NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton (FL): CRC Press/Taylor & Francis; 2011.
9.1. INTRODUCTION
From the piercing wail of an ambulance to the soothing melody of a Mozart sonata, it is clear that sound carries enormous meaning in daily life. Sound can be a reinforcing stimulus used to guide flexible behavior—as such, its meaning is often dependent on contextual cues. For example, the ringing of a bell at different times in one’s career may mean you are late for class or that your speaker time at a conference is over. Also, what we believe to be the inherent pleasing or aversive properties of sound are often counterintuitive. For example, psychophysical work suggests that it is the low-frequency spectral components that lead to the “chilling” effect of fingernails scratching on a blackboard, not the high frequencies that most people associate with the unpleasant nature of that sound (Halpern et al. 1986).
This chapter will focus on cortical aspects of sound processing in primates. However, due to the incomplete nature of primate literature, conclusions based on data from other species will be discussed. Insights from other mammalian species (i.e., cats, ferrets, rats) will be used to shape hypotheses. It is worth noting that important species differences exist in the auditory system, even within primates. For example, many species of Old and New World monkeys (e.g., macaques, capuchins) prefer silence over music (McDermott and Hauser 2007) (also see Chapter 19 in this volume). Another example is that language comprehension is something at which humans uniquely excel. However, as the specialized anatomy is not well understood, we will not review the pathways dedicated to language processing and production in humans.
9.2. EVOLUTIONARY CONSIDERATIONS: COMPARISON ACROSS MAMMALIAN TAXA
Sound has a necessarily complex role in guiding behavior and the systems that subserve this role have had a long time to evolve. To give some context for understanding the functions of the auditory system, we first briefly cover the evolutionary development of this system in mammals, and particularly primates.
The part of the auditory system that is most varied in mammals is auditory cortex (for review see Kaas and Hackett 2008). While reptiles have a dorsal cortex that is homologous to neocortex, this dorsal cortex does not have auditory inputs. Instead, the projections of the auditory thalamus are subcortical. Yet, all studied mammals have a region of temporal cortex that gets inputs from the auditory thalamus and is responsive to auditory stimuli. Thus, early mammals or the ancestors of mammals somehow acquired direct thalamic auditory projection to cortex. Most studied mammals have several areas of auditory cortex, including two or three primary or primary-like areas that are characterized by direct inputs from the tonotopically organized ventral nucleus of the medial geniculate complex (MGC), MGv, and are in turn also tonotopically organized. In addition, these primary fields are surrounded by a belt of secondary auditory areas, and additional, higher-level areas of auditory or multisensory processing may be present. For now, conclusions about how auditory cortex varies in organization across mammals need to be limited, as species in some of the major branches of mammalian evolution have not been studied, and studies have been few and incomplete for species in other major branches. Thus, we do not yet know how auditory cortex is organized in the major clades of Monotremata (platypus and echidna), Afrotheria (elephant, tenrec, etc.), and Xenarthra (armadillo, sloth, and anteater), and little is known about Marsupialia, except that possums have at least one primary area. Only one primary area has been demonstrated in Scandentia (tree shrews), which are close relatives of primates.
More progress has been made in studies of Carnivora (cats, dogs, ferrets), where at least two primary areas exist: an anterior auditory field (AAF) and the classical primary field (A1). A posterior field (P or PAF) has some of the characteristics of a primary field, and seven or eight secondary fields have been described. With more than one field having primary area features, identifying the same (homologous) areas across members of different orders of mammals has been challenging, but the characteristics of relative position, connections, architecture, and tonotopic organization have been helpful. While terms have been applied inconsistently, it now appears that rodents have primary AAF and A1 areas, and possibly a posterior field, that are homologous to those in carnivores, and comparable AAF and A1 areas have been identified in bats.
Primates also have three primary fields: a posterior A1, an anterior “rostral” area, R, and a more anterior rostrotemporal area, RT. Areas A1 and R share a common border representing low frequencies in primates, and A1 and AAF adjoin along a high-frequency representation in carnivores, rodents, and bats, so it is not clear that A1 and R in primates correspond to A1 and AAF in other mammals. One possibility is that if the expansion of the temporal lobe in primates rotated R from the posterior to the anterior border of A1, then AAF in carnivores could correspond to primate belt area CM. Correspondingly, both A1/AAF and A1/CM share a high-frequency border. Given these uncertainties about the identities of primary areas and the limited comparative evidence, there is little understanding of what secondary areas may be homologous, if any, across mammalian taxa. For now, we can surmise that early mammals had at least one primary area and a bordering secondary area or areas, and this organization has been partly retained, but variously elaborated, in the major lines leading to extant mammals. A likely common feature is that some of the secondary areas are bi- or multisensory.
In addition to taxa of mammals varying in the arrangement and number of areas of auditory cortex, auditory systems are sometimes obviously specialized in order to mediate unusual abilities. For example, echolocating bats have auditory systems that have expanded representations of the sound frequencies used in echolocation as well as specialized areas of auditory cortex for analyzing specific aspects of the echoes to provide information about target distance, size, and nature. The auditory systems of mice have specialized sectors devoted to the ultra-high frequencies used in social communication. Moreover, humans have greatly specialized auditory cortex of the left cerebral hemisphere for the processing of language.
9.3. PROPERTIES OF SOUND AND ETHOLOGICAL CONSIDERATIONS
To understand some basic properties of sound, consider a marmoset monkey twittering a “love song” to its mate in the dense tree cover of a Brazilian rain forest. It is plausible to suggest that this call is a rewarding stimulus, and it serves as a useful example. The first important question one has to ask (if one is a marmoset): Who is she? Identity cues, such as spectral frequency structure (i.e., which frequencies are in the call) and temporal modulation rates (i.e., how the amplitudes of the frequencies change in time), give rise to complex percepts such as pitch and harmonicity. These percepts, combined with other systems such as emotion and memory systems, in turn give rise to meaning and speaker identity (Moore 1997).
The second important question is: Where is she? Location cues include loudness (is she getting louder/approaching?), frequency structure (the outer ear, or pinna, filters sound in particular ways depending on their vertical location), and differences between the two ears in intensity (interaural intensity differences: IID) and time (interaural time differences: ITD). These features give rise to cues about direction of motion, vertical location, and horizontal location, respectively.
No animal processes sound outside of the confines of its surrounding environment, so ethology must be taken into consideration. Any forest, grassland, or classroom is like an auditory hall of mirrors, absorbing, reflecting, and distorting sound in characteristic ways (reviewed in Fitch 2002; Hauser 1996). The acoustic environment presents numerous challenges to the detection and discrimination of sounds, such as degradation (frequency-dependent attenuation, reverberation, and irregular amplitude fluctuations), and the levels and quality of ambient noise in the surroundings.
These degradations and distortions affect sound differently in various environments. For example, in a forest biome, reverberation off objects (such as trees) is more severe than in an open habitat, and reverberation effects are stronger for higher temporal modulation frequencies. Thus, the higher temporal frequencies of amplitude- and frequency-modulated sounds will be masked in a closed environment. In contrast, in open environments amplitude fluctuations from atmospheric inhomo-geneities are more likely to be a factor. These inhomogeneities are generally less than 50 Hz and so will affect low frequencies of temporal modulation of the sound (reviewed in Brown and Handford 2000; Wiley and Richards 1978).
In both environments, spectral frequency-dependent degradation gets worse with increasing spectral frequency, but for the forest environments, there appears to be a low-frequency pass-band window for which sound passes with less attenuation (Brown 1989; Morton 1975). This may be due to lower-frequency sound bouncing off the canopy or off the canopy’s midday thermal gradient. Thus, open environments propagate sound best with low spectral frequencies and high temporal modulation, and closed environments propagate sound best with low spectral frequencies (especially in the pass-band window) with slower rates of temporal modulation. Frequency-dependent ground attenuation is also a factor, but is similar in both environments. At 1 m above the earth, sounds in the range of 300–3000 Hz are attenuated the greatest. The higher the source is, the less attenuation occurs, especially at higher frequencies (Wiley and Richards 1978). Lastly, the source and frequency content of background masking noise is different for different habitats (Brown and Waser 1988).
The sound transmission effects described above are considerations in the design of communication sounds. The constraints of ecology on communication sounds have been well explored in fields such as birdsong. Songbirds are largely believed to have characteristic, highly structured calls. Numerous studies have looked at the correlation of habitat on birdsong and have shown numerous species whose calls operate within restricted ranges of spectral and temporal frequency. Acoustic environmental effects seem to show selection pressures, even at surprisingly short timescales. For example, the habitat for the California White-crowned Sparrow went from grassland to scrub over a 35-year period. In this time, the birds’ trill modulation rates also decreased (Derryberry 2009), presumably reflecting adaptation to increased selection pressure for better penetrating sound.
What can ethological considerations tell us about the constraints on the vocal behavior of primates? An influential hypothesis is that animals that do not have a complex social structure will communicate in a nongraded system (e.g., Marler 1975). A nongraded system is characterized by large feature distances between exemplars, which make it easier to distinguish between calls, even degraded ones. In contrast, the hypothesis predicts that animals that have close contact and easy visual access, such as in grassland, will develop graded vocalizations, where the calls have variability in a given exemplar. For these animals, there may be only subtle differences between different calls. This hypothesis also makes a second prediction that most long-range communication calls should exhibit a nongraded structure to counteract environmental degradation.
Consistent with this hypothesis, certain species of primates with complex social structures, such as macaques, exhibit graded vocalizations. However, even long-range calls, such as shrill barks, are graded when they are predicted to be nongraded (Fischer and Hammerschmidt 2002). What explains this discrepancy? Macaques live in highly variable habitats, from forest to semidesert, and many live in villages and towns. Because they need to be highly adaptable, we can conclude that ecological acoustics are not a primary factor in the evolution of production of the macaque vocal repertoire. However, there is at least one example where graded macaque calls appear to be optimized for long-range communication. Lost calls in toque macaques come in two basic types: one that is long duration and low frequency, better for long-distance propagation, and the other that is higher frequency with shorter repetitions, better to transmit location cues based on differences between the two ears (Dittus 1988).
For macaques it appears that Marler’s predictions are of limited usefulness. This is also true for other species of primates, both in Old World monkeys such as baboons (Fischer et al. 2001) and for New World arboreal species such as marmosets, squirrel monkeys, and tamarins (Hauser 1996; Schrader and Todt 1993). Given the risk of ambiguities introduced by sound degradation in a graded repertoire, why communicate in such a system? Precisely because they are graded, these repertoires may be able to carry more information (Hauser 1996). It appears that graded differences between similar calls are often the mark of individual voices (Hammerschmidt and Todt 1995). In monkeys, spectral peak patterns or differences in spectral composition in certain vocalizations help identify individual voices (Hauser and Fowler 1992; Rendall, Owren, and Rodman 1998; Rendall, Rodman, and Emond 1996). These are likened to human vocal tract resonances and are supposed to cue individual identity and morphology (e.g., size, gender) (Ghazanfar et al. 2007).
Following the school of thought pioneered by Barlow, the auditory system can be understood as a system that evolved to process behaviorally relevant sounds (Barlow 1961). To understand the mechanisms by which we process complex sounds to guide behavior, a natural place to start is by understanding the neural mechanisms of sound processing, including how these pathways interact with reward and emotional systems in the brain. From the earlier marmoset example we can see that both auditory object identity and location rely on partially overlapping sets of cues. The spectral frequency structure of the sound is important for both, and the changes of amplitude and frequency content over time are important for both. Auditory cues are critically time dependent, so a hallmark of the auditory system is its highly parallel nature. Thus, the architecture of auditory processing is characterized by multiple interacting streams even at its earliest levels. Also, context plays a large role in the relative importance of processing identity or space. Sometimes you need to know if it is your mate. At other times it is more important to know that an object is on a collision path with you than to specifically identify what it is you are dodging!
This chapter aims to review the main features and pathways of the auditory system in order to provide a foundation for the other chapters on reward. It describes auditory processing as sound waves hit the cochlea, travel up through the nuclei of the brainstem, further disseminate in multiple cortical streams, and finally arrive at associative areas such as the prefrontal and orbitofrontal cortices. Processing streams that have been identified anatomically are described, and physiological properties and possible functions are described where data are available. We then discuss how this system could interact with reward and limbic systems, providing examples where reward-based information influences auditory processing, and explore possible anatomical underpinnings of such activity. Because perceptual plasticity is so often based on reward, we will also briefly touch on the effects reward has on the processing of auditory stimuli in the context of learning. Lastly, we cover areas where auditory information is processed in some reward-based and limbic structures.
9.4. AUDITORY PROCESSING STREAMS AND PATHWAYS IN THE PRIMATE BRAIN
9.4.1. Early Auditory Processing: The Path to Cortex
9.4.1.1. Cochlea to the Inferior Colliculus
The most obvious and possibly most fundamental organizing principle of the auditory system is tonotopy, an orderly representation of sound frequency across a one-dimensional space. Tonotopy is first established at the level of the sensory epithelium (the cochlea). When sound waves hit the spiraled structure of the mammalian cochlea, the basilar membrane splits the sound into its frequency components. The basilar membrane has graded stiffness along its length, so wave amplitude changes in a frequency-dependent manner along the basilar membrane. Higher frequencies stimulate inner hair cells at the closest portion of the membrane (the base) and lower frequencies stimulate inner hair cells at the farthest portion (the apex). In mammals, the length of the basilar membrane is related to the range of high and low frequencies an animal can hear (West 1985). Thus, the cochlea establishes tonotopy, an organizational principle preserved though the levels of auditory processing through auditory cortex.
Responses from the cochlea project via the eighth nerve to the cochlear nucleus (Figure 9.1). The cochlear nucleus projects to structures in the superior olivary complex (SOC) such as the medial superior olive (MSO), the lateral superior olive (LSO), and the medial nucleus of the trapezoid body (MNTB) (reviewed in Pickles 1988; Rouiller 1997). It also projects to the nuclei of the lateral lemniscus. Again, each of these structures maintains a basic tonotopy established by the cochlea.

FIGURE 9.1
Major ascending connections of the subcortical auditory system. Selected ascending pathways from the cochlea to auditory cortex; major pathways are shown in thick lines. Divisions of subcortical nuclei are indicated in text.
Response properties of these structures show that neurons still faithfully represent sound by encoding spatial frequency at very high resolution. Neurons in these structures also demonstrate temporal modulation rate tuning (or how fast the neuron can synchronize with the temporal structure of the sound) at high rates. Nuclei of the superior olivary complex are especially important for the encoding of sound location, as they are the first place where ascending information from the two ears is combined (for review, see Kelly et al. 2002). From these structures and nuclei of the lateral lemniscus, responses reach the inferior colliculus (IC) of the midbrain. The inferior colliculus can be divided into two major portions: the central and external nuclei. Investigators also commonly distinguish the dorsal cortex, the dorsoventral nucleus, and the pericentral nucleus of the inferior colliculus.
The central nucleus (ICc) is considered to be the main relay nucleus of the inferior colliculus. It is tonotopically organized and receives a direct projection from the lateral lemniscus. Responses are tightly tuned to tones, modulation rate encoding shows synchronization up to 120 Hz, and response latencies are generally short (Langner and Schreiner 1988; Ryan and Miller 1978). In contrast, the external nucleus (ICx) is not tonotopically organized, its neurons have longer latencies, and it receives most of its inputs from sources other than the lateral lemniscus. Thus, there are two pathways: a fast, direct, tonotopically organized pathway (lemniscal pathway through the ICc) and a slower, indirect, nontonotopically organized pathway (nonlemniscal pathways through the ICx).
When compared to other sensory systems, the proliferation of brainstem nuclei in the auditory system is striking. The exact significance of this is not clear, but it may allow for more parallel processing, allowing greater and faster stimulus processing early to transform a simple one-dimensional representation of spectral frequency in time into complex percepts in space and time. This element of parallel processing is one of the hallmarks of the auditory system and is an architecture best suited to processing stimuli that occur on a very fast timescale, as in audition.
9.4.1.2. The Auditory Thalamus
Lying just medial and posterior to the lateral geniculate of the visual system, the MGC is a small and heterogeneous thalamic structure. The MGC is characterized as the primary feedforward auditory division because its inputs are dominated by the inferior colliculus (Jones 2003; Winer, Wenstrup, and Larue 1992). There is a multiplicity of pathways from the cochlea to the thalamus, but the MGC is an obligatory relay of auditory information into auditory cortex. Thus, it is useful to spend some time describing the organization and response properties of neurons in the MGC, since cortical responses can best be understood in the light of their thalamic inputs.
While the functional organization of the MGC has not been extensively explored, especially in primates, a general picture based on connectivity and microelectrode studies is emerging (reviewed in de Ribaupierre 1997). The MGC of primates consists of at least three main divisions: ventral (MGv), dorsal (MGd), and medial (MGm) (n.b., more than three divisions are distinguished in other mammalian species, such as cats). Based partly on the paucity of data, the nature and specialization of these divisions has been a matter of speculation for some time. Very early, Poljak (1926) posited that the MGv aided in localization and the MGd was involved in the discrimination of sounds. Later, Evans advanced a similar idea that the MGv was involved in localization and the MGd was involved in pattern recognition (Evans 1974). The current understanding of the MGC is that the divisions perhaps do not divide function so cleanly. What has become clear is that these divisions have different input connections and internal architecture, leading to neurons with different spectral frequency tuning properties, modulation rate tuning, response latencies, and sometimes multisensory properties.
The first division of the MGC, the MGv, receives tonotopically organized projections from both ipsi- and contralateral ICc, but ipsilateral input is stronger. This leads to a structure that is itself tonotopically organized. Neurons respond well to pure tones and are generally narrowly tuned to spectral frequency–they respond best to a small range of frequencies even at high intensities, perhaps only a quarter of an octave (Allon, Yeshurun, and Wollberg 1981; Calford 1983). Response latencies are quite short. In addition, temporal modulation rate tuning indicates that the responses of these neurons can follow and distinguish very rapid rates of stimulation (Allon, Yeshurun, and Wollberg 1981; Wang et al. 2008). In terms of selectivity to sound identity or location, the majority are primarily excited by sound coming from the contralateral ear, and sensitive to difference cues such as interaural intensity and time differences (Barone et al. 1996; Calford 1983; Starr and Don 1972). These MGv neurons are not selective for particular vocal stimuli (Symmes, Alexander, and Newman 1980), evidence that complex sound identity cues, such as call type, are not distinguished at this level.
A second division of the auditory thalamus, the MGd, receives most of its input from noncentral portions of the inferior colliculus. There is little evidence of tonotopy in MGd (but see Gross, Lifschitz, and Anderson 1974) and its neurons are generally poorly responsive to pure tones, with broad or multipeaked spectral frequency tuning. These neurons have long response latencies, consistent with their inputs from noncentral collicular nuclei. However, MGd neurons exhibit robust responses to complex sounds (Allon, Yeshurun, and Wollberg 1981; Calford 1983; He and Hu 2002). An important caveat is that MGd can be subdivided further in some species. It has been suggested that this region has two divisions in primates: anterior (MGad) and posterior (MGpd) (reviewed in Jones 2003). It is possible that the response properties differ between the two subdivisions, and it is suspected that the MGad may in fact be tonotopically organized and have neurons with short latencies, resembling MGv neurons.
A third division of the auditory thalamus, the MGm, receives inputs from both the central and external divisions of the inferior colliculus. MGm also receives significant projections from vestibular nuclei, the spinal cord, and the superior colliculus (SC) (Calford and Aitkin 1983; Rouiller 1997; Winer, Wenstrup, and Larue 1992). Connectivity of neurons within this nucleus may be highly variable, as MGm neurons project to all three core belt and parabelt regions of auditory cortex, as well as to other regions. There is also evidence that different cell classes within MGm also project to different cortical layers (Hashikawa et al. 1995; Molinari et al. 1995). There may be tonotopy in the rostral division of the MGm (Rouiller et al. 1989), but for the most part tonotopy through the entire structure is lacking. Much like the MGd, neurons are broadly spectrally tuned and are often multipeaked to tone stimuli. Response latencies in the MGm are also variable (Allon, Yeshurun, and Wollberg 1981; Calford 1983) and, consistent with its heterogeneous inputs, there is evidence for neurons with multisensory responses in at least some nonprimate species (e.g., Calford and Aitkin 1983; LeDoux et al. 1987; Rouiller et al. 1989).
There are other auditory-related nuclei in the primate thalamus, but their primary inputs are from structures such as cortical and nonprimary subcortical auditory structures, and cortical multisensory and brainstem nuclei. These include the posterior nuclear group (PO), medial pulvinar (PM), suprageniculate (SG), and limitans (Lim) (de la Mothe et al. 2006b; de Ribaupierre 1997; Rouiller and Durif 2004). The posterolateral section of the thalamic reticular nucleus is heavily implicated in mediating feedback from cortical structures. The posterior nuclear group lies dorsal and medial to the MGC. The medial pulvinar is the auditory-responsive region of the pulvinar and receives inputs from the superior colliculus, but whether it receives inputs from the inferior colliculus is not known. The medial pulvinar projects broadly to temporal, frontal, and cingulate cortex (Gutierrez et al. 2000). The thalamic reticular nucleus can be broken down into three parts: an anterior division that responds primarily to somatosensory inputs, a dorsal division that responds primarily to visual inputs, and a ventral division that responds primarily to auditory inputs. Neurons in the auditory sector are broadly tuned to tones, but can act in a frequency-specific manner mediated by connections with the MGC (Crabtree 1998).
The importance of the MGC cannot be overestimated in understanding auditory cortical processing: it is an obligatory relay to the cortex. In primates, each of the three divisions of the MGC transforms and modulates auditory neural responses in a different way. These three divisions project to different parts of auditory cortex in different degrees (see Figure 9.4), creating the firmament of the organization and response properties seen there.
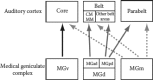
FIGURE 9.4
Connections of the MGC with regions of auditory cortex. A schematic of connections of the three subdivisions of the MGC (MGv, MGd, MGm) with the three regions of auditory cortex (core, belt, parabelt). For simplicity, only major connections are shown. (more...)
9.4.2. Auditory Cortex
Auditory cortex includes cortex that receives preferential projections from the MGC and is highly responsive to auditory stimuli. In humans, auditory cortex corresponds to Brodmann’s areas 41 and 42 located in the vicinity of Heschl’s gyrus on the superior temporal plane (Hackett et al. 2001). In macaques, auditory cortex is located on the caudal portion of the lower bank of the lateral sulcus and the superior temporal gyrus (Figure 9.2). Since only a small portion is visible on the surface of the macaque brain (upper brain), the parietal and frontal cortex has been “cut” away to reveal the areas of auditory cortex hidden deep in the lateral and circular sulcus (lower brain). From the figure, it is easy to appreciate one difficulty of studying auditory cortex: this part of cortex is almost completely covered by the parietal lobe in Old World primates such as macaques, chimpanzees, and humans.
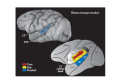
FIGURE 9.2
Location of primate auditory cortex (macaque). Upper figure: location of auditory cortex in macaque. Note that only the parabelt (blue: RPB, CPB) is exposed on the surface of the brain. Lower figure: the parietal and frontal cortices have been graphically (more...)
In this chapter, we emphasize a primate model of auditory cortical organization based on decades of anatomical and physiological research (Kaas and Hackett 1998, 2000; Hackett 2010). According to this working model, auditory cortex is first divided into three regions, which can be thought of as levels of processing (Figure 9.3). These regions are further subdivided into thirteen areas. Regions are subdivisions of auditory cortex and areas are subdivisions of regions.
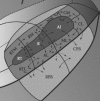
FIGURE 9.3
Organization of primate auditory cortex. A schematic of primate auditory cortex showing core, belt, and parabelt regions with areal subdivisions, and some short-range connections. For clarity, medial belt projections to parabelt are not pictured.
Distinctions between regions and areas are based on three features: connections (i.e., from thalamus and to other cortical areas), the cellular and histochemical architecture of cortical tissue, and functional organization, presumably reflected by specificity of neural response properties (such as patterns of tonotopic organization and differences in response properties). The anatomical differences in connections and architecture are thought to subserve the differences in function. In the following section, we will first describe general regional characteristics and then fill in details, where known, of areal characteristics.
In auditory cortex, three major regions are defined: core, a belt that wraps around the core, and a parabelt region lying lateral to the belt. Distinguishing architectonic features of auditory cortex can include markers for cellular and molecular features such as cytochrome oxidase (CO), acetylcholinesterase (AChE), parvalbumin, vesicular glutamate transporter 2 (vGluT2), and density of myelination. These markers change roughly stepwise as one progresses across regions in a medial to lateral direction (de la Mothe et al. 2006a; Hackett and de la Mothe 2009).
The core region receives its primary input from the MGv, and also receives a projection from the MGm (Figure 9.4). The core is densely myelinated, has a broad layer IV, and exhibits a high expression of CO, AChE, parvalbumin, and vGluT2, all consistent with receiving a dense and rapidly conducting projection from the thalamus (de la Mothe et al. 2006a, 2006b). This core region is densely connected within itself (between areas) and with areas in the adjoining belt region, but not with the parabelt (we will come back to this later). Compared to other regions, neurons in the core tend to have short response latencies (though this varies across areas, see below), narrow spectral frequency tuning functions, and relatively fast modulation frequency tuning (e.g., Bendor and Wang 2008; Kajikawa et al. 2008; Kusmierek and Rauschecker 2009; Merzenich and Brugge 1973; Recanzone 2000a; Recanzone, Guard, and Phan 2000a).
The belt region receives thalamic projections from the MGd and MGm, but not MGv (Figure 9.4). The belt has a less pronounced layer IV than the core, and it is also less myelinated and exhibits reduced expressions of the markers described above, consistent with a less robust projection from the MGC. The belt is divided into a medial and a lateral region, relative to its position in relation to the core. The medial belt region is most connected within itself and the adjoining core regions, and has additional connections to the parabelt (Figure 9.3). The lateral belt is most connected to itself, adjoining core, and adjoining parabelt. Neural responses in the belt have been less well studied electrophysiologically than in the core because most of the region responds poorly under anesthesia. Compared to core neurons, belt neurons appear to have wider spectral tuning functions, often with broad or multipeaked frequency tuning, and respond well to spectrally complex sounds (Kajikawa et al. 2008; Kusmierek and Rauschecker 2009; Recanzone 2008; Tian and Rauschecker 2004). Belt neurons also exhibit longer latencies and do not entrain as well to temporally modulated stimuli. Instead, temporal modulation rate may be encoded by firing rate (see Wang et al. 2008).
The third region, the parabelt, also receives thalamic projections from the MGd and MGm (Figure 9.4). The parabelt has a less pronounced layer IV than core or belt, and it is also less myelinated and exhibits further reduced expression of the markers described above. The parabelt region is most connected within itself and with the medial and lateral belt region (Figure 9.3). It may have a weak feedback projection to core, but no direct projection from it. The response properties of neurons have not been well studied, but based on patterns of connections and responses known thus far, parabelt neurons are expected to exhibit long latencies and respond extremely poorly to tones, with wide and probably complex frequency tuning functions. This region will probably be more responsive to sounds that are both spectrally and temporally complex, such as vocalizations.
Architectural distinctions between regions exist roughly stepwise in a medial to lateral direction, but it is also important to note that there is a distinct rostral to caudal gradient of the same molecular markers described above (i.e., markers for differences in cellular and molecular features, such as CO, AChE, parvalbumin, vGluT2, and density of myelination). In general, these features change gradually, where the strongest expression of these markers in a given region is caudal and the weakest expression is rostral.
Thus, upon this regional organization, regions are divided into areas (Figures 9.3 and 9.4) (Kaas and Hackett 1998, 2000).
The core region contains three areas, from caudal to rostral: the “primary” area A1, the rostral area R, and the rostral temporal area RT. The medial belt contains four areas, also named by location: the caudal medial area CM, the middle medial area MM, the rostral medial area RM, and the rostrotemporal medial area RTM. The lateral belt also has four areas: the caudal lateral area CL, the middle lateral area ML, the anterolateral area AL, and the rostrotemporal lateral area RTL. Lastly, the parabelt has at least two areas: the caudal parabelt area CPB and the rostral parabelt area RPB. Most, if not all, of these areas have their own, often crude, tonotopic map which flips representational order along caudorostral borders (see Petkov et al. 2006). Core area A1 has been best explored, followed next by belt area CM, and the rest are under active investigation. The functions of any of these areas have not been fully elucidated, partly because they must be interpreted in the context of the others. We do know something about specificities of neural responses in some areas and can make educated predictions about the rest.
Given these subdivisions of regions and areas, how does auditory information flow between and across regions and areas? Based on connectional anatomy and physiology, a picture of graded hierarchy of informational flow between regions is emerging. The MGv is the only subdivision of the MGC that has demonstrated strong tonotopy, so the tonotopy exhibited in the belt and parabelt (which do not receive projections from the MGv) is probably inherited from the core (Kaas and Hackett 2000; Rauschecker et al. 1997). Additionally, there is no direct projection from core to parabelt, further suggesting a high degree of serial processing from core to belt to parabelt. There is a convergence of inputs from each region to the next, which presumably leads to the wider frequency tuning and altered response specificity as one progresses across regions.
In further support of this direction of flow, latencies increase from core to lateral belt at the same rostrocaudal level (Issa and Wang 2008; Kusmierek and Rauschecker 2009; Rauschecker and Tian 2004; Recanzone 2008; Woods et al. 2006). Other support comes from sound-level functions. At lower levels, sound level (loudness) is encoded as a monotonic function (as sound level rises, so does neural firing rate). As one ascends the hierarchy of sound processing, one sees more complex, nonmonotonic cells, where cells reach peak firing at a certain sound level and then are less responsive at higher sound levels. Also, neural response thresholds (lowest sound level to elicit a response) get higher from core to belt. Tuning widths for tone frequencies also become wider for neurons from core to belt, presumably reflecting convergence in the belt of more tightly tuned inputs originating in the core or MGC (Rauschecker and Tian 2004; Woods et al. 2006). Temporal modulation tuning also progressively decreases and this is thought to be due to timing imprecision introduced by successive synaptic delays. Presumably to compensate for this, a nonsynchronized firing rate code for modulation rate also occurs at the level of auditory cortex (Bartlett and Wang 2007; reviewed in Wang et al. 2008). It should be noted, however, that while information flow across regions may be roughly serial, informational processing is not thought to occur in a strictly staged process. Instead, it is likely that many perceptual processes are occurring in parallel with each other in a graded serial fashion.
As mentioned before, the regions express dramatic medial to lateral stepwise changes in architecture and connectivity. Within each region, the architecture and response properties follow a less dramatic, but distinct, caudal to rostral decrease in molecular marker expression. Also, feedforward connectivity across areas within a region seems to have a preferential caudal to rostral flow, where connections within a region seem to be more robust caudal to rostral (e.g., A1 to R) than rostral to caudal (e.g., R to A1) (see Hackett 2010).
Rostrocaudal changes in architecture and differences in connectivity indicate that each area within a region has a unique profile of architecture and connectivity, which probably creates differences in functions between areas. For example, the most caudal core area (A1) is more myelinated than the most rostral core area (RT) (i.e., de la Mothe et al. 2006a) and demonstrates faster latencies than other core areas (Bendor and Wang 2008). Current evidence suggests that most of the belt areas exhibit slower latencies than the adjacent core area (i.e., ML slower than A1). Due to this rostral to caudal gradient it is not as easy to make predictions regarding response latencies between nonadjacent areas. There is growing evidence that the most caudal belt region, CM, has many neurons with response latencies that are as fast or faster than those in the most caudal core region, A1 (Kajikawa et al. 2005; Lakatos et al. 2005; Rauschecker and Tian 2004). However, CM receives most of its MGd inputs from the MGad nuclei, which, as discussed earlier, may exhibit tonotopy and fast latencies. Yet, physiological and lesion evidence seems to suggest that CM appears to depend completely on A1 inputs for its tone responses (Rauschecker et al. 1997). Clearly, these and other findings are presenting challenges to the present model in terms of information flow within auditory cortex.
As discussed in the introduction, auditory object location and identity share partially overlapping cues, whose coding is described above. To date, there has been little electrophysiological evidence of strong feature identity selectivity (e.g., for different calls) for neurons in core and belt areas of auditory cortex (Kusmierek and Rauschecker 2009; Recanzone 2008; Wang et al. 1995). There is a bias for recording in the larger and more accessible caudal core and belt areas, so the lack of selectivity found thus far may be simply due to this. One aspect of object identity is the subjective perception of pitch (irrespective of whether the frequency is actually present). A module of neurons that appears to be selective for pitch has been described on the lateral low frequency border of A1 with RT (Bendor and Wang 2005) and there seems to be converging evidence from functional imaging for a similar processing zone in human core auditory cortex, though it is often not as tightly localized (reviewed by Bendor and Wang 2006; but see Hall and Plack 2009).
The coding of object location in auditory cortex has been an area of intense interest. Many neurons appear to be sensitive to the spatial location of freefield sounds as well as headphone-based interaural intensity and time differences (IID and ITD), especially in the caudal belt fields (Ahissar et al. 1992; Miller and Recanzone 2009; Recanzone 2000b; Recanzone et al. 2000b; Scott, Malone, and Semple 2007; Woods et al. 2006). How this spatial selectivity is propagated is not well understood, as spatial selectivity in the MGC is virtually unknown. There has been little evidence for an ordered spatiotopic map in auditory cortex—instead, location in space is probably represented across a distributed population of neurons (Miller and Recanzone 2009). A co-registration of auditory information within an ordered spatiotopic map could occur with the lower layers of the superior colliculus (SC), which have significant inputs from auditory and multisensory areas of neocortex (e.g., Collins, Lyon, and Kaas 2005). Higher-order spatial perception such as the perception of auditory motion is also poorly understood, but belt areas have been shown to be sensitive to the presentation of approaching “looming” stimuli (Maier and Ghazanfar 2007). Areas of temporal and posterior parietal cortex that are sensitive to visual motion may be also sensitive to or modulated by auditory motion (Alink, Singer, and Muckli 2008).
9.4.3. Auditory-Responsive Cortex beyond Classical Auditory Cortex
9.4.3.1. Superior Temporal Gyrus
Areas of the superior temporal gyrus (STG) include Ts1, Ts2, the superior temporal polysensory region (STP), and parts of the temporoparietal temporal area (Tpt) (Figure 9.5). These areas have been shown to have dense reciprocal connections with belt and parabelt, and some have weaker auditory thalamic inputs from the MGC and multisensory nuclei of the posterior thalamus (Galaburda and Pandya 1983; Hackett et al. 2007a, 1998a, 1998b; Kosmal et al. 1997; Markowitsch et al. 1985; Pandya and Rosene 1993; Pandya, Rosene, and Doolittle 1994; Trojanowski and Jacobson 1975). Evidence from fMRI and PET studies in primates shows responsiveness to auditory stimuli, as well as to other modalities (Gil-da-Costa et al. 2006; Leinonen, Hyvarinen, and Sovijarvi 1980; Petkov et al. 2008; Poremba et al. 2004, 2003). Rostral STG has been shown to be particularly responsive to vocalizations and there is growing evidence for a voice identity processing area in the superior temporal region—one that responds preferentially to the vocal identity of particular callers (see also review of human literature by Belin 2006; Petkov et al. 2008; Poremba et al. 2004).

FIGURE 9.5
Auditory cortical projections to prefrontal, limbic structures. Long-range connections of auditory and auditory-related cortical fields to prefrontal and limbic structures projected on a macaque brain. Note the dorsoventral topography. For clarity, projections (more...)
9.4.3.2. Prefrontal Cortex and Ventral Premotor Cortex
Auditory cortical belt and parabelt project to areas in the prefrontal cortex, orbitofrontal cortex, and cingulate cortices in a topographic manner. Caudal parabelt primarily projects to dorsal prefrontal cortex and rostral parabelt primarily projects to ventral prefrontal cortex (Figure 9.5). Auditory-related areas on the STG described above project in a similar topographic manner (Barbas 2007; Barbas et al. 2005; Cavada et al. 2000; Morecraft et al. 2004; Pandya, Hallett, and Kmukherjee 1969; Petrides and Pandya 2002, 2007; Roberts et al. 2007; Saleem, Kondo, and Price 2008). These areas show auditory responsiveness to complex stimuli such as vocalizations or auditory responsiveness in a task-specific manner (Artchakov et al. 2007; Azuma and Suzuki 1984; Bodner, Kroger, and Fuster 1996; Cohen, Hauser, and Russ 2006; Cohen et al. 2007; Fuster, Bodner, and Kroger 2000; Ito 1982; Kikuchi-Yorioka and Sawaguchi 2000; Romanski 2007; Romanski, Averbeck, and Diltz 2005; Sugihara et al. 2006). For example, the dorsal-most portion of the frontal eye field responds to auditory stimuli and it is thought to mediate auditory-guided saccades. This is consistent with projection patterns described above showing a caudal projection from the caudal belt and adjoining association cortices to the portion of the frontal cortex containing the frontal eye fields. Auditory responsiveness has also been explored in the ventrolateral prefrontal cortex. Neurons in this part of cortex are not responsive to tones or noise, but are selectively responsive to different vocalizations (Cohen, Hauser, and Russ 2006, 2007; reviewed in Romanski and Averbeck 2009; Romanski, Averbeck, and Diltz 2005; Romanski and Goldman-Rakic 2002; Russ et al. 2008).
This dorsoventral topography of auditory belt and parabelt projections to the frontal cortex has led to the proposal that there exists a domain specificity of auditory processing in the auditory cortex (but see Recanzone and Cohen 2009; Romanski, Bates, and Goldman-Rakic 1999; Romanski, Tian, et al. 1999), much like the domain specificity described in the visual cortex (Ungerleider and Mishkin 1982). It is hypothesized that the dorsal prefrontal cortex receives projections from the dorsocaudal “where” stream of auditory processing and the ventral prefrontal cortex receives projections from the ventrocaudal “what” stream of auditory processing (Romanski, Tian, et al. 1999). This can be interpreted as functional specialization of prefrontal and auditory cortices (e.g., Rauschecker and Tian 2000; Romanski and Goldman-Rakic 2002; Tian et al. 2001).
In addition to prefrontal cortex being involved in auditory functions, ventral premotor cortex in monkeys contains neurons that respond to sounds signifying hand or mouth actions (Keysers et al. 2003). This region of cortex is also activated in humans when they listen to the sound of an action (Gazzola, Aziz-Zadeh, and Keysers 2006).
9.4.3.3. Insular Cortex
Another auditory-responsive region is insular cortex, lying medial to the medial belt of auditory cortex. This area is connected to the medial belt, and to a lesser extent lateral belt and parabelt, as well as areas of the superior temporal gyrus and prefrontal cortex (de la Mothe et al. 2006a; Hackett et al. 2007b). Early electrophysiological studies indicated it was responsive to both simple auditory stimuli (i.e., tones and clicks) and more complex stimuli (i.e., vocalizations) (Bieser 1998; Bieser and Muller-Preuss 1996; Sudakov et al. 1971) (see also Remedios, Logothetis, and Kayser 2009). Insular cortex also has been implicated in auditory functions in humans (Griffiths et al. 1997; Zatorre, Evans, and Meyer 1994). In a recent study, insular neurons responded preferentially to con-specific vocalizations over sounds with similar spectral or envelope structure, indicating that they are responding preferentially to the vocalization (Remedios, Logothetis, and Kayser 2009). How insular cortex fits into the “what vs. where” stream hypothesis has yet to be determined. The region of the anterior insula has been implicated in the ability to understand the emotional experiences of others (e.g. Peyron, Laurent, and Garcia-Larrea 2000), and auditory input to insular cortex may have an affective component and relate to emotional appreciation of music in humans (Craig 2008).
9.4.3.4. Corticofugal Projections
There are extensive corticofugal projections from auditory and auditory-related cortex (reviewed in Winer 2005), presumed to play a major role in top-down modulatory and learning effects. These connections have been most extensively explored in cats, but they have been demonstrated in primates as well (de la Mothe et al. 2006b; FitzPatrick and Imig 1978; Luethke, Krubitzer, and Kaas 1989; Morel and Kaas 1992). These corticofugal projections target primarily ipsilateral nuclei and structures. There are massive projections to the MGC (de la Mothe et al. 2006b; Winer et al. 2002), inferior colliculus (mostly outside of the central nucleus) (Winer et al. 1998), superior olivary complex (SOC), cochlear nucleus (CoN), pons (Brodal 1972), and basal ganglia (Reale and Imig 1983). Input to claustrum and endopiriform nucleus also arises from areas of auditory cortex (Beneyto and Prieto 2001). The dorsal putamen and caudate nucleus receive topographic projections from areas of auditory cortex (Reale and Imig 1983), and appear to have a role in sensory processing. Motor behavior could be modulated by inputs from auditory cortex to the basal ganglia (Beneyto and Prieto 2001). Auditory cortex also is positioned to affect autonomic function via its projections to amygdala (e.g., Romanski and LeDoux 1993) and central gray (Winer et al. 1998). Other inputs to the amygdala come directly from the auditory thalamus, at least in rats (Iwata et al. 1986).
9.5. REWARD-RELATED ACTIVITY IN AUDITORY CORTEX AND OTHER AUDITORY AREAS
9.5.1. Early Lesion Studies of Auditory Cortex
Having reviewed the basic framework for auditory processing, we now focus on roles of reward and emotion in auditory perception as well as connections that could be mediating these interactions. There have been a number of studies of the effects of cortical lesions on conditioned auditory discriminative behavior in cats (e.g., Butler, Diamond, and Neff 1957; Diamond, Goldberg, and Neff 1962; Jenkins and Merzenich 1984) and to a lesser extent in monkeys (e.g., Heffner and Heffner 1990a, 1990b). Lesions generally involved all of the primary core auditory cortex and much of the secondary auditory cortex of both hemispheres. The usual finding was that learned discriminations were lost immediately after such lesions. Simple discriminations, such as responding to a frequency change of a pulsating tone, were rapidly relearned, while more complex auditory tasks, such as responding to a change in a pattern of two pulsing tones, were not. Sound localization tasks were permanently impaired, while reflexive head-orienting responses remained, but were less accurate (Beitel and Kaas 1993; Jenkins and Merzenich 1984). Based on these studies, extensive bilateral lesions of auditory cortex did not seem to interfere with associating sounds with reward or punishment, but did produce deficits in complex auditory discriminations.
In rats, extensive bilateral lesions of the auditory region, including all known primary and secondary areas, did not abolish the ability to acquire auditory fear conditioning, but lesions of the auditory thalamus, particularly those that include MGm, did (Romanski and LeDoux 1992). The authors concluded that fear conditioning can be mediated by projections from the auditory thalamus to the amygdala (see below, and see also Edeline and Weinberger 1991, 1992). Quite possibly, auditory stimuli can be associated with reward or punishment using such subcortical pathways if the relevant auditory stimuli do not depend on cortical mechanisms for analysis.
9.5.2. Reward-Related Changes in the Responses of Neurons and Sensory Map Plasticity
In a developing animal, passive exposure to a sensory stimulus will increase that stimulus’ representation in cortex (Sanes and Bao 2009), but in intact adult animals the stimulus must be paired with a task to create the same changes. Large-scale reorganization of sensory topographic maps is highly reward dependent (Blake et al. 2006). In owl monkeys, training on a target frequency will expand the representation of that frequency in A1 (Blake et al. 2002; Recanzone, Schreiner, and Merzenich 1993), which is similar to results in rats (Blake et al. 2006; Polley, Steinberg, and Merzenich 2006). This phenomenon is commonly referred to as perceptual learning.
There have been many examples where pairing an auditory stimulus with reinforcement alters the receptive field organization of auditory cortical fields (e.g., Atiani et al. 2009; Beitel et al. 2003; Brosch, Selezneva, and Scheich 2005; Durif, Jouffrais, and Rouiller 2003; Hocherman, Itzhaki, and Gilat 1981), as well as the auditory thalamus (e.g., Edeline, Neuenschwander-el Massioui, and Dutrieux 1990; Edeline and Weinberger 1992; Gabriel, Saltwick, and Miller 1975; Halas et al. 1970; Komura et al. 2001, 2005; Ryugo and Weinberger 1978), the inferior colliculus (e.g., Disterhoft and Stuart 1977; Metzger et al. 2006; Nienhuis and Olds 1978; Olds, Nienhuis, and Olds 1978), and even the cochlear nucleus (e.g., Oleson, Ashe, and Weinberger 1975). When a stimulus (such as a tone) is paired with a reward, the neurons activated by that auditory stimulus generally increase their response rate, and/or auditory areas and nuclei devote larger proportions of their neurons to the representation of that rewarded sound.
Such behaviorally mediated plasticity is regulated by the basal cholinergic system that projects broadly to auditory and other forebrain structures (Kilgard and Merzenich 1998; Ma and Suga 2005; Weinberger 2003). Furthermore, lesions of the basal forebrain cholinergic system prevent the changes in sensory neuron responsiveness with reward (Conner, Chiba, and Tuszynski 2005; Juliano, Ma, and Eslin 1991; Prakash et al. 2004).
There are at least two proposed models for the basis of this plasticity (reviewed by Edeline 1999; Edeline and Weinberger 1992). The first model proposes that long-lasting effects in the nonlemniscal pathway contribute to cortical plasticity. This model postulates that plasticity effects are mainly at the level of the MGm, where acetylcholine released from the nucleus basalis creates long-lasting receptive field changes at the MGm, and possibly the MGv. These changes are perpetuated up through A1 (see Weinberger 1998, 2004; and see see Chapter 1 by Weinberger and Bieszczad in this volume). The second model proposes that cortical activity activates the amygdala, which activates the nucleus basalis, which in turn affects change in cortex. It implicates an association cortex-amygdala-nucleus basalis-auditory cortex loop (Gao and Suga 1998; Ma and Suga 2005). In the second model, changes in corticofugal pathways promote long-lasting thalamic plasticity (Weinberger et al. 1990). Regardless of the mechanism, plasticity is affected by a number of other neurotransmitters besides acetylcholine. Dopamine from the ventral tegmental area and norepinephrine from the locus coeruleus have been shown to induce auditory receptive field plasticity (see Bao, Chan, and Merzenich 2001; Edeline 1999; Seitz and Watanabe 2005).
Reward-based plasticity can reflect an increased processing of stimuli that show a predictive relationship to the reinforcing signal. For example, in a tone-conditioning task in rats, cortical expansion occurred at the representation corresponding to the target frequency, and also that corresponding to the low-frequency sounds emitted by the reward delivery system (Rutkowski and Weinberger 2005). Similarly, somatosensory and visual stimuli can evoke responses in auditory cortex when they are associated with an auditory stimulus and reward. After intensive training on an auditory task, cells in A1 of macaque responded to the visual cue that preceded the auditory stimulus and also responded to the touch of the bar that was used to indicate a response (Brosch, Selezneva, and Scheich 2005). Rather than propose that A1 is multisensory, it may be that in the context of overtraining A1 is sensitive to signals that predict reward. Lastly, there is also evidence to suggest that different topographic maps can reorganize independently without disturbing one another (Polley, Steinberg, and Merzenich 2006).
9.5.3. Connections with the Reward System
In order for auditory signals to motivate behavior, connections with brain structures involved in reward would appear to be necessary (reviewed in Schultz 2000). Thus, the next section seeks to identify connections of the auditory pathway with areas associated with the reward system, such as the basal ganglia, amygdala, basal forebrain, and frontal limbic cortices.
In monkeys, the proportion of auditory cortical projections to basal ganglia generally increases as one ascends from core to belt to parabelt to auditory-related cortices (Borgmann and Jurgens 1999; de la Mothe et al. 2006a; Selemon and Goldman-Rakic 1985; Smiley et al. 2007; Yeterian and Pandya 1998). The densest connections with the most striking topography emerge at the level of parabelt and auditory-related areas of the temporal lobe. Caudal areas project to dorsal parts of the caudate and putamen, and lateral and rostral areas project to the ventral portions of the caudate and putamen (Borgmann and Jurgens 1999; Forbes and Moskowitz 1974; Nauta and Whitlock 1956; Yeterian and Pandya 1998). Consistent with these projections, auditory-responsive neurons have been recorded in caudate, putamen, globus pallidus, and substantia nigra (Aosaki, Kimura, and Graybiel 1995; Chudler, Sugiyama, and Dong 1995; Hikosaka, Sakamoto, and Usui 1989; Hikosaka and Wurtz 1983; Nagy et al. 2005; Steinfels et al. 1983; Strecker et al. 1985).
The lateral nucleus of the amygdala (AL) receives highly processed visual and auditory information. It can receive auditory information from the thalamus (mainly, but not limited to, the MGm) or cortex (belt and parabelt of auditory cortex, and auditory-related areas such as rostral temporal cortices) (reviewed in Amaral et al. 1992; Romanski and LeDoux 1993). Most of these cortical projections arise from the rostral putative “what” pathway, similar to the visual system (see Amaral et al. 1992). The thalamoamygdala and corticoamygdala pathways have different but overlapping functions. It is believed that both pathways can mediate acquisition of simple auditory discriminations in fear-conditioning tasks, such as the acoustic startle. However, the corticoamygdala projections appear to be necessary to mediate acquisition of more complex auditory discriminations (Jarrell et al. 1987; LeDoux, Sakaguchi, and Reis 1984; Romanski and LeDoux 1992). The amygdala is responsive to auditory stimuli, especially in the context of a task, and is also responsive to emotional vocalizations in monkeys (Kuraoka and Nakamura 2007).
The basal forebrain nuclei are the main extrinsic sources of acetylcholine to auditory cortex (Jones and Burton 1976), a neurotransmitter previously discussed regarding its implications with plasticity and learning. The heavy AChE banding in layers 3 and 4 of auditory cortex, particularly in core and caudomedial areas (de la Mothe et al. 2006a; Winer and Lee 2007), presumably arises from basal forebrain. Basal forebrain projections to auditory cortex may be useful to mediate plasticity and memory effects. Consistent with this, the basal forebrain has demonstrated auditory activity when an auditory stimulus cues a reward (Wilson and Rolls 1990).
The prefrontal limbic cortices are located on the ventral and medial surface of the most anterior portion of the frontal lobe. Based on intrinsic and extrinsic connection patterns, they are generally considered to consist of two networks, the orbital (or ventral) and medial networks (Barbas 2007; Carmichael and Price 1996; Cavada et al. 2000; Price 2006). Areas on the ventral and ventromedial surface of the frontal lobe (e.g., 12, 11) have robust connections with rostral parabelt and rostral superior temporal gyrus (Barbas 1993; Hackett et al. 1999; Romanski, Bates, and Goldman-Rakic 1999; Saleem, Kondo, and Price 2008). These areas are auditory responsive and show responses to complex auditory stimuli, demonstrated both electrophysiologically (Rolls et al. 2006) and meta-bolically via 2-deoxyglucose (Poremba et al. 2003; Wiley and Richards 1978). Furthermore, these areas are important for responding to social auditory stimuli. Macaques with lesions of the OFC show less appropriate responses to vocal social cues such as threat and affiliation calls (Machado and Bachevalier 2006).
In the second network, areas on the medial surface of the frontal lobe and cingulate cortex have robust connections with rostral auditory parabelt and rostral superior temporal gyrus (Hackett et al. 1999; Romanski, Bates, and Goldman-Rakic 1999; Vogt and Pandya 1987). This region of frontal and cingulate cortex is also connected to brainstem vocalization centers and appears to be involved in emotional vocal processing and production (Alheid and Heimer 1996; Holstege 1991; Holstege, Bandler, and Saper 1996). It has long been known that vocalizations (among other things) can be elicited by electrical stimulation of anterior cingulate cortex in monkeys (e.g., Smith 1945) and that lesions of this cortex reduce or eliminate the productions of isolation calls (MacLean and Newman 1988).
9.6. CONCLUSIONS
This chapter reviews the auditory processing pathways, with particular emphasis on cortical and subcortical structures implicated in reward and reward-based plasticity. To provide a foundation for the other chapters on rewards, we have described the functional organization of auditory processing pathways. Where possible, we have described examples of reward-based activity within this pathway and described pathways that likely mediate this activity.
Analysis of the pathways of reward in the context of sensory systems is illuminating because it reveals ways that reward-based information could guide behavior. Additionally, analysis of these pathways also informs our evolving ideas about the basic structure of auditory processing. For example, perhaps the parts of the auditory system that are preferentially connected to reward structures are participating in different computations from those that are not. Analysis of the pathways of auditory informational flow is also useful to illuminate ideas about sensory organization. For example, if what/where is a global organizational principle of many sensory systems, then it is reasonable to expect that this organization is perpetuated into nonsensory systems, such as reward.
These pathways are also interesting from a comparative evolutionary perspective. In the course of primate evolution, the human brain underwent dramatic expansion, and the auditory cortices are no exception (Hackett et al. 2001). Comparing evidence from different primates such as marmosets, macaques, and humans inspires a search for similarities and differences between species. As discussed previously, differences may be due to evolutionary selection pressures in their specific ecological niche. Differences may also be due to constraints that emerge to minimize the metabolic cost and connection length of a larger brain (reviewed in Kaas 2000). For example, laterality of function in larger brains is hypothesized to have arisen from constraints to limit the number of long-range interhemispheric connections (Ringo et al. 1994). These connections are slower, temporally imprecise, and metabolically costly. Given the auditory system’s dependence on precise timing, it is no surprise that evidence for laterality in large brains is strongest in this sensory modality (e.g., language processing in humans).
There are still many fundamental questions left on the nature of auditory processing and reward processing within the auditory system. We need a more complete understanding of anatomical underpinnings, especially in close human relatives. We also need more studies of neuronal responses in the awake animal, especially in the context of task-guided behavior. Careful studies across different species will help elucidate global roles of reward and its specific interactions with the auditory system in primates.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Troy Hackett and Lisa de la Mothe of Vanderbilt University and Dr. Daniel Polley of Harvard Medical School for helpful comments on this chapter.
REFERENCES
- Ahissar M., Ahissar E., Bergman H., Vaadia E. Encoding of sound-source location and movement: Activity of single neurons and interactions between adjacent neurons in the monkey auditory cortex. J Neurophysiol. 1992;67:203–15. [PubMed: 1552320]
- Alheid G.F., Heimer L. Theories of basal forebrain organization and the “emotional motor system” Prog Brain Res. 1996;107:461–84. [PubMed: 8782537]
- Alink A., Singer W., Muckli L. Capture of auditory motion by vision is represented by an activation shift from auditory to visual motion cortex. J Neurosci. 2008;28:2690–97. [PMC free article: PMC6670665] [PubMed: 18337398]
- Allon N., Yeshurun Y., Wollberg Z. Responses of single cells in the medial geniculate body of awake squirrel monkeys. Exp Brain Res. 1981;41:222–32. [PubMed: 7215486]
- Amaral D., Price J., Pitkanen A., Carmichael S. Anatomical organization of the primate amygdaloid complex. In: Aggleton J., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 1–66.
- Aosaki T., Kimura M., Graybiel A.M. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–52. [PubMed: 7608768]
- Artchakov D., Tikhonravov D., Vuontela V., Linnankoski I., Korvenoja A., Carlson S. Processing of auditory and visual location information in the monkey prefrontal cortex. Exp Brain Res. 2007;180:469–79. [PubMed: 17390128]
- Atiani S., Elhilali M., David S.V., Fritz J.B., Shamma S.A. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–80. [PMC free article: PMC3882691] [PubMed: 19217382]
- Azuma M., Suzuki H. Properties and distribution of auditory neurons in the dorsolateral prefrontal cortex of the alert monkey. Brain Res. 1984;298:343–46. [PubMed: 6722560]
- Bao S., Chan V.T., Merzenich M.M. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. [PubMed: 11452310]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56:841–64. [PubMed: 8284038]
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Ann N Y Acad Sci. 2007;1121:10–32. [PubMed: 17698996]
- Barbas H., Hilgetag C.C., Saha S., Dermon C.R., Suski J.L. Parallel organization of contralateral and ipsilateral prefrontal cortical projections in the rhesus monkey. BMC Neurosci. 2005;6:32. [PMC free article: PMC1134662] [PubMed: 15869709]
- Barlow H. Possible principles underlying the transformation of sensory messages. In: Rosenblith W., editor. Sensory Communication. Cambridge: MIT Press; 1961. pp. 217–34.
- Barone P., Clarey J.C., Irons W.A., Imig T.J. Cortical synthesis of azimuth-sensitive single-unit responses with nonmonotonic level tuning: A thalamocortical comparison in the cat. J Neurophysiol. 1996;75:1206–20. [PubMed: 8867129]
- Bartlett E.L., Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol. 2007;97:1005–17. [PubMed: 17050830]
- Beitel R.E., Kaas J.H. Effects of bilateral and unilateral ablation of auditory cortex in cats on the unconditioned head orienting response to acoustic stimuli. J Neurophysiol. 1993;70:351–69. [PubMed: 8360719]
- Beitel R.E., Schreiner C.E., Cheung S.W., Wang X., Merzenich M.M. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci USA. 2003;100:11070–75. [PMC free article: PMC196928] [PubMed: 12941865]
- Belin P. Voice processing in human and non-human primates. Philos Trans R Soc Lond B Biol Sci. 2006;361:2091–107. [PMC free article: PMC1764839] [PubMed: 17118926]
- Bendor D., Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–65. [PMC free article: PMC1780171] [PubMed: 16121182]
- Bendor D., Wang X. Cortical representations of pitch in monkeys and humans. Curr Opin Neurobiol. 2006;16:391–99. [PMC free article: PMC4325365] [PubMed: 16842992]
- Bendor D., Wang X. Neural response properties of primary, rostral, and rostrotemporal core fields in the auditory cortex of marmoset monkeys. J Neurophysiol. 2008;100:888–906. [PMC free article: PMC2525707] [PubMed: 18525020]
- Beneyto M., Prieto J.J. Connections of the auditory cortex with the claustrum and the endopiriform nucleus in the cat. Brain Res Bull. 2001;54:485–98. [PubMed: 11397538]
- Bieser A. Processing of twitter-call fundamental frequencies in insula and auditory cortex of squirrel monkeys. Exp Brain Res. 1998;122:139–48. [PubMed: 9776512]
- Bieser A., Muller-Preuss P. Auditory responsive cortex in the squirrel monkey: Neural responses to amplitude-modulated sounds. Exp Brain Res. 1996;108:273–84. [PubMed: 8815035]
- Blake D.T., Heiser M.A., Caywood M., Merzenich M.M. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–81. [PMC free article: PMC2826987] [PubMed: 17046698]
- Blake D.T., Strata E., Churchland A.K., Merzenich M.M. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:10114–19. [PMC free article: PMC126633] [PubMed: 12119383]
- Bodner M., Kroger J., Fuster J.M. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7:1905–8. [PubMed: 8905689]
- Borgmann S., Jurgens U. Lack of cortico-striatal projections from the primary auditory cortex in the squirrel monkey. Brain Res. 1999;836:225–28. [PubMed: 10415425]
- Brodal P. The corticopontine projection in the cat. The projection from the auditory cortex. Arch Ital Biol. 1972;110:119–44. [PubMed: 5074166]
- Brosch M., Selezneva E., Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 2005;25:6797–806. [PMC free article: PMC6725347] [PubMed: 16033889]
- Brown C., Waser P. Environmental influences on the structure of primate vocalizations. In: Todt D., Goedeking P., Symmes D., editors. Primate Vocal Communication. Berlin: Springer-Verlag; 1988. pp. 51–66.
- Brown C.H. The acoustic ecology of east African primates and the perception of vocal signals by grey-cheeked mangabeys and blue monkeys. In: Dooling R., Hulse S., editors. The Comparative Psychology of Audition: Perceiving Complex Sounds. Hillsdale, NJ: Erlbaum; 1989. pp. 201–39.
- Brown T.J., Handford P. Sound design for vocalizations: Quality in the woods, consistency in the fields. Condor. 2000;102:81–92.
- Butler R.A., Diamond I.T., Neff W.D. Role of auditory cortex in discrimination of changes in frequency. J Neurophysiol. 1957;20:108–20. [PubMed: 13398855]
- Calford M.B. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983;3:2350–64. [PMC free article: PMC6564635] [PubMed: 6631485]
- Calford M.B., Aitkin L.M. Ascending projections to the medial geniculate body of the cat: Evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983;3:2365–80. [PMC free article: PMC6564620] [PubMed: 6313877]
- Carmichael S.T., Price J.L. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. [PubMed: 8835726]
- Cavada C., Company T., Tejedor J., Cruz-Rizzolo R.J., Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–42. [PubMed: 10731218]
- Chudler E.H., Sugiyama K., Dong W.K. Multisensory convergence and integration in the neostriatum and globus pallidus of the rat. Brain Res. 1995;674:33–45. [PubMed: 7773693]
- Cohen Y.E., Hauser M.D., Russ B.E. Spontaneous processing of abstract categorical information in the ventrolateral prefrontal cortex. Biol Lett. 2006;2:261–65. [PMC free article: PMC1618918] [PubMed: 17148378]
- Cohen Y.E., Theunissen F., Russ B.E., Gill P. Acoustic features of rhesus vocalizations and their representation in the ventrolateral prefrontal cortex. J Neurophysiol. 2007;97:1470–84. [PubMed: 17135477]
- Collins C.E., Lyon D.C., Kaas J.H. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat Rec A Discov Mol Cell Evol Biol. 2005;285:619–27. [PubMed: 15912524]
- Conner J.M., Chiba A.A., Tuszynski M.H. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–79. [PubMed: 15848797]
- Crabtree J.W. Organization in the auditory sector of the cat’s thalamic reticular nucleus. J Comp Neurol. 1998;390:167–82. [PubMed: 9453662]
- Craig A. Interoception and emotion, a neuroanatomical perspective. In: Lewis M., Haviland-Jones J., Barrett L., editors. Handbook of Emotions. New York: Guilford Press; 2008. pp. 272–88.
- de la Mothe L.A., Blumell S., Kajikawa Y., Hackett T.A. Cortical connections of the auditory cortex in marmoset monkeys: Core and medial belt regions. J Comp Neurol. 2006a;496:27–71. [PubMed: 16528722]
- de la Mothe L.A., Blumell S., Kajikawa Y., Hackett T.A. Thalamic connections of the auditory cortex in marmoset monkeys: Core and medial belt regions. J Comp Neurol. 2006b;496:72–96. [PMC free article: PMC4419740] [PubMed: 16528728]
- de Ribaupierre F. Acoustical information processing in the auditory thalamus and cerebral cortex. In: Ehret G., Romand R., editors. The Central Auditory System. New York: Oxford University Press; 1997. pp. 317–98.
- Derryberry E.P. Ecology shapes birdsong evolution: Variation in morphology and habitat explains variation in White-crowned Sparrow song. Am Naturalist. 2009;174:24–33. [PubMed: 19441960]
- Diamond I.T., Goldberg J.M., Neff W.D. Tonal discrimination after ablation of auditory cortex. J Neurophysiol. 1962;25:223–35. [PubMed: 13886108]
- Disterhoft J.F., Stuart D.K. Differentiated short latency response increases after conditioning in inferior colliculus neurons of alert rat. Brain Res. 1977;130:315–33. [PubMed: 884527]
- Dittus W. An analysis of toque macaque cohesion calls from an ecological perspective. In: Todt D., Goedeking P., Symmes D., editors. Primate Vocal Communication. Berlin: Springer-Verlag; 1988. pp. 31–49.
- Durif C., Jouffrais C., Rouiller E.M. Single-unit responses in the auditory cortex of monkeys performing a conditional acousticomotor task. Exp Brain Res. 2003;153:614–27. [PubMed: 14578996]
- Edeline J.M. Learning-induced physiological plasticity in the thalamo-cortical sensory systems: A critical evaluation of receptive field plasticity, map changes and their potential mechanisms. Prog Neurobiol. 1999;57:165–224. [PubMed: 9987805]
- Edeline J.M., Neuenschwander-el Massioui N., Dutrieux G. Discriminative long-term retention of rapidly induced multiunit changes in the hippocampus, medial geniculate and auditory cortex. Behav Brain Res. 1990;39:145–55. [PubMed: 2167693]
- Edeline J.M., Weinberger N.M. Subcortical adaptive filtering in the auditory system: Associative receptive field plasticity in the dorsal medial geniculate body. Behav Neurosci. 1991;105:154–75. [PubMed: 2025387]
- Edeline J.M., Weinberger N.M. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: Receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. [PubMed: 1554440]
- Evans E. Neural processes for the detection of acoustic patterns and for sound localization. In: Schmidt F., Wordern F., editors. The Neurosciences, Third Study Program. Cambridge: MIT Press; 1974. pp. 131–45.
- Fischer J., Hammerschmidt K. An overview of the barbary macaque, Macaca sylvanus, vocal repertoire. Folia Primatol (Basel). 2002;73:32–45. [PubMed: 12065939]
- Fischer J., Hammerschmidt K., Cheney D.L., Seyfarth R.M. Acoustic features of female chacma baboon barks. Ethology. 2001;107:33–54.
- Fitch W.T.S. Primate vocal production and its implications for auditory research. In: Ghazanfar A., editor. Primate Audition: Ethology and Neurobiology. London: CRC Press; 2002. pp. 87–108.
- FitzPatrick K.A., Imig T.J. Projections of auditory cortex upon the thalamus and midbrain in the owl monkey. J Comp Neurol. 1978;177:573–55. [PubMed: 415070]
- Forbes B.F., Moskowitz N. Projections of auditory responsive cortex in the squirrel monkey. Brain Res. 1974;67:239–54. [PubMed: 4220029]
- Fuster J.M., Bodner M., Kroger J.K. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–51. [PubMed: 10830963]
- Gabriel M., Saltwick S.E., Miller J.D. Conditioning and reversal of short-latency multiple-unit responses in the rabbit medial geniculate nucleus. Science. 1975;189:1108–9. [PubMed: 1162365]
- Galaburda A.M., Pandya D.N. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol. 1983;221:169–84. [PubMed: 6655080]
- Gao E., Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA. 1998;95:12663–70. [PMC free article: PMC22888] [PubMed: 9770543]
- Gazzola V., Aziz-Zadeh L., Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824–29. [PubMed: 16979560]
- Ghazanfar A.A., Turesson H.K., Maier J.X., van Dinther R., Patterson R.D., Logothetis N.K. Vocal-tract resonances as indexical cues in rhesus monkeys. Curr Biol. 2007;17:425–30. [PMC free article: PMC2361420] [PubMed: 17320389]
- Gil-da-Costa R., Martin A., Lopes M.A., Munoz M., Fritz J.B., Braun A.R. Species-specific calls activate homologs of Broca’s and Wernicke’s areas in the macaque. Nat Neurosci. 2006;9:1064–70. [PubMed: 16862150]
- Griffiths T.D., Rees A., Witton .C., Cross P.M., Shakir R.A., Green G.G. Spatial and temporal auditory processing deficits following right hemisphere infarction. A psychophysical study. Brain. 1997;120(5):785–94. [PubMed: 9183249]
- Gross N.B., Lifschitz W.S., Anderson D.J. The tonotopic organization of the auditory thalamus of the squirrel monkey (Saimiri sciureus). Brain Res. 1974;65:323–32. [PubMed: 4214444]
- Gutierrez C., Cola M.G., Seltzer B., Cusick C. Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. J Comp Neurol. 2000;419:61–86. [PubMed: 10717640]
- Hackett T.A. Information flow in the auditory cortical network. Hear Res. 2010 doi:10.1016/j. heares. 2010.01.011. [PMC free article: PMC3022347] [PubMed: 20116421]
- Hackett T.A., de la Mothe L.A. Regional and laminar distribution of the vesicular glutamate transporter, VGluT2, in the macaque monkey auditory cortex. J Chem Neuroanat. 2009;38:106–16. [PMC free article: PMC2774764] [PubMed: 19446630]
- Hackett T.A., De La Mothe L.A., Ulbert I., Karmos G., Smiley J., Schroeder C.E. Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol. 2007a;502:924–52. [PubMed: 17444488]
- Hackett T.A., Preuss T.M., Kaas J.H. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol. 2001;441:197–222. [PubMed: 11745645]
- Hackett T.A., Smiley J.F., Ulbert I., Karmos G., Lakatos P., de la Mothe L.A., Schroeder C.E. Sources of somatosensory input to the caudal belt areas of auditory cortex. Perception. 2007b;36:1419–30. [PubMed: 18265825]
- Hackett T.A., Stepniewska I., Kaas J.H. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998a;394:475–95. [PubMed: 9590556]
- Hackett T.A., Stepniewska I., Kaas J.H. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998b;400:271–86. [PubMed: 9766404]
- Hackett T.A., Stepniewska I., Kaas J.H. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Res. 1999;817:45–58. [PubMed: 9889315]
- Halas E.S., Beardsley J.V., Sandlie M.E. Conditioned neuronal responses at various levels in conditioning paradigms. Electroencephalogr Clin Neurophysiol. 1970;28:468–77. [PubMed: 4192813]
- Hall D.A., Plack C.J. Pitch processing sites in the human auditory brain. Cereb Cortex. 2009;19:576–85. [PMC free article: PMC2638814] [PubMed: 18603609]
- Halpern D.L., Blake R., Hillenbrand J. Psychoacoustics of a chilling sound. Percept Psychophys. 1986;39:77–80. [PubMed: 3725541]
- Hammerschmidt K., Todt D. Individual-differences in vocalizations of young barbary macaques (macaca sylvanus) – a multi-parametric analysis to identify critical cues in acoustic signaling. Behaviour. 1995;132:381–99.
- Hashikawa T., Molinari M., Rausell E., Jones E.G. Patchy and laminar terminations of medial geniculate axons in monkey auditory cortex. J Comp Neurol. 1995;362:195–208. [PubMed: 8576433]
- Hauser M.D. The Evolution of Communication. Cambridge, MA: MIT Press; 1996.
- Hauser M.D., Fowler C.A. Fundamental frequency declination is not unique to human speech: Evidence from nonhuman primates. J Acoust Soc Am. 1992;91:363–69. [PubMed: 1737885]
- He J., Hu B. Differential distribution of burst and single-spike responses in auditory thalamus. J Neurophysiol. 2002;88:2152–56. [PubMed: 12364537]
- Heffner H.E., Heffner R.S. Effect of bilateral auditory cortex lesions on absolute thresholds in Japanese macaques. J Neurophysiol. 1990a;64:191–205. [PubMed: 2388065]
- Heffner H.E., Heffner R.S. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990b;64:915–31. [PubMed: 2230934]
- Hikosaka O., Sakamoto M., Usui S. Functional properties of monkey caudate neurons II. Visual and auditory responses. J Neurophysiol. 1989;61:799–813. [PubMed: 2723721]
- Hikosaka O., Wurtz R.H. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49:1230–53. [PubMed: 6864248]
- Hocherman S., Itzhaki A., Gilat E. The response of single units in the auditory cortex of rhesus monkeys to predicted and to unpredicted sound stimuli. Brain Res. 1981;230:65–86. [PubMed: 6797681]
- Holstege G. Descending motor pathways and the spinal motor system: Limbic and non-limbic components. Prog Brain Res. 1991;87:307–421. [PubMed: 1678191]
- Holstege G., Bandler R., Saper C.B. The emotional motor system. Prog Brain Res. 1996;107:3–6. [PubMed: 8782510]
- Issa E.B., Wang X. Sensory responses during sleep in primate primary and secondary auditory cortex. J Neurosci. 2008;28:14467–80. [PMC free article: PMC3844765] [PubMed: 19118181]
- Ito S.I. Prefrontal unit activity of macaque monkeys during auditory and visual reaction time tasks. Brain Res. 1982;247:39–47. [PubMed: 7127120]
- Iwata J., LeDoux J.E., Meeley M.P., Arneric S., Reis D.J. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. [PubMed: 3768689]
- Jarrell T.W., Gentile C.G., Romanski L.M., McCabe P.M., Schneiderman N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardiac conditioning to acoustic conditioned stimuli in rabbits. Brain Res. 1987;412:285–94. [PubMed: 3607469]
- Jenkins W.M., Merzenich M.M. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–47. [PubMed: 6512590]
- Jones E.G. Chemically defined parallel pathways in the monkey auditory system. Ann N Y Acad Sci. 2003;999:218–33. [PubMed: 14681146]
- Jones E.G., Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol. 1976;168:197–247. [PubMed: 821974]
- Juliano S.L., Ma W., Eslin D. Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Natl Acad Sci USA. 1991;88:780–84. [PMC free article: PMC50897] [PubMed: 1992469]
- Kaas J. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain Mind. 2000;1:7–23.
- Kaas J., Hackett T. The functional neuroanatomy of the auditory cortex. In: Doallos P., Oertel D., editors. The Senses. Amsterdam: Elsevier; 2008. pp. 765–80.
- Kaas J.H., Hackett T.A. Subdivisions of auditory cortex and levels of processing in primates. Audiol Neurootol. 1998;3:73–85. [PubMed: 9575378]
- Kaas J.H., Hackett T.A. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–99. [PMC free article: PMC34351] [PubMed: 11050211]
- Kajikawa Y., de La Mothe L., Blumell S., Hackett T.A. A comparison of neuron response properties in areas A1 and CM of the marmoset monkey auditory cortex: Tones and broadband noise. J Neurophysiol. 2005;93:22–34. [PubMed: 15342713]
- Kajikawa Y., de la Mothe L.A., Blumell S., Sterbing-D’Angelo S.J., D’Angelo W., Camalier C.R., Hackett T.A. Coding of FM sweep trains and twitter calls in area CM of marmoset auditory cortex. Hear Res. 2008;239:107–25. [PMC free article: PMC2581800] [PubMed: 18342463]
- Kelly K., Metzger R., Mulette-Gillman O., Werner-Reiss U., Groh J. Representation of sound location in the primate brain. In: Ghazanfar A., editor. Primate Audition: Ethology and Neurobiology. London: CRC Press; 2002. pp. 177–98.
- Keysers C., Kohler E., Umilta M.A., Nanetti L., Fogassi L., Gallese V. Audiovisual mirror neurons and action recognition. Exp Brain Res. 2003;153:628–36. [PubMed: 12937876]
- Kikuchi-Yorioka Y., Sawaguchi T. Parallel visuospatial and audiospatial working memory processes in the monkey dorsolateral prefrontal cortex. Nat Neurosci. 2000;3:1075–76. [PubMed: 11036261]
- Kilgard M.P., Merzenich M.M. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–18. [PubMed: 9497289]
- Komura Y., Tamura R., Uwano T., Nishijo H., Kaga K., Ono T. Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature. 2001;412:546–49. [PubMed: 11484055]
- Komura Y., Tamura R., Uwano T., Nishijo H., Ono T. Auditory thalamus integrates visual inputs into behavioral gains. Nat Neurosci. 2005;8:1203–9. [PubMed: 16116444]
- Kosmal A., Malinowska M., Kowalska D.M. Thalamic and amygdaloid connections of the auditory association cortex of the superior temporal gyrus in rhesus monkey (Macaca mulatta). Acta Neurobiol Exp (Wars). 1997;57:165–88. [PubMed: 9407703]
- Kuraoka K., Nakamura K. Responses of single neurons in monkey amygdala to facial and vocal emotions. J Neurophysiol. 2007;97:1379–87. [PubMed: 17182913]
- Kusmierek P., Rauschecker J.P. Functional specialization of medial auditory belt cortex in the alert rhesus monkey. J Neurophysiol. 2009;102:1606–22. [PMC free article: PMC2746772] [PubMed: 19571201]
- Lakatos P., Pincze Z., Fu K.M., Javitt D.C., Karmos G., Schroeder C.E. Timing of pure tone and noise-evoked responses in macaque auditory cortex. Neuroreport. 2005;16:933–37. [PubMed: 15931064]
- Langner G., Schreiner C.E. Periodicity coding in the inferior colliculus of the cat I. Neuronal mechanisms. J Neurophysiol. 1988;60:1799–1822. [PubMed: 3236052]
- LeDoux J.E., Ruggiero D.A., Forest R., Stornetta R., Reis D.J. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987;264:123–46. [PubMed: 2445791]
- LeDoux J.E., Sakaguchi A., Reis D.J. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–98. [PMC free article: PMC6564820] [PubMed: 6707732]
- Leinonen L., Hyvarinen J., Sovijarvi A.R. Functional properties of neurons in the temporo-parietal association cortex of awake monkey. Exp Brain Res. 1980;39:203–15. [PubMed: 6772459]
- Luethke L.E., Krubitzer L.A., Kaas J.H. Connections of primary auditory cortex in the New World monkey, Saguinus. J Comp Neurol. 1989;285:487–513. [PubMed: 2474584]
- Ma X., Suga N. Long-term cortical plasticity evoked by electric stimulation and acetylcholine applied to the auditory cortex. Proc Natl Acad Sci USA. 2005;102:9335–40. [PMC free article: PMC1166631] [PubMed: 15961542]
- Machado C.J., Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippo-campal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav Neurosci. 2006;120:761–86. [PubMed: 16893284]
- MacLean P.D., Newman J.D. Role of midline frontolimbic cortex in production of the isolation call of squirrel monkeys. Brain Res. 1988;450:111–23. [PubMed: 3401705]
- Maier J.X., Ghazanfar A.A. Looming biases in monkey auditory cortex. J Neurosci. 2007;27:4093–100. [PMC free article: PMC6672548] [PubMed: 17428987]
- Markowitsch H.J., Emmans D., Irle E., Streicher M., Preilowski B. Cortical and subcortical afferent connections of the primate’s temporal pole: A study of rhesus monkeys, squirrel monkeys, and marmosets. J Comp Neurol. 1985;242:425–58. [PubMed: 4086670]
- Marler P. On the origin of speech from animal sounds. In: Kavanaugh J., Cutting J., editors. The Role of Speech in Language. Cambridge: MIT Press; 1975. pp. 11–37.
- McDermott J., Hauser M.D. Nonhuman primates prefer slow tempos but dislike music overall. Cognition. 2007;104:654–68. [PubMed: 16935277]
- Merzenich M.M., Brugge J.F. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res. 1973;50:275–96. [PubMed: 4196192]
- Metzger R.R., Greene N.T., Porter K.K., Groh J.M. Effects of reward and behavioral context on neural activity in the primate inferior colliculus. J Neurosci. 2006;26:7468–76. [PMC free article: PMC6674197] [PubMed: 16837595]
- Miller L.M., Recanzone G.H. Populations of auditory cortical neurons can accurately encode acoustic space across stimulus intensity. Proc Natl Acad Sci USA. 2009;106:5931–35. [PMC free article: PMC2667094] [PubMed: 19321750]
- Molinari M., Dell’Anna M.E., Rausell E., Leggio M.G., Hashikawa T., Jones E.G. Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. J Comp Neurol. 1995;362:171–94. [PubMed: 8576432]
- Moore B. The Psychology of Hearing. Four. San Diego, CA: Academic Press; 1997.
- Morecraft R.J., Cipolloni P.B., Stilwell-Morecraft K.S., Gedney M.T., Pandya D.N. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469:37–69. [PubMed: 14689472]
- Morel A., Kaas J.H. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol. 1992;318:27–63. [PubMed: 1583155]
- Morton E.S. Ecological sources of selection on avian sounds. Am Naturalist. 1975;109:17–34.
- Nagy A., Paroczy Z., Norita M., Benedek G. Multisensory responses and receptive field properties of neurons in the substantia nigra and in the caudate nucleus. Eur J Neurosci. 2005;22:419–24. [PubMed: 16045495]
- Nauta W.J., Whitlock D.G. Subcortical projections from the temporal neocortex in Macaca mulatta. J Comp Neurol. 1956;106:183–212. [PubMed: 13398494]
- Nienhuis R., Olds J. Changes in unit responses to tones after food reinforcement in the auditory pathway of the rat: Intertrial arousal. Exp Neurol. 1978;59:229–42. [PubMed: 639917]
- Olds J., Nienhuis R., Olds M.E. Patterns of conditioned unit responses in the auditory system of the rat. Exp Neurol. 1978;59:209–28. [PubMed: 639916]
- Oleson T.D., Ashe J.H., Weinberger N.M. Modification of auditory and somatosensory system activity during pupillary conditioning in the paralyzed cat. J Neurophysiol. 1975;38:1114–39. [PubMed: 1177008]
- Pandya D.N., Hallett M., Kmukherjee S.K. Intra- and interhemispheric connections of the neocortical auditory system in the rhesus monkey. Brain Res. 1969;14:49–65. [PubMed: 4977327]
- Pandya D.N., Rosene D.L. Laminar termination patterns of thalamic, callosal, and association afferents in the primary auditory area of the rhesus monkey. Exp Neurol. 1993;119:220–34. [PubMed: 7679356]
- Pandya D.N., Rosene D.L., Doolittle A.M. Corticothalamic connections of auditory-related areas of the temporal lobe in the rhesus monkey. J Comp Neurol. 1994;345:447–71. [PubMed: 7929912]
- Petkov C.I., Kayser C., Augath M., Logothetis N.K. Functional imaging reveals numerous fields in the monkey auditory cortex. PLoS Biol. 2006;4:e215. [PMC free article: PMC1479693] [PubMed: 16774452]
- Petkov C.I., Kayser C., Steudel T., Whittingstall K., Augath M., Logothetis N.K. A voice region in the monkey brain. Nat Neurosci. 2008;11:367–74. [PubMed: 18264095]
- Petrides M., Pandya D.N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Eur J Neurosci. J Neurosci. 2002;2007;1627:291–310. 11573–86. [PubMed: 12169111]
- Peyron R., Laurent B., Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–88. [PubMed: 11126640]
- Pickles J. An Introduction to the Physiology of Hearing. Second. San Diego, CA: Academic Press; 1988.
- Poljak S. The connections of the acoustic nerve. J Anat. 1926;60:465–69.
- Polley D.B., Steinberg E.E., Merzenich M.M. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–82. [PMC free article: PMC6674159] [PubMed: 16672673]
- Poremba A., Malloy M., Saunders R.C., Carson R.E., Herscovitch P., Mishkin M. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature. 2004;427:448–51. [PubMed: 14749833]
- Poremba A., Saunders R.C., Crane A.M., Cook M., Sokoloff L., Mishkin M. Functional mapping of the primate auditory system. Science. 2003;299:568–72. [PubMed: 12543977]
- Prakash N., Cohen-Cory S., Penschuck S., Frostig R.D. Basal forebrain cholinergic system is involved in rapid nerve growth factor (NGF)-induced plasticity in the barrel cortex of adult rats. J Neurophysiol. 2004;91:424–37. [PubMed: 14507983]
- Price J.L. Connections of orbital cortex. In: Zald D., Rauch S., editors. The Orbitofrontal Cortex. New York: Oxford University Press; 2006. pp. 39–55.
- Rauschecker L.P., Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–6. [PMC free article: PMC34352] [PubMed: 11050212]
- Rauschecker L.P., Tian B. Processing of band-passed noise in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;91:2578–89. [PubMed: 15136602]
- Rauschecker J.P., Tian B., Pons T., Mishkin M. Serial and parallel processing in rhesus monkey auditory cortex. J Comp Neurol. 1997;382:89–103. [PubMed: 9136813]
- Reale R.A., Imig T.J. Auditory cortical field projections to the basal ganglia of the cat. Neuroscience. 1983;8:67–86. [PubMed: 6835523]
- Recanzone G.H. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 2000a;150:104–18. [PubMed: 11077196]
- Recanzone G.H. Spatial processing in the auditory cortex of the macaque monkey. Proc Natl Acad Sci USA. 2000b;97:11829–35. [PMC free article: PMC34356] [PubMed: 11050216]
- Recanzone G.H. Representation of con-specific vocalizations in the core and belt areas of the auditory cortex in the alert macaque monkey. J Neurosci. 2008;28:13184–93. [PMC free article: PMC2614135] [PubMed: 19052209]
- Recanzone G.H., Cohen Y.E. Serial and parallel processing in the primate auditory cortex revisited. Behav Brain Res. 2009;206:1–7. [PMC free article: PMC2783172] [PubMed: 19686779]
- Recanzone G.H., Guard D.C., Phan M.L. Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. J Neurophysiol. 2000a;83:2315–31. [PubMed: 10758136]
- Recanzone G.H., Guard D.C., Phan M.L., Su T.K. Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. J Neurophysiol. 2000b;83:2723–39. [PubMed: 10805672]
- Recanzone G.H., Schreiner C.E., Merzenich M.M. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. [PMC free article: PMC6576321] [PubMed: 8423485]
- Remedios R., Logothetis N.K., Kayser C. An auditory region in the primate insular cortex responding preferentially to vocal communication sounds. J Neurosci. 2009;29:1034–45. [PMC free article: PMC6665141] [PubMed: 19176812]
- Rendall D., Owren M.J., Rodman P.S. The role of vocal tract filtering in identity cueing in rhesus monkey (Macaca mulatta) vocalizations. J Acoust Soc Am. 1998;103:602–14. [PubMed: 9440345]
- Rendall D., Rodman P.S., Emond R.E. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim Behav. 1996;51:1007–15.
- Ringo J.L., Doty R.W., Demeter S., Simard P.Y. Time is of the essence: A conjecture that hemi spheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–43. [PubMed: 7950307]
- Roberts A.C., Tomic D.L., Parkinson C.H., Roeling T.A., Cutter D.J., Robbins T.W., Everitt B.J. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): An antero grade and retrograde tract-tracing study. J Comp Neurol. 2007;502:86–112. [PubMed: 17335041]
- Rolls E.T., Critchley H.D., Browning A.S., Inoue K. Face-selective and auditory neurons in the primate orbitofrontal cortex. Exp Brain Res. 2006;170:74–87. [PubMed: 16328289]
- Romanski L.M. Representation and integration of auditory and visual stimuli in the primate ventra lateral prefrontal cortex. Cereb Cortex. 2007;17 Suppl 1:i61–69. [PMC free article: PMC2778283] [PubMed: 17634387]
- Romanski L.M., Averbeck B.B. The primate cortical auditory system and neural representation of conspecific vocalizations. Annu Rev Neurosci. 2009;32:315–46. [PMC free article: PMC2767298] [PubMed: 19400713]
- Romanski L.M., Averbeck B.B., Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2005;93:734–47. [PubMed: 15371495]
- Romanski L.M., Bates J.F., Goldman-Rakic P.S. Auditory belt and parabelt projections to the pre frontal cortex in the rhesus monkey. J Comp Neurol. 1999;403:141–57. [PubMed: 9886040]
- Romanski L.M., Goldman-Rakic P.S. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–16. [PMC free article: PMC2793092] [PubMed: 11753413]
- Romanski L.M., LeDoux J.E. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–9. [PMC free article: PMC6575992] [PubMed: 1331362]
- Romanski L.M., LeDoux J.E. Information cascade from primary auditory cortex to the amygdala: Corticocortical and corti coamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–32. [PubMed: 7511012]
- Romanski L.M., Tian B., Fritz J., Mishkin M., Goldman-Rakic P.S., Rauschecker J.P. Dua streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosc. 1999;2:1131–36. [PMC free article: PMC2778291] [PubMed: 10570492]
- Rouiller E. Functional organization of the auditory pathways. In: Ehret G., Romand R., editors. The Central Auditory System. New York: Oxford University Press; 1997. pp. 3–65.
- Rouiller E.M., Durif C. Neurosci Lett. Vol. 358. 2004. The dual pattern of corticothalamic projection of the primary auditory cortex in macaque monkey; pp. 49–52. [PubMed: 15016432]
- Rouiller E.M., Rodrigues-Dagaeff C., Simm G., De Ribaupierre Y., Villa A., De Ribaupierre F. Functional organization of the medial division of the medial geniculate body of the cat: Tonotopic organization, spatial distribution of response properties and cortical connections. Hear Res. 1989;39:127–42. [PubMed: 2737960]
- Russ B.E., Ackelson A.L., Baker A.E., Cohen Y.E. Coding of auditory-stimulus identity in the auditory non-spatial processing stream. J Neurophysiol. 2008;99:87–95. [PMC free article: PMC4091985] [PubMed: 18003874]
- Rutkowski R.G., Weinberger N.M. Encoding of learned importance of sound by magnitude of rep resentational area in primary auditory cortex. Proc Natl Acad Sci USA. 2005;102:13664–69. [PMC free article: PMC1200094] [PubMed: 16174754]
- Ryan A., Miller J. Single unit responses in the inferior colliculus of the awake and performing rhesus monkey. Exp Brain Res. 1978;32:389–407. [PubMed: 98341]
- Ryugo D.K., Weinberger N.M. Differential plasticity of morphologically distinct neuron populations in the medical geniculate body of the cat during classical conditioning. Behav Biol. 1978;22:275–301. [PubMed: 626625]
- Saleem K.S., Kondo H., Price J.L. Complementary circuits connecting the orbital and media prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–93. [PubMed: 18067141]
- Sanes D.H., Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol. 2009;19:188–99. [PMC free article: PMC2717554] [PubMed: 19535241]
- Schrader L., Todt D. Contact call parameters covary with social-context in common marmosets Callithrix-j-jacchus. Anim Behav. 1993;46:1026–28.
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. [PubMed: 11257908]
- Scott B.H., Malone B.J., Semple M.N. Effect of behavioral context on representation of a spatial cue in core auditory cortex of awake macaques. J Neurosci. 2007;27:6489–99. [PMC free article: PMC6672434] [PubMed: 17567810]
- Seitz A., Watanabe T. A unified model for perceptual learning. Trends Cogn Sci. 2005;9:329–34. [PubMed: 15955722]
- Selemon L.D., Goldman-Rakic P.S. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–94. [PMC free article: PMC6565017] [PubMed: 2983048]
- Smiley J.F., Hackett T.A., Ulbert I., Karmas G., Lakatos P., Javitt D.C., Schroeder C.E. Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol. 2007;502:894–923. [PubMed: 17447261]
- Smith W. The functional significance of the rostral cingular cortex as revealed by its responses to electrical excitation. J Neurophysiol. 1945;8:241–55.
- Starr A., Don M. Responses of squirrel monkey (Samiri sciureus) medial geniculate units to binaural click stimuli. J Neurophysiol. 1972;35:501–17. [PubMed: 4624738]
- Steinfels G.F., Heym J., Strecker R.E., Jacobs B.L. Response of dopaminergic neurons in cat to auditory stimuli presented across the sleep-waking cycle. Brain Res. 1983;277:150–54. [PubMed: 6640288]
- Strecker R.E., Steinfels G.F., Abercrombie E.D., Jacobs B.L. Caudate unit activity in freely moving cats: Effects of phasic auditory and visual stimuli. Brain Res. 1985;329:350–53. [PubMed: 3978457]
- Sudakov K., MacLean P.D., Reeves A., Marino R. Unit study of exteroceptive inputs to claustro-cortex in awake, sitting, squirrel monkey. Brain Res. 1971;28:19–34. [PubMed: 4997565]
- Sugihara T., Diltz M.D., Averbeck B.B., Romanski L.M. Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J Neurosci. 2006;26:11138–47. [PMC free article: PMC2767253] [PubMed: 17065454]
- Symmes D., Alexander G.E., Newman J.D. Neural processing of vocalizations and artificial stimuli in the medial geniculate body of squirrel monkey. Hear Res. 1980;3:133–46. [PubMed: 7419482]
- Tian B., Rauschecker J.P. Processing of frequency-modulated sounds in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;92:2993–3013. [PubMed: 15486426]
- Tian B., Reser D., Durham A., Kustov A., Rauschecker J.P. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–93. [PubMed: 11303104]
- Trojanowski J.Q., Jacobson S. A combined horseradish peroxidase-autoradiographic investigation of reciprocal connections between superior temporal gyrus and pulvinar in squirrel monkey. Brain Res. 1975;85:347–53. [PubMed: 1111845]
- Ungerleider L., Mishkin M. Two cortical visual systems. In: Ingle D.J., Goodale M.A., Mansfield R.J.W., editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–86.
- Vogt B.A., Pandya D.N. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–89. [PubMed: 3624555]
- Wang X., Lu T., Bendor D., Bartlett E. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience. 2008;157:484–94. [PubMed: 19143093]
- Wang X., Merzenich M.M., Beitel R., Schreiner C.E. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: Temporal and spectral characteristics. J Neurophysiol. 1995;74:2685–706. [PubMed: 8747224]
- Weinberger N., Ashe J., Metherate R., McKenna T., Diamond D., Bakin J. Retuning auditory cortex by learning: A preliminary model of receptive field plasticity. Concepts Neurosci. 1990;1:91–132.
- Weinberger N.M. Physiological memory in primary auditory cortex: Characteristics and mechanisms. Neurobiol Learn Mem. 1998;70:226–51. [PubMed: 9753599]
- Weinberger N.M. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–84. [PubMed: 14521869]
- Weinberger N.M. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–90. [PMC free article: PMC3590000] [PubMed: 15034553]
- West C.D. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am. 1985;77:1091–1101. [PubMed: 3980863]
- Wiley R.H., Richards D.G. Physical constraints on acoustic communication in atmosphere – implications for evolution of animal vocalizations. Behav Ecol Sociobiol. 1978;3:69–94.
- Wilson F.A., Rolls E.T. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Res. 1990;509:213–31. [PubMed: 2322819]
- Winer J.A. Decoding the auditory corticofugal systems. Hear Res. 2005;207:1–9. [PubMed: 16091301]
- Winer J.A., Chernock M.L., Larue D.T., Cheung S.W. Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey. Hear Res. 2002;168:181–95. [PubMed: 12117520]
- Winer J.A., Larue D.T., Diehl J.J., Hefti B.J. Auditory cortical projections to the cat inferior colliculus. J Comp Neurol. 1998;400:147–74. [PubMed: 9766397]
- Winer J.A., Lee C.C. The distributed auditory cortex. Hear Res. 2007;229:3–13. [PMC free article: PMC2637155] [PubMed: 17329049]
- Winer J.A., Wenstrup J.J., Larue D.T. Patterns of GABAergic immunoreactivity define subdivisions of the mustached bat’s medial geniculate body. J Comp Neurol. 1992;319:172–90. [PubMed: 1592903]
- Woods T.M., Lopez S.E., Long J.H., Rahman J.E., Recanzone G.H. Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey. J Neurophysiol. 2006;96:3323–37. [PubMed: 16943318]
- Yeterian E.H., Pandya D.N. Corticostriatal connections of the superior temporal region in rhesus monkeys. J Comp Neurol. 1998;399:384–402. [PubMed: 9733085]
- Zatorre R.J., Evans A.C., Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci. 1994;14:1908–19. [PMC free article: PMC6577137] [PubMed: 8158246]
- Nonhuman primates prefer slow tempos but dislike music overall.[Cognition. 2007]Nonhuman primates prefer slow tempos but dislike music overall.McDermott J, Hauser MD. Cognition. 2007 Sep; 104(3):654-68. Epub 2006 Aug 28.
- Review A primer of primate pathology: lesions and nonlesions.[Toxicol Pathol. 2003]Review A primer of primate pathology: lesions and nonlesions.Lowenstine LJ. Toxicol Pathol. 2003 Jan-Feb; 31 Suppl:92-102.
- [Dissociations between music and language functions after cerebral resection: A new case of amusia without aphasia].[Can J Exp Psychol. 1997][Dissociations between music and language functions after cerebral resection: A new case of amusia without aphasia].Peretz I, Belleville S, Fontaine S. Can J Exp Psychol. 1997 Dec; 51(4):354-68.
- Review Music and language: relations and disconnections.[Handb Clin Neurol. 2015]Review Music and language: relations and disconnections.Kraus N, Slater J. Handb Clin Neurol. 2015; 129:207-22.
- Review Primate vocal communication: a useful tool for understanding human speech and language evolution?[Hum Biol. 2011]Review Primate vocal communication: a useful tool for understanding human speech and language evolution?Fedurek P, Slocombe KE. Hum Biol. 2011 Apr; 83(2):153-73.
- Sound - Neurobiology of Sensation and RewardSound - Neurobiology of Sensation and Reward
- PREDICTED: Bombus terrestris RNA-binding protein Pasilla (LOC100651952), transcr...PREDICTED: Bombus terrestris RNA-binding protein Pasilla (LOC100651952), transcript variant X1, mRNAgi|2245585471|ref|XM_003395519.4|Nucleotide
- Profile neighbors for GEO Profiles (Select 77906011) (151)GEO Profiles
- Profile neighbors for GEO Profiles (Select 77909746) (200)GEO Profiles
- Chromosome neighbors for GEO Profiles (Select 77909751) (20)GEO Profiles
Your browsing activity is empty.
Activity recording is turned off.
See more...
