strategies used in conventional carbohydrate synthesis (see Chapter 39).
Therapeutic Applications of Proteoglycans, Proteoglycan-modifying Enzymes, and Other Glycan Polymers (28–34)
Control of Blood Coagulation
Unfractionated heparin has been used for several decades as an anticoagulant. Its efficacy is based on heparin binding and activating antithrombin, a critical protease inhibitor in the coagulation cascade. Activation of antithrombin leads to rapid inhibition of thrombin and Factor Xa, shutting down the production of fibrin clots. Heparin is produced by autodigestion of pig mucosa, followed by graded fractionation of the material (see Chapter 11). Annual production is probably about 150,000 pounds, which translates into more than a billion doses per year. Unfractionated heparin binds to several plasma, platelet, and endothelial proteins, producing a highly variable anticoagulant response. Therefore, much interest exists in producing heparin preparations with more predictable outcomes. One class of derivatives is the low-molecular-weight heparins, which involve chemical cleavage of the chains into smaller fragments. The pharmacological properties and the relative efficacy of the various low-molecular-weight heparins are still under investigation, but studies have already indicated better pharmacological properties (i.e., reduced clearance) and fewer secondary complications than unfractionated heparin. The enzymes of heparin biosynthesis are now being cloned, and it should thus become possible to produce recombinant heparins as well.
Recombinant heparinases are under development to remove circulating heparin from blood in order to avoid excessive bleeding. The conventional approach is to use the basic protein protamine, which binds to heparin, neutralizes its activity, and results in clearance of the complex in the kidney and liver. Heparinase I is being developed as an alternative neutralization agent because enzyme-mediated neutralization preserves the antithrombotic properties of heparin and does not cause hemodynamic perturbations.
Surgical Applications of Hyaluronan
Hyaluronan is a naturally occurring vertebrate polysaccharide which has found extensive use in surgery. Because of its viscoelastic properties, hyaluronan has lubricating and cushioning properties that have made it useful for protecting the corneal endothelium during ocular surgery. Intra-articular injection of hyaluronan also has a palliative effect on patients with osteoarthritis. Hyaluronan has anti-adhesive properties and therefore might be useful in postsurgical wound-healing as well. The mechanism of action is not well understood, but it may involve hyaluronan-binding proteins that mediate cell adhesion (see Chapter 29). Other carbohydrate-based anti-adhesion substances are under development as well. The FDA recently approved a carbohydrate-containing gel (ADCON-L) to treat postsurgical adhesions that occur frequently after lumbar disc surgery. The ECM is potentially a rich source of bioactive glycoconjugates for treating disorders and diseases dependent on cell adhesion.
β-Glucan-mediated Immune Stimulation
A variety of carbohydrate-containing compounds are also being used as immune enhancers and vaccines (Table 41.2). For example, particulate and soluble β1–3 glucans with β1–6 branches are effective in preventing infections by enhancing macrophage and neutrophil functions. The mechanisms triggering these enhanced cellular responses are essentially unknown, but some evidence exists that the glucans bind to the complement receptor, CR3. Glucan binding in combination with interaction with iC3b on opsonized bacteria or yeast leads to phagocytosis and degranulation. In contrast, when CR3 on phagocytes or natural killer cells adheres to iC3b on erythrocytes or tumor cells that lack CR3-binding membrane polysaccharides, neither lysis nor cytotoxicity is stimulated. These ideas may explain the mechanism of tumoricidal glucans used for immunotherapy.
Future Directions (20, 35–39)
Medical applications of glycobiology have to date focused largely on roles of carbohydrates known for several decades, including mediating adhesion of pathogens and toxins to host cells, the appearance of tumor-associated antigens, the regulation of blood coagulation, and the circulatory half-life of plasma glycoproteins. More recently, novel families of carbohydrate-specific cell adhesion proteins have been identified that are being recognized to mediate important functions in the immune system. Although the carbohydrate ligands of the selectin family of leukocyte adhesion proteins were only identified in this decade, several companies are developing compounds to block selectin-mediated functions as novel agents to treat acute and chronic inflammation. It is likely that additional therapeutic opportunities will emerge as the roles of newly described and yet to be discovered carbohydrate-binding proteins are elucidated.
Although the approaches to modulating biological systems involving carbohydrates are varied, most involve the use of glycans or glycomimetics to modulate the activity of a carbohydrate-binding protein. A promising alternative is to use inhibitors of the enzymes that degrade or synthesize carbohydrates as a way of modulating the functions or properties they impart (see Table 41.3). Sialic acid analogs being developed as potent inhibitors of influenza virus sialidase are showing promising results in clinical trials and may be available within a few years (see Chapter 40). Other glycosidase inhibitors being evaluated target endogenous glycosidases. For exmple, Acarbose, an intestinal α-glucosidase inhibitor, is being used to treat diabetes mellitus. Inhibition of α-glucosidase in the gut blocks the uptake of glucose from glucose-containing polysaccharides in the diet (e.g., dietary glycogen) and thereby reduces the load of exogenous glucose derived from a meal. Glucosylceramide synthetase is an example of a glycosyltransferase that is a target for inhibitor development since inhibition of this enzyme might decrease the accumulation of glycosphingolipids in lysosomal storage disorders. Another example already on the consumer product market is Lufenuron, a benzoylphenyl urea chitin synthesis inhibitor that is effective against certain insects such as fleas. When adult female fleas feed on an animal that has been treated with Lufenuron, egg development does not occur normally, since chitin is not deposited correctly. Thus, the life cycle of the flea is interrupted.
Such creative approaches to modulating the many structural and biological functions of carbohydrates set the stage for creating products of commercial importance even as the myriad of functions of this class of compounds are still being discovered.
References
- 1.
- Misaizu T, Matsuki S, Strickland T W, Takeuchi M, Kobata A, Takasaki S. Role of antennary structure of N-linked sugar chains in renal handling of recombinant human erythropoietin. Blood. 1995;86:4097–4104. [PubMed: 7492766]
- 2.
- Takeuchi M, Inoue N, Strickland T W, Kubota M, Wada M, Shimizu R, Hoshi S, Kozutsumi H, Takasaki S, Kobata A. Relationship between sugar chain structure and biological activity of recombinant human erythropoietin produced in Chinese hamster ovary cells. Proc. Natl. Acad. Sci. 1989;86:7819–7822. [PMC free article: PMC298162] [PubMed: 2813359]
- 3.
- Ferrari J, Gunson J, Lofgren J, Krummen L, Warner T G. Chinese hamster ovary cells with constitutively expressed sialidase antisense RNA produce recombinant DNase in batch culture with increased sialic acid. Biotechnol. Bioeng. 1998;60:589–595. [PubMed: 10099467]
- 4.
- Goochee C F, Monica T. Environmental effects on protein glycosylation. Bio/Technology. 1990;8:421–427. [PubMed: 1366536]
- 5.
- Andersen D C, Goochee C F. The effect of ammonia on the O-linked glycosylation of granulocyte colony-stimulating factor produced by Chinese hamster ovary cells. Biotechnol. Bioeng. 1995;47:96–105. [PubMed: 18623371]
- 6.
- Grammatikos S I, Valley U, Nimtz M, Conradt H S, Wagner R. Intracellular UDP-N-acetylhexosamine pool affects N-glycan complexity: A mechanism of ammonium action on protein glycosylation. Biotechnol. Prog. 1998;14:410–419. [PubMed: 9622521]
- 7.
- Nimtz M, Martin W, Wray V, Klöppel K -D, Augustin J, Conradt H S. Structures of sialylated oligosaccharides of human erythropoietin expressed in recombinant BHK-21 cells. Eur. J. Biochem. 1993;213:39–56. [PubMed: 8477709]
- 8.
- Parekh R B, Patel T P. Comparing the glycosylation patterns of recombinant glycoproteins. Trends Biotechnol. 1992;10:276–280. [PubMed: 1368380]
- 9.
- Wright A, Morrison S L. Effect of glycosylation on antibody function: Implications for genetic engineering. Trends Biotechnol. 1997;15:26–32. [PubMed: 9032990]
- 10.
- Neufeld E F. Lysosomal storage diseases. Annu. Rev. Biochem. 1991;60:257–280. [PubMed: 1883197]
- 11.
- Furbish F S, Steer C J, Krett N L, Barranger J A. Uptake and distribution of placental glucocerebrosidase in rat hepatic cells and effects of sequential deglycosylation. Biochim. Biophys. Acta. 1981;673:425–434. [PubMed: 6784774]
- 12.
- Kaye E M. Therapeutic approaches to lysosomal storage diseases. Curr. Opin. Pediatr. 1995;7:650–654. [PubMed: 8776014]
- 13.
- Whittington R, Goa K L. Alglucerase. A review of its therapeutic use in Gaucher's disease. Drugs. 1992;44:72–93. [PubMed: 1379912]
- 14.
- Von Itzstein M, Wu W -Y, Kok G B, Pegg M S, Dyason J C, Jin B, Van Phan T, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. [PubMed: 8502295]
- 15.
- Sahasrabudhe A, Lawrence L, Epa V C, Varghese J N, Colman P M, McKimm-Breschkin J L. Substrate, inhibitor, or antibody stabilizes the Glu 119 Gly mutant influenza virus neuraminidase. Virology. 1998;247:14–21. [PubMed: 9683567]
- 16.
- Calfee D P, Hayden F G. New approaches to influenza chemotherapy—Neuraminidase inhibitors. Drugs. 1998;56:537–553. [PubMed: 9806102]
- 17.
- Mendel D B, Tai C Y, Escarpe P A, Li W X, Sidwell R W, Huffman J H, Sweet C, Jakeman K J, Merson J, Lacy S A, Lew W, Williams M A, Zhang L J, Chen M S, Bischofberger N, Kim C U. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob. Agents Chemother. 1998;42:640–646. [PMC free article: PMC105511] [PubMed: 9517945]
- 18.
- Li W X, Escarpe P A, Eisenberg E J, Cundy K C, Sweet C, Jakeman K J, Merson J, Lew W, Williams M, Zhang L J, Kim C U, Bischofberger N, Chen M S, Mendel D B. Identification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 1998;42:647–653. [PMC free article: PMC105512] [PubMed: 9517946]
- 19.
- Vetvicka V, Thornton B P, Ross G D. Soluble β-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Invest. 1996;98:50–61. [PMC free article: PMC507400] [PubMed: 8690804]
- 20.
- Glaser V. 1998. Carbohydrate drugs move closer to market. Genet. Eng. News18.
- 21.
- Goldberg L, Lee J, Cairns T, Cook T, Lin C K S, Palmer A, Simpson P, Taube D. Inhibition of the human antipig xenograft reaction with soluble oligosaccharides. Transplant. Proc. 1995;27:249–250. [PubMed: 7878988]
- 22.
- Sharma A, Okabe J, Birch P, McClellan S B, Martin M J, Platt J L, Logan J S. Reduction in the level of Gal(α1,3)Gal in transgenic mice and pigs by the expression of an α(1,2)fucosyltransferase. Proc. Natl. Acad. Sci. 1996;93:7190–7195. [PMC free article: PMC38958] [PubMed: 8692967]
- 23.
- Platt J L. New directions for organ transplantation. Nature. 1998;392:11–17. [PubMed: 9579856]
- 24.
- Lowe J B, Ward P A. Therapeutic inhibition of carbohydrate-protein interactions in vivo. J. Clin. Invest. 1997;99:822–826. [PMC free article: PMC507887] [PubMed: 9062337]
- 25.
- Park I Y, Lee D S, Song M H, Kim W, Won J M. Cylexin: A P-selectin inhibitor prolongs heart allograft survival in hypersensitized rat recipient. Transplant. Proc. 1998;30:2927–2928. [PubMed: 9838290]
- 26.
- Buerke M, Weyrich A S, Zheng Z, Gaeta F C A, Forrest M J, Lefer A M. Sialyl Lewisx-containing oligosaccharide attenuates myocardial reperfusion injury in cats. J. Clin. Invest. 1994;93:1140–1148. [PMC free article: PMC294061] [PubMed: 7907602]
- 27.
- Ichikawa Y, Wang R, Wong C -H. Regeneration of sugar nucleotide for enzymatic oligosaccharide synthesis. Methods Enzymol. 1994;247:107–127. [PubMed: 7898347]
- 28.
- Hirsh J. Drug therapy: Heparin. N. Engl. J. Med. 1991;324:1565–1574. [PubMed: 2027360]
- 29.
- Hirsh J. Comparison of the relative efficacy and safety of low molecular weight heparin and unfractionated heparin for the treatment of deep venous thrombosis. Semin. Hematol. 1997;34:20–25. [PubMed: 9399406]
- 30.
- Korutla L N, Stewart G J, Lasz E C, Maione T E, Niewiarowski S. Evaluation of recombinant platelet factor 4 and protamine sulfate for heparin neutralization: Clotting and clearance studies in rat. Thromb. Haemost. 1994;71:609–614. [PubMed: 8091389]
- 31.
- Petersen J, Russell L, Andrus K, MacKinnon M, Silver J, Kliot M. Reduction of Extraneural Scarring by Adcon-t/n After Surgical Intervention. Neurosurgery. 1996;38:976–983. [PubMed: 8727824]
- 32.
- Frederickson R C. ADCON-L: A review of its development, mechanism of action, and preclinical data. Eur. Spine J. 1996;5 Suppl 1:S7–S9. [PubMed: 8915644]
- 33.
- Silver P J, Broughton R, Bouthillier J, Quinn T A, Wallace A M, Weishaar R E. Neutralase reverses the anti-coagulant but not the anti-thrombotic activity of heparin in a rabbit model of venous thrombosis. Thromb. Res. 1998;91:143–150. [PubMed: 9733158]
- 34.
- Goa K L, Benfield P. Hyaluronic acid: A review of its pharmacology and use as a surgical aid in opthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47:536–566. [PubMed: 7514978]
- 35.
- Platt F M, Butters T D. New therapeutic prospects for the glycosphingolipid lysosomal storage diseases. Biochem. Pharmacol. 1998;56:421–430. [PubMed: 9763217]
- 36.
- Baptista J A, Goss P, Nghiem M, Krepinsky J J, Baker M, Dennis J W. Measuring swainsonine in serum of cancer patients: Phase I clinical trial. Clin. Chem. 1994;40:426–430. [PubMed: 8131279]
- 37.
- Goss P E, Baptiste J, Fernandes B, Baker M, Dennis J W. A phase I study of swainsonine in patients with advanced malignancies. Cancer Res. 1994;54:1450–1457. [PubMed: 8137247]
- 38.
- Hink W F, Drought D C, Barnett S. Effect of an experimental systemic compound, CGA-184699, on life stages of the cat flea (Siphonaptera: Pulicidae). J. Med. Entomol. 1991;28:424–427. [PubMed: 1875371]
- 39.
- Bischoff H. The mechanism of α-glucosidase inhibition in the management of diabetes. Clin. Invest. Med. 1995;18:303–311. [PubMed: 8549017]
- Glycobiology in Biotechnology and Medicine - Essentials of GlycobiologyGlycobiology in Biotechnology and Medicine - Essentials of Glycobiology
- Historical Background and Overview - Essentials of GlycobiologyHistorical Background and Overview - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
