NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014.
13.1. INTRODUCTION
Adult neurogenesis is a striking form of neural plasticity occurring in restricted regions of the mammalian brain. The past decades have witnessed tremendous research efforts in this field providing significant information regarding the anatomical, molecular, and functional mechanisms underlying neurogenesis in the adult brain. New neuron production regulates integrated brain functions, learning and memory, and adapts the brain to the changing world. Recent data in rodents indicates a link between adult neurogenesis and reproductive and social behavior. This provides the opportunity to unravel the function of this form of neural plasticity in ethologically relevant contexts and opens new perspectives to explore how the brain processes social stimuli. In this chapter we will summarize some of the major key points regarding the cues and mechanisms modulating adult neurogenesis during social interaction and possible role/s played by newborn neurons in this context. To achieve this goal we will give an overview of past and ongoing literature showing this link, with particular emphasis on our recent studies on two examples of sexual behavior: mate pheromonal imprinting in female mice, and paced mating in rats.
The early conception of the function of the brain postulated that once the brain developed it became stable and no new neurons were added in adulthood. This dogma has gradually been dropped over the past 40 years with the clear demonstration that adult neurogenesis is a striking form of structural remodeling characterizing the brain of vertebrates, though with significant differences between groups (Lindsey and Tropepe 2006; Bonfanti and Peretto 2011). In the 1970s, the issue of adult neurogenesis was regarded with skepticism although during the previous decade some proliferative activity was reported in the brain by Altman and colleagues (Altman 1963; Altman and Das 1965). Only years later, with the progress of neuroanatomical techniques and the demonstration of genesis and integration of new neurons in the adult brain of canaries (Nottebohm 1985), adult neurogenesis regained attention.
A new era in this field occurred starting from two simultaneous findings: the occurrence of a massive cell migration toward the rodents’ olfactory bulb (Luskin 1993; Lois and Alvarez-Buylla 1994) and the first isolation of adult neural stem cells (Reynolds and Weiss 1992). These studies strengthened the idea that neural plasticity in adult mammals not only occurs through synaptic remodeling but also through the addition of new neurons in the mature preexisting circuits. During the last two decades, this intriguingly persisting process in the mammalian brain was intensely investigated, and several review articles progressively made the point on its extension, features, and significance under physiological and pathological conditions (Emsley et al. 2005; Sohur et al. 2006; Gould 2007; Migaud et al. 2010; Bonfanti and Peretto 2011; Curtis et al. 2011; Fuentealba et al. 2012).
It is now clear that a constitutive/physiologic neurogenesis in adult mammals mostly occurs within two telencephalic regions, the subventricular zone–olfactory bulb system (SVZ-OB) (Lois and Alvarez-Buylla 1994) and the subgranular zone (SGZ) of dentate gyrus (DG) of the hippocampus (Kempermann et al. 2004). The neurogenic process in these regions is orchestrated by a complex interplay between intrinsic and extrinsic environmental cues. Several developmental signals, morphogens, growth factors, neurotransmitters, hormones, transcription factors, and epigenetic regulators have been described to tightly regulate specification and activity of proliferating progenitors, as well as the migration and integration of neuronal precursors within functional circuits (Hagg 2005; Faigle and Song 2013). The signaling mechanisms supporting adult neurogenesis are dynamically regulated by many environmental cues that can either positively or negatively influence the neurogenic process at the level of progenitor cells and during the integration of newborn neurons within circuits (Ma et al. 2009). This activity-dependent regulation is only beginning to be unraveled. Importantly, adult-born neurons in neurogenic regions exhibit critical periods of plasticity during a specific time window of their maturation (Nissant et al. 2009; Ming and Song 2011), and high responsiveness toward the same stimuli driving their integration/selection into circuits (Magavi et al. 2005; Kee et al. 2007). This supports a role of newborn neurons in sensory processing in DG and OB. Accordingly, several sources of data indicate that adult neurogenesis contributes to mechanisms of learning and memory (Lazarini et al. 2009; Moreno et al. 2009), and more recent hypotheses suggest that it also contributes to enhancing pattern separation (Aimone et al. 2011; Sahay et al. 2011). In this view, the continuous addition of new neurons in the olfactory bulb and hippocampus rather than being a simple mechanism of renewal of preexisting cells expands the capacity for plasticity in these regions.
In this chapter we will describe recent findings (see Feierstein et al. 2012 for review) that link reproductive and social stimuli to adult neurogenesis, particularly in the olfactory system. We will address first a brief description of the main results supporting this connection, and then, taking into account our recent work (Oboti et al. 2009, 2011; Corona et al. 2011; Portillo et al. 2012), we will focus on two striking examples of sexual behavior: (1) mate pheromonal imprinting to avoid pregnancy block in female mice and (2) paced mating in rats, namely the ability to control the sexual interaction. In both cases we demonstrate a link between adult neurogenesis and social activities underlying the reproductive function.
13.2. REPRODUCTIVE AND SOCIAL BEHAVIOR MODULATES ADULT NEUROGENESIS
A growing number of studies indicates that reproductive and social behavior modulates adult neurogenesis (Smith et al. 2001; Huang and Bittman 2002; Shingo et al. 2003; Mak et al. 2007; Larsen et al. 2008; Ruscio et al. 2008; Oboti et al. 2009; Furuta and Bridges 2009; Feierstein et al. 2010; Mak and Weiss 2010; Corona et al. 2011; Oboti et al. 2011; Sakamoto et al. 2011; Portillo et al. 2012; Brus et al. 2013). This provides the opportunity to unravel the function of this form of adult neural plasticity in ethologically relevant contexts, and in parallel, the chance to investigate how sensory stimuli underlying reproductive and social behavior are processed in the adult brain. Although several unanswered questions on the link between reproductive/social stimuli and adult neurogenesis remain to be clarified, some major/common key points can be extrapolated from the current data.
Pheromonal cues in rodents convey information about species-specificity, gender, social status, health, genetic advantage and individual recognition (see Tirindelli et al. 2009 for review). Accordingly, they have been demonstrated as major stimuli to enhance neurogenesis in both the olfactory bulb region and hippocampus (Mak et al. 2007; Larsen et al. 2008; Oboti et al. 2009, 2011; Mak and Weiss 2010). Nevertheless, other sensory stimuli/pathways are potentially involved in the modulation of adult neurogenesis occurring during reproductive and social experiences. Indeed, neurogenesis is also enhanced in pregnancy or pseudopregnancy and lactation (Shingo 2003; Larsen 2010) in male mice upon interaction with their offspring (Mak and Weiss 2010) and during pacing behavior (Corona et al. 2011; Portillo et al. 2012). These conditions and activities are driven by multiple sensory pathways and involve complex levels of brain integration and elaboration.
A direct contact (fully exploration of pheromonal cues and/or pairing) between subjects or with the stimulus (bedding or urine) is necessary to affect adult neurogenesis (Huang and Bittman 2001; Smith et al. 2001; Shingo et al. 2003; Mak et al. 2007; Larsen et al. 2008; Ruscio et al. 2008; Furuta and Bridges 2009; Oboti et al. 2009; Brus et al. 2010; Feierstein et al. 2010; Mak and Weiss 2010; Corona et al. 2011; Oboti et al. 2011; Sakamoto et al. 2011; Portillo et al. 2012). This implies activation of both the main olfactory and vomeronasal systems, which cooperate in the control of social/reproductive behavior (Keller et al. 2006). However, their relative contribution to adult neurogenesis in different social contexts still remains largely unexplored.
Interestingly, reproductive/social stimuli affect neurogenesis at two different levels of the neurogenic process, increasing proliferation of progenitor cells in the neurogenic niches and survival/integration of newborn neurons within the functional circuits. The anterior pituitary hormone prolactin (PRL) appears as a key factor promoting proliferation of SVZ progenitors during social interaction (Shingo et al. 2003; Mak et al. 2007; Larsen et al. 2008, 2010; Mak and Weiss 2010). Such enhanced proliferation per se results in increased incorporation of newborn neurons into the olfactory bulb circuits 15–20 days after the social experience (Shingo et al. 2003; Mak et al. 2007; Larsen et al. 2008). PRL in female rodents rises during mating (Erskine 1995), pregnancy and lactation (Grattan and Kokay 2008) and after prolonged exposure to male pheromones (Larsen 2008). This is crucial for adaptation and survival of the mother and fetus during pregnancy and postpartum period (Grattan and Kokay 2008). Thus, based on these functions it has been proposed that PRL-induced neurogenesis in the maternal brain early in gestation favors the parental care (Larsen et al. 2010). Similarly, PRL also increases neurogenesis in the paternal brain, possibly influencing paternal offspring recognition (Mak and Weiss 2010). Accordingly, inactivation of the PRL-enhanced neurogenesis seems to negatively affect some aspects of the parental behavior (Larsen et al. 2010; Mak and Weiss 2010), although conflicting results have been obtained after disruption of olfactory bulb neurogenesis via x-ray irradiation (Feierstein et al. 2010) or through genetically targeted ablation of newborn neurons (Sakamoto et al. 2011). Further work needs to clarify how PRL acts to stimulate proliferation in adult SVZ neurogenic niche (see for example Mak et al. 2007; Larsen et al. 2008), and to explore the involvement of other predictable factors influencing/mediating such activity during social interaction (see the following paragraphs describing the putative role of opioids released in pacing behavior).
As mentioned above, social stimuli and in particular pheromonal cues can also promote adult neurogenesis favoring the survival/integration of newborn neurons during a critical time window of their maturation. This sensory-driven activity, as detailed in the next paragraphs, appears independent from the proliferative effect exerted on progenitor cells and it is prominent in the accessory olfactory bulb (AOB) of female mice. Importantly, in this region newborn neurons are preferentially activated (show higher level of c-Fos expression) shortly after their integration by the same pheromonal cues that enhance their survival (Oboti et al. 2011). This supports a rapid functional recruitment of these cells in circuits activated by the social experience. Accordingly, depletion of these “young and excitable” neurons leads to abnormal social interaction between sexes (Feierstein et al. 2010; Sakamoto et al. 2011) and inability to recognize the mating partner (Oboti et al. 2011). Thus, during reproductive and social experiences neurogenesis increases through a double mechanism: (1) enhancing proliferation of progenitor cells, which later on provide a pool of newborn neurons potentially involved in the parental behavior, and (2) favoring survival of integrating neurons, which are rapidly involved in mechanisms of individual/partner recognition. Although further analyses are needed to clarify this mechanism, it appears committed to optimize reproductive success.
In the following paragraphs we will give two striking examples showing (1) how social stimuli/interaction can influence neurogenesis affecting SVZ progenitors proliferation and survival of newborn neurons, (2) how this process significantly increases incorporation of new cells in the AOB of rodents, and (3) how newborn neurons in the AOB of female mice are involved in processing male pheromonal cues.
13.3. ROLE OF AOB NEWBORN NEURONS IN THE PHEROMONAL MATING-INDUCED IMPRINTING
13.3.1. Olfactory Pregnancy Block in Mice
In mice, when a recently mated female is exposed to chemosignals from an unfamiliar male, a neuroendocrine reflex leads the female to pregnancy block and in turn a return to estrous.
This reflex known as the Bruce effect (Bruce 1959) is one of the best-known examples of behavior driven by pheromones that involves the vomeronasal system (Brennan and Zufall 2006). Indeed, the Bruce effect is mediated by vomeronasal (VN) excitatory projections from the accessory olfactory bulb to the medial amygdala (MeA), the bed nucleus of the stria terminalis, the medial hypothalamus, and ultimately the dopaminergic neurons of the arcuate nucleus that control prolactin release by the anterior pituitary (Li et al. 1989). Prolactin in mice is luteotrophic and its dopaminergic inhibition, mediated by the arcuate nucleus during critical postmating stages, prevents blastocyst implantation (Brennan 2009). Pregnancy block depends on male chemosensory cues contained in urine since both direct exposure to male soiled bedding or application of male urine to the nose of a recently mated female induces pregnancy failure (Rosser et al. 1989; Leinders-Zufall et al. 2004). Notably, exteroceptive estrous induction is lost by stud-male odors through enhancement of granule-to-mitral synaptic inhibition occurring in the AOB (see also Chapter 11) during a sensitive period around mating (Brennan et al. 1990; Matsuoka et al. 1997). This male-specific pheromone recognition/imprinting process involves a restricted pool of granule cells in the AOB, which actually inhibits mitral cell signal transmission to the forebrain areas involved in estrous induction for several weeks (50–60 days) (Brennan et al. 1990; Matsuoka et al. 1997). Long-term maintenance of this inhibition implies that, since pairings may occur at shorter intervals, different or partially overlapping cohorts of cells may be necessary to each imprinting process. These features drove us to hypothesize a role of adult olfactory bulb neurogenesis in the Bruce effect since our early studies showing that SVZ-derived neuroblasts also reach the AOB (Bonfanti et al. 1997; Peretto et al. 1999). Indeed, adult neurogenesis continuously refills this region with pools of young newborn inhibitory interneurons that show unique functional properties such as enhanced synaptic plasticity and increased responsiveness to recently experienced odors (Magavi et al. 2005; Nissant et al. 2009).
13.3.2. Male Pheromones Affect Integration of Newborn Neurons in the AOB of Adult Female Mice
Preliminary investigation of olfactory bulb neurogenesis in adult mice confirmed that a subpopulation of SVZ-derived neuroblasts acquires proper neurochemical and morphological profiles of mature inhibitory GABAergic interneurons in the AOB of both sexes (Figure 13.1) (Oboti et al. 2009). This data definitely demonstrated the AOB, just as the MOB and the hippocampus, represents a site of constitutive adult neurogenesis. Then, we showed that chronic exposure (28 days) to male soiled bedding, which contains semiochemicals present in urine and exocrine glands secretion (Brennan and Keverne 2004), significantly increases the number of new neurons in the AOB of adult females (Oboti et al. 2009). This effect was elicited only by direct contact with male bedding and not by its volatile compounds, thus supporting such experience-dependent regulation of neurogenesis requires vomeronasal organ activity.

FIGURE 13.1
Adult-generated SVZ-derived neurons in the AOB of mice: morphological analysis. 3-D reconstruction of EGFP-positive SVZ-derived precursors at 60 days after homotopic engraftment. EGFP-positive cells can be observed in the AOB layers and in the MOB GrL. (more...)
One major point was to clarify whether AOB-enhanced neurogenesis induced by long-term exposure to male pheromones depends on early proliferative effects on SVZ progenitors, as shown in other studies (Shingo et al. 2003; Mak et al. 2007; Larsen et al. 2008), or by increased survival of newborn neurons. According to the occurrence of critical periods for sensory experience-dependent survival of newly generated granule cells in MOB (Petreanu and Alvarez-Buylla 2002; Yamaguchi and Mori 2005), we found that 1-week-long familiarization/exposure to male bedding/urine enhances survival of newborn neurons during their selection/integration within circuits (affecting cells aged between 7 and 14 days) (Figure 13.2a). This effect was most prominent in the granule cell layer of the AOB and much weaker in the MOB. In addition, it was restricted to postpubertal females (absent in prepubertal females or in adult males) (Figure 13.2b) (Oboti et al. 2011). This indicated that neuronal integration in the AOB of adult female mice tightly correlates with the activity elicited in this region by exposure to male odors.
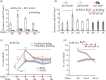
FIGURE 13.2
Sensory-driven integration and function of newborn neurons in the AOB of adult female mice. (a) Quantification of BrdU-labeled cells at 28 dpi in multiple AOB layers of p45 female mice after familiarization with male bedding performed in four different (more...)
13.3.3. AOB Newborn Neurons Are Functionally Recruited by Individual Male Odors
In order to establish whether newborn neurons in the AOB could represent a competent cellular substrate responding specifically to male individual odors, we combined immunostaining for c-Fos as a marker for neuronal excitation (Morgan et al. 1987) and BrdU labeling. Such an approach was previously used to visualize the functional recruitment of newborn cells in defined sensory tasks in the main olfactory bulb and hippocampus (Magavi et al. 2005; Kee et al. 2007). We found that the percentage of c-Fos/BrdU coexpression induced by familiar (experienced for 1 week) versus unfamiliar (never experienced before) male soiled bedding was significantly higher in AOB newborn cells aged 3 weeks (Figure 13.2c). Moreover, this activity was transient since it started to vanish after 7 days (Figure 13.2d) (Oboti et al. 2011). These results indicate that, in female mice, AOB newborn granule cells are functionally recruited by male pheromones soon after their integration and preferentially by experienced male cues. The transient nature of this activation is consistent with a privileged involvement of young newborn neurons in the elaboration of specific chemosensory signals. Interestingly, exposure to experienced male cues, but not unfamiliar ones, elicited attenuated responses in nuclei of the VNS involved in estrous induction such as the medial hypothalamus, medial amygdala, and arcuate nucleus (Oboti et al. 2011). This supported the theory that the enhanced response shown by AOB new neurons to male individual odors could impact on the forebrain activity induced by these cues.
13.3.4. Molecules and Pathways Affecting Sensory-Driven Survival of AOB Newborn Neurons
In order to investigate the nature of the cues affecting enhanced integration of newborn neurons in the AOB, we employed molecules present in the low molecular weight (LMW) fraction of urine (containing small organic molecules and small peptides) as well as high molecular weight (HMW) major urinary proteins (MUPs) contained in the HMW fraction) (Brennan and Zufall 2006). We found that urine deprived of MUPs by protease treatment was still capable of inducing newborn cell survival in the AOB. Accordingly, the HMW fraction of male urine treated with menadion to deprive it from all volatile ligands (Chamero et al. 2007) and loaded onto female LMW urine fraction to stimulate investigation was uneffective. Importantly, these effects were specific for the AOB and not MOB (Oboti et al. 2011). These results indicated the cues affecting adult neurogenesis in the AOB of females mice are comprised in the LMW fraction of male urine, which is primarily important for mate recognition/Bruce effect (Peele et al. 2003; Leinders-Zufall et al. 2004). Pheromones contained in the LMW fraction of male urine are sensed by both the main and accessory olfactory systems (Brennan and Zufall 2006). Therefore, to investigate which way male olfactory cues influence AOB neurogenesis in female mice, we compared the effects of genetic deletion of the Trpc2 cation channel (which leads to impaired VN function (Leypold et al. 2002) with lesions of the MOE caused by intranasal irrigation of zinc sulfate (ZnSO4) (McBride et al. 2003). We found that enhanced AOB granule cell survival was absent in trpc2-/- mice. By contrast, ZnSO4 lesions of the MOE did not abolish enhanced neuronal survival in the AOB, confirming that vomeronasal contact is necessary and sufficient to increase new AOB granule cells. Since AOB activity can also be induced by centrifugal inputs from the medial amygdala, even in absence of VNO stimulation (Pankevich et al. 2006; Martel and Baum 2009), we delivered excitotoxic lesions to the MeA by injections of ibotenic acid (Chauveau et al. 2008), a glutamatergic agonist, and stimulated both lesioned and sham-lesioned mice with male bedding. Exposures increased cell survival in the AOB of sham-lesioned mice but not in lesioned ones. Notably, granule cell survival in the MOB was unaffected by either bedding exposure or lesioning with ibotenic acid (Oboti et al. 2011). Together these results indicated that male pheromones trigger integration of new AOB granule cells through VN centripetal and centrifugal sensory activity.
13.3.5. Functional Role of AOB Newborn Neurons
Sensory mechanisms and molecular cues driving survival and functional recruitment of newborn granule cells in the AOB of female mice support that adult neurogenesis in this region could be involved in the mate pheromonal imprinting. To test this hypothesis, we blocked the renewal of adult-born interneurons by administrating the antimitotic drug Ara-C using osmotic minipumps, as previously done in other studies (Breton-Provencher et al. 2009). After a 4-week-long delivery of the antimitotic drugs (a period of time covering the pick responsiveness to familiar stimuli of AOB newborn neurons), which completely eliminated newborn cells in both the main and accessory olfactory bulbs, we tested the ability of adult female mice to recognize their mating partners in order to avoid exteroceptive implantation failure (Figure 13.2e). In other words, we tested whether Ara-C-treated females exposed during the postmating critical period (3 days after the beginning of mating in coincidence with the prolactin peaks) (Peele et al. 2003; Leinders-Zufall et al. 2004) to the same partners undergo pregnancy block.
In contrast to saline-treated females, we found a high rate of pregnancy failure in Ara-C-treated mice, meaning the treatment switched the effect of familiar odor to that of an unfamiliar one. To rule out that this effect of Ara-C was caused by induced infertility, we analyzed the pregnancy failure rate after Ara-C treatment without subsequent exposure to familiar odor. In this case, the pregnancy failure rate was low, indicating that the high pregnancy failure was due to odor exposure after mating and not to Ara-C-induced infertility. These experiments demonstrated that ablation of bulbar neurogenesis compromises the formation of the stud male olfactory memory in female mice. To rule out a potential involvement of MOB newborn neurons in this memory, we tested Ara-C-treated females after surgical lesion of the vomeronasal nerves, a condition known to eliminate exteroceptive pregnancy block alone (Bellringer et al. 1980; Matsuoka et al. 2005). This procedure was sufficient to prevent the high rate of pregnancy block by stud male exposure (Oboti et al. 2011), further supporting the key role of AOB newborn interneurons in this process (Figure 13.2f).
13.4. PACED MATING AND NEUROGENESIS IN THE AOB OF ADULT RATS
13.4.1. Mating Behavior in Rats
As should be evident by now, it is clear that pheromones induce neurogenesis in the adult brain of mammals and that these neurons can be involved in the modulation of reproductive and social behavior. In the following section we will describe another type of stimuli that also induces neurogenesis in the olfactory bulb and which is also crucial for reproduction and paced mating. There are only a few studies that have evaluated the effects of sexual behavior on neurogenesis and most of them have been done in rats. A detailed description of mating behavior is beyond the scope of this chapter but some important aspects will be briefly described. In rats, mating consists of a series of stereotyped behavioral patterns that are easily distinguishable. In the case of the male, they display several mounts and intromissions that would eventually lead to ejaculation. If the female is receptive she would display proceptive behaviors characterized by ear wiggling, hoping, and darting. Another important component of the behavior displayed by the female is the receptive posture in response to a mount from the male (a detailed description of the rat male and female sexual behavior can be found in Blaustein and Erskine 2002 and Meisel and Sachs 1994). A key factor in the sexual interaction is the ability that the females have to space the sexual interaction depending on the stimulation they receive. The vaginal stimulation that the female receives during mating is more intense after ejaculation than after intromission and mounts (Erskine 1989). Classic studies have shown that if given the opportunity, females will escape from the male side with a higher frequency after ejaculation than intromission and mounts. The possibility to space or control the sexual interaction is known as paced mating (Erskine 1989) and this is what usually occurs when rats mate in seminatural and natural conditions (Robitaille and Bouvet 1976; McClintock and Adler 1978; McClintock and Anisko 1982; McClintock et al. 1982).
Mating in rats is highly promiscuous and it occurs in groups where several males and females mate at the same time. In this way a female may receive several mounts, intromissions, and ejaculations from different males. As well, males could display mounts, intromissions, and ejaculations with several females (McClintock and Anisko 1982) and hence both males and females pace the sexual interaction (see Martinez and Paredes 2001 for a discussion). The possibility to pace the sexual interaction has several physiological and behavioral advantages over nonpaced mating (Erskine and Baum 1982; Erskine 1989; Erskine et al. 1989; Paredes and Alonso 1997; Paredes and Vazquez 1999). For example, only when males and females pace the sexual interaction does sexual behavior induce a reward state as evaluated by conditioned place preference (Paredes and Vazquez 1999; Martinez and Paredes 2001; Paredes 2009) that assures that the behavior will be repeated in the future. Under laboratory conditions paced mating can be easily observed when the mating cage is divided by a partition, with a hole in the bottom large enough to allow the female, but not the male, to move back and forth to the compartment in which the male is confined (Erskine 1989). In this way the female controls or paces the sexual interactions; when the animals mate without the partition the male controls the sexual interaction. In the following section we will describe how our studies suggest that paced mating, but not nonpaced mating, induces an increase in the number of new neurons in the adult brain by affecting proliferation of SVZ progenitors.
13.4.2. Female Sexual Behavior and Neurogenesis
Few studies have evaluated the effects of mating on neurogenesis (see Table 13.1). One of the first studies that evaluated if the new cells generated in the adult are activated during sexual behavior was done in hamsters. For that purpose male hamsters were injected with BrdU either 10 days, 3 weeks, or 7 weeks before they were allowed to mate with a receptive female. After 90 minutes of mating subjects were sacrificed and c-Fos expression and BrdU labeling analyzed. The new neurons in the olfactory bulbs expressed Fos in those males injected with the synthetic marker 3 and 7 weeks before mating. No significant increase in the number of double-labeled neurons was observed when male hamsters were exposed to female hamster vaginal secretions, to an aggressive male, or to peppermint odor, suggesting that neurons born in adulthood are incorporated in the functional circuits that control mating behavior in the hamster (Huang and Bittman 2002).
TABLE 13.1
Studies That Have Evaluated Cell Proliferation and Neurogenesis with Different Aspects of Mating
Since the olfactory bulbs and the processing of chemosensory cues are crucial for a successful reproduction we decided to investigate if mating itself can induce the formation of new neurons in the adult olfactory bulb region. In the first study ovariectomized females, hormonally primed to induce sexual behavior, were tested under different behavioral conditions. One group of females was exposed to a sexually active male, another group of females mated in a condition where they were not able to pace the sexual interaction, and one group of females that paced the sexual interaction. All females received BrdU injections 1 hour before, immediately after, and 1 hour after the behavioral tests and sacrificed 15 days later. This would let us determine the survival of neurons produced around the time of mating. We observed a significant increase in the density of BrdU-positive cells in the granular layer of AOB when females were allowed to pace the sexual interaction in comparison to the other groups. No differences in cell density in the main olfactory bulb were found. These results indicate that pacing behavior promotes an increase in SVZ proliferation that in turn leads to a higher density of the new cells in the accessory olfactory bulb (Corona et al. 2011). This effect is specifically associated with the ability of controlling and pacing the sexual interaction since nonpaced mating did not induce changes in cell density. As well, the increase in cell density is not associated with different levels of estradiol and progesterone or behavioral differences because all groups had the same hormone (Arzate et al. 2013) and behavioral levels.
In a followup study we tested if the repetition of the stimulus could increase the number of new neurons in the olfactory bulbs after the first sexual encounter. For that purpose females were randomly assigned to one of the following groups: (1) females without sexual contact, (2) females that were given one session of paced mating, (3) females given four sessions of nonpaced mating, and (4) females given four sessions of paced mating. Some sections were analyzed for BrdU immunohistochemistry and others were double-labeled with immunofluorescence for BrdU and NeuN to label mature neurons. As in our previous experiment all groups were injected with BrdU 1 hour before, immediately after, and 1 hour after the behavioral tests. Females were sacrificed 15 days later and the density of new neurons was analyzed in the olfactory bulbs. The results of this experiment further confirmed our previous result; that is, the females that paced the sexual interaction in one session showed a higher number of mature (NeuN-positive) neurons in the granular cell layer of the AOB. The group that mated four times also had a higher number of neurons in the granular cell layer. Moreover, the group that mated four times pacing the sexual interaction also showed a significant increase in the number of neurons in the granular layer of the MOB (Arzate et al. 2013). It is clear then that paced mating induces plastic changes that eventually result in an increase in the number of new neurons in the OB 15 days after the first mating encounter. If the female mates once the increase is observed in the granular cell layer of the AOB, and if the stimulation is repeated an increase in the number of neurons is observed in the AOB and the MOB.
In our design females were sacrificed 15 days after copulation and BrdU injection, suggesting that a higher number of neurons survive the 2-week period. As already described, there is clear evidence indicating that pheromones induce cell proliferation in the adult SVZ. For example, female prairie voles exposed to a sexually mature male in tests where they could smell, see, and hear but had limited physical contact with the male for 48 hours showed an increase in the proliferation of new cells with a neuronal phenotype in the SVZ. Ovariectomized females exposed to males or intact females exposed to females showed no increase in BrdU labeling (Smith et al. 2001). It has also been shown that pheromones of the preferred dominant male stimulate cellular proliferation in the SVZ and neuronal production in the MOB (Mak et al. 2007). Since we sacrificed the females 15 days after copulation, it is possible that the time frame to observe differences in the number of cells in the SVZ had passed. Therefore, studies are now underway to evaluate cell proliferation in the SVZ and the rostral migratory stream (RMS) after exposure to sexually relevant olfactory cues and after mating. Preliminary data indicates that 2 days after females are exposed to a sexually experienced male or to amyl acetate there is a significant increase in the number of BrdU labeled cells in the SVZ (Paredes et al. 2013, manuscript in preparation). These results could indicate that in our experimental conditions, 1 hour of exposure to male pheromones or to amyl acetate is sufficient to induce cell proliferation in the SVZ. These results are in agreement with observations indicating that after the new neurons area born, their survival is activity-dependent (Alonso et al. 2006; Mandairon et al. 2006; Lazarini et al. 2009; Moreno et al. 2009). For example, it has been shown that a spaced learning paradigm as opposed to a massed paradigm increased the survival of adult-born neurons in the olfactory bulb, allowing long-term consolidation of the olfactory task (Kermen et al. 2010). As well, discrimination learning increases the number of newborn neurons in the adult OB, prolonging their survival (Alonso et al. 2006). In male mice the prolonged exposure (40 days) to an odor-enriched environment increases the number of new cells in the glomerular cell layer of the MOB, facilitating odor discrimination (Rochefort et al. 2002; Rochefort and Lledo 2005). It is also documented that the survival of newborn cells is significantly reduced in mice unilaterally deprived from sensory input by naris occlusion, suggesting that the survival of the new cells in the OB depends on sensory input (Mandairon et al. 2006). Future studies will need to determine if repeated exposures to male pheromones or to amyl acetate increases the survival of the cells observed in the SVZ. We also need to determine the functional integration of the BrdU-labeled neurons 45 days after paced mating once the new neurons integrate into functional circuits.
13.4.3. Male Sexual Behavior and Neurogenesis
The first studies that evaluated if sexual behavior in males could induce cell proliferation in the adult brain were done in the hamster (Antzoulatos et al. 2008). Sexually experienced males were injected with BrdU and mated weekly for 7 weeks. No enhancement of cell proliferation was found in the medial preoptic area or the medial amygdala, two structures crucial for the expression of male sexual behavior. In another study male rats were exposed to sexually receptive females with whom they could copulate for 1 day or for 14 consecutive days. Male rats that mated once (acute) or for 14 consecutive days (chronic) with receptive females showed an increase in cell proliferation and neurogenesis in the dentate gyrus of the hippocampus. No changes were observed in males exposed to receptive females, suggesting that a rewarding experience, sex, promotes neurogenesis (Leuner et al. 2010). In both of these studies, the authors did not evaluate cell proliferation in the SVZ, the RMS, or the olfactory bulbs. Therefore, we decided to investigate if sexual behavior can induce SVZ neurogenesis in males. Our design was similar to what we have done in females. Basically, BrdU was injected 1 hour before, at the end, and 1 hour after the behavioral tests, sacrificing the subjects 15 days later. The groups included (1) males without sexual stimulation, (2) males exposed to female odors, and (3) males that mated for 1 hour without pacing the sexual interaction and males that paced the sexual interaction until achieving 1 or 3 ejaculations. As in the case of the female, we observed a significant increase in the number of newborn neurons in the granular cell layer of the AOB in the groups of males that ejaculated once or three times pacing (controlling) the sexual interaction. No differences between groups were found in the other layers of the AOB or in the MOB. We also showed that around 40% of the new cells differentiated into neurons. The group of males not allowed to pace the sexual interaction ejaculated a mean of 3.4 times during the 1-hour test. Despite the fact that the nonpaced group had more ejaculations than the groups that ejaculated 1 or 3 times no increase in the number of new neurons was observed. These results clearly indicate the quality of the stimulation received during paced mating but not the intensity of the stimulation (number of intromissions) is a crucial factor to induce neurogenesis in the AOB (Portillo et al. 2012).
13.4.4. Opioids and Neurogenesis
Another important difference between paced and nonpaced mating is the rewarding value of the sexual interaction. We have repeatedly shown that only if subjects, both males and females, pace the sexual interaction, a conditioned place preference indicative of a reward state is developed (Martinez and Paredes 2001; Camacho et al. 2009). This reward state is mediated by a common opioid system because administration of the opioid antagonist naloxone blocks the rewarding effects induced by sexual behavior in both males (Agmo and Gomez 1993) and females (Paredes and Martinez 2001). In fact several lines of evidence suggest that opioids are released during sexual behavior thereby reducing the aversive consequences of repeated sexual stimulation and enabling the eventual development of a reward or positive affective estate (Agmo 2007; Paredes and Fernández-Guasti 2008; Paredes 2009). Other appetitive behaviors also induce cell proliferation in the hippocampus. Rats emit 50-KHz ultrasonic vocalizations in appetitive situations, like tickle, while 22-KHz calls are associated with aversive situations (see Wohr et al. 2009 and references therein). The rate of hippocampal cell proliferation was analyzed in rats that perceived tickling as appetitive or aversive and in nontickled rats. Repeated tickling increased cell proliferation in the hippocampus in the rats that experienced tickling as appetitive (Wohr et al. 2009). Another activity that could be considered appetitive and is mediated by opioids is exercise. It has also been shown that exercise, either treadmill or swimming, stimulates neurogenesis in the hippocampus of adult rats (Chae et al. 2012). Further studies need to address whether cell proliferation and neurogenesis in appetitive behaviors are mediated by opioids. This indeed is a possibility considering that opioids are also involved in the proliferation and survival of the new cells in the SVZ (Sargeant et al. 2008) and that morphine treatment increases the number of new cells in the SVZ of adult male rats (Messing et al. 1979).
13.5. CONCLUDING REMARKS
Here we have shown that social stimuli underlying reproductive behavior in rodents enhance incorporation of newborn neurons in adult neurogenic regions, particularly in the olfactory bulb region. This occurs through a double action that influences proliferation of progenitors/precursors and incorporation of newborn neurons within functional circuits. Pheromonal cues contained in urine and hormones such as PRL are important mediators of this mechanism. Nevertheless, additional studies are needed to better characterize the nature and source of the sensory cues driving increased incorporation of newborn neurons as well as the molecular players and brain circuits/systems mediating this mechanism during social interaction. For example, our experiments of urine fractionation suggest the survival of new neurons in the AOB is regulated by molecules included in the LMW urine fraction, but whether MHC peptide ligands (already known to convey individuality in the Bruce effect) or MUPs testosterone-dependent volatile ligands are involved in such activity remains to be determined. Similarly, the possible role of opioids and the involvement of the reward state in controlling proliferation of SVZ progenitors deserve further investigation.
Concerning the role played by OB newborn neurons, the data reviewed in this chapter supports that they are involved in recognizing the mating partner, which is critical to avoiding pregnancy block in mice, and learning the odor of the offspring, to favor selective care and preventing inbreeding. Thus, waiting for further feasible confirmations, the occurrence of adult neurogenesis in key regions controlling social stimuli appears of extraordinary functional importance in the context of the reproductive behavior.
ACKNOWLEDGMENTS
This chapter was supported by PRIN 2010-2011 to Paolo Peretto and by CONACyT 167101 and DGAPA IN200512 to Raúl G. Paredes.
REFERENCES
- Agmo A. Functional and Dysfunctional Sexual Behavior. London: Academic Press; 2007. Learning and Sex: Sexual Activity as Reinforcement and Reward.
- Agmo A, Gomez M. Sexual reinforcement is blocked by infusion of naloxone into the medial preoptic area. Behav. Neurosci. 1993;107:812–18. [PubMed: 8280390]
- Aimone J.B, Deng W, Gage F.H. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70(4):589–96. [PMC free article: PMC3240575] [PubMed: 21609818]
- Alonso M, Viollet C, Gabellec M.M, Meas-Yedid V, Olivo-Marin J.C, Lledo P.M. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J. Neurosci. 2006;26:10508–13. [PMC free article: PMC6674694] [PubMed: 17035535]
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat. Rec. 1963;145:573–91. [PubMed: 14012334]
- Altman J, Das G.D. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–56. [PubMed: 5886931]
- Antzoulatos E, Magorien J.E, Wood R.I. Cell proliferation and survival in the mating circuit of adult male hamsters: Effects of testosterone and sexual behavior. Horm. Behav. 2008;54:735–40. [PMC free article: PMC2588138] [PubMed: 18775431]
- Arzate D.M, Portillo W, Corona R, Paredes R.G. Repeated paced mating promotes the arrival of more newborn neurons in the main and accessory olfactory bulbs of adult female rats. Neuroscience. 2013;232:151–60. [PubMed: 23262235]
- Bellringer J.F, Pratt H.P, Keverne E.B. Involvement of the vomeronasal organ and prolactin in pheromonal induction of delayed implantation in mice. J. Reprod. Fertil. 1980;59:223–28. [PubMed: 7401039]
- Blaustein J.D, Erskine M.S. Feminine Sexual Behavior: Cellular Integration of Hormonal and Afferent Information in the Rodent Brain. New York: Academic Press. 2002
- Bonfanti L, Peretto P. Adult neurogenesis in mammals: A theme with many variations. Eur. J. Neurosci. 2011;34:930–50. [PubMed: 21929626]
- Bonfanti L, Peretto P, Merighi A, Fasolo A. Newly-generated cells from the rostral migratory stream in the accessory olfactory bulb of the adult rat. Neuroscience. 1997;81:489–502. [PubMed: 9300436]
- Brennan P.A. Outstanding issues surrounding vomeronasal mechanisms of pregnancy block and individual recognition in mice. Behav. Brain Res. 2009;200:287–94. [PubMed: 19071163]
- Brennan P.A, Keverne E.B. Something in the air? New insights into mammalian pheromones. Curr. Biol. 2004;14:81–89. [PubMed: 14738757]
- Brennan P.A, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–15. [PubMed: 17108955]
- Brennan P, Kaba H, Keverne E.B. Olfactory recognition: A simple memory system. Science. 1990;250:1223–26. [PubMed: 2147078]
- Breton-Provencher V, Lemasson M, Peralta M.R, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 2009;29:15245–57. [PMC free article: PMC6665973] [PubMed: 19955377]
- Bruce H. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. [PubMed: 13805128]
- Brus M, Meurisse M, Gheusi G, Keller M, Lledo P.M, Levy F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J. Comp. Neur. 2013;521:169–88. [PubMed: 22700217]
- Camacho F.J, Portillo W, Quintero-Enriquez O, Paredes R.G. Reward value of intromissions and morphine in male rats evaluated by conditioned place preference. Physiol. Behav. 2009;98:602–27. [PubMed: 19799920]
- Chae C.H, Lee H.C, Jung S.L, et al. Swimming exercise increases the level of nerve growth factor and stimulates neurogenesis in adult rat hippocampus. Neuroscience. 2012;212:30–37. [PubMed: 22516011]
- Chamero P, Marton T.F, Logan D.W, et al. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. [PubMed: 18064011]
- Chauveau F, Pierard C, Coutan M, et al. Prefrontal cortex or basolateral amygdala lesions blocked the stress-induced inversion of serial memory retrieval pattern in mice. Neurobiol. Learn. Mem. 2008;90:395–403. [PubMed: 18572424]
- Corona R, Larriva-Sahd J, Paredes R.G. Paced-mating increases the number of adult new born cells in the internal cellular (granular) layer of the accessory olfactory bulb. PloS One. 2011;6:e19380. [PMC free article: PMC3103495] [PubMed: 21637743]
- Curtis M.A, Kam M, Faull R.L. Neurogenesis in humans. Eur. J. Neurosci. 2011;33:1170–74. [PubMed: 21395861]
- Emsley J.G, Mitchell B.D, Kempermann G, Macklis J.D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog. Neurobiol. 2005;75:321–41. [PubMed: 15913880]
- Erskine M.S. Solicitation behavior in the estrous female rat: A review. Horm. Behav. 1989;23:473–502. [PubMed: 2691387]
- Erskine M.S. Prolactin release after mating and genitosensory stimulation in females. Endocr. Rev. 1995;16(4):508–28. [PubMed: 8521792]
- Erskine M. S, Baum M.J. Effects of paced coital stimulation on termination of estrus and brain indoleamine levels in female rats. Pharmacol. Biochem. Behav. 1982;17:857–61. [PubMed: 6184734]
- Erskine M.S, Kornberg E, Cherry J.A. Paced copulation in rats: Effects of intromission frequency and duration on luteal activation and estrus length. Physiol. Behav. 1989;45:33–39. [PubMed: 2727140]
- Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta. 2013;1830(2):2435–48. [PMC free article: PMC3541438] [PubMed: 22982587]
- Feierstein C.E. Linking adult olfactory neurogenesis to social behavior. Front. Neurosci. 2012;6:173. [PMC free article: PMC3510682] [PubMed: 23226115]
- Feierstein C.E, Lazarini F, Wagner S, et al. Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front. Behav. Neurosci. 2010;4:176. [PMC free article: PMC3001759] [PubMed: 21160552]
- Fuentealba L.C, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698–708. [PMC free article: PMC3726005] [PubMed: 22704510]
- Furuta M, Bridges R.S. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res. Bull. 2009;80(6):408–13. [PMC free article: PMC2764823] [PubMed: 19712726]
- Gould E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007;8:481–88. [PubMed: 17514200]
- Grattan D.R, Kokay I.C. Prolactin: A pleiotropic neuroendocrine hormone. J. Neuroendocrinol. 2008;20(6):752–63. [PubMed: 18601698]
- Hagg T. Molecular regulation of adult CNS neurogenesis: An integrated view. Trends Neurosci. 2005;28(11):589–95. [PubMed: 16153715]
- Huang L, Bittman E.L. Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm. Behav. 2002;41:343–50. [PubMed: 11971669]
- Kee N, Teixeira C.M, Wang A.H, Frankland P.W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007;10:355–62. [PubMed: 17277773]
- Keller M, Douhard Q, Baum M.J, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem. Senses. 2006;31(4):315–23. [PMC free article: PMC2263131] [PubMed: 16484502]
- Kempermann G, Wiskott L, Gage F.H. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004;14:186–91. [PubMed: 15082323]
- Kermen F, Sultan S, Sacquet J, Mandairon N, Didier A. Consolidation of an olfactory memory trace in the olfactory bulb is required for learning-induced survival of adult-born neurons and long-term memory. PloS One. 2010;5:e12118. [PMC free article: PMC2921340] [PubMed: 20730099]
- Larsen C. M, Grattan D.R. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151(8):3805–14. [PubMed: 20484459]
- Larsen C.M, Kokay I.C, Grattan D.R. Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Horm. Behav. 2008;53:509–17. [PubMed: 18258236]
- Lazarini F, Mouthon M.A, Gheusi G, et al. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PloS One. 2009;4:e7017. [PMC free article: PMC2737283] [PubMed: 19753118]
- Leinders-Zufall T, Brennan P, Widmayer P, et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–37. [PubMed: 15528444]
- Leuner B, Glasper E.R, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PloS One. 2010;5:e11597. [PMC free article: PMC2904381] [PubMed: 20644737]
- Leypold B.G, Yu C.R, Leinders-Zufall T, et al. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. U S A. 2002;99:6376–81. [PMC free article: PMC122956] [PubMed: 11972034]
- Li C.S, Kaba H, Saito H, Seto K. Excitatory influence of the accessory olfactory bulb on tuberoinfundibular arcuate neurons of female mice and its modulation by oestrogen. Neuroscience. 1989;29:201–8. [PubMed: 2710344]
- Lindsey B. W, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog. Neurobiol. 2006;80:281–307. [PubMed: 17218052]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–48. [PubMed: 8178174]
- Luskin M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–89. [PubMed: 8338665]
- Ma D.K, Kim W.R, Ming G.L, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. N. Y. Acad. Sci. 2009;1170:664–73. [PMC free article: PMC2729764] [PubMed: 19686209]
- Magavi S.S, Mitchell B.D, Szentirmai O, Carter B.S, Macklis J.D. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J. Neurosci. 2005;25:10729–39. [PMC free article: PMC6725839] [PubMed: 16291946]
- Mak G. K, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat. Neurosci. 2010;13:753–58. [PubMed: 20453850]
- Mak G.K, Enwere E.K, Gregg C, et al. Male pheromone-stimulated neurogenesis in the adult female brain: Possible role in mating behavior. Nat. Neurosci. 2007;10:1003–11. [PubMed: 17603480]
- Mandairon N, Sacquet J, Jourdan F, Didier A. Long-term fate and distribution of newborn cells in the adult mouse olfactory bulb: Influences of olfactory deprivation. Neuroscience. 2006;141:443–51. [PubMed: 16713121]
- Martel K. L, Baum M.J. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur. J. Neurosci. 2009;29:368–76. [PMC free article: PMC2754263] [PubMed: 19077123]
- Martinez I, Paredes R.G. Only self-paced mating is rewarding in rats of both sexes. Horm. Behav. 2001;40:510–17. [PubMed: 11716580]
- Matsuoka M, Kaba H, Mori Y, Ichikawa M. Synaptic plasticity in olfactory memory formation in female mice. Neuroreport. 1997;8:2501–4. [PubMed: 9261816]
- Matsuoka M, Norita M, Costanzo R.M. A new surgical approach to the study of vomeronasal system regeneration. Chem. Senses. 2005 30Suppl:129–30. [PubMed: 15738074]
- McBride K, Slotnick B, Margolis F.L. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem. Senses. 2003;28:659–70. [PubMed: 14627534]
- McClintock M. K, Adler N.T. The role of the female during copulation in wild and domestic Norway rats (Rattus norvegicus). Behaviour. 1978;67:67–96.
- McClintock M.K, Anisko J.J. Group mating among Norway rats. I. Sex differences in the pattern and neuroendocrine consequences of copulation. Anim. Behav. 1982;30:398–409.
- McClintock M.K, Anisko J.J, Adler N.T. Group mating among Norway rats. II. The social dynamics of copulation: Competition, cooperation, and mate choice. Anim. Behav. 1982;30:410–25.
- Meisel R.L, Sachs B.D. The physiology of male sexual behavior. In: Knobil E, Neill J.D, editors. In The Physiology of Reproduction. New York: Raven Press; 1994. pp. 3–105.
- Messing R.B, Dodge C, Waymire J.C, Lynch G.S, Deadwyler S.A. Morphine induced increases in the incorporation of 3H-thymidine into brain striatal DNA. Brain Res. Bulletin. 1979;4:615–19. [PubMed: 487217]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci. 2010;32:2042–52. [PubMed: 21143659]
- Ming G. L, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70(4):687–702. [PMC free article: PMC3106107] [PubMed: 21609825]
- Moreno M.M, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc. Natl. Acad. Sci. U S A. 2009;106:17980–5. [PMC free article: PMC2764902] [PubMed: 19815505]
- Morgan J.I, Cohen D.R, Hempstead J.L, Curran T. Mapping patterns of c-Fos expression in the central nervous system after seizure. Science. 1987;237:192–97. [PubMed: 3037702]
- Nissant A, Bardy C, Katagiri H, Murray K, Lledo P.M. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat. Neurosci. 2009;12:728–30. [PubMed: 19412168]
- Nottebohm F. Neuronal replacement in adulthood. Ann. N. Y. Acad. Sci. 1985;457:143–61. [PubMed: 3913361]
- Oboti L, Savalli G, Giachino C, et al. Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. Eur. J. Neurosci. 2009;29:679–92. [PubMed: 19200078]
- Oboti L, Schellino R, Giachino C, et al. Newborn interneurons in the accessory olfactory bulb promote mate recognition in female mice. Front. Neurosci. 2011;5:113. [PMC free article: PMC3182443] [PubMed: 21994486]
- Pankevich D.E, Cherry J.A, Baum M.J. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiol. Behav. 2006;87:781–88. [PMC free article: PMC2263135] [PubMed: 16516252]
- Paredes R.G. Evaluating the neurobiology of sexual reward. ILAR J. 2009;50:15–27. [PubMed: 19106449]
- Paredes R. G, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav. Neurosci. 1997;111:123–28. [PubMed: 9109630]
- Paredes R. G, Fernández-Guasti A. Rewarding properties of mating. In: Méndez M, Mondragón-Ceballos R, editors. Neural Mechanisms of Drugs of Abuse and natural Reinforcers. Trivandrum Kerala, India: Research Signpost; 2008. pp. 159–170.
- Paredes R.G, Vazquez B. What do female rats like about sex? Paced mating. Behav. Brain Res. 1999;105:117–27. [PubMed: 10553695]
- Peele P, Salazar I, Mimmack M, Keverne E.B, Brennan P.A. Low molecular weight constituents of male mouse urine mediate the pregnancy block effect and convey information about the identity of the mating male. Eur. J. Neurosci. 2003;18:622–28. [PubMed: 12911758]
- Peretto P, Merighi A, Fasolo A, Bonfanti L. The subependymal layer in rodents: A site of structural plasticity and cell migration in the adult mammalian brain. Brain Res. Bull. 1999;49:221–43. [PubMed: 10424843]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: Role of olfaction. J. Neurosci. 2002;22:6106–13. [PMC free article: PMC6757952] [PubMed: 12122071]
- Portillo W, Unda N, Camacho F.J, et al. Sexual activity increases the number of newborn neurons in the accessory olfactory bulb of male rats. Front. Neuroanat. 2012;6 Art 25. [PMC free article: PMC3390685] [PubMed: 22783170]
- Reynolds B. A, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;225:1707–10. [PubMed: 1553558]
- Robitaille J. A, Bouvet J. Field observations on the social behavior of the Norway rat, Rattus norvegicus (Berkenhout). Biol Behav. 1976;1:289–308.
- Rochefort C, Lledo P.M. Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. Eur. J. Neurosci. 2005;22:2863–70. [PubMed: 16324121]
- Rochefort C, Gheusi G, Vincent J.D, Lledo P.M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 2002;22:2679–89. [PMC free article: PMC6758329] [PubMed: 11923433]
- Rosser A.E, Remfry C.J, Keverne E.B. Restricted exposure of mice to primer pheromones coincident with prolactin surges blocks pregnancy by changing hypothalamic dopamine release. J. Reprod. Fertil. 1989;87:553–59. [PubMed: 2513390]
- Ruscio M.G, Sweeny T.D, Hazelton J.L, Suppatkul P, Boothe E, Carter C.S. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav. Brain Res. 2008;187(1):9–16. [PMC free article: PMC2699755] [PubMed: 17913255]
- Sahay A, Wilson D.A, Hen R. Pattern separation: A common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70(4):582–8. [PMC free article: PMC3109085] [PubMed: 21609817]
- Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, Kageyama R. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc. Natl. Acad. Sci. U S A. 2011;108:8479–84. [PMC free article: PMC3100923] [PubMed: 21536899]
- Sargeant T.J, Miller J.H, Day D.J. Opioidergic regulation of astroglial/neuronal proliferation: Where are we now? J. Neurochem. 2008;107:883–97. [PubMed: 18786168]
- Shingo T, Gregg C, Enwere E, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–20. [PubMed: 12511652]
- Smith M.T, Pencea V, Wang Z, Luskin M.B, Insel T.R. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm. Behav. 2001;39:11–21. [PubMed: 11161879]
- Sohur U.S, Emsley J.G, Mitchell B.D, Macklis J.D. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1477–97. [PMC free article: PMC1664671] [PubMed: 16939970]
- Tirindelli R, Dibattista M, Pifferi S, Menini A. From pheromones to behavior. Physiol Rev. 2009;89(3):921–56. [PubMed: 19584317]
- Wohr M, Kehl M, Borta A, Schanzer A, Schwarting R.K, Hoglinger G.U. New insights into the relationship of neurogenesis and affect: Tickling induces hippocampal cell proliferation in rats emitting appetitive 50-kHz ultrasonic vocalizations. Neuroscience. 2009;163:1024–30. [PubMed: 19638303]
- Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. U S A. 2005;102:9697–702. [PMC free article: PMC1157102] [PubMed: 15976032]
- Review Neurogenesis in the Adult Olfactory Bulb.[The Neurobiology of Olfaction....]Review Neurogenesis in the Adult Olfactory Bulb.Pignatelli A, Belluzzi O. The Neurobiology of Olfaction. 2010
- Review The interplay between reproductive social stimuli and adult olfactory bulb neurogenesis.[Neural Plast. 2014]Review The interplay between reproductive social stimuli and adult olfactory bulb neurogenesis.Peretto P, Schellino R, De Marchis S, Fasolo A. Neural Plast. 2014; 2014:497657. Epub 2014 Jul 22.
- Review Influence of Cat Odor on Reproductive Behavior and Physiology in the House Mouse: (Mus Musculus).[Neurobiology of Chemical Commu...]Review Influence of Cat Odor on Reproductive Behavior and Physiology in the House Mouse: (Mus Musculus).Voznessenskaya VV. Neurobiology of Chemical Communication. 2014
- Review Engineering Aspects of Olfaction.[Neuromorphic Olfaction. 2013]Review Engineering Aspects of Olfaction.Persaud KC. Neuromorphic Olfaction. 2013
- Review Performance of a Computational Model of the Mammalian Olfactory System.[Neuromorphic Olfaction. 2013]Review Performance of a Computational Model of the Mammalian Olfactory System.Benjaminsson S, Herman P, Lansner A. Neuromorphic Olfaction. 2013
- Social Cues, Adult Neurogenesis, and Reproductive Behavior - Neurobiology of Che...Social Cues, Adult Neurogenesis, and Reproductive Behavior - Neurobiology of Chemical Communication
Your browsing activity is empty.
Activity recording is turned off.
See more...
