Due to the lack of evidence described in the main Provisional Peer-Reviewed Toxicity Value (PPRTV) assessment, it is inappropriate to derive provisional toxicity values for perylene. However, some information is available for this chemical, which although insufficient to support derivation of provisional toxicity values under current guidelines, may be of use to risk assessors. In such cases, the Center for Public Health and Environmental Assessment (CPHEA) summarizes available information in an appendix and develops a “screening value.” Appendices receive the same level of internal and external scientific peer review as the provisional reference values to ensure their appropriateness within the limitations detailed in the assessment. Users of screening toxicity values in an appendix to a PPRTV assessment should understand that there could be more uncertainty associated with the derivation of an appendix screening toxicity value than for a value presented in the body of the assessment. Questions or concerns about the appropriate use of screening values should be directed to the CPHEA.
APPLICATION OF AN ALTERNATIVE ANALOGUE APPROACH (METHODS)
The analogue approach allows for the use of data from related compounds to calculate screening values when data for the compound of interest are limited or unavailable. Details regarding searches and methods for analogue analysis are presented in Wang et al. (2012). Three types of potential analogues (structural, metabolic, and toxicity-like) are identified to facilitate the final analogue chemical selection. The analogue approach may or may not be route-specific or applicable to multiple routes of exposure. All information is considered together as part of the final weight-of-evidence (WOE) approach to select the most suitable analogue both toxicologically and chemically.
An expanded analogue identification approach Wang et al. (2012) was developed to collect a more comprehensive set of candidate analogues for the compounds undergoing U.S. Environmental Protection Agency (U.S. EPA) PPRTV screening-level assessment. As described below, this method includes application of a variety of tools and methods for identifying candidate analogues that are similar to the target chemical based on chemical structure and key features, metabolic relationships, or related toxic effects and mechanisms of action.
To identify structurally-related compounds, an initial pool of analogues is identified using automated tools, including ChemIDplus (NLM, 2022a), the CompTox Chemicals Dashboard (U.S. EPA, 2022c), and the Organisation for Economic Co-operation and Development (OECD) Quantitative Structure-Activity Relationship (QSAR) Toolbox (OECD, 2022), to conduct structural similarity searches. Additional analogues identified as ChemIDplus-related substances, parent, salts, and mixtures, and CompTox-related substances are considered. CompTox Generalized Read-Across (GenRA) analogues are collected using the methods available on the publicly available GenRA Beta version, which may include Morgan fingerprints, Torsion fingerprints, ToxPrints and ToxCast, Tox21, and ToxRef data. For compounds that have very few analogues identified by structure similarity using a similarity threshold of 0.8 or 80%, substructure searches in the QSAR Toolbox may be performed, or similarity searches may be rerun using a reduced similarity threshold (e.g., 70 or 60%). The compiled list of candidate analogues is batch run through the CompTox Chemicals Dashboard where QSAR-ready simplified molecular-input line-entry system (SMILES) notations are collected and toxicity data availability is determined (e.g., from the Agency for Toxic Substances and Disease Registry [ATSDR], California Environmental Protection Agency [CalEPA], U.S. EPA Integrated Risk Information System [IRIS], PPRTV assessments). The batch output information is then uploaded into the Chemical Assessment Clustering Engine (ChemACE) (U.S. EPA, 2011a), which clusters the chemicals based on chemical fragments and displays the toxicity data availability for each candidate. The ChemACE output is reviewed by an experienced chemist, who narrows the list of structural analogues based on known or expected structure-toxicity relationships, reactivity, and known or expected metabolic pathways.
During the development of a systematic evidence map (SEM), toxicokinetic studies were identified and tagged as potentially relevant supplemental material. These studies were used to identify metabolic analogues (metabolites and metabolic precursors). Metabolites were also identified from the two OECD QSAR Toolbox metabolism simulators (in vivo rat metabolism simulator and rat liver S9 metabolism simulator). Targeted PubMed searches were conducted to identify metabolic precursors and other compounds that share any of the observed or predicted metabolites identified for the target chemical. Metabolic analogues are then added to the pool of candidate analogues and toxicity data availability is determined (e.g., from ATSDR, CalEPA, U.S. EPA IRIS, PPRTV assessments).
In vivo toxicity data for the target chemical (if available from the SEM) are evaluated to determine whether specific or characteristic toxicity was observed (e.g., cholinesterase inhibition, inhibition of oxidative phosphorylation). In addition, in vitro mechanistic data tagged as potentially relevant supplemental material from the SEM or obtained from tools including GenRA, ToxCast/Tox21, and Comparative Toxicogenomics Database (CTD) (CTD, 2022; Davis et al., 2021) were evaluated for this purpose. Data from CompTox Chemicals Dashboard ToxCast/Tox21 are collected to determine bioactivity of the target chemical in in vitro assays that may indicate potential mechanism(s) of action. The GenRA option within the Dashboard also offers an option to search for analogues based on similarities in activity in ToxCast/Tox21 in vitro assays. Using the ToxCast/Tox21 bioactivity data, nearest neighbors identified with similarity indices of ≥0.5 may be considered potential candidate analogues. The CTD (CTD, 2022; Davis et al., 2021) is searched to identify compounds with gene interactions similar to interactions induced by the target chemical; compounds with gene interactions similar to the target chemical (with a similarity index >0.5) may be considered potential candidate analogues. These compounds are then added to the pool of candidate analogues, and toxicity data availability is determined (e.g., from ATSDR, CalEPA, U.S. EPA IRIS, PPRTV assessments).
The tools used for the expanded analogue searches were selected because they are publicly available, which allows for transparency and reproducibility of the results, and because they are supported by U.S. and OECD agencies, updated regularly, and widely used. The application of a variety of different tools and methods to identify candidate analogues serves to minimize the limitations of any individual tool with respect to the pool of chemicals included, chemical fragments considered, and methods for assessing similarity. Further, the inclusion of techniques to identify analogues based on metabolism and toxicity or bioactivity expands the pool of candidates beyond those based exclusively on structural similarity.
Analogue Search Results for Perylene
Candidate analogues for perylene were identified based on metabolic relationships, structural relationships, and toxicodynamic relationships. For candidates identified through these approaches, the U.S. EPA (IRIS and PPRTV assessments), ATSDR, and CalEPA sources were searched for subchronic, intermediate, and chronic oral and inhalation toxicity values. Details are provided below (see “Identification of Structural Analogues with Established Toxicity Values”).
Identification of Structural Analogues with Established Toxicity Values
Perylene is not a member of an existing OECD or New Chemical category. Candidate structural analogues for perylene were identified using similarity searches in the OECD Toolbox, the U.S. EPA CompTox Chemicals Dashboard, and ChemIDplus tools (NLM, 2022a; OECD, 2022; U.S. EPA, 2022a). A total of 521 unique structural analogues were identified for perylene in the Dashboard, OECD QSAR Toolbox, and ChemIDplus (80% similarity threshold).
The list of potential analogues was reviewed by a chemist with expertise in read-across. Criteria for including/excluding candidates were as follows:
- Exclude compounds with elements other than carbon and hydrogen.
- Include compounds with four, five, or six fused six-carbon aromatic rings.
- Exclude compounds with smaller or larger rings (greater than six or less than six carbon rings).
- Include only compact, condensed compounds:
- compounds containing the pyrene fragment (17 compounds); or
- at least one ring shares bonds with three or more other rings (12 compounds).
Using these criteria, a total of 29 candidate structural analogues for perylene were identified, as shown in Table A-1. Two of the candidate structural analogues had relevant toxicity values: benzo[a]pyrene (BaP) and pyrene.
Table A-1Candidate Structural Analogues Identified for Perylene based on Tools and Expert Judgment
| Tool (method)a | Analogue (CASRNs) Selected for Toxicity Value Searchesb | Structure |
|---|---|---|
| Dashboard (Tanimoto method), OECD Toolbox (Dice method), and ChemIDplus (method not described) | Benzo[a]pyrene (CASRN 50-32-8) |
![Diagram of Chemical Structure for Benzo[a]pyrene (CASRN 50-32-8) a structural analogue identified for Perylene.](/books/NBK601584/bin/appaif2.jpg)
|
| Benzo[e]pyrene (CASRN 192-97-2) |
![Diagram of Chemical Structure Benzo[e]pyrene (CASRN 192-97-2) a structural analogue identified for Perylene.](/books/NBK601584/bin/appaif3.jpg)
| |
| Dibenzo(a,e)pyrene (CASRN 192-65-4) |
| |
| Dibenzo[a,h]pyrene (CASRN 189-64-0) |
| |
| Dibenzo[a,i]pyrene (CASRN 189-55-9) |
![Diagram of Chemical Structure for Dibenzo[a,i]pyrene (CASRN 189-55-9) a structural analogue identified for Perylene.](/books/NBK601584/bin/appaif6.jpg)
| |
| Dibenzo[a,l]pyrene (CASRN 191-30-0) |
![Diagram of Chemical Structure for Dibenzo[a,l]pyrene (CASRN 191-30-0) a structural analogue identified for Perylene.](/books/NBK601584/bin/appaif7.jpg)
| |
| Naphtho(2,1,8-qra)naphthacene (CASRN 196-42-9) |

| |
| Dashboard (Tanimoto method) and ChemIDplus (method not described) | Anthanthrene (CASRN 191-26-4) |

|
| Benzo(g)chrysene (CASRN 196-78-1) |
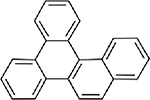
| |
| Benzo(h)pentaphene (CASRN 214-91-5) |

| |
| Benzo(a)perylene (CASRN 191-85-5) |
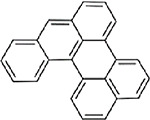
| |
| Benzo[b]perylene (CASRN 197-70-6) |
![Diagram of Chemical Structure Benzo[b]perylene (CASRN 197-70-6) a structural analogue identified for Perylene based on tools. Tool Method: Dashboard (Tanimoto method) and ChemIDplus (method not described)](/books/NBK601584/bin/appaif13.jpg)
| |
| Benzo(g,h,i)perylene (CASRN 191-24-2) |
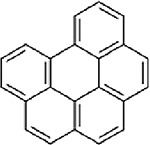
| |
| Benzo(pqr)picene (CASRN 189-96-8) |
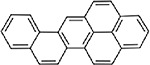
| |
| Dibenz[a,c]anthracene (CASRN 215-58-1) |
![Diagram of Chemical Structure for Dibenz[a,c]anthracene (CASRN 215-58-1) candidate structural analogues identified for Perylene.](/books/NBK601584/bin/appaif16.jpg)
| |
| Dibenzo[c,g]chrysene (CASRN 53156-66-4) |
![Diagram of Chemical Structure for Dibenzo[c,g]chrysene (CASRN 53156-66-4) candidate structural analogues identified for Perylene.](/books/NBK601584/bin/appaif17.jpg)
| |
| Dibenzo(c,mno)chrysene (CASRN 196-28-1) |
![Diagram of Chemical Structure for Dibenzo[c,mno]chrysene (CASRN 196-28-1) candidate structural analogues identified for Perylene.](/books/NBK601584/bin/appaif18.jpg)
| |
| Dibenzo(c,p)chrysene (CASRN 196-52-1) |
![Diagram of Chemical Structure for Dibenzo[c,p]chrysene (CASRN 196-52-1) candidate structural analogues identified for Perylene.](/books/NBK601584/bin/appaif19.jpg)
| |
| Dibenzo(g,p)chrysene (CASRN 191-68-4) |
![Diagram of Chemical Structure for Dibenzo[g,p]chrysene (CASRN 191-68-4) candidate structural analogues identified for Perylene.](/books/NBK601584/bin/appaif20.jpg)
| |
| Dibenzo(a,c)naphthacene (CASRN 216-00-2) |

| |
| Dibenzo(de,qr)naphthacene (CASRN 193-09-9) |
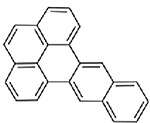
| |
| Dibenzo(fg,op)naphthacene (CASRN 192-51-8) |

| |
| Naphtho(2,3-g)chrysene (CASRN 196-64-5) |
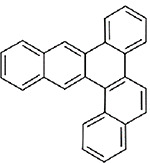
| |
| Pyrene (CASRN 129-00-0) |
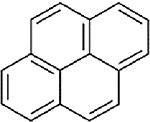
| |
| Tribenz(a,c,h)anthracene (8CI)(9CI) (CASRN 215-26-9) |
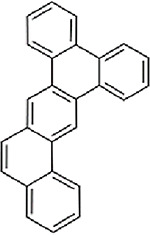
| |
| Triphenylene (CASRN 217-59-4) |
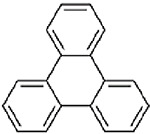
| |
| Dashboard (Tanimoto) | Dibenzo[de,mn]tetracene (CASRN 214-63-1) |
![Diagram of Chemical Structure for Dibenzo[de,mn]tetracene (CASRN 214-63-1) a structural analogue identified for Perylene.](/books/NBK601584/bin/appaif28.jpg)
|
| Dibenzo[ij,no]tetraphene (CASRN 143214-92-0) |
![Diagram of Chemical Structure for Dibenzo[ij,no]tetraphene (CASRN 143214-92-0) a structural analogue identified for Perylene.](/books/NBK601584/bin/appaif29.jpg)
| |
| Naphtho[1,2-g]chrysene (CASRN 112772-15-3) |
![Diagram of Chemical Structure for Naphtho[1,2-g]chrysene (CASRN 112772-15-3) a structural analogue identified for Perylene.](/books/NBK601584/bin/appaif30.jpg)
|
- a
80% similarity threshold was applied.
- b
Bold shows compounds with oral or inhalation toxicity values.
Identification of Toxicokinetic Precursors or Metabolites with Established Toxicity Values
Experimental studies identifying metabolites of perylene were not identified in the scientific literature. Metabolism of other polycyclic aromatic hydrocarbons (PAHs) has been investigated extensively in both in vitro and in vivo studies (U.S. EPA, 2017a, b; ATSDR, 1995). Metabolites resulting from oxidation of PAHs include epoxides, dihydrodiols, phenols, and quinones (U.S. EPA, 2017a, b; ATSDR, 1995). Oxidized forms of perylene are candidate metabolites based on known metabolic pathways involving cytochrome P450 (CYP450) oxidation of other PAHs such as naphthalene, pyrene, and BaP (Shimada and Fujii-Kuriyama, 2004; ATSDR, 1995). Predicted metabolites of perylene were collected from the OECD QSAR Toolbox (OECD, 2022). PubMed searches (searching “perylene” or “198-55-0” and “metabolite”) were conducted to identify metabolic precursors to perylene. No metabolic precursors were identified. PubMed was also searched to identify other compounds that are metabolized to any of the observed or predicted metabolites of perylene (searching the metabolite name or [CASRN if available] and “metabolite”). No compounds that share at least one metabolite with perylene were identified in these searches.
Table A-2 summarizes the candidate metabolic analogues for perylene. The predicted metabolites of perylene are a result of CYP450 oxidation occurring at the 1- (bay) or 3- (peri) positions of the aromatic rings. Searches for relevant toxicity values for the candidate metabolic analogues of perylene did not identify oral or inhalation toxicity values for any of the observed or predicted metabolites.
Table A-2Candidate Metabolic Analogues of Perylene
| Relationship to Perylene | Compound |
|---|---|
| Metabolic precursor | None identified |
| Predicted metabolitesa | 3-Hydroxy-peryleneb |
| 1-Hydroxy-peryleneb | |
| Shares common metabolite(s) | None identified |
- a
Predicted metabolites of perylene were collected from the OECD QSAR Toolbox (OECD, 2022).
- b
CASRN not available for this metabolite.
OECD = Organisation for Economic Co-operation and Development; QSAR = quantitative structure-activity relationship.
Identification of Analogues on the Basis of Toxicity/Mechanistic/MOA Information and Established Toxicity Values
The toxicological data for perylene, described in the main PPRTV assessment above, do not suggest any specific, characteristic toxicity (e.g., cholinesterase inhibition, inhibition of oxidative phosphorylation) that could be used to identify candidate analogues. One study examining immunosuppression after short-term subcutaneous administration of perylene in mice (White et al., 1985) showed no evidence of immunosuppression. No other relevant toxicity or mechanistic studies on perylene were identified.
Perylene was active in 11 PubChem bioactivity assays reported in the Dashboard (U.S. EPA, 2020b) but was not active in the three available ToxCast/Tox21 assays (U.S. EPA, 2020a). The GenRA option within the Dashboard offers an option to search for analogues based on similarities in activity in ToxCast/Tox21 in vitro assays; however, because perylene was not active in any ToxCast assays, there were no results (U.S. EPA, 2020c).
The CTD (2022) identified several compounds with gene interactions similar to interactions induced by perylene. In the CTD, similarity is measured by the Jaccard index, calculated as the size of the intersection of interacting genes for chemical A and chemical B divided by the size of the union of those genes (range from 0 [no similarity] to 1 [complete similarity]). Among the compounds with gene interactions similar to perylene, the numbers of common gene interactions ranged from 1 to 2 and similarity indices ranged from 0.33 to 0.67. Although there was one compound with a similarity index over 0.5 (1-methylfluorene, CASRN 1730-37-6, similarity index of 0.67), the similarity was based on only two gene interactions and there were no relevant toxicity values available for this chemical, so it was not considered a candidate analogue.
Summary
Searches for structural, metabolic, and toxicity/mechanistic analogues for perylene yielded a total of 31 unique candidate analogues: 2 metabolites and 29 structural analogues. Neither of the identified metabolic analogues had oral or inhalation noncancer toxicity values. Of the 29 structural candidates, 1 had relevant oral and inhalation noncancer toxicity values (BaP) and 1 had relevant oral noncancer toxicity values (pyrene).
Structural Analogues
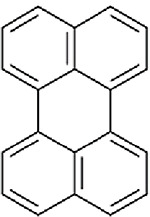
BaP and pyrene were identified as candidate analogues of perylene based on structural similarity. Table A-3 illustrates the chemical structures and summarizes the physicochemical properties for perylene and the two candidate analogues. All three compounds are members of the PAH class of chemicals and are nonsubstituted PAHs. Perylene and BaP consist of five benzene rings, while pyrene contains only four benzene rings. Due to the aromatic ring number, perylene and BaP share the same molecular weight, while the molecular weight of pyrene is lower. Some physicochemical properties are similar for the target compound and both analogues. For example, melting points show that all the compounds are solids at room temperature. In addition, perylene and both candidate analogues have the potential for moderate volatilization from water to air based on their Henry’s law constant values. Considering vapor pressure, water solubility and, octanol-water partition coefficient (log Kow) values presented in Table A-3, BaP appears to be more similar to perylene than pyrene. Although all three compounds are expected to have low volatility from dry surfaces and will exist as particulates in the atmosphere, perylene and BaP, with vapor pressures of > 5 × 10−9 mm Hg, have lower potential for inhalation exposure as gases or vapors than does pyrene. Water solubility is considerably lower for perylene and BaP, compared to pyrene, and the log Kow values for perylene and BaP are higher than that of pyrene. In general, compounds with log Kow values >4 are considered hydrophobic chemicals, which are likely to partition to fat compartments in the body following absorption (Schwarzenbach et al., 2016). Consequently, partitioning to fat will be greater for perylene and BaP, compared to pyrene. Based on structural characteristics (i.e., five- vs. four-ring PAHs) and some physicochemical properties (vapor pressure, water solubility, log Kow), BaP is a more similar candidate structural analogue to perylene than is pyrene.
Table A-3Physical-Chemical Properties of Perylene and Candidate Structural Analogues
| Chemical | Perylenea | BaPb | Pyrenec |
|---|---|---|---|
| Structure |
|
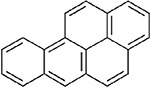
|
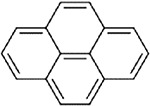
|
| CASRN | 198-55-0 | 50-32-8 | 129-00-0 |
| Molecular weight (g/mol) | 252.32 | 252.32 | 202.26 |
| Melting point (°C) | 274 | 177 | 150 |
| Boiling point (°C) | >350 (estimated range 443–487) | 495 | 399 |
| Vapor pressure (mm Hg at 25°C) | 5.25 × 10−9 | 5.49 × 10−9 | 4.5 × 10−6 |
| Henry’s law constant (atm-m3/mole) | 1.06 × 10−6 (predicted average) | 4.57 × 10−7 | 1.19 × 10−5 |
| Water solubility (mg/L) | 1.58 × 10−9 | 8.40 × 10−9 | 6.65 × 10−7 |
| Octanol-water partition coefficient (log Kow) | 6.04 | 6.13 | 4.88 |
- a
The U.S. EPA CompTox Chemicals Dashboard (perylene CASRN 198-55-0) (U.S. EPA, 2022c). Accessed on January 18, 2023.
- b
The U.S. EPA CompTox Chemicals Dashboard (benzo[a]pyrene CASRN 50-32-8) (U.S. EPA, 2022b). Accessed on January 18, 2023.
- c
The U.S. EPA CompTox Chemicals Dashboard (pyrene CASRN 129-00-0) (U.S. EPA, 2022d). Accessed on January 18, 2023.
All values are experimental averages unless otherwise specified.
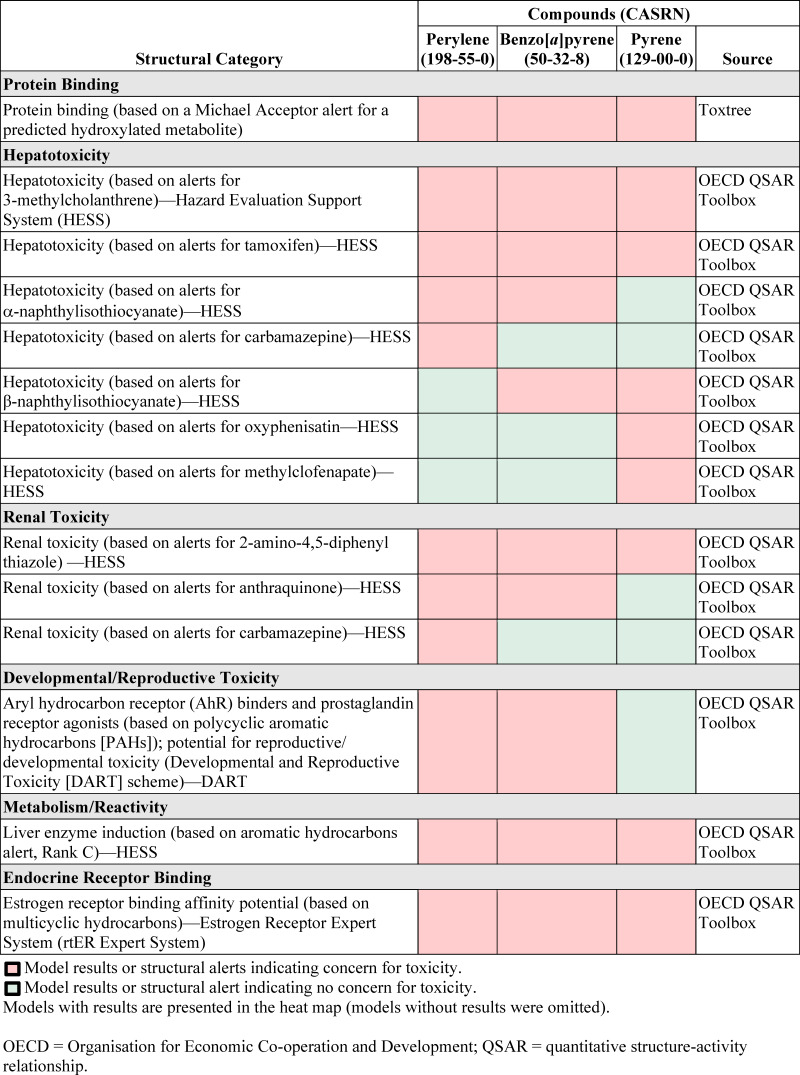
Structural alerts and predictions for genotoxicity and carcinogenicity were identified using computational tools as follows. Relevant structural alerts and toxicity predictions for noncancer health effects were identified using computational tools from the OECD (2022) QSAR Toolbox profilers, OCHEM (2022) ToxAlerts, and IDEAconsult (2018) Toxtree. The model results for perylene and its candidate analogue compounds are shown in Figure A-1. Concerns for protein binding, hepatotoxicity, renal toxicity, developmental/reproductive toxicity, metabolism/reactivity, and endocrine receptor binding are indicated for perylene and its candidate analogues. Based on structural alerts, BaP is a more similar candidate analogue to perylene than is pyrene.
Figure A-1Structural Alerts for Perylene and Candidate Analogues
OECD = Organisation for Economic Co-operation and Development; QSAR = quantitative structure-activity relationship.
Toxtree indicated the potential for protein binding based on predicted hydroxylated metabolites for perylene and both candidate analogues. PAHs, in general, are metabolized in multiple tissues in the body into more soluble metabolites, including dihydrodiols, phenols, quinones, and epoxides, that form conjugates with glucuronide, glutathione (GSH), or sulfate (U.S. EPA, 2017b; IARC, 2010; ATSDR, 1995). There is also limited experimental evidence of perylene metabolism (see Section 2.3.3)
The OECD QSAR Toolbox Hazard Evaluation Support System (HESS) model showed a concern for hepatotoxicity for perylene and both analogues based on structural similarity to 3-methylcholanthrene (inducer of hepatic enzymes) and tamoxifen (hepatic steatosis). There is supportive evidence for hepatic enzyme induction for both analogues and limited experimental evidence of perylene inducing various hepatic enzymes including AHH, “BaP hydrolase,” 7-ethoxyresorufin O-deethylase (EROD), 7-ethoxycoumarin O-deethylase (ECOD), benzphetamine n-demethylase (BPD), and other CYP450 enzymes (see Section 2.3.3). The HESS model also showed a concern for hepatotoxicity for perylene and BaP based on structural similarity to α-naphthylisothiocyanate, which induces cholestasis, hyper-bilirubinemia, and necrotic injury in biliary epithelial cells. Concerns for perylene were also predicted based on a structural alert for carbamazepine, which is associated with vanishing bile duct syndrome; neither candidate analogue showed this alert. Other structural alerts showing concern for hepatotoxicity for BaP and/or pyrene (based on structural similarity to β-naphthylisothiocyanate, oxyphenisatin, or methylclofenapate) were not alerts for perylene.
The OECD QSAR Toolbox HESS model showed a concern for renal toxicity for perylene and both analogues based on structural similarity to 2-amino-4,5-diphenyl thiazole (renal polycystic disease). The HESS model also showed a concern for renal toxicity for perylene and BaP based on structural similarity to anthraquinone (renal degeneration). Concerns for perylene were also predicted based on a structural alert for carbamazepine, which is associated with acute renal failure, hyponatremia, and immunologically mediated acute interstitial nephritis without nephrotic syndrome; neither candidate analogue showed this alert.
The OECD QSAR Toolbox Developmental and Reproductive Toxicity (DART) model showed a concern for developmental and/or reproductive toxicity for perylene and BaP, but not pyrene, based on aryl hydrocarbon receptor (AhR) binders and prostaglandin receptor agonists. The structural alert applies to PAH compounds containing three to five fused aromatic rings aligned in line, such as BaP and benz[a]anthracene. Pyrene’s aromatic rings are grouped together, which made it inactive for this particular model alert. There is also limited experimental evidence of perylene binding AhR (see Section 2.3.3 for additional context).
Other alerts from the OECD QSAR Toolbox include an alert from the HESS model for potential for liver enzyme induction for perylene and both candidate analogues based on the aromatic hydrocarbon structure and an alert from the Estrogen Receptor Expert System (rtER Expert System) for potential estrogen receptor binding affinity based on a multicyclic hydrocarbons structure. Although there are some inconsistencies that varied by model system, some predictive structure-activity relationship (SAR) tools support concern for mutagenicity/carcinogenicity.
Metabolic Analogues
Table A-4 summarizes available toxicokinetic data for perylene and the structurally similar compounds identified as potential analogues.
Table A-4Comparison of ADME Data for Perylene (CASRN 198-55-0) and Candidate Analogues
| Chemical | Perylene | BaP | Pyrene |
|---|---|---|---|
| Structure |
|
|
|
| CASRN | 198-55-0 | 50-32-8 | 129-00-0 |
| Absorption | |||
| Rate and extent of absorption |
|
Laboratory animals (all routes):
|
Laboratory animals (oral, i.t., dermal):
|
| Distribution | |||
| Extent of distribution |
|
Laboratory animals (all routes):
|
Laboratory animals (all routes):
|
| Metabolism | |||
| Rate; Primary reactive metabolites |
|
Laboratory animals (all routes), in vitro:
|
Laboratory animals (i.p.)
|
| Enzyme induction |
|
|
|
| Excretion | |||
| Elimination half-time; route of excretion |
|
Laboratory animals (all routes):
|
Laboratory animals (oral, dermal):
|
| References | OECD (2022); Harris et al. (1988); Asokan et al. (1986); Piskorska-Pliszczynska et al. (1986); Mukhtar et al. (1982); Neubert and Tapken (1978) | U.S. EPA (2017a); IARC (2010); ATSDR (1995); Harris et al. (1988); Piskorska-Pliszczynska et al. (1986); Mukhtar et al. (1982); Neubert and Tapken (1978) | OECD (2022); IARC (2010); ATSDR (1995); Lipniak and Brandys (1993); Asokan et al. (1986); Piskorska-Pliszczynska et al. (1986); Mukhtar et al. (1982); Boyland and Sims (1964) |
ADME = absorption, distribution, metabolism, excretion; AhR = aryl hydrocarbon receptor; AHH = aryl hydrocarbon hydroxylase; BaP = benzo[a]pyrene; CYP450 = cytochrome P450; EROD = 7-ethoxyresorufin O-deethylase; GSH = glutathione; i.t. = intratracheal; Kow = octanol-water partition coefficient; PAH = polycyclic aromatic hydrocarbon; OECD = Organisation for Economic Cooperation and Development; QSAR = quantitative structure-activity relationship.
No data on absorption or distribution are available for perylene. Both candidate analogues are absorbed via oral, inhalation, and dermal routes and show initial widespread distribution followed by accumulation and retention in fat (U.S. EPA, 2017a; IARC, 2010; ATSDR, 1995; Lipniak and Brandys, 1993). Rate and extent of absorption vary depending on exposure medium (i.e., enhanced in the presence of oils and fats), and oral absorption is dependent on presence of bile in the small intestines. Pyrene is absorbed more readily and completely than BaP. The candidate analogues are widely distributed in the body with preferential accumulation in fat as suggested by the log Kow values >4. High levels are also observed in the gut (following exposure via any route) due to mucociliary clearance from the respiratory tract and hepatobiliary excretion of metabolites. Because absorption and distribution of a chemical in the body are determined largely by physical and chemical properties related to chemical size and general structure (e.g., lipophilicity [log Kow values >4], vapor pressure, molecular weight, etc.) (Schwarzenbach et al., 2016), it is reasonable to assume that perylene will be absorbed and distributed similarly to the candidate analogues based on similar size, structure, and physicochemical properties.
No in vivo or in vitro metabolism data are available for perylene. In silico predictions from the OECD QSAR Toolbox predict metabolism of perylene via oxidation to 1- and 3-hydroxy-perylene. The metabolism of BaP has been extensively reviewed by the U.S. EPA (2017a), IARC (2010), and ATSDR (1995). BaP is oxidized by CYP450 in multiple tissues in the body to more soluble metabolites, including dihydrodiols, phenols, quinones, and epoxides, which then form conjugates with glucuronide, glutathione (GSH), or sulfate. Limited in vivo evidence also indicates that pyrene undergoes oxidative metabolism to 1- and 4-hydroxypyrene, 1,6- and 1,8-dihydroxypyrene, 1,6- and 1,8-pyrenequinone, and trans-4,5-dihydro-4,5-dihydroxypyrene (Boyland and Sims, 1964). Based on analogy to other PAHs (ATSDR, 1995), oxidative metabolism of pyrene is expected to be mediated by CYP450s.
As discussed in the main PPRTV assessment, some studies reported weak induction of the monooxygenase enzyme, aryl hydrocarbon hydroxylase (AHH), following in vivo exposure to perylene (Asokan et al., 1986; Mukhtar et al., 1982; Neubert and Tapken, 1978). Of the candidate analogues, BaP is a known inducer of monooxygenase enzymes, particularly AHH (ATSDR, 1995; Neubert and Tapken, 1978). Like perylene, evidence for AHH induction in vivo is mixed for pyrene (Asokan et al., 1986; Mukhtar et al., 1982; Neubert and Tapken, 1978). In vitro, perylene and pyrene are weak AhR binders and BaP is a strong AhR binder (Piskorska-Pliszczynska et al., 1986).
No elimination data are available for perylene. Elimination is rapid via all routes in laboratory animals (22–30 hours, see Table A-4) for BaP and via oral and dermal routes for pyrene (there are no data on elimination following inhalation exposure to pyrene) (U.S. EPA, 2017a, b; IARC, 2010; ATSDR, 1995; Lipniak and Brandys, 1993). For BaP, the primary route of elimination is via feces, with lesser amounts excreted via urine and small amounts excreted in breast milk. For all routes, BaP is excreted mainly as conjugated metabolites. Data for pyrene are less robust but indicate that similar amounts are eliminated via feces and urine.
In summary, there are no experimental toxicokinetic data for the target chemical, perylene. Absorption and distribution are similar for the candidate analogues. Similarities in log Kow values suggest a similar potential accumulation in fat tissues for the target and analogue compounds. Experimental metabolism data are available for both candidate analogues, and metabolic pathways (CYP450 oxidation) are expected to be similar for the target compound based on in silico predictions from the OECD QSAR Toolbox. There is evidence of enzyme induction by perylene and both candidate analogues, although the magnitude of induction varies across compounds. Elimination rate is similar for both candidate analogues, with a higher portion of elimination occurring via feces for BaP. Based on the limited data available, both candidate analogues appear to be suitable metabolic analogues for perylene.
Toxicity-Like Analogues
Oral Exposure
No repeat-dose oral toxicity studies evaluating noncancer effects or acute oral lethality studies are available for perylene. The available oral toxicity values for candidate analogues are summarized in Table A-5. Critical effects for candidate analogues include neurodevelopmental effects (BaP) (U.S. EPA, 2017a) and kidney effects (pyrene) (U.S. EPA, 2007, 1990). Numerous additional toxicity targets have been identified for BaP at doses higher than the lowest lowest-observed-adverse-effect level (LOAEL) associated with neurodevelopmental effects (0.2 mg/kg-day), which corresponds to the BMDL1SD of 0.092 mg/kg-day presented in Table A-5. The lowest LOAELs for additional toxicity targets of BaP include: reproductive system (1 mg/kg-day), adult nervous system (2 mg/kg-day), cardiovascular system (12.5 mg/kg-day), immunological system (15 mg/kg-day), and the kidney and liver (30 mg/kg-day) (U.S. EPA, 2017a, b). Repeat-dose oral exposure data for pyrene are limited, and do not identify additional toxicity targets other than the kidney, for which effects were observed at ≥125 mg/kg-day (U.S. EPA, 2007). Reproductive and/or developmental toxicity have not been evaluated following oral exposure to pyrene.
Table A-5Comparison of Available Oral Toxicity Values for Perylene (CASRN 198-55-0) and Candidate Structural Analogues
| Chemical | Perylene | BaP | Pyrene |
|---|---|---|---|
| Structure |

|
|
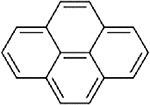
|
| CASRN | 198-55-0 | 50-32-8 | 129-00-0 |
| Subchronic oral toxicity values | |||
| POD (mg/kg-d) | ND | The POD for the chronic RfD (0.092 mg/kg-d) is also applicable to subchronic exposure because it is based on a developmental study (see further details below) | 75 |
| POD type | ND | ND | NOAEL |
| Subchronic UFC | ND | ND | 300 (UFH, UFA, UFD) |
| Subchronic p-RfD (mg/kg-d) | ND | ND | 3 × 10−1 |
| Critical effects | ND | ND | Kidney effects (renal tubular pathology, decreased kidney weights) at ≥125 mg/kg-d |
| Species | ND | ND | Mouse |
| Duration | ND | ND | 13 wk |
| Route (method) | ND | ND | Oral (gavage) |
| Source | NA | NA | U.S. EPA (2007) (PPRTV) |
| Chronic oral toxicity values | |||
| POD (mg/kg-d) | ND | 0.092 | 75 |
| POD type | ND | BMDL1SD | NOAEL |
| Chronic UFc | ND | 300 (UFH, UFA, UFD) | 3,000 (UFH, UFA, UFS, UFD) |
| Chronic RfD/p-RfD (mg/kg-d) | ND | 3 × 10−4 | 3 × 10−2 |
| Critical effects | ND | Neurodevelopmental effects (open field crossed squares at PND 69; elevated plus maze open arm entries at PND 70; Morris water maze hidden platform trial escape latency at PNDs 71–74) | Kidney effects (renal tubular pathology, decreased kidney weights) at ≥125 mg/kg-d |
| Species | ND | Rat | Mouse |
| Duration | ND | PNDs 5–11 | 13 wk |
| Route (method) | ND | Oral (gavage) | Oral (gavage) |
| Source | NA | U.S. EPA (2017a, 2017b) (IRIS) | U.S. EPA (1990) (IRIS) |
| Acute oral lethality data | |||
| Oral LD50 (mg/kg) | ND | ND | ND |
| Toxicity at LD50 | ND | ND | ND |
| Source | NLM (2022c) | NLM (2022b); U.S. EPA (2017a) | NLM (2022d); U.S. EPA (2007) |
BaP = benzo[a]pyrene; BMDL = benchmark dose lower confidence limit; IRIS = Integrated Risk Information System; LD50 = median lethal dose; NA = not applicable; ND = no data; NOAEL = no-observed-adverse-effect level; PND = postnatal day; POD = point of departure; p-RfD = provisional reference dose; RfD = reference dose; SD = standard deviation; UFA = interspecies uncertainty factor; UFC = composite uncertainty factor; UFD = database uncertainty factor; UFH = intraspecies uncertainty factor; UFS = subchronic-to-chronic uncertainty factor.
Of the candidate analogues, BaP provides the lowest candidate point of departure (POD) based on neurodevelopment (0.092 mg/kg-day, which is nearly 3 orders of magnitude lower than the POD of 75 mg/kg-day for pyrene based on kidney effects). Additionally, the lowest LOAEL identified for kidney effects following repeat-dose oral exposure to BaP is 30 mg/kg-day (U.S. EPA, 2017a), which is lower than the POD for pyrene based on kidney effects. In the absence of repeated exposure oral toxicity data for perylene, there is no information with which to clearly identify the most suitable candidate analogue based on toxicity comparisons. From available toxicity data, BaP provides the most conservative candidate POD, which is based on neurodevelopmental effects. In development of the reference dose (RfD) for BaP, the U.S. EPA compared candidate values for developmental, reproductive, and immunological effects (U.S. EPA, 2017a, b). The overall RfD, based on neurobehavioral effects in rats exposed during the early postnatal period, was supported by numerous human and animal studies and was considered protective of all types of health effects. The MOA for neurodevelopmental effects of BaP is not fully understood; however, possible mechanisms may involve oxidative stress in the brain or disrupted gene and/or protein expression levels of neurotransmitters or receptors, resulting in altered neurotransmitter signaling in the brain (U.S. EPA, 2017a). Available data are inadequate to determine whether these possible mechanistic events might occur following exposure to perylene.
Inhalation Exposure
No repeat-exposure inhalation toxicity studies or acute inhalation lethality studies are available for perylene. Inhalation toxicity values for candidate analogues are presented in Table A-6. Of the candidate analogues, only BaP has an inhalation toxicity value, which is based on developmental effects (decreased embryo/fetal survival) (U.S. EPA, 2017a). No inhalation toxicity values were developed for pyrene due to lack of adequate data (U.S. EPA, 2007). Additional adverse toxicological effects have been identified for BaP at concentrations higher than the lowest LOAEL associated with developmental effects (0.025 mg/m3), including reproductive toxicity at ≥0.075 mg/m3 and neurodevelopmental effects at 0.1 mg/m3 (only concentration evaluated for neurodevelopmental effects) (U.S. EPA, 2017a, b).
Table A-6Comparison of Available Inhalation Toxicity Values for Perylene (CASRN 198-55-0) and Candidate Structural Analogues
| Chemical | Perylene | BaP | Pyrene |
|---|---|---|---|
| Structure |

|
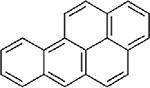
|
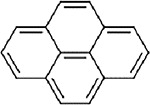
|
| CASRN | 198-55-0 | 50-32-8 | 129-00-0 |
| Subchronic inhalation toxicity values | |||
| POD (mg/m3) | ND | The POD for the chronic RfC (0.0046 mg/m3) is also applicable to subchronic exposure because it is based on a developmental study (see further details below) | ND |
| POD type | ND | ND | ND |
| Subchronic UFc | ND | ND | ND |
| Subchronic p-RfC (mg/m3) | ND | ND | ND |
| Critical effects | ND | ND | ND |
| Species | ND | ND | ND |
| Duration | ND | ND | ND |
| Route (method) | ND | ND | ND |
| Source | NA | NA | U.S. EPA (2007) (PPRTV) |
| Chronic inhalation toxicity values | |||
| POD (mg/m3) | ND | 0.0046 | ND |
| POD type | ND | LOAEL | ND |
| Chronic UFc | ND | 3,000 (UFH, UFA, UFL, UFD) | ND |
| Chronic p-RfC/RfC (mg/m3) | ND | 2 × 10−6 | ND |
| Critical effects | ND | Decreased embryo/fetal survival | ND |
| Species | ND | Rat | ND |
| Duration | ND | GDs 11–20 | ND |
| Route (method) | ND | Inhalation (nose-only) | ND |
| Source | NA | U.S. EPA (2017a) (IRIS) | U.S. EPA (2007) (PPRTV) |
| Acute inhalation lethality data | |||
| Oral LC50 (mg/m3) | ND | ND | ND |
| Toxicity at LC50 | ND | ND | ND |
| Source | NLM (2022c) | NLM (2022b); U.S. EPA (2017a) | NLM (2022d); U.S. EPA (2007) |
BaP = benzo[a]pyrene; GD = gestation day; IRIS = Integrated Risk Information System; LC50 = median lethal concentration; LOAEL = lowest-observed-adverse-effect level; NA = not applicable; ND = no data; POD = point of departure; NOAEL = no-observed-adverse-effect level; PPRTV = Provisional Peer-Revised Toxicity Value; p-RfC = provisional reference concentration; RfC = reference concentration; UFA = interspecies uncertainty factor; UFC = composite uncertainty factor; UFD = database uncertainty factor; UFH = intraspecies uncertainty factor; UFL = LoAEL-to-NOAEL uncertainty factor.
As described in U.S. EPA (2017a, 2017b), the U.S. EPA IRIS assessment derived candidate reference concentration (RfC) values for BaP for both reproductive and developmental effects. The overall RfC, based on decreased embryo/fetal survival following prenatal inhalation exposure, was considered protective of all types of health effects. The MOA for BaP-mediated developmental effects is unknown. Possible mechanisms may include covalent protein binding of oxidative metabolites, oxidative stress, and formation of reactive oxygen species; AhR-mediated effects on cell growth and differentiation; stimulation of apoptosis; disrupted development of fetal vascular system (resulting in impaired fetal nutrition); developmental immune dysfunction; and alterations in maternal hormones/hormone receptors following exposure during gestation (U.S. EPA, 2017a; IARC, 2010; Archibong et al., 2002; ATSDR, 1995). Based on structural alerts only, perylene would also be expected to show covalent protein binding of oxidative metabolites, AhR-mediated effects, and alterations in progesterone and estrogen binding. However, in vitro data suggest that perylene is a weak AhR inducer, whereas BaP is a strong AhR inducer (see Section 2.3). In the absence of repeated-exposure inhalation toxicity data for perylene, there is no information with which to clearly identify or rule out BaP as an appropriate analogue based on toxicity comparisons.
Weight-of-Evidence Approach
A tiered WOE approach as described in Wang et al. (2012) was used to select the overall best analogue chemical. The approach focuses on identifying a preferred candidate for three types of analogues: structural analogues, toxicokinetic or metabolic analogues, and toxicity-like analogues. Selection of the overall best analogue chemical is then based on all of the information from the three analogue types, and the following considerations used in a WOE approach: (1) lines of evidence from the U.S. EPA assessments are preferred; (2) biological and toxicokinetic data are preferred over the structural similarity comparisons; (3) lines of evidence that indicate pertinence to humans are preferred; (4) chronic studies are preferred over subchronic studies when selecting an analogue for a chronic value; (5) chemicals with more conservative/health-protective toxicity values may be favored; and (6) if there are no clear indications as to the best analogue chemical based on the other considerations, then the candidate analogue with the most structural similarity may be preferred.
BaP is the closest analogue based on structural characteristics and physicochemical properties. Perylene and BaP both contain five benzene rings, whereas pyrene contains only four benzene rings. Physicochemical properties (see Table A-3) suggest that pyrene is more volatile and water soluble than perylene or BaP. In addition, log Kow values suggest that partitioning to fat is greater for perylene and BaP compared to pyrene. Perylene and BaP also share more common structural alerts from models than perylene and pyrene. There are no toxicokinetic data available for perylene, but in general, PAHs as a class show similar toxicokinetic profiles (e.g., CYP450 oxidation). Therefore, both BaP and pyrene are suitable toxicokinetic analogues. There are no oral or inhalation data available for perylene. Therefore, toxicity comparisons provide little basis for assessment of candidate analogues. For oral exposure, critical targets of toxicity were neurodevelopmental effects for BaP and kidney effects for pyrene. The neurodevelopmental effects of BaP provide the most sensitive measure of toxicity among the candidate analogue compounds, making BaP a health-protective analogue. For inhalation exposure, BaP is the only candidate analogue compound with an inhalation toxicity value, which is based on developmental effects.
In summary, both BaP and pyrene are considered suitable analogues. However, BaP is the closest analogue based on structural and physicochemical properties and provides the most health-protective oral toxicity value and the only inhalation toxicity value. Therefore, BaP is selected as the analogue compound for both oral and inhalation screening toxicity values.
ORAL NONCANCER TOXICITY VALUES
Derivation of a Screening Subchronic Provisional Reference Dose
Based on the overall analogue approach presented in this PPRTV assessment, BaP is selected as the analogue for perylene for derivation of screening subchronic and chronic provisional reference doses (p-RfDs). The study used for the U.S. EPA screening subchronic and chronic p-RfD values for perylene is an early postnatal gavage study of BaP in rats (U.S. EPA, 2017a; Chen et al., 2012). The Toxicological Review of Benzo[a]pyrene (CASRN 50-32-8): Supplemental Information (U.S. EPA, 2017b) provided the following summary:
Chen et al. (2012) treated male and female neonatal Sprague-Dawley rats (10/sex/group) with benzo[a]pyrene (unspecified purity) dissolved in peanut oil by gavage daily on PNDs 5–11, at doses of 0.02, 0.2, or 2 mg/kg in 3 mL vehicle/kg body weight, determined individually based upon daily measurements. This time period was described as representing the brain growth spurt in rodents, analogous to brain developmental occurring from the third trimester to 2 years of age in human infants. Breeding was performed by pairs of 9-week-old rats, with delivery designated as PND 0. Litters were culled to eight pups/dam (four males and four females, when possible) and randomly redistributed at PND 1 among the nursing dams; dams themselves were rotated every 2–3 days to control for caretaking differences, and cage-side observations of maternal behavior were made daily. One male and female from each litter were assigned per treatment group, and the following physical maturation landmarks were assessed daily in all treatment groups until weaning at PND 21: incisor eruption, eye opening, development of fur, testis decent, and vaginal opening.
Neonatal sensory and motor developmental tests were administered to pups during the preweaning period at PNDs 12, 14, 16, and 18, and were behavioral tests administered to rats as adolescents (PNDs 35 and 36) or as adults (PNDs 70 and 71): each rat was only tested during one developmental period. All dosing was performed from 1,300 to 1,600 hours, and behavioral testing was during the “dark” period from 1,900 to 2,300 hours, although tests were performed in a lighted environment. Pups were observed individually and weighed daily, the order of testing litters was randomized each day, and all observations were recorded by investigators blinded to group treatment.
Sensory and motor developmental tests, including the surface righting reflex test, negative geotaxis test, and cliff aversion test, were performed only once, while the forelimb grip strength test was assessed during three 60-second trials on PND 12. Rat movements during the open-field test were recorded by camera, and two blinded investigators scored movement and rearing separately during a 5-minute evaluation period. Blinded investigators directly observed video monitoring of rat movements during the elevated plus maze, and after a 5-minute free exploration period, recorded number of entries into the closed and open arms, time spent in the open arms, and latency to the first arm entry.
Assessment of the Morris water maze was slightly different, in that the rats were habituated to the testing pool by a 60-second swim without a platform on the day prior to testing. The rats were then tested during a 60-second swim with a hidden platform present at a constant position each day for 4 days; on the 5th day, the rats were evaluated during a 60-second probe swim without a platform. The number of times each animal crossed the original platform location and the duration of time spent in the platform quadrant were recorded during this final evaluation. One pup/sex/litter were assigned for behavioral testing to each of four tracks: Track 1, surface righting reflex test, cliff aversion test, and open-field test (PNDs 12–18); Track 2, negative geotaxis test, forelimb grip strength test, and open-field test (PNDs 12–20); Track 3, elevated plus maze, Morris water maze, and open-field test (PNDs 34–36); and Track 4, elevated plus maze, Morris water maze, and open-field test (PNDs 69–71). All results were presented in graphic form only.
No significant effects on pup body weight were observed during the 7-day treatment period (PNDs 5–11). Three-way ANOVA (time × benzo[a]pyrene treatment × sex) indicated that effects of benzo[a]pyrene were not sex-dependent throughout the 71-day experiment, so both sexes were pooled together. From this pooled analysis, pups in the 2 mg/kg-day treatment group gained significantly less weight at both PND 36 and 71. There were no differences among treatment groups in incisor eruption, eye opening, development of fur, testis decent, or vaginal opening.
For all measurements of neonatal sensory and motor development, results from both sexes were analyzed together since benzo[a]pyrene was reported to have no significant interaction with sex by 3-way ANOVA. No significant differences were observed in either the cliff aversion or forelimb grip strength tests. In the surface righting reflex test, latency was increased in the 0.2 mg/kg-day group at PND 12, in the 0.02 and 2 mg/kg-day groups at PND 14, and in only the high-dose group at PND 16; latency was not significantly different in any group at PND 18. At PND 12, there was a dose-related increase in negative geotaxis latency associated with 0.02, 2, and 2 mg/kg-day benzo[a]pyrene, which was also present in the 2 mg/kg-day group at PND 14, but returned to control levels at PND 16 and 18. In the open field test, there were no significant differences in either locomotion or rearing activity at PND 18 or 20. At PND 34, the 2 mg/kg-day group exhibited significantly increased movement, but increases in rearing were not significant. At PND 69, increased locomotion was observed in both the 0.2 and 2 mg/kg-day groups, while rearing was significantly increased in only the 2 mg/kg-day treatment group.
The elevated plus maze performance was only evaluated in adolescent and adult rats. Unlike the previous tests, 3-way ANOVA revealed a statistically significant interaction between neonatal benzo[a]pyrene treatment and sex, so male and female performance was analyzed independently. No significant differences in PND 35 males were observed, and the only significant observation in PND 35 females was increased time spent in the open maze arms by the 2 mg/kg-day treatment group. Significantly decreased latency time to first open arm entry was observed in PND 70 males and females in both 0.2 and 2 mg/kg-day treatment groups; these groups also spent significantly more time in open maze arms, along with the 0.02 mg/kg-day female group. At PND 70, the 2 mg/kg-day males, along with the 0.2 and 2 mg/kg-day females, entered more frequently into open arms and less frequently into closed arms than the vehicle controls. In the Morris water maze, escape latency (time to reach the platform during each of the four testing days) was consistently increased in the 2 mg/kg-day treatment group of both sexes, in both adolescent and adult animals. These increases were statistically significant in both males and females treated with 2 mg/kg-day benzo[a]pyrene at both PNDs 39 and 74, and were also significantly elevated in 0.2 mg/kg-day animals of both sexes at PND 74. Likewise, performance during the 5th test day, in the absence of the escape platform, was significantly adversely affected by both metrics (decreased time spent in the target quadrant and decreased number of attempts to cross the platform location) in 2 mg/kg-day rats of both sexes at both PNDs 40 and 75. PND 75 females treated with 0.2 mg/kg-day benzo[a]pyrene also showed significant decreases in both performance metrics, while PND 75 0.2 mg/kg-day males only demonstrated significant differences in “time spent in target quadrant.” Swim speed was also assessed, but there were no differences among any treatment group at either age evaluated.
The benchmark dose lower confidence limit with one standard deviation (BMDL1SD) of 0.092 mg/kg-day was identified as the POD for BaP based on neurobehavioral effects during a susceptible life stage (U.S. EPA, 2017a). This POD is selected from among a suite of available endpoints because it represents multiple neurobehavioral endpoints from several behavioral tests (i.e., Morris water maze, elevated plus maze, and open field test). Similar effects were replicated among numerous additional studies. As described in U.S. EPA (2017a): “modeling for each of the three endpoints resulted in BMDL1SD values that clustered in the range 0.092–0.16 mg/kg-day. The lower end of this range of BMDLs, 0.092 mg/kg-day, was selected to represent the point of departure (POD) from these three endpoints for RfD derivation.” Thus, the BMDLs representing different behavioral manifestations of neurotoxicity were considered together to define the POD for neurobehavioral changes. The U.S. EPA (2017a) did not convert the POD into a human equivalent dose (HED) using BW3/4 because the critical study evaluated developmental toxicity in early postnatal animals directly exposed to BaP. BW3/4 scaling was determined to be inappropriate because: (1) it is unknown if allometric scaling derived from adult animals is appropriate for extrapolating doses in neonates in the absence of quantitative toxicokinetic and toxicodynamic differences; and (2) differences in temporal patterns of development across species result in complications for interspecies dose extrapolation.
The RfD for BaP is derived using a composite uncertainty factor (UFC) of 300, reflecting 10-fold uncertainty factors for interspecies extrapolation and intraspecies variability (UFA and UFH, respectively) and a 3-fold uncertainty factor for database uncertainties (UFD) (U.S. EPA, 2017a). Wang et al. (2012) indicated that the uncertainty factors typically applied in deriving a toxicity value for the chemical of concern are the same as those applied to the analogue unless additional information is available. To derive the screening subchronic p-RfD for perylene from the BaP data, the UFD of 3 is increased to 10 to account for the absence of repeat-dose oral toxicity data for perylene.
Table A-7 summarizes the uncertainty factors for the screening subchronic p-RfD for perylene.
Table A-7Uncertainty Factors for the Screening Subchronic p-RfD for Perylene (CASRN 198-55-0)
| UF | Value | Justification |
|---|---|---|
| UFA | 10 | A UFA of 10 is applied to account for uncertainty associated with extrapolating from animals to humans when no cross-species dosimetric adjustment (HED calculation) is performed. |
| UFD | 10 | A UFD of 10 is applied to reflect database limitations for the BaP analogue and the absence of repeat-dose and reproductive/developmental toxicity data for perylene. |
| UFH | 10 | A UFH of 10 is applied for interindividual variability to account for human-to-human variability in susceptibility in the absence of quantitative information to assess the toxicokinetics and toxicodynamics of perylene in humans. |
| UFL | 1 | A UFL of 1 is applied for LOAEL-to-NOAEL extrapolation because the POD is a BMDL. |
| UFS | 1 | A UFS of 1 is applied because a developmental study was selected as the principal study. The developmental period is recognized as a susceptible life stage when exposure during a time window of development is more relevant to the induction of developmental effects than lifetime exposure (U.S. EPA, 1991). |
| UFC | 1,000 | Composite UF = UFA × UFH × UFD × UFL × UFS. |
BaP = benzo[a]pyrene; BMDL = benchmark dose lower confidence limit; HED = human equivalent dose; LOAEL = lowest-observed-adverse-effect level; NOAEL = no-observed-adverse-effect level; POD = point of departure; p-RfD = provisional reference dose; UF = uncertainty factor; UFA = interspecies uncertainty factor; UFC = composite uncertainty factor; UFD = database uncertainty factor; UFH = intraspecies uncertainty factor; UFL = LOAEL-to-NOAEL uncertainty factor; UFS = subchronic-to-chronic uncertainty factor.
Derivation of a Screening Chronic Provisional Reference Dose
BaP is also selected as the analogue for perylene for derivation of a screening chronic p-RfD. The key study and calculation of the POD are described above for the subchronic p-RfD. In deriving the screening chronic p-RfD for perylene, the same uncertainty factors used for the screening subchronic p-RfD (UFA of 10, UFH of 10, and UFD of 10) are applied. An additional uncertainty factor for study duration is not applied because a developmental study is used as the principal study.
Table A-8 summarizes the uncertainty factors for the screening chronic p-RfD for perylene.
Table A-8Uncertainty Factors for the Screening Chronic p-RfD for Perylene (CASRN 198-55-0)
| UF | Value | Justification |
|---|---|---|
| UFA | 10 | A UFA of 10 is applied to account for uncertainty associated with extrapolating from animals to humans when no cross-species dosimetric adjustment (HED calculation) is performed. |
| UFD | 10 | A UFD of 10 was applied to reflect database limitations for the BaP analogue and the absence of repeat-dose and reproductive/developmental toxicity data for perylene. |
| UFH | 10 | A UFH of 10 is applied for interindividual variability to account for human-to-human variability in susceptibility in the absence of quantitative information to assess the toxicokinetics and toxicodynamics of perylene in humans. |
| UFL | 1 | A UFL of 1 is applied for LOAEL-to-NOAEL extrapolation because the POD is a BMDL. |
| UFS | 1 | A UFS of 1 is applied because a developmental study was selected as the principal study. The developmental period is recognized as a susceptible life stage when exposure during a time window of development is more relevant to the induction of developmental effects than lifetime exposure (U.S. EPA, 1991). |
| UFC | 1,000 | Composite UF = UFA × UFH × UFD × UFL × UFS. |
BaP = benzo[a]pyrene; BMDL = benchmark dose lower confidence limit; HED = human equivalent dose; LOAEL = lowest-observed-adverse-effect level; NOAEL = no-observed-adverse-effect level; POD = point of departure; p-RfD = provisional reference dose; UF = uncertainty factor; UFA = interspecies uncertainty factor; UFC = composite uncertainty factor; UFD = database uncertainty factor; UFH = intraspecies uncertainty factor; UFL = LOAEL-to-NOAEL uncertainty factor; UFS = subchronic-to-chronic uncertainty factor.
INHALATION NONCANCER TOXICITY VALUES
Derivation of a Screening Subchronic Provisional Reference Concentration
Based on the overall analogue approach presented in this PPRTV assessment, BaP is selected as the analogue for perylene for deriving the screening subchronic and chronic provisional reference concentrations (p-RfCs). The study used for the U.S. EPA screening subchronic and chronic p-RfC values for perylene is a prenatal inhalation study of BaP in rats (U.S. EPA, 2017a; Archibong et al., 2002). The Toxicological Review of Benzo[a]pyrene (CASRN 50-32-8): Supplemental Information (U.S. EPA, 2017b) provided the following summary:
Archibong et al. (2002) evaluated the effect of exposure to inhaled benzo[a]pyrene on fetal survival and luteal maintenance in timed-pregnant F344 rats. Prior to exposure on GD 8, laparotomy was performed to determine the number of implantation sites, and confirmed pregnant rats were divided into three groups, consisting of rats that had four to six, seven to nine, or more than nine conceptuses in utero. Rats in these groups were then assigned randomly to the treatment groups or control groups to ensure a similar distribution of litter sizes. Animals (10/group) were exposed to benzo[a]pyrene:carbon black aerosols at concentrations of 25, 75, or 100 μg/m3 via nose-only inhalation, 4 hours/day on GDs 11–20. Control animals were either sham-exposed to carbon black or remained entirely unexposed. Results of particle size analysis of generated aerosols were reported by several other reports from this laboratory (Inyang et al., 2003; Ramesh et al., 2001a; Hood et al., 2000). Aerosols showed a trimodal distribution (average of cumulative mass, diameter) <95%, 15.85 μm; 89%, <10 μm; 55%, <2.5 μm; and 38%, <1 μm (Inyang et al., 2003). Ramesh et al. (2001a) reported that the MMAD (± geometric SD) for the 55% mass fraction with diameters <2.5 μm was 1.7 ± 0.085. Progesterone, estradiol-17β, and prolactin concentrations were determined in plasma collected on GDs 15 and 17. Fetal survival was calculated as the total number of pups divided by the number of all implantation sites determined on GD 8. Individual pup weights and crown-rump length per litter per treatment were determined on PND 4 (PND 0 = day of parturition).
Archibong et al. (2002) reported that exposure of rats to benzo[a]pyrene caused biologically and statistically significant (p ≤ 0.05) reductions in fetal survival compared with the two control groups; fetal survival rates were 78.3, 38.0, and 33.8% per litter at 25, 75, and 100 μg/m3, respectively, and 96.7% with carbon black or 98.8% per litter in untreated controls (see Table D-30). Consequently, the number of pups per litter was also decreased in a concentration-dependent manner. The decrease was ∼50% at 75 μg/m3 and ∼65% at 100 μg/m3, compared with sham-exposed and unexposed control groups. No effects on hormone levels were observed on GDs 15 or 17 at the low dose. Biologically significant decreases in mean pup weights (expressed as g per litter) of >5% relative to the untreated control group were observed at doses ≥75 μg/m3 (14 and 16% decreases at 75 and 100 μg/m3, respectively, p < 0.05). There were no statistically significant differences from the control groups in crown-rump length (see Table D-30).
Benzo[a]pyrene exposure at 75 μg/m3 caused a statistically significant decrease in plasma progesterone, estradiol, and prolactin on GD 17; these levels were not determined in the rats exposed to 100 μg/m3 (Archibong et al., 2002). Plasma prolactin is an indirect measure of the activity of decidual luteotropin, a prolactin-like hormone whose activity is necessary for luteal maintenance during pregnancy in rats. Control levels of prolactin increased from GD 15 to 17, but this increase did not occur in the rats exposed to 75 μg/m3. Although the progesterone concentration at 75 μg/m3 was significantly lower than in controls on GD 17, the authors thought that the circulating levels should have been sufficient to maintain pregnancy; thus, the increased loss of fetuses was thought to be caused by the lower prolactin levels rather than progesterone deficiency. The reduced circulating levels of progesterone and estradiol-17β among benzo[a]pyrene-treated rats were thought to be a result of limited decidual luteotropic support for the corpora lutea. The authors proposed the following mechanism for the effects of benzo[a]pyrene on fertility: benzo[a]pyrene or its metabolites decreased prolactin and decidual luteotropin levels, compromising the luteotropic support for the corpora lutea and thereby decreasing the plasma levels of progesterone and estradiol-17β. The low estradiol-17β may decrease uterine levels of progesterone receptors, thereby resulting in fetal mortality. Based on biologically and statistically significant decreases in pups/litter and percent fetal survival/per litter, the LOAEL was 25 μg/m3; no NOAEL was identified.
The LOAEL of 25 μg/m3 for decreased embryo/fetal survival was selected as the POD for BaP (U.S. EPA, 2017a). The POD was converted into a LOAELHEC of 4.6 μg/m3 (0.0046 mg/m3) by the U.S. EPA (2017a):
By definition, the RfC is intended to apply to continuous lifetime exposures for humans (U.S. EPA, 1994a). EPA recommends that adjusted continuous exposures be used for developmental toxicity studies by the inhalation route as well as for inhalation studies of longer durations (U.S. EPA, 2002). The PODs were adjusted to account for the discontinuous daily exposure as follows:
Next, the human equivalent concentration (HEC) was calculated from the PODADJ by multiplying by a DAF, which, in this case, was the regional deposited dose ratio (RDDRER) for extrarespiratory (i.e., systemic) effects as described in Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry (U.S. EPA, 1994a). The observed developmental effects are considered systemic in nature (i.e., extrarespiratory) and the normalizing factor for extrarespiratory effects of particles is body weight (i.e., the equivalent dose across species is mass deposited in the entire respiratory tract per unit body weight). The RDDRER was calculated as follows:
where:
The total fractional deposition includes particle deposition in the nasal-pharyngeal, tracheobronchial, and pulmonary regions. FTOT for both animals and humans was calculated using the Multi-Path Particle Dosimetry (MPPD) model, a computational model used for estimating human and rat airway particle deposition (MPPD; Version 2.0 © 2006, as accessed through the former Hamner Institute; now publicly available through Applied Research Associates). FTOT was based on the average particle size of 1.7 ± 0.085 μm (mass median aerodynamic diameter [MMAD] ± geometric SD) as reported in Wu et al. (2003a) for the exposure range 25–100 μm3. For the model runs, the Yeh-Schum 5-lobe model was used for the human and the asymmetric multiple path model was used for the rat (see Appendix E for MPPD model output). Both models were run under nasal breathing scenarios after adjusting for inhalability. A geometric SD of 1 was used as the default by the model because the reported geometric SD of 0.085 was ≤1.05.
The human parameters used in the model for calculating FTOT and in the subsequent calculation of the PODHEC were as follows: human body weight, 70 kg; VE, 13.8 L/minute; breathing frequency, 16 per minute; tidal volume, 860 mL; functional residual capacity, 3,300 mL; and upper respiratory tract volume, 50 mL. Although the most sensitive population in Archibong et al. (2002) is the developing fetus, the adult rat dams were directly exposed. Thus, adult rat parameters were used in the calculation of the HEC. The parameters used for the rat were body weight, 0.25 kg (a generic weight for male and female rats); VE, 0.18 L/minute; breathing frequency, 102 per minute; tidal volume, 1.8 mL; functional residual capacity, 4 mL; and upper respiratory tract volume, 0.42 mL. All other parameters were set to default values (see Appendix E).
Under these conditions, the MPPD model calculated FTOT values of 0.621 for the human and 0.181 for the rat. Using the above equation, the RDDRER was calculated to be 1.1.
From this, the PODHEC was calculated as follows:
The RfC for BaP is derived from the LOAELHEC of 0.0046 mg/m3 using a UFC of 3,000, reflecting a 10-fold LOAEL-to-no-observed-adverse-effect level (NOAEL) uncertainty factor (UFL), UFH, and UFD and a 3-fold UFA (U.S. EPA, 2017a). Wang et al. (2012) indicated that the uncertainty factors typically applied in deriving a toxicity value for the chemical of concern are the same as those applied to the analogue unless additional information is available. Given the limitations of the current database, the uncertainty factors for BaP were adopted for perylene.
Table A-9 summarizes the uncertainty factors for the screening subchronic p-RfC for perylene.
Table A-9Uncertainty Factors for the Screening Subchronic p-RfC for Perylene (CASRN 198-55-0)
| UF | Value | Justification |
|---|---|---|
| UFA | 3 | A UFA of 3 (100.5) is applied to account for uncertainty associated with extrapolating from animals to humans, using toxicokinetic cross-species dosimetric adjustment (HEC calculation) as specified in the U.S. EPA (1994) guidelines. |
| UFD | 10 | A UFD of 10 is applied to reflect the database limitations for the BaP analogue and the absence of repeat-dose and reproductive/developmental toxicity data for perylene. |
| UFH | 10 | A UFH of 10 is applied for interindividual variability to account for human-to-human variability in susceptibility in the absence of quantitative information to assess the toxicokinetics and toxicodynamics of perylene in humans. |
| UFL | 10 | A UFL of 10 is applied for LOAEL-to-NOAEL extrapolation because the POD is a LOAEL. |
| UFS | 1 | A UFS of 1 is applied because a developmental study was selected as the principal study. The developmental period is recognized as a susceptible life stage when exposure during a time window of development is more relevant to the induction of developmental effects than lifetime exposure (U.S. EPA, 1991). |
| UFC | 3,000 | Composite UF = UFA × UFH × UFD × UFL × UFS. |
BaP = benzo[a]pyrene; HEC = human equivalent concentration; LOAEL = lowest-observed-adverse-effect level; NOAEL = no-observed-adverse-effect level; POD = point of departure; p-RfC = provisional reference concentration; UF = uncertainty factor; UFA = interspecies uncertainty factor; UFC = composite uncertainty factor; UFD = database uncertainty factor; UFH = intraspecies uncertainty factor; UFL = LOAEL-to-NOAEL uncertainty factor; UFS = subchronic-to-chronic uncertainty factor.
Derivation of a Screening Chronic Provisional Reference Concentration
BaP is also selected as the analogue for perylene for derivation of a screening chronic p-RfC. The key study and calculation of the POD are described above for the subchronic p-RfC. In deriving the screening chronic p-RfC for perylene, the same uncertainty factors used for the screening subchronic p-RfC (UFA of 3, UFH of 10, UFD of 10, UFL of 10) are applied. An additional uncertainty factor for study duration is not applied because a developmental study is used as the principal study.
Table A-10 summarizes the uncertainty factors for the screening chronic p-RfC for perylene.
Table A-10Uncertainty Factors for the Screening Chronic p-RfC for Perylene (CASRN 198-55-0)
| UF | Value | Justification |
|---|---|---|
| UFA | 3 | A UFA of 3 is applied to account for uncertainty associated with extrapolating from animals to humans when a cross-species dosimetric adjustment (HEC calculation) as specified in the U.S. EPA (1994) guidelines. |
| UFD | 10 | A UFD of 10 is applied to reflect the database limitations for the BaP analogue and the absence of repeat-dose and reproductive/developmental toxicity data for perylene. |
| UFH | 10 | A UFH of 10 is applied for interindividual variability to account for human-to-human variability in susceptibility in the absence of quantitative information to assess the toxicokinetics and toxicodynamics of perylene in humans. |
| UFL | 10 | A UFL of 10 is applied for LOAEL-to-NOAEL extrapolation because the POD is a LOAEL. |
| UFS | 1 | A UFS of 1 is applied because a developmental study was selected as the principal study. The developmental period is recognized as a susceptible life stage when exposure during a time window of development is more relevant to the induction of developmental effects than lifetime exposure (U.S. EPA, 1991). |
| UFC | 3,000 | Composite UF = UFA × UFH × UFD × UFL × UFS. |
BaP = benzo[a]pyrene; HEC = human equivalent concentration; LOAEL = lowest-observed-adverse-effect level; NOAEL = no-observed-adverse-effect level; POD = point of departure; p-RfC = provisional reference concentration; UF = uncertainty factor; UFA = interspecies uncertainty factor; UFC = composite uncertainty factor; UFD = database uncertainty factor; UFH = intraspecies uncertainty factor; UFL = LOAEL-to-NOAEL uncertainty factor; UFS = subchronic-to-chronic uncertainty factor.
- SCREENING NONCANCER PROVISIONAL VALUES - Provisional Peer-Reviewed Toxicity Valu...SCREENING NONCANCER PROVISIONAL VALUES - Provisional Peer-Reviewed Toxicity Values for Perylene (CASRN 198-55-0)
- COMMONLY USED ABBREVIATIONS AND ACRONYMS - Provisional Peer-Reviewed Toxicity Va...COMMONLY USED ABBREVIATIONS AND ACRONYMS - Provisional Peer-Reviewed Toxicity Values for Perylene (CASRN 198-55-0)
- DERIVATION OF PROVISIONAL VALUES - Provisional Peer-Reviewed Toxicity Values for...DERIVATION OF PROVISIONAL VALUES - Provisional Peer-Reviewed Toxicity Values for Perylene (CASRN 198-55-0)
- REVIEW OF POTENTIALLY RELEVANT DATA (NONCANCER AND CANCER) - Provisional Peer-Re...REVIEW OF POTENTIALLY RELEVANT DATA (NONCANCER AND CANCER) - Provisional Peer-Reviewed Toxicity Values for Perylene (CASRN 198-55-0)
- Homo sapiens protein phosphatase 1 regulatory subunit 8 (PPP1R8), transcript var...Homo sapiens protein phosphatase 1 regulatory subunit 8 (PPP1R8), transcript variant 1, mRNAgi|1519245064|ref|NM_014110.5|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...