Clinical Description
Cold-induced sweating syndrome (CISS) can present to the clinician in infancy as Crisponi syndrome, or from age three years onward as cold-induced sweating syndrome. Interviews with mothers of children or adults with CISS, review of a targeted questionnaire completed by caregivers of the infants, and review of early medical records revealed that probably all individuals had findings of CS in infancy, some more severe than others [Hahn et al 2010].
To date, 99 individuals have been identified with biallelic pathogenic variants in either CLCF or CRLF1 [Hahn et al 2010; Buers et al 2020a; Buers et al 2020b; Schierz et al 2020; Authors, personal observations]. The following description of the phenotypic features associated with this condition is based on these reports.
Table 2.
Cold-Induced Sweating Syndrome / Crisponi Syndrome (CISS/CS): Frequency of Select Features
View in own window
| Feature | % of Persons with Feature | Comment |
|---|
Infantile
period
| Dysmorphic facial features | 100% | |
| Poor suck & swallow, micrognathia, restricted jaw movements | 100% | |
| Excessive startling when crying, being handled, or w/loud noises | 90% | Assoc w/an opisthotonos-like posture, tightly pursed lips, & excessive foamy salivation |
| Facial-oral-laryngospasms assoc w/respiratory distress | ~60% | Assoc w/startle |
| Bouts of hyperthermia | 90% | Isolated temperature spikes up to 42°C (108° F) w/o infections |
| Camptodactyly &/or flexion deformity at elbows | 80% | |
| Misshapen feet, overriding toes | ~50% | |
| Scaly erythematous skin rash | ~20% | |
Childhood/
Adulthood
| Gradual improvement of swallowing & feeding by end of 1st yr | ~100% | |
| Persistent lower facial weakness | ~90% | |
| Impaired thermoregulation | 100% | |
| Paradoxic cold-induced sweating | 100% | |
| Heat intolerance | 100% | |
| Progressive thoracolumbar kyphoscoliosis | 90% | |
Presentation in Infancy (Crisponi Syndrome)
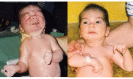
Typical dysmorphic features (see Suggestive Findings) are noted at birth and are accompanied by an inability to suckle and swallow due to facial and bulbar weakness and the presence of micrognathia and restricted jaw movements. Intermittently, infants show characteristic facial-oral contractions, with tightly pursed lips and excessive salivation; these are not observed when relaxed or during sleep (, ).
Excessive startle. When crying or being handled or with loud noises, infants tend to startle excessively; they transiently assume an opisthotonus-like posture with extended neck, flexed arms and fisted hands. At the same time, they show characteristic facial contractions and may also develop laryngospasm and difficulty breathing, with circumoral cyanosis as a sign of anoxia.
Life-threatening events. Respiratory difficulties and unexplained high fevers up to 42º C [108º F] can lead to seizures and sudden death. Crisponi [1996] originally described this presentation in 17 newborns from 12 families of southern Sardinia. As 15 of the infants died in the first few months, the condition was considered to have a poor prognosis. However, it is now recognized that survival past infancy can be expected with attentive care, as most early-infantile problems disappear gradually over the first two years of life.
Feeding issues. Affected infants usually begin to feed normally by age one to two years. Occasionally, dysphagia persists throughout the first decade and may be associated with lack of interest in food or liquids. Gastroesophageal reflux may occur.
Neurodevelopmental findings. Excessive startling disappears by age two years. Psychomotor and speech development can be delayed due to manual difficulties caused by camptodactyly and orofacial weakness, respectively [Hahn et al 2010].
It is important to note that the seizures noted in infants with this disorder occur in association with hyperthermia and not as a primary seizure disorder.
Corneal injury. Decreased blink rate and incomplete eye closure in sleep due to facial muscle weakness predisposes for exposure keratitis and corneal scarring.
Orthopedic findings include camptodactyly of the fingers and flexion contractures of the elbows; hands are often fisted.
Skin findings. A scaly erythematous rash on the face, trunk and limbs is present in infancy and persists through early childhood.
Early dental decay to the point of complete loss of teeth in the second decade has been observed.
Presentation in Childhood and Adulthood
Persistent lower facial weakness. Decreased mimical expression, open mouth position, horizontal smile, and nasal speech have been observed in childhood and beyond.
Impaired thermoregulation
Cold-induced sweating, the most disabling symptom in adulthood, is recognized during the first decade (from age 3 years onward). At environmental temperatures of ≤22°C (72º F), affected individuals sweat profusely on their face, arms (sparing the palms) and upper body, accompanied by intense shivering and dermal vasoconstriction, so that the fingers appear cold and cyanotic.
Profuse sweating is also triggered by apprehension or nervousness or by sweet gustatory stimuli, in particular by chocolate.
Heat intolerance. In contrast, affected individuals sweat very little in heat and only in the lumbar region, the groin, and the anterior thigh. They become flushed and unpleasantly overheated in hot climates [
Hahn et al 2006,
Hahn et al 2010]. Although the hyperhidrosis can be effectively treated [
Hahn et al 2006,
Hahn et al 2010,
Herholz et al 2010], heat intolerance is a lifelong problem.
Scoliosis. Toward the end of the first decade, affected children develop progressive thoracolumbar kyphoscoliosis that requires either bracing or spinal instrumentation.
Decreased tactile, temperature, and pain perception. Decrease in perception of light touch, temperature, and painful stimuli and atrophy of small foot muscles have been observed in some individuals [Knappskog et al 2003], indicating a mild chronic axonal sensory and motor peripheral neuropathy. In a study by Hahn et al [2006], sensory and motor nerve conduction velocities recorded from sural nerves and from peroneal nerves were normal, but the evoked sensory and motor action potential amplitudes were reduced [Hahn et al 2006]. This altered sensory perception may be accounted for by the findings of length-dependent derangement of somatic sensory innervation found in skin biopsies derived from fingers and distal legs [Di Leo et al 2010].
Other Findings
Laboratory tests, metabolic studies, and detailed studies of autonomic functions are normal, with the exception of sudomotor functions [Hahn et al 2006]. EEG and brain MRI are normal. Rarely a thin corpus callosum and delayed white matter myelination has been described.
Prognosis
Once the difficulties of early childhood have been overcome, individuals with CISS/CS are for the most part able to lead a fairly normal and productive life, obtain a secondary education, and have children. Life expectancy is probably normal; to date only one individual has been followed to the eighth decade [Hahn et al 2006].
Nomenclature
The term "cold-induced sweating syndrome" was coined [Knappskog et al 2003] from the title of the Lancet report "cold-induced profuse sweating on back and chest" [Sohar et al 1978].
Following the demonstration of locus heterogeneity, the abbreviations CISS1 and CISS2 were introduced [Hahn et al 2006]. As CISS1 and CISS2 are clinically indistinguishable, the term "CISS" covers both disorders until a molecular diagnosis is made.
With time, survivors of infantile-onset Crisponi syndrome [Crisponi 1996] will develop CISS, substantiated by the identification of pathogenic variants in CRLF1 [Buers et al 2020a] or CLCF1 [Hahn et al 2006, Hahn et al 2010]. Thus, CISS and Crisponi syndrome are not "allelic disorders," but rather Crisponi syndrome is the infantile presentation of CISS [Dagoneau et al 2007, Hahn et al 2010].