NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings R, Esko J, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1999.

Essentials of Glycobiology.
Show detailsPrimary contributions to this chapter were made by A. Varki (University of California at San Diego).
THIS CHAPTER PRESENTS SOME HISTORICAL BACKGROUND regarding the discovery of animal lectins and a general introduction to the topic and then discusses the current classification of these molecules, based on sequence homologies and probable evolutionary relatedness. Some general principles regarding the structure and function of animal lectins are also considered, as well as some aspects of their carbohydrate-binding properties. Further information regarding each of the major classes of animal lectins is covered in Chapters 23–27. For details regarding the analysis of carbohydrate-protein interactions and the physical principles involved, see Chapter 4.
Historical Background of the Discovery of Animal Lectins (1–31)
Lectins are carbohydrate-binding proteins. Although they were first discovered more than 100 years ago in plants (see Chapter 30), they are now known to be present throughout nature (including the microbial world, wherein they tend to be called by other names, such as hemagglutinins, adhesins, and toxins; see Chapter 28). Interactions between animal cells involving carbohydrates were first discovered by observing the phenomenon of reaggregation of dissociated marine sponge cells, a form of species-specific recognition. There was also evidence for the presence of hemagglutinins (red cell agglutinating activity) in the body fluids of various crustaceans and arachnids. However, until the 1960s, such interactions had not been discovered in complex multicellular animals such as vertebrates. The first inkling that there might be lectins endogenous to a vertebrate system came from the studies of Ginsburg and colleagues in which blood leukocytes from the rat were treated with bacterial glycosidases and then injected back into the circulation. The treatments resulted in changes in the homing of cells to different internal organs. Given the nature of the tools available at that time, it was difficult to ascertain whether these phenomena were mediated by an endogenous lectin. In retrospect, these studies were probably demonstrating the generation of ligands for a hepatic galactose receptor (due to sialidase action) and/or possibly the selectins (due to fucosidase action). The first direct evidence for a mammalian lectin arose serendipitously during work by Ashwell and colleagues, who were studying the mechanisms that controlled the turnover of glycoproteins in the blood circulation. Looking for an improved way to introduce a radioactive tracer into purified glycoproteins before reinjection, they attempted to transfer the tritium label from tritiated borohydride into the sugar chains of these proteins, which contain the terminal sequence Sia-Gal-GlcNAc. This was successfully done either following mild periodate oxidation of sialic acid side chains (which left the rest of the sialic acid molecule intact) or following oxidation of the 6-position of galactose residues (which required removing the outer sialic acid residues). To their surprise, there was a dramatic difference between the circulating half-lives of these two preparations, which terminated either in sialic acid or galactose residues. The molecules that retained labeled sialic acids remained in the circulation for many days, whereas those that had lost them (and now terminated in galactose residues) disappeared within minutes. The importance of these terminal β-linked galactose residues was confirmed by in vitro resialylation or by β-galactosidase treatment, both of which partially restored stability in circulation. The site of accumulation of the desialylated glycoproteins was found to be primarily the liver. This led to the discovery of the “asialoglycoprotein receptor,” a hepatocyte membrane protein complex that specifically recognizes terminal β-linked galactose or GalNAc residues on circulating glycoproteins or cells. It was subsequently found that this lectin activity was markedly inhibited by plasma from birds and reptiles. This, in turn, led to the discovery of a chicken hepatic lectin, which preferentially recognizes terminal GlcNAc residues. Thus, chickens have a GlcNAc-specific hepatic receptor instead of a galactose-specific one, permitting the circulation of asialoglycoproteins, but not glycoproteins that also have their galactose residues removed.
Affinity columns of immobilized asialoglycoproteins were then used to purify the hepatic asialoglycoprotein receptor. The same approach was applied by investigators working in other systems, uncovering a variety of galactose-binding lectins in cell types, ranging from the slime mold Dictyostelium discoideum to mammalian tissues such as the heart. Most of these lectins eventually proved to be quite different from the original asialoglycoprotein receptor, being water soluble and of relatively low molecular weight (now known as the galectins, Chapter 27). Meanwhile, Hill and colleagues reported yet another type of hepatic uptake system that seemed to involve recognition of fucose moieties.
In the early 1970s, Neufeld and colleagues reported a carbohydrate-dependent system mediating the uptake of lysosomal enzymes by fibroblasts. In 1977, Sly and colleagues demonstrated specific blockade of this uptake by the monosaccharide Man-6-P. Further work by the groups of Kornfeld, Jourdian, Von Figura, and others led to the discovery of Man-6-P receptors, which recognize phosphorylated high-mannose-type oligosaccharides that are selectively expressed on lysosomal enzymes. Encouraged by the prospect of using this uptake system to correct lysosomal enzyme deficiency diseases in humans, other investigators infused intact labeled lysosomal enzymes into intact animals and followed their fate. As it turned out, mature lysosomal enzymes were not actually rich in Man-6-P (the phosphate esters are mostly removed after initial targeting to lysosomes; see Chapter 23); instead, the clearance predominantly involved recognition of terminal mannose and GlcNAc residues. This led to the discovery of the macrophage mannose receptor. Thus, by the beginning of the 1980s, the concept of vertebrate lectins that could recognize specific endogenous ligands had become firmly established.
Meanwhile, several circulating soluble lectins were discovered in the blood plasma of various species, with varied carbohydrate-binding specificities. Initially, it had been thought that sialic acids, while serving as ligands for exogenous microbial pathogens, generally acted as “masks” within vertebrates, preventing binding by endogenous lectins that recognized underlying saccharides. The discovery of some arachnid and crustacean lectins that could recognize sialic acids in vitro did not change this impression, since these organisms did not themselves express endogenous sialic acids. The first indication that sialic acids might serve as endogenous ligands within vertebrates came from Fearon and Austen, who showed that the binding of the complement regulatory H protein to “self” cell surfaces was dependent on sialic acid residues. Rosen and colleagues then showed that sialidase treatment of the rat lymph node sections abolished binding of lymphocytes to high endothelial venules; this was the first demonstration of the involvement of carbohydrates in recognition by what turned out to be the selectin family of vascular receptors (see Chapter 26). Meanwhile, the discoveries that the anticoagulant effects of heparin chains were mediated by a structural sequence specific for antithrombin III and that the cartilage link protein could bind to hyaluronan established the concept that protein interactions with polyanionic glycosaminoglycan chains could also be based on highly specific recognition (see Chapter 29). In the late 1980s, it became evident that the primary amino acid sequence of a protein could be used to predict carbohydrate recognition properties of proteins (see Current Classification of Animal Lectins below). This led to the recognition of the hyaluronan-binding properties of CD44 and the correct prediction that selectins would recognize carbohydrates. In the current decade, the discovery of sialic-acid-dependent binding by the B-cell molecule CD22 and the cloning of the macrophage receptor sialoadhesin led to the definition of another new family of lectins (the Siglecs) belonging to the immunoglobulin superfamily (see Chapter 24). A specific clearance system was also discovered that recognized the sulfated GalNAc residues on pituitary glycoprotein hormones. Most recently, several lectins have been discovered within the ER-Golgi pathway itself, where sugar chain biosynthesis occurs.
Current Classification of Animal Lectins (7,15,19,23,26,31–40)
For a while after their discovery, animal lectins were classified according to the carbohydrate sequences to which they bound best (e.g., β-galactoside-binding lectins). Only with the advent of molecular cloning did a more consistent classification emerge, based on amino acid sequence homology and evolutionary relatedness of these lectins (for classification, see Table 22.1, and for schematic examples, see Figure 22.1). The first such classification was proposed by Drickamer, based on some highly conserved amino acid sequence motifs in the carbohydrate recognition domains of two groups of lectins: The first group required calcium for recognition and was therefore called C-type lectins, and the other group required “free” thiols for stability and was termed S-type lectins. Meanwhile, the two lectins that recognized Man-6-P were sequenced and found to be homologous, but distinct from all the others, justifying their recognition as P-type lectins. Although some classes of lectins such as the P-type and S-type seemed to recognize a single class of sugars (Man-6-P and β-galactosides, respectively), others like the C-type encompassed a variety of molecules that shared in common only a lectin protein module. A major breakthrough occurred when the independent cloning of three homologous vascular adhesin receptors revealed a common amino-terminal C-type lectin motif; these three molecules eventually turned out to be the selectins. This was the first time that carbohydrate recognition had been predicted on the basis of the primary amino acid sequence of a cloned protein, validating the concept of classification based on sequence homology. The cloning of a variety of circulating soluble lectins also led to the recognition of a subset of C-type lectins designated as the “collectin family.” In addition, two calcium-binding lectins (calnexin and calcireticulin) are unrelated to the C-type lectins (not all calcium-requiring lectins are C-type lectins) and specifically recognize glucose residues on newly synthesized glycoproteins. Studies in the 1990s have also revealed that immunoglobulin superfamily members can recognize carbohydrates, leading to a new group of I-type lectins (see Chapter 24). A subgroup of these molecules that specifically recognize sialic acids have been recently designated the “Siglecs” (for sialic acid/immunoglobulin superfamily/lectins). Another class of evolutionarily very ancient circulating soluble lectins called the pentraxins is recognized not so much by primary sequence homologies, but by a consistent pentameric structural organization and a probable role in the primary host immune response.
Table 22.1
Animal lectin classes and families.

Figure 22.1
Schematic examples of major groupings of animal lectins, based on protein structure. Examples of some of the major families are shown. The emphasis is on the extracellular domain structure and topology. The following are the defined carbohydrate-binding (more...)
These general groupings are based primarily on sequence homologies and probable evolutionary relatedness and include the majority of known animal lectins. However, many others do not show any obvious sequence homologies or evolutionary relationships (Table 22.1). Another large group that defies easy classification based on sequence data or general structure are the proteins that bind glycosaminoglycans such as heparin (GAG-binding proteins; see Chapter 29). Unlike other animal lectins that tend to recognize specific terminal aspects of sugar chains by fitting them into shallow but relatively well-defined binding pockets, GAG-protein interactions seem to involve surface clusters of positively charged amino acids that line up against internal regions of the anionic GAG chains. Thus, despite the fact that the GAG structural motifs recognized can be quite specific, and many involve the general motifs XBBXBX, XBBBXXBX, or TXXBXXTBXXXTBB (where B is a basic residue and X is a hydropathic residue), most GAG-binding proteins do not seem to be evolutionarily related to each other. Partly for these reasons, the term “lectin” is not even commonly applied to GAG-binding proteins.
For further details regarding many of these different classes of animal lectins, see Chapters 23–27, and for details regarding the principles of carbohydrate:protein interactions, see Chapter 4. The rest of this chapter provides an overview of general principles regarding the biosynthesis, physical structure, binding properties, and regulation of animal lectins.
Biosynthesis, Trafficking, and Regulation of Animal Lectins (12–13,15,21,23,25–26,30,34,37,41)
Some lectin genes are expressed constitutively, whereas others are induced by gene activation under specific biological circumstances. All membrane-bound and many soluble lectins are synthesized on ER-bound ribosomes and delivered to their eventual destinations via the ER-Golgi pathway. Thus, the lectins themselves are often glycoproteins. However, a significant subset of soluble lectins (galectins, heparin-binding growth factors, and some cytokines) are synthesized on free ribosomes and delivered directly to the exterior of the cell by an as yet poorly understood mechanism involving extrusion through the plasma membrane. This makes some teleological sense, since several of these lectins can recognize biosynthetic intermediates that occur in the Golgi-ER pathway (e.g., galactosides and high-mannose oligosaccharides). By circumventing the conventional pathway of secretion, these molecules can avoid unwanted premature interactions with potential ligands that are synthesized within the same cell. In addition, some of these lectins (such as the galectins) are sensitive to the redox state of the environment and can remain active only in the reducing environment of the cytosol. Upon entering the oxidizing environment of the extracellular space, they must therefore immediately bind to ligands or become progressively inactivated. Another form of regulation occurs when the lectin binds to cognate sugar chains present on the same molecule or the same cell surface and hence becomes functionally inactive (e.g., the Siglecs, where sialic-acid-bearing ligands from the same cell surface must be removed before the lectin can be active). Some membrane-bound lectins are internalized upon binding to ligands, with delivery to internal acidic compartments (endosomes). There the cargo is released, and some of the receptors can recycle back to their original location.
Soluble and Membrane-bound Forms of Animal Lectins (42–43)
From a functional point of view, it is worth considering these carbohydrate-binding proteins in two physical classes: soluble and membrane-bound. Cell-membrane-bound lectins are more likely to be involved in endocytosis or cell adhesion and to stay confined to the cell type of their original synthesis. On the other hand, soluble lectins are capable of diffusing locally in tissues and/or entering the blood circulation. Although useful in functional terms, this type of physical classification is confounded by two issues: First, lectins that start out life as membrane-bound proteins can be proteolytically shed into the extracellular fluid, and second, soluble multivalent lectins can become attached to cell surfaces via their carbohydrate-binding sites. Figure 22.2 indicates some examples of these relationships and the nature of the potential interactions with natural ligands, which, in turn, can also be soluble or membrane-bound.
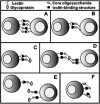
Figure 22.2
Possible mechanisms of regulation of an animal lectin by cognate ligands. Potential ligands can be on the cell surface and/or on soluble glycoproteins (including sugar chains attached to the lectin itself). As discussed in the text, the “lectin-binding (more...)
Nature of Lectin-Ligand Interactions (42–43)
Several crystal structures of animal lectins with their cognate ligands have been elucidated, allowing an understanding of these interactions at the level of atomic resolution. These can be divided into two general groups: those involving GAG chains (mostly mediated by ordered arrays of surface charge contacts; Chapter 29) and those involving N- and O-glycans. The principles that have emerged about the latter are as follows: First, the binding sites are of relatively low affinity and are found in shallow indentations on the surface of the proteins. Second, selectivity is mostly achieved via a combination of hydrogen bonds (involving the hydroxyl groups of the sugars) and by van der Waals' packing of the hydrophobic face of monosaccharide rings against aromatic amino acid side chains. Third, further selectivity can be achieved by additional contacts between the saccharide and the protein, sometimes involving bridging water molecules or divalent cations. Finally, the actual region of contact between the saccharide and the polypeptide typically involves only one to three monosaccharide residues. As a consequence of all of the above, these lectin-binding sites tend to be of relatively low affinity, but of high specificity. The ability of such low-affinity sites to mediate biologically relevant interactions in the intact system thus appears to require multivalency.
Animal Lectins Are Generally Multivalent (14,21,26,31,34,44–46)
Until the 1990s, all of the animal lectins discovered were found to be naturally multivalent, either because of their defined multisubunit structure or by virtue of having multiple carbohydrate-binding sites within a single polypeptide. Indeed, high avidity generated by multivalent binding of low-affinity single sites appears to be a common mechanism for optimizing lectin function in nature, and a traditional definition for a lectin was “a multivalent carbohydrate-binding protein that is not an antibody.” The first exception to this general rule appeared to be the selectins, which have only a single CRD site within their extracellular polypeptide domains (see Chapter 26). The same situation applies to the Siglecs (see Chapter 24). However, in both these instances, evidence is appearing that the molecules become functionally multimeric either by noncovalent association or by clustering on cell surfaces. It remains to be seen whether biologically significant binding by any animal lectin can arise from a strictly monovalent interaction. It is also of note that a single lectin can carry multiple binding sites for multiple ligands, e.g., the macrophage mannose receptor is now known to bind not only to mannans, but also via a distinct CRD to the 4-O-sulfated GalNAc residues of pituitary glycoprotein hormones.
Nature of the Ligands for Animal Lectins (6,12,14–15,17–18,21,23,27,29,31,34,41,43–45,47–48)
Details about the natural ligands for animal lectins can be found in other chapters in this volume. In the few instances where crystal structures of animal lectins with their cognate ligands are available, it is evident that the contact regions between the two usually occupy only one to three monosaccharide units (see Chapter 4). On the other hand, despite their stereospecificity, monosaccharides or small oligosaccharide units tend to be weak inhibitors of lectin interactions. The natural ligands for most lectins are typically complex glycoconjugates that carry clustered arrays of the cognate carbohydrate, thus cooperating with clustered lectin-binding sites to generate high-avidity binding, which is further enhanced by mass transport effects (high local concentrations of ligands). In some instances (e.g., the selectins), the nature of this clustering is not easily defined, and cooperation with other aspects of the underlying polypeptide may be necessary to generate optimal binding. Partly for this reason, it is common to see the names of the underlying polypeptide backbone used to define the nature of a ligand, e.g., PSGL-1 is the ligand for P-selectin. However, it should be recognized that unless it is correctly glycosylated and/or otherwise modified (e.g., sulfated), the polypeptide is not itself the ligand. Typically, these polypeptides are simply carriers of the true ligands for lectins, which are made up of combinations of glycan units. In addition, recombinant lectins that are often used to identify potential biological ligands are usually multimeric in structure and/or are presented in multivalent clustered arrays in soluble complexes or on solid supports. Thus, although a variety of molecules may be found to bind to a given recombinant lectin in a glycosylation-dependent manner, only a few of these “ligands” may be actually involved in mediating biologically significant interactions. The challenge then is to tell the difference between what can bind to a recombinant lectin in an in vitro experiment, and what actually does bind in vivo to the native lectin in a biologically relevant manner. Indeed, the term ligand should probably be reserved for the latter type of biologically relevant structures. It should also be kept in mind that the natural ligands of some animal lectins may be present primarily on foreign invaders (e.g., the circulating soluble mannan-binding protein may serve to bind and opsonize microorganisms bearing high densities of mannose, such as yeasts and other fungi).
Types of Functions Mediated by Animal Lectins (6,11–13,15,20–21,23–25,28–29,31,37,49–51)
Animal lectins provide one functional explanation for the enormous diversity of glycan structures found on animal cells (i.e., recognition of endogenous ligands). Details about the function of various animal lectins can be found in later chapters. Table 22.2 lists some examples of specific known or putative functions. It can be seen that nature has capitalized on the versatility and diversity of carbohydrate binding to generate a wide variety of functional outcomes. In some of these instances, the biological significance of the interactions is evident from the consequences of natural or experimental genetic mutations in the expression of the lectin or its ligand. Of course, there are still several other instances wherein the lectin and its specificities are well defined, but the biological functions of the interaction remain elusive. In these cases (e.g., the Siglecs), the highly restricted cell-type-specific expression of the lectins makes it reasonable to predict that they do serve highly specific biological functions.
Table 22.2
Some examples of biological functions of animal lectins.
Future Directions
The discovery of new animal lectins will likely continue as it has in the past, i.e., arising from serendipitous observations, unexpected sequence homologies of newly cloned molecules to known lectins, or directed discovery attempts using defined carbohydrate probes as affinity ligands. The ultimate goal must be not only the identification and structural characterization of all of their natural ligands, and the elucidation of the nature of the relevant binding interactions at atomic resolution, but also a full understanding of the biological roles of these molecules. The latter must eventually be confirmed by natural or genetically manipulated mutations in the expression of the lectins and/or their ligands in intact animals.
References
- 1.
- Van Den Hamer C J, Morell A G, Scheinberg I H, Hickman J, Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J. Biol. Chem. 1970;245:4397–4402. [PubMed: 4322435]
- 2.
- Morell A G, Gregoriadis G, Scheinberg I H, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J. Biol. Chem. 1971;246:1461–1467. [PubMed: 5545089]
- 3.
- Lindahl U, Backstrom G, Hook M, Thunberg L, Fransson L A, Linker A. Structure of the antithrombin-binding site in heparin. Proc. Natl. Acad. Sci. 1979;76:3198–3202. [PMC free article: PMC383791] [PubMed: 226960]
- 4.
- Rosenberg R D, Lam L. Correlation between structure and function of heparin. Proc. Natl. Acad. Sci. 1979;76:1218–1222. [PMC free article: PMC383221] [PubMed: 286307]
- 5.
- Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem. 1982;51:531–554. [PubMed: 6287920]
- 6.
- Schauer R. Sialic acids and their role as biological masks. Trends Biochem. Sci. 1985;10:357–360.
- 7.
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 1988;263:9557–9560. [PubMed: 3290208]
- 8.
- Stoolman L M. Adhesion molecules controlling lymphocyte migration. Cell. 1989;56:907–910. [PubMed: 2647304]
- 9.
- Yamashita K, Kobata A, Suzuki T, Umetsu K. Allomyrina dichotoma lectins. Methods Enzymol. 1989;179:331–340. [PubMed: 2622357]
- 10.
- Kornfeld S. Lysosomal enzyme targeting. Biochem. Soc. Trans. 1990;18:367–374. [PubMed: 2164980]
- 11.
- Drickamer K. Clearing up glycoprotein hormones. Cell. 1991;67:1029–1032. [PubMed: 1662115]
- 12.
- Stahl P D. The mannose receptor and other macrophage lectins. Curr. Opin. Immunol. 1992;4:49–52. [PubMed: 1317711]
- 13.
- Knudson C B, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed: 7691670]
- 14.
- Sharon N. Lectin-carbohydrate complexes of plants and animals: An atomic view. Trends Biochem. Sci. 1993;18:221–226. [PubMed: 8346557]
- 15.
- Barondes S H, Cooper D N W, Gitt M A, Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. [PubMed: 8063692]
- 16.
- Bergeron J J M, Brenner M B, Thomas D Y, Williams D B. Calnexin: A membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 1994;19:124–128. [PubMed: 8203019]
- 17.
- Rosen S D, Bertozzi C R. The selectins and their ligands. Curr. Opin. Cell Biol. 1994;6:663–673. [PubMed: 7530461]
- 18.
- Spillmann D, Lindahl U. Glycosaminoglycan-protein interactions: A question of specificity. Curr. Opin. Struct. Biol. 1994;4:677–682.
- 19.
- Stamenkovic I, Aruffo A. Hyaluronic acid receptors. Methods Enzymol. 1994;245:195–218. [PubMed: 7539092]
- 20.
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. [PubMed: 7736583]
- 21.
- McEver R P, Moore K L, Cummings R D. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J. Biol. Chem. 1995;270:11025–11028. [PubMed: 7538108]
- 22.
- Nelson R M, Venot A, Bevilacqua M P, Linhardt R J, Stamenkovic I. Carbohydrate-protein interactions in vascular biology. Annu. Rev. Cell Dev. Biol. 1995;11:601–631. [PubMed: 8689570]
- 23.
- Powell L D, Varki A. I-type lectins. J. Biol. Chem. 1995;270:14243–14246. [PubMed: 7782275]
- 24.
- Stockert R J. The asialoglycoprotein receptor: Relationships between structure, function, and expression. Physiol. Rev. 1995;75:591–609. [PubMed: 7624395]
- 25.
- Baenziger J U. Glycosylation: To what end for the glycoprotein hormones? Endocrinology. 1996;137:1520–1522. [PubMed: 8612480]
- 26.
- Crocker P R, Feizi T. Carbohydrate recognition systems: Functional triads in cell-cell interactions. Curr. Opin. Struct. Biol. 1996;6:679–691. [PubMed: 8913692]
- 27.
- Crocker P R, Kelm S, Hartnell A, Freeman S, Nath D, Vinson M, Mucklow S. Sialoadhesin and related cellular recognition molecules of the immunoglobulin superfamily. Biochem. Soc. Trans. 1996;24:150–156. [PubMed: 8674645]
- 28.
- Hooper L V, Manzella S M, Baenziger J U. From legumes to leukocytes: Biological roles for sulfated carbohydrates. FASEB J. 1996;10:1137–1146. [PubMed: 8751716]
- 29.
- Kansas G S. Selectins and their ligands: Current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed: 8896391]
- 30.
- Kasai K, Hirabayashi J. Galectins: A family of animal lectins that decipher glycocodes. J. Biochem. 1996;119:1–8. [PubMed: 8907168]
- 31.
- Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. [PubMed: 9068613]
- 32.
- Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. [PubMed: 2463827]
- 33.
- Bevilacqua M, Butcher E, Furie B, Gallatin M, Gimbrone M, Harlan J, Kishimoto K, Lasky L, McEver R, Paulson J, Rosen S, Seed B, Siegelman M, Springer T, Stoolman L, Tedder T, Varki A, Wagner D, Weissman I, Zimmerman G. Selectins: A family of adhesion receptors. Cell. 1991;67:233. [PubMed: 1717161]
- 34.
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 1992;61:307–330. [PubMed: 1323236]
- 35.
- Barondes S H, Castronovo V, Cooper D N W, Cummings R D, Drickamer K, Feizi T, Gitt M A, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu F -T, Lotan R, Mercurio A M, Monsigny M, Pillai S, Poirer F, Raz A, Rigby P W J, Rini J M, Wang J L. Galectins: A family of animal β-galactoside-binding lectins. Cell. 1994;76:597–598. [PubMed: 8124704]
- 36.
- Kelm S, Schauer R, Crocker P R. The sialoadhesins—A family of sialic acid-dependent cellular recognition molecules within the immunoglobulin superfamily. Glycoconj. J. 1996;13:913–926. [PubMed: 8981082]
- 37.
- Helenius A, Trombetta E S, Hebert D N, Simons J F. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. [PubMed: 17708944]
- 38.
- Taylor M E. Evolution of a family of receptors containing multiple C-type carbohydrate-recognition domains. Glycobiology. 1997;7:v–viii. [PubMed: 9147037]
- 39.
- Glycosaminoglycan-protein interactions: Definition of consensus sites in glycosaminoglycan binding proteins. BioEssays. 20:156–167. [PubMed: 9631661]
- 40.
- Crocker P R, Clark E A, Filbin M, Gordon S, Jones Y, Kehrl J H, Kelm S, Le Douarin N, Powell L, Roder J, Schnaar R L, Sgroi D C, Stamenkovic K, Schauer R, Schachner M, van den Berg T K, van der Merwe P A, Watt S M, Varki A. Siglecs: A family of sialic-acid binding lectins [letter] Glycobiology. 1998;8:v. [PubMed: 9498912]
- 41.
- Kjellén L, Lindahl U. Proteoglycans: Structures and interactions. Annu. Rev. Biochem. 1991;60:443–475. [PubMed: 1883201]
- 42.
- Rini J M. Lectin structure. Annu. Rev. Biophys. Biomol. Struct. 1995;24:551–577. [PubMed: 7663127]
- 43.
- Weis W I, Drickamer K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996;65:441–473. [PubMed: 8811186]
- 44.
- Lee Y C. Biochemistry of carbohydrate-protein interaction. FASEB J. 1992;6:3193–3200. [PubMed: 1397841]
- 45.
- Drickamer K, Taylor M E. Biology of animal lectins. Annu. Rev. Cell Biol. 1993;9:237–264. [PubMed: 8280461]
- 46.
- Mahoney J A, Schnaar R L. Ganglioside-based neoglycoproteins. Methods Enzymol. 1994;242:17–27. [PubMed: 7891574]
- 47.
- Varki A. Selectin ligands. Proc. Natl. Acad. Sci. 1994;91:7390–7397. [PMC free article: PMC44407] [PubMed: 7519775]
- 48.
- Varki A. Selectin ligands: Will the real ones please stand up? J. Clin. Invest. 1997;99:158–162. [PMC free article: PMC507781] [PubMed: 9005982]
- 49.
- Fearon D T. Activation of the alternative complement pathway. CRC. Crit. Rev. Immunol. 1979;1:1–32. [PubMed: 162484]
- 50.
- Weiss P, Ashwell G. The asialoglycoprotein receptor: Properties and modulation by ligand. Prog. Clin. Biol. Res. 1989;300:169–184. [PubMed: 2674962]
- 51.
- Roberts D D, Haverstick D M, Dixit V M, Frazier W A, Santoro S A, Ginsburg V. The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J. Biol. Chem. 1985;260:9405–9411. [PubMed: 3926767]
- Historical Background of the Discovery of Animal Lectins
- Current Classification of Animal Lectins
- Biosynthesis, Trafficking, and Regulation of Animal Lectins
- Soluble and Membrane-bound Forms of Animal Lectins
- Nature of Lectin-Ligand Interactions
- Animal Lectins Are Generally Multivalent
- Nature of the Ligands for Animal Lectins
- Types of Functions Mediated by Animal Lectins
- Future Directions
- References
- Discovery and Classification of Animal Lectins - Essentials of GlycobiologyDiscovery and Classification of Animal Lectins - Essentials of Glycobiology
- Glycobiology of Protozoal and Helminthic Parasites - Essentials of GlycobiologyGlycobiology of Protozoal and Helminthic Parasites - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
