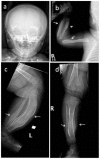
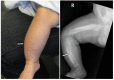
Clinical Description
Caffey disease is characterized by massive subperiosteal new bone formation usually involving the diaphyses of the long bones, as well as the ribs, mandible, scapulae, and clavicles [Caffey & Silverman 1945, Caffey 1957].
Presentation. Typically the skeletal manifestations of Caffey disease first appear with fever, joint swelling, and pain between birth and age five months, and resolve before age two years [Kamoun-Goldrat & le Merrer 2008, Cerruti-Mainardi et al 2011, Ranganath et al 2011].
Recurrence. Episodes of recurrence of the manifestations of Caffey disease have been reported multiple times, in individuals with the classic infantile presentation [Navarre et al 2013]. Etiology and precipitating factors for recurrence remain unclear [Navarre et al 2013].
Other findings. In a family described by Gensure et al [2005], an individual with the defining COL1A1 pathogenic variant had a history of Caffey disease as a child and joint laxity and skin hyperextensibility with a history of hernias and multiple fractures in adulthood. Subsequent clinical examination of other individuals in that family who also had the defining COL1A1 pathogenic variant revealed varying degrees of joint laxity and hyperextensibility. Skin biopsy of affected individuals showed collagen fibrils that were larger, more variable in shape, and less densely packed than age- and sex-matched controls. Granulofilamentous material was also visible in the matrix along the collagen fibrils. Cultured fibroblasts showed a mix of normal type I collagen and abnormal disulfide crosslinking, either within or between mutated collagen fibrils. The findings reported by Gensure et al [2005] have not been found in other families with the same pathogenic variant [Cho et al 2008, Cerruti-Mainardi et al 2011, Ranganath et al 2011]
Long-term outcome. Although anecdotal evidence suggests that the manifestations of Caffey disease resolve spontaneously by age two years and do not predispose to long-term bone abnormalities, the literature on Caffey disease does not directly address long-term outcomes. The study of a single family suggested that individuals who have the defining pathogenic variant may be prone to short stature and residual bone deformities [Suphapeetiporn et al 2007]. In addition, it has been suggested that fractures (possibly related to decreased bone mineral density) may be more common in these individuals [Gensure et al 2005, Suphapeetiporn et al 2007].
Other bone-related complications may potentially occur: in one case report a child with Caffey disease developed tumoral calcinosis (thought to be due to constant remodeling) after repeated inflammatory events [Issa El Khoury et al 2012].
Laboratory findings observed in a few affected individuals:
Serum biochemical markers of inflammation (white blood cell count, erythrocyte sedimentation rate, C-reactive protein) have been elevated [
Gensure et al 2005].
Bone and muscle biopsy of affected sites in a few individuals have demonstrated an inflammatory reaction [
Katz et al 1981].