Corresponding author: Dorairaj Prabhakaran, Centre for Chronic Disease Control, Public Health Foundation of India, Gurgaon, India; gro.aidnicdcc@narakahbarpd.
Introduction
Current and Projected Burden of Tobacco and Cardiovascular Diseases
Tobacco use is a leading global cause of death, accounting for more than 6 million deaths annually or at least 12 percent of deaths among people age 30 years and older (16 percent for men, 7 percent for women) (WHO 2012, 2013). It is the single most preventable cause of cardiovascular diseases (CVDs), which comprise a large number of conditions and are the leading cause of death globally, accounting for an estimated 17.3 million to 17.5 million deaths yearly (Naghavi and others 2015; WHO 2015a). Tobacco is also the leading cause of premature death from CVD (deaths before age 70 years), accounting for an estimated 5.9 million premature deaths in 2013 (Roth, Nguyen, and others 2015). Such deaths deprive families of productive members, and communities and economies of a productive workforce (Rigotti and Clair 2013). Tobacco use also causes substantial morbidity and results in tremendous health care costs related to CVD. Although tobacco use affects all countries regardless of their level of economic or health system development, the impact is most profound in low- and middle-income countries (LMICs), which shoulder the largest share of total and premature deaths from CVD globally (WHO 2015a). Future projections are alarming, with LMICs accounting for much of the future global burden of tobacco use and related CVD mortality and morbidity (Ezzati and Lopez 2003). China (with 301 million tobacco users) and India have the highest burden of tobacco use in the world (WHO n.d.).
Generally, high rates of tobacco use mean a higher burden of CVD. This association is compounded by population growth and aging, both of which are major contributors to the absolute number of CVD sufferers (Roth, Nguyen, and others 2015).
Reducing tobacco use is thus crucial to averting tobacco deaths, which are projected to increase to 10 million annually by 2030 if current trends continue (WHO 2013, 2015a). Premature deaths from CVDs are also projected to increase to 7.8 million in 2025 if business as usual continues, including in the approach to controlling tobacco use and preventing noncommunicable diseases (Roth, Nguyen, and others 2015). Urgent action is needed to halt and reverse this course.
Mandate and Opportunity for Action
The global mandate for reducing tobacco use is stronger than ever, with the World Health Assembly’s adoption of a 30 percent global target for relative reduction of tobacco use by 2025. This goal is one among several targets to reduce premature mortality from four noncommunicable diseases, including CVD, by 25 percent by 2025 (the 25×25 target). Unfortunately, many countries are not on course to meet this target (Bilano and others 2015).
Studies clearly show that reducing tobacco use is key to achieving these targets (Kontis and others 2014; Kontis and others 2015). Reducing tobacco use would offset some of the increase in the absolute number of cardiovascular deaths caused by population growth and aging, especially in LMICs (Roth, Forouzanfar, and others 2015). Indeed, several studies demonstrate the need to achieve more ambitious targets for reducing tobacco use (50 percent relative to 2010) if countries are to reach the 25×25 target (Kontis and others 2014; Kontis and others 2015; Roth, Nguyen, and others 2015).
This chapter reviews the literature to synthesize key knowledge on the links between tobacco use and CVD. The introduction on burden of CVD attributable to tobacco is followed by a brief review of the main pathophysiological mechanisms by which tobacco use causes CVD. The third section highlights the role of tobacco and other CVD risk factors, and the fourth reviews tobacco-related CVDs that are most important from a public health and health systems perspective. The focus is on cigarette smoking, but other forms are also discussed in the fifth section. This is followed by a section on the socioeconomic dimensions of tobacco use. The seventh section highlights the cardiovascular health benefits of stopping tobacco use. The concluding section calls for enhanced engagement and cooperation of public health and health care providers to stem the rise of tobacco-related CVDs, especially in LMICs.
Tobacco Use and Cardiovascular Disease: Pathophysiology and Mechanisms
Tobacco use has myriad effects on the cardiovascular system that contribute to CVD pathophysiology. Box 4.1 reviews some of the terms used to explain the mechanisms by which tobacco use can cause CVD. The effects of cigarette smoking and exposure to secondhand smoke have been studied most, but many of the effects are common to other forms of use, including smokeless tobacco.

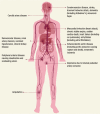
Burning tobacco products produce two forms of smoke: mainstream and sidestream. Mainstream smoke is inhaled and exhaled by the smoker, whereas sidestream smoke comes from the burning end of the cigarette (Ambrose and Barua 2004) and is even more toxic than mainstream smoke (Schick and Glantz 2005). Among the more than 7,000 chemicals in cigarette smoke, many components are known to mediate the pathophysiology of CVD (Borgerding and Klus 2005). Toxic chemicals such as carbon monoxide, polycyclic aromatic hydrocarbons, nicotine, and heavy metals and their oxides have profound effects on vascular endothelium (cells lining the blood vessels), blood lipids (fats), and clotting (thrombotic) factors causing atherosclerosis (plaque buildup). The latter affects arteries (vessels carrying oxygenated blood to organs across various vascular beds). These effects can lead to adverse cardiovascular events such as myocardial infarction (heart attack), stroke (brain attack), and aortic dissection (rupture of the aorta, the main artery emanating from the heart). illustrates the pathophysiological mechanisms implicated in tobacco-associated atherosclerosis.
Pathophysiological Mechanisms of Tobacco-Associated Atherosclerosis.
The mechanisms by which cigarette smoking induces and promotes atherogenesis and, consequently, atherosclerosis and atherothrombosis are complex and interconnected. The key pathways are inflammation, endothelial dysfunction, prothrombosis, altered lipid metabolism, insulin resistance, and increased demand for but diminished supply of myocardial oxygen and blood (demand-supply mismatch) (U.S. Department of Health and Human Services 2014). Smoking is also known to be responsible for increased release of catecholamines, which exert cardiovascular effects such as increased heart rate, vasoconstriction, and increased cardiac output (Cryer and others 1976). displays the key constituents involved in some of these mechanisms.
Overview of Pathophysiological Mechanisms of Tobacco in the Development of Cardiovascular Disease.
Briefly, atherogenesis starts when smoking-activated inflammatory cells adhere to the inner vessel wall (endothelium) that has been damaged by smoking and accumulate under the vessel surface (subendothelium), causing chronic inflammation. This and other mechanisms contribute to endothelial dysfunction. Subendothelial inflammatory cells secrete substances that promote the development and growth of plaque through the accumulation of cholesterol-rich cells. Continued inflammation destabilizes and ruptures some of these plaques, causing vasoconstriction (acute narrowing of arteries) and thrombosis (clots, which are made up mainly of platelets, or thrombocytes, which are components of blood responsible for stopping bleeding). This process can lead to occlusion of blood vessels, causing cardiovascular events such as heart or brain attacks.
Endothelial Dysfunction
A healthy vascular endothelium is crucial to cardiovascular functioning and health. The blood vessels normally dilate in response to external or internal stress and increased demands for flow caused by the endothelium’s production and release of nitric oxide (a vessel relaxant), thus maintaining blood flow. A healthy endothelium also fights thrombosis and inflammation. Smoking undermines all of these functions, making endothelial dysfunction (decreased dilatation and ability to fight thrombosis and inflammation) a central mechanism in CVD pathophysiology.
Nicotine, oxidants, and free radicals in smoke—and free radicals generated by endothelial cells themselves in response to smoke—reduce the availability of nitric oxide; thus, there is either no response to stress or vasoconstriction (Barua and others 2001; Ichiki and others 1996; Kugiyama and others 1996; Salahuddin, Prabhakaran, and Roy 2012; U.S. Department of Health and Human Services 2014; Wolf and Baynes 2007). Vasoconstriction can, in turn, increase the prothrombotic (clotting) response, although this is not the only mechanism for thrombosis. Smoking-induced damage to the endothelium also alters the interaction with flowing blood cells, thus increasing the chances that inflammatory substances and platelets will stick to the vessel wall. In addition, this damage decreases the ability of the endothelium to regulate the local levels of clot-forming versus clot-dissolving substances in favor of clotting (Nowak and others 1987; U.S. Department of Health and Human Services 2014). Smoking also reduces the elasticity of arteries, resulting in stiffening and trauma to their walls and reducing coronary flow reserve (Celermajer and others 1993; Celermajer and others 1996; Stefanadis and others 1997).
Other components are also implicated in endothelial dysfunction, including heavy metals such as lead, arsenic, and mercury, which catalyze the oxidation of cellular proteins and may lead to structural cellular damage and endothelial dysfunction. In addition to free radicals and oxidants, further endothelial dysfunction may be mediated by polycyclic aromatic hydrocarbons (Salahuddin, Prabhakaran, and Roy 2012; Wolf and Baynes 2007). These compounds also enhance oxidation of low-density lipoprotein (LDL), as discussed later in this chapter.
The adverse effects of smoking on endothelial function occur early, with recent studies showing that even brief exposure (one hour or less) to smoke, including secondhand smoke, results in endothelial damage and can potentially be long lasting (Juonala and others 2012). Fortunately, quitting smoking is associated with improved endothelial function (Johnson and others 2010).
Prothrombotic Effects of Smoking
Smoking promotes thrombosis through two mechanisms strongly implicated in adverse cardiovascular events: (1) activation and aggregation (clumping together) of platelets and (2) activation of the coagulation (clotting) system (U.S. Department of Health and Human Services 2014). Although the latter mechanism is important and occurs through increased production of thrombosis factors—such as thrombin, fibrinogen, and von Willebrand factor—and decreased dissolution of blood clots (fibrinolysis), the former is especially critical in CVD pathophysiology. This mechanism is largely responsible for thrombi that form in coronary arteries following plaque rupture and cause heart attacks by blocking arterial blood supply to the myocardium.
Several mechanisms explain the platelet-activating effects of smoking. These mechanisms include elevated levels of platelet-activating substance, which are partly caused by oxidation of phospholipids; impaired release of nitric oxide, which inhibits platelet activation, caused by oxidative stress and endothelial dysfunction (Owens and Mackman 2010; Ruggeri 2000); and increased production of substances that promote platelet aggregation (U.S. Department of Health and Human Services 2014). Fibrinogen levels are known to vary with the number of cigarettes smoked, as do high red blood cell count and blood viscosity (Glantz and Parmley 1991; Kannel, D’Agostino, and Belanger 1987; Powell 1998; Smith and Fischer 2001; Smith and others 1997). Smoking also leads to more binding of platelets to white blood cells, a process that is both proinflammatory and prothrombotic and changes the structure of platelets to make them more susceptible to aggregation.
Lipid Oxidation and Insulin Resistance
Although smoking can enhance the endothelial dysfunction caused directly by elevated cholesterol, smoking produces its major impact through lipid oxidation. Cigarette smoking enhances oxidation of plasma LDL cholesterol, the “bad cholesterol” that is proatherogenic and known to impair endothelial function (Frei and others 1991; Heitzer and others 1996; Pech-Amsellem and others 1996). Evidence from animal models supports smoking-induced atherosclerosis through oxidized LDL products (Yamaguchi and others 2000). Simultaneously, the release of neuromodulators such as catecholamines may result in lipolysis, producing free fatty acids in the blood stream (Muscat and others 1991). These modified lipid products are rapidly engulfed by circulating macrophages to form foam cells. These foam cells are an integral part of the atherosclerosis plaque.
Another product of lipid peroxidation (caused by tobacco) is acrolein, an aldehyde that reacts with lipoproteins in high-density lipoprotein (HDL), the “good cholesterol,” and modifies them, making them unavailable to remove cholesterol from cells lining the vessels (Shao and others 2005). This process undermines a key mechanism that the body uses to fight atherosclerosis.
Smoking is also associated with increased insulin resistance and hyperinsulinemia, which has been implicated in the link with diabetes and the acceleration of atherosclerosis. Insulin resistance often co-occurs with derangement in lipid metabolism. Chronic smoking influences the accumulation of visceral fat, which further aggravates insulin resistance (Chiolero and others 2008). Insulin secretion may also be affected by the direct action of nicotine on beta cells, which secrete insulin (Bruin and others 2008; Stadler and others 2014; Yoshikawa, Hellström-Lindahl, and Grill 2005). Tobacco smoke dysregulates and imbalances other endocrine secretions (catecholamines, growth hormone) that counter the effect of insulin (Kapoor and Jones 2005).
Proinflammatory Effects of Smoking
CVD is now understood to be an inflammatory condition, with inflammation playing a major role in the initiation and progression of atherosclerosis and the development of cardiovascular events. Inflammatory markers are a harbinger of damage to blood vessels and contribute to all of the pathways already mentioned (Kannel, D’Agostino, and Belanger 1987; Matetzky and others 2000; Newby and others 1999).
Large, well-conducted population studies demonstrate that markers of inflammation, including white blood cells, fibrinogens, interleukin-6, and other proteins, are elevated in smokers (Bermudez and others 2002; Smith and Fischer 2001; Tracy and others 1997). Such markers return to normal baseline levels within five years of quitting smoking, as demonstrated in the Northwick Park Heart Study and the Monitoring of Trends and Determinants in Cardiovascular Disease study (Dobson and others 1991; Meade, Imeson, and Stirling 1987). Using data from 15,489 individuals who participated in the third National Health and Nutrition Examination Survey, Bakhru and Erlinger (2005) demonstrated that inflammatory markers—including C-reactive protein, fibrinogen, white cell count, and albumin—have a dose-dependent, temporal relationship to smoking and smoking cessation, with the markers returning to baseline levels five years after smoking cessation. This finding suggests that the inflammatory pathway of smoking-related CVD may be reversible with smoking cessation and reduced exposure to secondhand smoke.
Oxygen Supply-Demand Mismatch
Nicotine and carbon monoxide, among other components of tobacco smoke, also contribute to CVD by affecting the myocardial (heart muscle) oxygen demand-supply balance (the first two pathways in ). Nicotine exerts its cardiometabolic effects through sympathetic stimulation (the adrenaline system) (U.S. Department of Health and Human Services 1988). It increases myocardial oxygen demand by increasing heart rate, blood pressure, and myocardial contractility (pumping), while reducing myocardial blood supply through vasoconstriction and endothelial dysfunction (Salahuddin, Prabhakaran, and Roy 2012). At the same time, stiffness of peripheral arteries and the effect mediated by catecholamines lead to increased myocardial workload. This process results in ischemia (reduced blood and oxygen supply, which, when symptomatic, can produce angina or heart attacks). Carbon monoxide also produces ischemia because it competes with oxygen to combine with hemoglobin, the blood component responsible for carrying oxygen to tissues. Carbon monoxide binds more tightly to hemoglobin and compromises the availability of oxygen to the myocardium (Aronow 1974; Glantz and Parmley 1991).
Role of Genes
Most of the harmful CVD effects of smoking are attributed to the poisonous substances in cigarette smoke. However, genes also influence the impact of smoking, altering the metabolism of the by-products of smoke and playing an intermediate role in other pathophysiologic pathways leading to CVD (Winkelmann, von Holt, and Unverdorben 2009). While genes may play a marginal role in the addiction to smoking and the relationship to CVD, the epigenetic modification of genes may play a larger role in increasing the risk of CVD. Epigenetic modifications by tobacco smoke of several cells of the body lead to damage of the vessel wall, increasing the tendency toward clotting and inflammation, all of which contribute to CVD (Ambrose and Barua 2004; Breitling 2013; Freson, Izzi, and Van Geet 2012; Schleithoff and others 2012; Vinci, Polvani, and Pesce 2013).
In summary, the interplay of alterations in coronary vasoconstriction, endothelial dysfunction, and altered lipids stimulates a cascade of events leading to atherothrombosis and, subsequently, cardiovascular events such as heart attacks.
Tobacco Use and Cardiovascular Disease Risk Factors
Smoking and Dyslipidemia
Compared with nonsmokers, smokers have higher levels of bad cholesterol (LDL) and lower serum concentrations of good cholesterol (HDL). Smokers have 3 percent more cholesterol, 9 percent more triglycerides, and 5.7 percent less HDL (Craig, Palomaki, and Haddow 1989). A clinical trial showed that stopping smoking improved total HDL and the amount of large HDL particles, but that it did not affect LDL cholesterol levels or LDL size (Gepner and others 2011). The combination of smoking and dyslipidemia significantly increases the risk of coronary atherosclerotic disease.
Smoking and Hypertension
Smoking unequivocally increases the cardiovascular risks associated with hypertension. However, the role of smoking in altering blood pressure itself remains unclear, given that observational studies in diverse populations, mostly in high-income countries (HICs), have found no association between smoking and blood pressure (Brummett and others 2011; Green, Jucha, and Luz 1986). Blood pressure rises abruptly after smoking starts, but it returns to presmoking levels within a few hours (Tachmes, Fernandez, and Sackner 1978). Age may modify the link between smoking and blood pressure. A large cross-sectional study from a nationally representative sample of adults in the United Kingdom reported higher systolic blood pressure among older male smokers after adjusting for covariates. This was not the same for young smokers, however, or for diastolic blood pressure levels (Primatesta and others 2001). Ambulatory daytime diastolic blood pressure was also significantly higher, by 5 millimeters of mercury (mmHg, a measure of pressure) among tobacco users over “never-users” age 45 years and older. When daytime heart rates of tobacco users and nonusers were compared, those of the former were significantly higher. The increase in heart rate associated with smoking may be the key factor in the added cardiovascular risk associated with smoking in people with high blood pressure.
Smoking and Diabetes
Smokers have more insulin resistance and are more hyperinsulinemic (higher levels of insulin are postulated to be a precursor of type 2 diabetes) compared with nonsmokers (Facchini and others 1992). Cigarette smoking is a risk factor for the development of type 2 diabetes through two pathways (Eliasson 2003). The first is mediated directly through hyperinsulinemia and insulin resistance. The second is mediated through the accumulation of visceral fat, and the effect is confounded by low physical activity and unhealthy diet (Chiolero and others 2008). Evidence is increasing that smoking causes greater accumulation of visceral fat. Several cross-sectional studies indicate that the waist-to-hip ratio is higher in smokers than in nonsmokers (Bamia and others 2004; Canoy and others 2005). Smokers with diabetes have higher hemoglobin A1c levels (glycated hemoglobin, which indicates long-term control of blood sugar), require more insulin, and have increased risk of vascular complications of diabetes such as kidney disease, blindness, and CVD (Zhu and others 2011).
Insulin resistance, central obesity, and dyslipidemia caused by smoking increase the risk of metabolic syndrome—a constellation of metabolic abnormalities that includes high waist circumference, high blood pressure, abnormal blood sugar, and high lipid levels. The mechanistic link between cigarette smoking and insulin resistance has not been fully established, but evidence exists of a role for nicotine. Sympathetic activation and release of corticosteroids and growth hormone by nicotine may contribute to insulin resistance. A systematic review comprehensively investigated the association between smoking and diabetes using compiled results from 88 prospective studies with more than 5 million participants. The pooled relative risk (RR) of diabetes was 1.37 (95 percent confidence interval [CI] 1.33–1.42) among current active smokers and 1.22 (95 percent CI 1.10–1.35) among passive smokers. The study also highlighted the long-term benefits of cessation in reducing diabetes risk to the same level as that of nonsmokers after 10 or more years of abstinence (RR 1.11, 95 percent CI 1.02–1.20) (Pan and others 2015).
Will and others (2001) found a positive association between frequency of smoking and incidence of diabetes in a large cohort study, the Cancer Prevention Study I. The adjusted incidence density ratio (the ratio of the incidence rate among exposed to the incidence rate among unexposed) increased as the number of packs per day increased, from 1.05 for persons smoking less than one pack a day (95 percent CI 0.98–1.12) to 1.45 for persons smoking two or more packs a day (95 percent CI 1.34–1.57). Results of similar magnitude were reported among women. The risk reversed with an increase in the duration of cessation.
Smoking multiplies the risk of CVD in the presence of each of the three main risk factors. For example, the presence of both high blood pressure and smoking results in a striking 15-fold higher risk of stroke. This relationship is graded and consistent across all levels of blood pressure (Neaton and others 1993). Similar effects have been observed for noncigarette and smokeless forms of tobacco use (Boffetta and Straif 2009; Gupta and Asma 2008; Yusuf and others 2004).
Cardiovascular Disease Outcomes and Manifestations of Tobacco Use
This review focuses on the major CVDs caused by tobacco use, primarily through atherosclerosis of various vessel beds (). The latter include the aorta and vessels originating from it: the coronary arteries (supplying blood to the heart muscle), the carotid and cerebral arteries (supplying blood to the head and brain), and the renal and peripheral arteries (supplying blood to the kidneys and limbs, respectively). Box 4.2 lists the main CVDs and their complications (Ambrose and Barua 2004; Aronow 1974; Borgerding and Klus 2005; Glantz and Parmley 1991; Salahuddin, Prabhakaran, and Roy 2012; U.S. Department of Health and Human Services 1988).
Diagram Representing Cardiovascular Manifestations of Tobacco Use.

Major Cardiovascular Diseases Caused by Tobacco Use.
Concerns about the harmful effects of smoking initially centered on lung diseases, but vascular diseases occur earlier in life and contribute to a substantial number of deaths (Lopez, Collishaw, and Piha 1994; U.S. Department of Health and Human Services 1983). Cigarette smoking is known to increase the risk of CVD, just as it is known to increase the risk of hypertension, hypercholesterolemia, and diabetes (U.S. Department of Health and Human Services 1983).
Smoking and Cardiovascular Mortality
Cigarette smoking increases the rates of all-cause and CVD death (Brummett and others 2011; Qiao and others 2000). The risk of 35-year all-cause mortality and 35-year heart disease mortality is nearly 60 percent higher in smokers than in nonsmokers (Qiao and others 2000). Smoking is an even stronger independent predictor of all-cause and cardiovascular mortality in older adults. Pooled data on more than 500,000 older adults (older than age 60 years) from 25 cohort studies indicate more than a doubling of risk for current smokers and a 37 percent increased risk for former smokers compared with never-smokers (Mons and others 2015).
The seminal work of Doll and others (2004) on the relationship between smoking and cardiovascular mortality followed a large cohort of doctors from 1951 until 2001 to monitor cause-specific mortality and attributed 25 percent of excess risk of death to coronary heart disease (in that cohort). Similarly, in a large cohort of construction workers, age-adjusted coronary heart disease mortality was higher among smokers than nonsmokers and highest among heavy smokers, with almost a doubling of risk (Bolinder and others 1994). Young smokers (younger than age 50 years) were found to have five to six times higher death rates than nonsmokers (Parish and others 1995).
Smoking and Coronary Heart Disease
Coronary (ischemic) heart disease is the most common form of atherosclerotic CVD and is responsible for the largest share of cardiovascular morbidity and mortality. It is also associated with increased risk of sudden death (Aronow 1974; Bolinder and others 1994). Cigarette smoking has been consistently and causally linked to coronary heart disease in prospective studies (Njølstad, Arnesen, and Lund-Larsen 1996; Prescott and others 1998; U.S. Department of Health and Human Services 2010). The INTERHEART study (Yusuf and others 2004), a large international case-control study, showed that smoking tripled the risk of heart attack. The risk was highest among younger patients, in whom tobacco use increased risk more than sevenfold. The risk had a dose-response relationship, increasing linearly with an increase in the number of cigarettes smoked per day and duration of use (Yusuf and others 2004). In addition, women using tobacco lose the gender protection against heart disease noted among women younger than age 50 years (U.S. Department of Health and Human Services 2001). In the Nurses’ Health Study (Kawachi and others 1994), heart disease increased more than fourfold (RR 4.23, 95 percent CI 3.6–4.96) among female nurse smokers over never-smokers. This risk was greatest among those who started smoking before age 15 years (Kawachi and others 1994). In a pooled analysis of more than 2.4 million people, female smokers were 25 percent more likely to develop coronary heart disease than male smokers (RR 1.25, 95 percent CI 1.12–1.39) (King 2011).
Smoking and Heart Failure
Heart failure is a rising global public health challenge. It is associated with either reduced myocardial relaxation (stiffness resulting from ischemia or uncontrolled hypertension) or reduced myocardial pumping (because of previous heart attacks). It may also be associated with smoking-related lung conditions, such as chronic obstructive pulmonary disease. Heart failure is often characterized by dyspnea, fluid retention, weight gain, and peripheral edema. Coronary (ischemic) heart disease, in which smoking plays a major part, is among the most common causes of heart failure. Heart failure is especially prevalent in the elderly and is associated with significant mortality and morbidity, requiring repeated hospitalization. It ranks among the top causes of health care costs in HICs. This portends trouble for LMICs currently experiencing a rise in cardiovascular risk factors and diseases known to increase the risk of heart failure.
A systematic review found that smoking is associated with a 60 percent increased risk of incident heart failure (Yang and others 2015). A similar association was found among older people, with the highest risk among current smokers and a dose-effect association among former smokers. The risk of heart failure increases with the number of cigarettes smoked. Men who smoked more than 15 cigarettes a day were at 2.5 times the risk of heart failure compared with never-smokers (odds ratio 2.31, 95 percent CI 1.58–3.37) (Wilhelmsen and others 2008). Continued cigarette smoking is also associated with increased risk of recurrent heart failure. A study retrospectively charting the admission status in a U.S. Veterans Administration facility found that noncompliance with smoking cessation interventions was a significant predictor of multiple readmissions for heart failure, with 80 percent excess risk (Evangelista, Doering, and Dracup 2000).
Smoking and Carotid and Cerebrovascular Diseases
Similar to its association with heart attack, cigarette smoking is causally associated with stroke (Ambrose and Barua 2004; U.S. Department of Health and Human Services 2014). The INTERSTROKE study—a study involving 3,000 cases and controls in 22 countries and designed to establish associations between various risk factors and stroke—revealed that the odds of having ischemic stroke (resulting from occlusion of blood supply to the brain) was 2.3 (99 percent CI 1.9–2.8) and that of having hemorrhagic stroke (bleeding into the brain) was 1.4 (99 percent CI 1.1–1.9) times higher among smokers than among nonsmokers (O’Donnell and others 2010).
Smoking in conjunction with hypertension was the main risk factor identified for stroke, and a dose-response relationship exists with the number of cigarettes smoked per day. A meta-analysis using 32 cohorts and case-control studies involving more than 11,000 stroke events showed a 50 percent overall increase in risk of stroke in smokers. This risk appears to be higher among women, young smokers, and heavy smokers (Shinton and Beevers 1989). Smoking is also strongly implicated in transient ischemic attacks, a transient and milder form of stroke with symptoms typically resolving within 24 hours. Smoking has also been shown to increase the risk of dementia, another increasing global health challenge, including dementia caused by Alzheimer’s disease (Cataldo, Prochaska, and Glantz 2010; WHO 2015b).
Smoking and Arrhythmia
Although substantial evidence exists on the role of tobacco in the initiation and progression of atherosclerosis, its role in arrhythmias is less defined and likely to be complex (D’Alessandro and others 2011). Two of the most common conditions associated with smoking—coronary heart disease and chronic lung disease—are both strongly associated with arrhythmias, making it difficult to tease out the direct pro-arrhythmic effects of smoking and its components. Nonetheless, D’Alessandro and others (2011) argue strongly that smoking has direct and acute toxic pro-arrhythmia effects. They propose several mechanisms that mediate the risk: nicotine-induced catecholamine release; myocardial profibrotic effects of nicotine, which can increase susceptibility to catecholamine; oxidative stress; and ischemia or hypoxia, especially associated with carbon monoxide (D’Alessandro and others 2011).
We have alluded to the increased risk of sudden cardiac death in smokers. Smoking has been linked in several studies to ventricular arrhythmia (lethal heart rhythm disorder arising from the lower chambers of the heart) in people with coronary heart disease (Engström and others 1999; Goldenberg and others 2006; Vlietstra and others 1986). Smoking is also associated with increasing risk of supraventricular arrhythmia (often benign fast heart rhythm from the upper chambers of the heart), as documented in a study of the Multicenter Automated Defibrillator Implantation Trial (Goldenberg and others 2006). In addition, smoking is associated with atrial fibrillation, the most common form of supraventricular arrhythmia, with a review suggesting a doubling of risk of atrial fibrillation in smokers (Morris and others 2015). Both the Atherosclerosis Risk in Communities Study (Chamberlain and others 2011), with 15,000 participants, and the Rotterdam Study (Heeringa and others 2008), with 5,668 subjects, showed increased incidence of atrial fibrillation in current smokers (RR 2.05, 95 percent CI 1.71–2.47 and RR 1.51, 95 percent CI 1.07–2.12) and former smokers (RR 1.32, 95 percent CI 1.10–1.57 and RR 1.49, 95 percent CI 1.14–1.97) (Chamberlain and others 2011; Heeringa and others 2008). The risk was dose dependent. The risk also increased in the Manitoba Follow-up Study (Krahn and others 1995). Limitations in older studies may have masked the association between smoking and atrial fibrillation (D’Alessandro and others 2011). Evidence for the link between smoking and arrhythmias also exists in studies showing less arrhythmia in persons who stop smoking (Kinoshita and others 2009; Peters and others 1995).
Smoking and Aortic and Peripheral Artery Disease
It is well established that various risk factors differ in their association with atherosclerosis. Studies confirm that smoking is associated more strongly with aortic disease—particularly abdominal aortic and peripheral arterial disease—than any other risk factor and that the association of smoking with abdominal aortic aneurysm and peripheral arterial disease is much stronger than its association with any other CVD. The EPIC-Norfolk prospective population study, with 21,798 participants, quantified the magnitude of association: smoking increased risk of abdominal aortic aneurysm more than sevenfold (RR 7.66, 95 percent CI 4.50–13.04) and peripheral arterial disease more than fourfold (RR 4.66, 95 percent CI 3.29–6.61) (Stoekenbroek and others 2015). In a large study in the United Kingdom involving 1,937,360 people, the corresponding relative risks were 5.18 (95 percent CI 4.61–5.82) for abdominal aortic aneurysm and 5.16 (95 percent CI 4.80–5.54) for peripheral arterial disease (Pujades-Rodriguez and others 2015). These results are consistent with the findings of a meta-analysis of smoking and peripheral arterial disease involving 55 studies (Lu, Mackay, and Pell 2014). In countries with a high proportion of smokers, nearly 50 percent of peripheral arterial disease can be attributed to smoking (Willigendael and others 2004). Smoking is also strongly associated with the risk of expansion and rupture of an abdominal aortic aneurysm, suggesting that screening for abdominal aortic aneurysm should be restricted to smokers (Howard and others 2015; Sweeting and others 2012). Former smokers have a lower risk of both abdominal aortic aneurysm and peripheral arterial disease.
Smoking and Chronic Kidney Disease
Chronic kidney disease, a condition associated with increased mortality, morbidity, and health care costs, is a growing global public health concern. It is strongly linked to CVD and its risk factors, including smoking. Smokers have a higher risk of macroalbuminuria (elevated urinary albumin excretion) (Halimi and others 2000; Pinto-Sietsma and others 2000). Halimi and colleagues found that renal function, estimated by creatinine clearance, was reduced among smokers. Chronic smoking may result in proteinuria and irreversible kidney damage: the adjusted relative risk was 3.26 (95 percent CI 1.66–6.80) and 2.69 (95 percent CI 1.24–5.99), respectively, among current and former smokers (Halimi and others 2000). Several factors affect the association between smoking and chronic kidney disease, including hemodynamic mechanisms such as cardiorenal syndrome in people with heart failure, and increase in blood pressure, which is known to promote progression of chronic kidney disease (Orth 2004). A large population-based cohort with a median follow-up of 10.3 years demonstrated that smokers younger than age 70 years were at fourfold higher risk of chronic kidney disease than never-smokers (Hallan and Orth 2011). Similarly, in a large population study in Japan, the risk of developing chronic kidney disease was greater among smokers (Yamagata and others 2007). Current male and female smokers had a relative risk of 1.26 (95 percent CI 1.14–1.41) and 1.40 (95 percent CI, 1.16–1.69), respectively, for developing chronic kidney disease over nonsmokers. Persons with preexisting chronic kidney disease have a substantial risk of CVD mortality. In the Cardiovascular Health Study, the group of elderly persons (older than age 65 years) with chronic kidney disease had a CVD risk rate of 32 deaths per 1,000 person-years, compared with 16 per 1,000 person-years in the group without chronic kidney disease, with smoking a major predictor of the same (Shlipak and others 2005).
Cardiovascular Disease Associated with Other Forms of Tobacco Use
Other aspects of cigarette smoking, such as secondhand smoke, also have harmful cardiovascular effects. As detailed in , tobacco is consumed worldwide in many forms other than cigarettes, both smoked and smokeless (Saleheen, Zhao, and Rasheed 2014). Although most other forms have been documented to affect cardiovascular health, differences exist in their magnitude and nature of cardiovascular impact (Katsiki and others 2013).
Secondhand Smoke
Also known as environmental tobacco smoke, secondhand smoke has physiological effects similar to those of active smoke: oxidized lipids, increased arterial thickening, and decreased coronary flow velocity (Howard and others 1998; Otsuka and others 2001). Detrimental cardiovascular effects are even demonstrated in exposed children (Raghuveer and other 2016). Many studies have examined the association of secondhand smoke with CVD outcomes and results. An influential meta-analysis of 10 cohort studies and 8 case-control studies showed a 25 percent higher risk (RR 1.25, 95 percent CI 1.17–1.32) of coronary heart disease among nonsmokers exposed to secondhand smoke with a dose-response relationship (He and others 1999). An updated meta-analysis came to a similar conclusion, finding a 27 percent increased risk (RR 1.27, 95 percent CI 1.10–1.48) (Fischer and Kraemer 2015). Similarly, the INTERHEART study, which was not included in the updated meta-analysis, found a 24 percent to 62 percent increased risk with secondhand smoke, depending on the dose of exposure (Teo and others 20067). In focusing on LMICs, Olasky, Levy, and Moran (2012) found a high prevalence of secondhand smoke and increased risk of both ischemic heart disease and stroke. The consistency of these findings after taking account of other (confounding) factors suggests that the association is causal and definitive (Glantz and Parmley 1991). The ill effects of secondhand smoke can be confirmed by the presence of physiological markers of tobacco smoke. Compared with men not exposed to secondhand smoke, those exposed had higher levels of carbon monoxide and lower pulmonary function (Svendsen and others 1987). Biomarkers of CVD, such as C-reactive protein, homocysteine, fibrinogen, and white cell count, were assessed among never-smoking adults participating in the third National Health and Nutrition Examination Survey. It was found that the levels of fibrinogen (mean difference 14.39 milligrams per deciliter, 95 percent CI 5.7–23.1, p = 0.002) and homocysteine (mean difference 1.02 micromoles per liter, 95 percent CI 0.6–1.4, p < 0.001) were significantly raised among passive smokers with higher cotinine levels when compared with those with no cotinine levels (Venn and Britton 2007).
Thirdhand Smoke
The lasting or residual tobacco smoke contamination that persists after the cigarette is extinguished is referred to as thirdhand smoke (Winickoff and others 2009). It reflects the contamination of surfaces with tobacco smoke. Surface-mediated reactions of tobacco smoke products (hydrogen cyanide, butane, toluene, polonium-201, and others) may form carcinogenic compounds that accumulate over time to become progressively toxic (Petrick and Dubowski 2011; Sleiman and others 2010). Animal studies have found thirdhand smoke to be associated with increased lipid levels, inflammatory cytokine production, and collagen stimulation, all of which potentially contribute to CVD (Martins-Green and others 2014). Although preliminary research suggests that thirdhand smoke may be associated with a risk of heart disease, the magnitude of risk needs further research.
Other Forms of Tobacco Smoking
Bidi, Cigar, and Pipe
Bidi smoking is practiced in India and other countries in South Asia. Bidi smoking is more common among low-socioeconomic-status groups, raising equity issues related to its use and health effects. Tobacco is wrapped in leaves that are then thinly rolled and secured with threads. Bidi rolling is an unregulated business that includes households, small workshops, and cooperatives that evade regulations and taxes (Gupta and Asma 2008). Bidis have concentrations of nicotine, tar, and other toxic ingredients equal to or greater than those in cigarettes. Given the increased risk of CVD at a young age among the Indian population, the adverse effects of this and other forms of tobacco use have large population-level health impacts. The INTERHEART study revealed that, in addition to cigarette smoking, use of other forms of tobacco such as bidi and chewing tobacco (also common in South Asian countries) was associated with a significantly higher risk of heart attack. The risk with bidi use was as high as the risk with cigarettes, but that of chewed tobacco was slightly lower. However, the risk was highest (fourfold compared with those who never smoked) in individuals who used both smoked and smokeless forms of tobacco (Teo and others 2006). Another hospital-based case-control study confirmed nine times greater risk (RR 9.1, 95 percent CI 4.7–17.7) of myocardial infarction among those who smoked more than 10 bidis a day compared with nonsmokers; the risk was slightly less for persons smoking an equal number of cigarettes per day (RR 7.3, 95 percent CI 3.9–13.8) (Rastogi and others 2005).
Cigars and pipes for smoking tobacco are also associated with risk of CVD. Cigars, tightly rolled bundles of dried and fermented tobacco leaves, are commonly smoked in Brazil, Cameroon, Mexico, and Africa. The relative risk of coronary heart disease among cigar smokers when compared with nonsmokers was 1.27 (95 percent CI 1.12–1.45) (Iribarren and others 1999). The risk was of similar magnitude among British men who smoked either pipes or cigars. The relative risk was 1.59 for coronary heart disease (95 percent CI 1.05–2.39) and 1.83 for stroke (95 percent CI 0.98–3.42) among pipe or cigar smokers when compared with never-smokers (Shaper 2003). Therefore, it is an underestimation to count cigarettes as the only form of tobacco smoking.
Waterpipe Tobacco
Waterpipe (hookah, shisha, or narghile) smoking has been popular among Asians, Arabs, and in other Middle Eastern and some African countries for a long time. This mode of tobacco use has seen a global resurgence recently, especially among youth (Maziak and others 2004). Waterpipe smoking varies widely across the globe. Among men, the prevalence is highest in Vietnam (13 percent) followed by the Arab Republic of Egypt (6.2 percent); among women, it is the highest in the Russian Federation (3.2 percent) (Morton and others 2014). A common misconception is that the smoke gets filtered and becomes less toxic after passing through a water receptacle (Kandela 2000; Varsano and others 2003). Waterpipe smokers engage in longer smoking sessions, which exposes them to more smoke (Shihadeh 2003). Although the health risks associated with waterpipe smoking have not been fully determined, the cardiovascular effects are clear and range from increased heart rate, higher blood pressure, and increased serum fibrinogen to ischemic heart disease, angina, aneurysms, and stroke (El-Zaatari, Chami, and Zaatari 2015). Cumulative exposure to waterpipe smoking is also significantly associated with coronary artery disease. Exposure as high as 40 waterpipe-years (reflecting both duration and dose) was associated with almost a threefold increase in the risk of obstructive coronary disease compared with nonsmokers (Sibai and others 2014). A dose-response relationship exists between heart disease and waterpipe smoking. Among more than 50,000 residents of Golestan, the Islamic Republic of Iran, heavy waterpipe smokers had significantly greater risk of heart disease than nonsmokers (Islami and others 2013).
Smokeless Tobacco
Commonly used in South and Southeast Asia, Sub-Saharan Africa, and Northern Europe, smokeless tobacco has been associated with CVD (Gupta, Gupta, and Khedar 2013). A pooled analysis of both cohort and case-control studies estimated a 13 percent higher risk of fatal heart attack and 40 percent higher risk of fatal stroke among users compared with nonusers, with evidence of a dose-response relationship. The increased risk of fatal myocardial infarction in this meta-analysis appears to be small, but the effect is large at the population level, especially given the consistency of results and robust study designs and analysis (Boffetta and Straif 2009). This finding has prompted strong calls for discouraging the use of smokeless tobacco (Gupta, Gupta, and Khedar 2013; Piano and others 2010).
Electronic Nicotine Delivery Systems (Electronic Cigarettes)
Electronic cigarettes typically involve converting liquid into an aerosol, facilitated by a battery circuit. The liquid contents include nicotine, propylene glycol, flavoring agents, and other substances. Growing awareness and use of electronic cigarettes has been observed among the young and high-income groups, and popularity is growing through advertising (Adkison and others 2013; Regan and others 2013). Electronic cigarettes do not fall under the ambit of regulatory authorities in most countries. Although some have advocated electronic cigarettes as a smoking cessation aid, many public health experts have spoken strongly against popularizing e-cigarettes. This is not only because of concerns about limited evidence of e-cigarettes’ aiding cessation; it is also because of evidence of e-cigarettes’ toxicity and of their potential to induce smoking in nonsmokers, especially youth, and to perpetuate nicotine addiction among users, thereby jeopardizing users’ attempts to quit smoking. The research on the chemical constituents and toxicity of e-cigarettes is growing. This toxicity is particularly concerning in people with CVD (Benowitz and Burbank 2016). This and other reviews (Morris and others 2015) document the many negative cardiovascular effects of e-cigarettes. Furthering the results of chemical and toxicological studies, preclinical research shows that compared with cigarettes, e-cigerettes also have detrimental effects on vascular function, specifically oxidative stress and endothelial function (Carnevale and others 2016). Clinical studies of the chronic cardiovascular effects of e-cigarettes are forthcoming.
Socioeconomic Dimensions of Tobacco-Use-Related Cardiovascular Diseases
Many socioeconomic variables modify the relationship between tobacco use and CVD. We have alluded to some of these and their interactions, for example, age (young male smokers are at higher risk of sudden death), gender (women have more risk for coronary heart disease), and ethnicity (South Asians face greater risks) (Huxley and Woodward 2011). However, it is crucial to consider many other socioeconomic dimensions, such as the impact of heterogeneous macroeconomic and human development, income inequality, and socioeconomic status. The tobacco industry quickly takes advantage of the opportunities provided by urbanization and improved social standards. Their ubiquitous packaging and advertisements reach out to all segments of society, making tobacco products available and accessible (Bhan and others 2016). In most settings, strong socioeconomic gradients have persisted, with smoking being more prevalent among the poor and disadvantaged groups. This outcome can be explained by lack of knowledge, inadequate penetration of behavioral interventions, and inability to choose healthy options (Laaksonen and others 2005). The expenses associated with smoking often divert the resources of vulnerable groups from basic necessities. Although the links between each of these variables and either tobacco use or CVD have been well researched, their complex interactions as modifiers of the link between tobacco use and CVD have been less studied, especially in LMICs. This is an important agenda for future research. It is critical to understand the effects of these factors because they determine temporal patterns, quit attempts, and inequalities in morbidity and mortality, and to direct specific interventions to target groups.
Cardiovascular Benefits of Tobacco Use Cessation
The profound cardiovascular harm of tobacco use and its global toll, especially in LMICs, indicates that prevention of tobacco initiation and lifelong avoidance of all tobacco products are the best strategies. The link has prompted calls for a tobacco-free world (Beaglehole and others 2015). The evidence for the cardiovascular benefits of tobacco cessation, particularly cigarette smoking, is compelling (box 4.3). Smoking cessation benefits all users, irrespective of form, duration, and age. Cardiovascular benefits are consistent and set in early after tobacco cessation (Bakhru and Erlinger 2005; Gratziou 2009; Hatsukami and others 2005).

Time to Cardiovascular Benefit of Smoking Cessation after Last Cigarette.
In general population studies, smoking cessation has clearly been shown to prolong life, especially when it occurs early in life. For example, in a cohort of doctors followed for 50 years, smokers who continued to smoke lost, on average, 10 years of life (Doll and others 2004). The years of life gained were three, six, and nine for those who stopped at ages 60, 50, and 40, respectively. However, this relationship does not set a threshold age for quitting, and age should not be a refraining factor for quitting—even the elderly benefit from smoking cessation (Burns 2000).
In people diagnosed with CVD, persistent smoking is associated with a significant increase in cardiovascular events and mortality (Buckley and others 2009; Simpson and others 2011). Tobacco cessation is a key intervention for preventing further cardiovascular events, particularly heart attacks, and for prolonging survival. A meta-analysis of 12 cohort studies reported a reduction in mortality, irrespective of gender, duration of follow-up, or time of assessment of smoking status after the cardiovascular event. The overall reduction in mortality after a mean follow-up of 4.8 years was nearly 50 percent (odds ratio 0.54, 95 percent CI 0.46–0.62) (Wilson and others 2000). The systematic review by Critchley and Capewell (2003) of 20 cohort studies of patients with coronary heart disease, with at least two years of follow-up, estimated that one-third of CVD outcomes—such as coronary artery bypass surgery, angioplasty, and recurrent heart attacks—can be prevented by quitting smoking (Critchley and Capewell 2003). Unfortunately, numerous studies in diverse populations (HICs and LMICs) have documented persistent smoking in people who have been diagnosed with CVD, including those who have suffered heart attacks. Several barriers are described in the literature, including lack of support to quit, lack of resources to quit, cultural norms, and stressful living conditions (Rosenthal and others 2013; Twyman and others 2014). In addition to the barriers perceived by the smokers, physicians perceive an additional set of barriers, including their own disbelief in the effectiveness of lifestyle intervention, lack of adequate counseling skills, and scarcity of time during their practice (Ockene and Miller 1997). These barriers indicate a tremendous missed opportunity for secondary prevention, especially among patients with CVD. Because strong advice by health care providers, particularly physicians, leads to more quitting, there have been calls for cardiovascular specialists to pay greater attention to smoking cessation (Jabbour and others 2002). Patient-centered advice does not take much time and helps the patient gain confidence to attempt and maintain cessation.
Although interventions targeted at individual smokers are important, policy interventions, particularly for persons at high risk of or with existing CVD, are also crucial for reducing overall rates of tobacco use at the population level. The World Health Organization (WHO) Framework Convention on Tobacco Control is a comprehensive, binding convention that provides member countries, now numbering 180, with measures and corresponding guidelines to reduce both demand and supply (see Jha and others [2015] for a more detailed discussion of strategies of tobacco control). The cardiovascular impact of implementing various policy measures in the framework has been studied, with smoke-free legislation and policies banning smoking in public spaces receiving the most attention. A meta-analysis of 31 studies of cardiovascular impact in 47 locations showed a 12 percent reduction in hospitalization for acute coronary events (RR 0.88, 95 percent CI 0.85–0.90). At places where the reduction in smoking prevalence was more than the mean (2.1 percent reduction) there was a 14 percent reduction in events after the enforcement of legislation (Jones and others 2014). This impact translates into large benefits at the population level. Although it is difficult to know with certainty what proportion of the benefit comes from reduced exposure to secondhand smoke in nonsmokers as opposed to reduced consumption or more quitting among smokers, evidence suggests that both smokers and nonsmokers benefit (Seo and Torabi 2007).
Conclusions
Robust evidence indicates that tobacco use causes atherosclerotic CVD. The link is strong with various forms of tobacco use, and the magnitude is substantial and consistent across all cardiovascular manifestations.
Tobacco use acts both independently of and synergistically with other risk factors common to CVD. Complex mechanisms underlie the pathophysiology of tobacco-attributed CVD. The risk appears to be higher among younger age groups who are smoking more cigarettes a day, among women than among men, and in certain ethnicities such as South Asians.
The most common manifestations of tobacco-related CVD include myocardial infarction, angina, stroke, aortic aneurysm, and peripheral artery disease. However, heart failure, chronic kidney disease, and atrial fibrillation are emerging as global health issues. These manifestations lead to morbidity, premature mortality, loss of productive years of life, and tremendous health care costs, burdening already stretched health systems, especially in LMICs.
Tobacco cessation protects against CVD at all ages and adds years to life. Population- and individual-level interventions to reduce the number of people starting to smoke and getting more people to quit have great promise, especially for those with or at high risk of CVD. Implementation of the full provisions of the Framework Convention on Tobacco Control provides a clear path toward a world free of tobacco use and where tobacco-related CVD becomes a thing of the past.
Notes
Professor Dorairaj Prabhakaran is supported through a research grant from the Wellcome Trust and the National Heart, Lung, and Blood Institute of the United States (Contract no. HHSN268200900026C).
World Bank Income Classifications as of July 2014 are as follows, based on estimates of gross national income per capita for 2013:
Low-income countries = US$1,045 or less
Middle-income countries are subdivided:
lower-middle-income = US$1,046 to US$4,125
upper-middle-income = US$4,126 to US$12,745
High-income countries = US$12,746 or more.
References
Adkison S E, O’Connor R J, Bansal-Travers M, Hyland A, Borland R., and others. 2013. “Electronic Nicotine Delivery Systems: International Tobacco Control Four-Country Survey.” American Journal of Preventive Medicine
44 (3): 207–15. [
PMC free article: PMC3627474] [
PubMed: 23415116]
Ambrose J A, Barua R S. 2004. “The Pathophysiology of Cigarette Smoking and Cardiovascular Disease: An Update.” Journal of the American College of Cardiology
43 (10): 1731–37. [
PubMed: 15145091]
Aronow W S.
1974. “Tobacco and the Heart.” JAMA
229 (13): 1799–800. [
PubMed: 4479316]
Bakhru A, Erlinger T P. 2005. “Smoking Cessation and Cardiovascular Disease Risk Factors: Results from the Third National Health and Nutrition Examination Survey.” PLoS Medicine
2 (6): e160. [
PMC free article: PMC1160573] [
PubMed: 15974805]
Bamia C, Trichopoulou A, Lenas D, Trichopoulos D. 2004. “Tobacco Smoking in Relation to Body Fat Mass and Distribution in a General Population Sample.” International Journal of Obesity and Related Metabolic Disorders
28 (8): 1091–96. [
PubMed: 15197410]
Barua R S, Ambrose J A, Eales-Reynolds L J, DeVoe M C, Zervas J G, Saha D C. 2001. “Dysfunctional Endothelial Nitric Oxide Biosynthesis in Healthy Smokers with Impaired Endothelium-Dependent Vasodilatation.” Circulation
104 (16): 1905–10. [
PubMed: 11602492]
Beaglehole R, Bonita R, Yach D, Mackay J, Reddy K S. 2015. “A Tobacco-Free World: A Call to Action to Phase Out the Sale of Tobacco Products by 2040.” The Lancet
385 (9972): 1011–18. [
PubMed: 25784348]
Bermudez E A, Rifai N, Buring J E, Manson J E, Ridker P M. 2002. “Relation between Markers of Systemic Vascular Inflammation and Smoking in Women.” American Journal of Cardiology
89 (9): 1117–19. [
PubMed: 11988205]
Bhan N, Karan A, Srivastava S, Selvaraj S, Subramanian S V., and others. 2016. “Have Socioeconomic Inequalities in Tobacco Use in India Increased over Time? Trends from the National Sample Surveys (2000–2012).” Nicotine and Tobacco Research (April): ii. [
PMC free article: PMC4941603] [
PubMed: 27048274]
Bilano V, Gilmour S, Moffiet T, d’Espaignet E T, Stevens G A. 2015. “Global Trends and Projections for Tobacco Use, 1990–2025: An Analysis of Smoking Indicators from the WHO Comprehensive Information Systems for Tobacco Control.” The Lancet
385 (9972): 966–76. [
PubMed: 25784347]
Bolinder G, Alfredsson L, Englund A, Faire U de. 1994. “Smokeless Tobacco Use and Increased Cardiovascular Mortality among Swedish Construction Workers.” American Journal of Public Health
84 (3): 399–404. [
PMC free article: PMC1614817] [
PubMed: 8129055]
Borgerding M, Klus H. 2005. “Analysis of Complex Mixtures—Cigarette Smoke.” Experimental and Toxicologic Pathology
57 (Suppl 1): 43–73. [
PubMed: 16092717]
Breitling L P.
2013. “Current Genetics and Epigenetics of Smoking/Tobacco-Related Cardiovascular Disease.” Arteriosclerosis, Thrombosis, and Vascular Biology
33 (7): 1468–72. [
PubMed: 23640490]
Bruin J E, Gerstein H C, Morrison K M, Holloway A C. 2008. “Increased Pancreatic Beta-Cell Apoptosis Following Fetal and Neonatal Exposure to Nicotine Is Mediated via the Mitochondria.” Toxicological Sciences
103 (2): 362–70. [
PubMed: 18203686]
Brummett B H, Babyak M A, Siegler I C, Shanahan M, Harris K M., and others. 2011. “Systolic Blood Pressure, Socioeconomic Status, and Biobehavioral Risk Factors in a Nationally Representative U.S. Young Adult Sample.” Hypertension
58 (2): 161–66. [
PMC free article: PMC3160108] [
PubMed: 21730296]
Buckley B S, Simpson C R, McLernon D J, Murphy A W, Hannaford P C. 2009. “Five-Year Prognosis in Patients with Angina Identified in Primary Care: Incident Cohort Study.” BMJ
339 (August): b3058. [
PMC free article: PMC2722695] [
PubMed: 19661139]
Burns D M.
2000. “Cigarette Smoking among the Elderly: Disease Consequences and the Benefits of Cessation.” American Journal of Health Promotion
14 (6): 357–61. [
PubMed: 11067570]
Canoy D, Wareham N, Luben R, Welch A, Bingham S., and others. 2005. “Cigarette Smoking and Fat Distribution in 21,828 British Men and Women: A Population-Based Study.” Obesity Research
13 (8): 1466–75. [
PubMed: 16129730]
Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L., and others. 2016. “Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function.” Chest
150 (3): 606–12 [
PubMed: 27108682]
Cataldo J K, Prochaska J J, Glantz S A. 2010. “Cigarette Smoking Is a Risk Factor for Alzheimer’s Disease: An Analysis Controlling for Tobacco Industry Affiliation.” Journal of Alzheimer’s Disease
19 (2): 465–80. [
PMC free article: PMC2906761] [
PubMed: 20110594]
Celermajer D S, Adams M R, Clarkson P, Robinson J, McCredie R., and others. 1996. “Passive Smoking and Impaired Endothelium-Dependent Arterial Dilatation in Healthy Young Adults.” New England Journal of Medicine
334 (3): 150–54. [
PubMed: 8531969]
Celermajer D S, Sorensen K E, Georgakopoulos D, Bull C, Thomas O., and others. 1993. “Cigarette Smoking Is Associated with Dose-Related and Potentially Reversible Impairment of Endothelium-Dependent Dilation in Healthy Young Adults.” Circulation
88 (5): 2149–55. [
PubMed: 8222109]
Chamberlain A M, Agarwal S K, Folsom A R, Duval S, Soliman E Z., and others. 2011. “Smoking and Incidence of Atrial Fibrillation: Results from the Atherosclerosis Risk in Communities Study.” Heart Rhythm
8 (8): 1160–66. [
PMC free article: PMC3139831] [
PubMed: 21419237]
Chiolero A, Faeh D, Paccaud F, Cornuz J. 2008. “Consequences of Smoking for Body Weight, Body Fat Distribution, and Insulin Resistance.” American Journal of Clinical Nutrition
87 (4): 801–9. [
PubMed: 18400700]
Critchley J A, Capewell S. 2003. “Mortality Risk Reduction Associated with Smoking Cessation in Patients with Coronary Heart Disease: A Systematic Review.” JAMA
290 (1): 86–97. [
PubMed: 12837716]
Cryer P E, Haymond M W, Santiago J V, Shah S D. 1976. “Norepinephrine and Epinephrine Release and Adrenergic Mediation of Smoking-Associated Hemodynamic and Metabolic Events.” New England Journal of Medicine
295 (11): 573–77. [
PubMed: 950972]
D’Alessandro A, Boeckelmann I, Hammwhoner M, Goette A. 2011. “Nicotine, Cigarette Smoking, and Cardiac Arrhythmia: An Overview.” European Journal of Preventive Cardiology
19 (3): 297–305. [
PubMed: 22779085]
Dobson A J, Alexander H M, Heller R F, Lloyd D M. 1991. “How Soon after Quitting Smoking Does Risk of Heart Attack Decline?” Journal of Clinical Epidemiology
44 (11): 1247–53. [
PubMed: 1941018]
Eliasson B., 2003. “Cigarette Smoking and Diabetes.” Progress in Cardiovascular Diseases
45 (5): 405–13. [
PubMed: 12704597]
Engström G, Hedblad B, Janzon L, Juul-Möller S. 1999. “Ventricular Arrhythmias during 24-H Ambulatory ECG Recording: Incidence, Risk Factors, and Prognosis in Men with and without a History of Cardiovascular Disease.” Journal of Internal Medicine
246 (4): 363–72. [
PubMed: 10583707]
Evangelista L S, Doering L V, Dracup K. 2000. “Usefulness of a History of Tobacco and Alcohol Use in Predicting Multiple Heart Failure Readmissions among Veterans.” American Journal of Cardiology
86 (12): 1339–42. [
PubMed: 11113409]
Ezzati M, Lopez A D. 2003. “Estimates of Global Mortality Attributable to Smoking in 2000.” The Lancet
362 (9387): 847–52. [
PubMed: 13678970]
Facchini F S, Hollenbeck C B, Jeppesen J, Chen Y D, Reaven G M. 1992. “Insulin Resistance and Cigarette Smoking.” The Lancet
339 (8802) 1128–30. [
PubMed: 1349365]
Fischer F, Kraemer A. 2015. “Meta-Analysis of the Association between Second-Hand Smoke Exposure and Ischaemic Heart Diseases, COPD, and Stroke.” BMC Public Health
15 (December): 1202. [
PMC free article: PMC4667413] [
PubMed: 26627181]
Frei B, Forte T M, Ames B N, Cross C E. 1991. “Gas Phase Oxidants of Cigarette Smoke Induce Lipid Peroxidation and Changes in Lipoprotein Properties in Human Blood Plasma: Protective Effects of Ascorbic Acid.” Biochemical Journal
277 (Pt 1):133–38. [
PMC free article: PMC1151201] [
PubMed: 1854329]
Freson K, Izzi B, Geet C Van. 2012. “From Genetics to Epigenetics in Platelet Research.” Thrombosis Research
129 (3): 325–29. [
PubMed: 22192152]
Gepner A D, Piper M E, Johnson H M, Fiore M C, Baker T B. and others. 2011. “Effects of Smoking and Smoking Cessation on Lipids and Lipoproteins: Outcomes from a Randomized Clinical Trial.” American Heart Journal
161 (1): 145–51. [
PMC free article: PMC3110741] [
PubMed: 21167347]
Glantz S A, Parmley W W. 1991. “Passive Smoking and Heart Diseas Epidemiology, Physiology, and Biochemistry.” Circulation
83 (1): 1–12. [
PubMed: 1984876]
Goldenberg I, Moss A J, McNitt S, Zareba W, Daubert J P., and others. 2006. “Cigarette Smoking and the Risk of Supraventricular and Ventricular Tachyarrhythmias in High-Risk Cardiac Patients with Implantable Cardioverter Defibrillators.” Journal of Cardiovascular Electrophysiology
17 (9): 931–36. [
PubMed: 16759297]
Gratziou C., 2009. “Respiratory, Cardiovascular, and Other Physiological Consequences of Smoking Cessation.” Current Medical Research and Opinion
25 (2): 535–45. [
PubMed: 19193001]
Green M S, Jucha E, Luz Y. 1986. “Blood Pressure in Smokers and Nonsmokers: Epidemiologic Findings.” American Heart Journal
111 (5): 932–40. [
PubMed: 3706114]
Gupta P C, Asma S. eds. 2008. Bidi Smoking and Public Health. New Delhi: Ministry of Health and Family Welfare, Government of India.
Halimi J M, Giraudeau B, Vol S, Cacès E, Nivet H. and others. 2000. “Effects of Current Smoking and Smoking Discontinuation on Renal Function and Proteinuria in the General Population.” Kidney International
58 (3): 1285–92. [
PubMed: 10972692]
Hallan S I, Orth S R. 2011. “Smoking Is a Risk Factor in the Progression to Kidney Failure.” Kidney International
80 (5): 516–523. [
PubMed: 21677635]
Hatsukami D K, Kotlyar M, Allen S, Jensen J, Li S., and others. 2005. “Effects of Cigarette Reduction on Cardiovascular Risk Factors and Subjective Measures.” Chest
128 (4): 2528–37. [
PubMed: 16236919]
He J, Vupputuri S, Allen K, Prerost M R, Hughes J., and others. 1999. “Passive Smoking and the Risk of Coronary Heart Disease: A Meta-Analysis of Epidemiologic Studies.” New England Journal of Medicine
340 (12): 920–26. [
PubMed: 10089185]
Heeringa J, Kors J A, Hofman A, Rooij F J A van, Witteman J C M. 2008. “Cigarette Smoking and Risk of Atrial Fibrillation: The Rotterdam Study.” American Heart Journal
156 (6): 1163–69. [
PubMed: 19033014]
Heitzer T, Ylä-Herttuala S, Luoma J, Kurz S, Münzel T., and others. 1996. “Cigarette Smoking Potentiates Endothelial Dysfunction of Forearm Resistance Vessels in Patients with Hypercholesterolemia: Role of Oxidized LDL.” Circulation
93 (7): 1346–53. [
PubMed: 8641023]
Howard D P J, Banerjee A, Fairhead J F, Handa A, Silver L E., and others. 2015. “Population-Based Study of Incidence of Acute Abdominal Aortic Aneurysms with Projected Impact of Screening Strategy.” Journal of the American Heart Association
4 (8): e001926. [
PMC free article: PMC4599457] [
PubMed: 26289347]
Howard G, Wagenknecht L E, Burke G L, Diez-Roux A, Evans G W., and others. 1998. “Cigarette Smoking and Progression of Atherosclerosis: The Atherosclerosis Risk in Communities Study.” Journal of the American Medical Association
279 (2): 119–24. [
PubMed: 9440661]
Huxley R R, Woodward M. 2011. “Cigarette Smoking as a Risk Factor for Coronary Heart Disease in Women Compared with Men: A Systematic Review and Meta-Analysis of Prospective Cohort Studies.” The Lancet
378 (9799): 1297–305. [
PubMed: 21839503]
Ichiki K, Ikeda H, Haramaki N, Ueno T, Imaizumi T. 1996. “Long-Term Smoking Impairs Platelet-Derived Nitric Oxide Release.” Circulation
94 (12): 3109–14. [
PubMed: 8989117]
Iribarren C, Tekawa I S, Sidney S, Friedman G D. 1999. “Effect of Cigar Smoking on the Risk of Cardiovascular Disease, Chronic Obstructive Pulmonary Disease, and Cancer in Men.” New England Journal of Medicine
340 (23): 1773–80. [
PubMed: 10362820]
Islami F, Pourshams A, Vedanthan R, Poustchi H, Kamangar F., and others. 2013. “Smoking Water-Pipe, Chewing Nass, and Prevalence of Heart Disease: A Cross-Sectional Analysis of Baseline Data from the Golestan Cohort Study, Iran.” Heart
99 (4): 272–78. [
PMC free article: PMC3671096] [
PubMed: 23257174]
Jabbour S, Reddy K S, Muna W F T, Achutti A. 2002. “Cardiovascular Disease and the Global Tobacco Epidemic: A Wake-Up Call for Cardiologists.” International Journal of Cardiology
86 (2–3): 185–92. [
PubMed: 12419555]
Jha P, MacLennan M, Chaloupka F J, Yureki A, Ramasundarahettige C., and others. 2015. “Global Hazards of Tobacco and the Benefits of Smoking Cessation and Tobacco Taxes.” In Disease Control Priorities (third edition): Volume 3, Cancer, edited by Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. . Washington, DC: World Bank. [
PubMed: 26913345]
Johnson H M, Gossett L K, Piper M E, Aeschlimann S E, Korcarz C E., and others. 2010. “Effects of Smoking and Smoking Cessation on Endothelial Function: 1-Year Outcomes from a Randomized Clinical Trial.” Journal of the American College of Cardiology
55 (18): 1988–95. [
PMC free article: PMC2947952] [
PubMed: 20236788]
Jones M R, Barnoya J, Stranges S, Losonczy L, Navas-Acien A. 2014. “Cardiovascular Events Following Smoke-Free Legislations: An Updated Systematic Review and Meta-Analysis.” Current Environmental Health Reports
1 (3): 239–49. [
PMC free article: PMC4198310] [
PubMed: 25328861]
Juonala M, Magnussen C G, Venn A, Gall S, Kähönen M., and others. 2012. “Parental Smoking in Childhood and Brachial Artery Flow-Mediated Dilatation in Young Adults: The Cardiovascular Risk in Young Finns Study and the Childhood Determinants of Adult Health Study.” Arteriosclerosis, Thrombosis, and Vascular Biology
32 (4): 1024–31. [
PubMed: 22345167]
Kandela P., 2000. “Nargile Smoking Keeps Arabs in Wonderland.” The Lancet
356 (9236): 1175. [
PubMed: 11030308]
Kannel W B, D’Agostino R B, Belanger A J. 1987. “Fibrinogen, Cigarette Smoking, and Risk of Cardiovascular Disease: Insights from the Framingham Study.” American Heart Journal
113 (4): 1006–10. [
PubMed: 3565227]
Kapoor D, Jones T H. 2005. “Smoking and Hormones in Health and Endocrine Disorders.” European Journal of Endocrinology
152 (4): 491–99. [
PubMed: 15817903]
Katsiki N, Papadopoulou S K, Fachantidou A I, Mikhailidis D P. 2013. “Smoking and Vascular Risk: Are All Forms of Smoking Harmful to All Types of Vascular Disease?” Public Health
127 (5): 435–41. [
PubMed: 23453194]
Kawachi I, Colditz G A, Stampfer M J, Willett W C, Manson J E., and others. 1994. “Smoking Cessation and Time Course of Decreased Risks of Coronary Heart Disease in Middle-Aged Women.” Archives of Internal Medicine
154 (2): 169–75. [
PubMed: 8285812]
King A., 2011. “Risk Factors: Cigarette Smoking Increases the Risk of Coronary Heart Disease in Women More than in Men.” Nature Reviews Cardiology
8 (November): 612. [
PubMed: 21878881]
Kinoshita M, Herges R M, Hodge D O, Friedman L, Ammash N M., and others. 2009. “Role of Smoking in the Recurrence of Atrial Arrhythmias after Cardioversion.” American Journal of Cardiology
104 (5): 678–82. [
PubMed: 19699344]
Kontis V, Mathers C D, Bonita R, Stevens G A, Rehm J., and others. 2015. “Regional Contributions of Six Preventable Risk Factors to Achieving the 25×25 Non-Communicable Disease Mortality Reduction Target: A Modelling Study.” The Lancet Global Health
3 (12): e746–57. [
PubMed: 26497599]
Kontis V, Mathers C D, Rehm J, Stevens G A, Shield K D., and others. 2014. “Contribution of Six Risk Factors to Achieving the 25×25 Non-Communicable Disease Mortality Reduction Target: A Modelling Study.” The Lancet
384 (9941): 427–37. [
PubMed: 24797573]
Krahn A D, Manfreda J, Tate R B, Mathewson F A, Cuddy T E. 1995. “The Natural History of Atrial Fibrillation: Incidence, Risk Factors, and Prognosis in the Manitoba Follow-Up Study.” American Journal of Medicine
98 (5): 476–84. [
PubMed: 7733127]
Kugiyama K, Yasue H, Ohgushi M, Motoyama T, Kawano H., and others. 1996. “Deficiency in Nitric Oxide Bioactivity in Epicardial Coronary Arteries of Cigarette Smokers.” Journal of the American College of Cardiology
28 (5): 1161–67. [
PubMed: 8890810]
Laaksonen M, Rahkonen O, Karvonen S, Lahelma E. 2005. “Socioeconomic Status and Smoking: Analysing Inequalities with Multiple Indicators.” European Journal of Public Health
15 (3): 262–69. [
PubMed: 15755781]
Lopez A D, Collishaw N E, Piha T. 1994. “A Descriptive Model of the Cigarette Epidemic in Developed Countries.” Tobacco Control
3 (3): 242–47.
Lu L, Mackay D F, Pell J P. 2014. “Meta-Analysis of the Association between Cigarette Smoking and Peripheral Arterial Disease.” Heart
100 (5): 414–23. [
PubMed: 23922053]
Martins-Green M, Adhami N, Frankos M, Valdez M, Goodwin B., and others. 2014. “Cigarette Smoke Toxins Deposited on Surfaces: Implications for Human Health.” PLoS One
9 (1): e86391. [
PMC free article: PMC3906039] [
PubMed: 24489722]
Matetzky S, Tani S, Kangavari S, Dimayuga P, Yano J., and others. 2000. “Smoking Increases Tissue Factor Expression in Atherosclerotic Plaques: Implications for Plaque Thrombogenicity.” Circulation
102 (6): 602–04. [
PubMed: 10931797]
Meade T W, Imeson J, Stirling Y. 1987. “Effects of Changes in Smoking and Other Characteristics on Clotting Factors and the Risk of Ischaemic Heart Disease.” The Lancet
2 (8566): 986–88. [
PubMed: 2889958]
Mons U, Müezzinler A, Gellert C, Schöttker B, Abnet C C., and others. 2015. “Impact of Smoking and Smoking Cessation on Cardiovascular Events and Mortality among Older Adults: Meta-Analysis of Individual Participant Data from Prospective Cohort Studies of the Chances Consortium.” BMJ
350 (April): h1551. [
PMC free article: PMC4413837] [
PubMed: 25896935]
Morris P B, Ference B A, Jahangir E, Feldman D N, Ryan J J., and others. 2015. “Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes.” Journal of the American College of Cardiology
66 (12): 1378–91. [
PubMed: 26383726]
Morton J, Song Y, Fouad H, Awa F El, Naga R Abou El., and others. 2014. “Cross-Country Comparison of Waterpipe Use: Nationally Representative Data from 13 Low- and Middle-Income Countries from the Global Adult Tobacco Survey (GATS).” Tobacco Control
23 (5): 419–27. [
PMC free article: PMC4145417] [
PubMed: 23760609]
Muscat J E, Harris R E, Haley N J, Wynder E L. 1991. “Cigarette Smoking and Plasma Cholesterol.” American Heart Journal
121 (1, Pt 1):141–47. [
PubMed: 1985356]
Naghavi M, Wang H, Lozano R, Davis A, Liang X., and others. 2015. “Global, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013.” The Lancet
385 (9963): 117–71. [
PMC free article: PMC4340604] [
PubMed: 25530442]
Neaton J D, Wentworth D N, Cutler J, Stamler J, Kuller L. 1993. “Risk Factors for Death from Different Types of Stroke.” Annals of Epidemiology
3 (5): 493–99. [
PubMed: 8167825]
Newby D E, Wright R A, Labinjoh C, Ludlam C A, Fox K A., and others. 1999. “Endothelial Dysfunction, Impaired Endogenous Fibrinolysis, and Cigarette Smoking: A Mechanism for Arterial Thrombosis and Myocardial Infarction.” Circulation
99 (11): 1411–15. [
PubMed: 10086962]
Njølstad I, Arnesen E, Lund-Larsen P G. 1996. “Smoking, Serum Lipids, Blood Pressure, and Sex Differences in Myocardial Infarction. A 12-Year Follow-Up of the Finnmark Study.” Circulation
93 (3): 450–56. [
PubMed: 8565161]
Nowak J, Murray J J, Oates J A, Fitzgerald G A. 1987. “Biochemical Evidence of a Chronic Abnormality in Platelet and Vascular Function in Healthy Individuals Who Smoke Cigarettes.” Circulation
76 (1): 6–14. [
PubMed: 3297389]
Ockene I S, Miller N H. 1997. “Cigarette Smoking, Cardiovascular Disease, and Stroke: A Statement for Healthcare Professionals from the American Heart Association.” Circulation
96 (9): 3243–47. [
PubMed: 9386200]
O’Donnell M J, Xavier D, Liu L, Zhang H, Chin S L., and others. 2010. “Risk Factors for Ischaemic and Intracerebral Haemorrhagic Stroke in 22 Countries (the INTERSTROKE Study): A Case-Control Study.” The Lancet
376 (9735): 112–23. [
PubMed: 20561675]
Orth S R.
2004. “Effects of Smoking on Systemic and Intrarenal Hemodynamics: Influence on Renal Function.” Journal of the American Society of Nephrology
15 (1): S58–63. [
PubMed: 14684675]
Otsuka R, Watanabe H, Hirata K, Tokai K, Muro T., and others. 2001. “Acute Effects of Passive Smoking on the Coronary Circulation in Healthy Young Adults.” JAMA
286 (4): 436–41. [
PubMed: 11466122]
Pan A, Wang Y, Talaei M, Hu F B, Wu T. 2015. “Relation of Active, Passive, and Quitting Smoking with Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis.” The Lancet Diabetes and Endocrinology
3 (12): 958–67. [
PMC free article: PMC4656094] [
PubMed: 26388413]
Parish S, Collins R, Peto R, Youngman L, Barton J., and others. 1995. “Cigarette Smoking, Tar Yields, and Non-Fatal Myocardial Infarction: 14,000 Cases and 32,000 Controls in the United Kingdom; the International Studies of Infarct Survival (ISIS) Collaborators.” BMJ
311 (7003): 471–77. [
PMC free article: PMC2550542] [
PubMed: 7647641]
Pech-Amsellem M A, Myara I, Storogenko M, Demuth K, Proust A., and others. 1996. “Enhanced Modifications of Low-Density Lipoproteins (LDL) by Endothelial Cells from Smokers: A Possible Mechanism of Smoking-Related Atherosclerosis.” Cardiovascular Research
31 (6): 975–83. [
PubMed: 8759254]
Peters R W, Brooks M M, Todd L, Liebson P R, Wilhelmsen L. 1995. “Smoking Cessation and Arrhythmic Death: The CAST Experience.” Journal of the American College of Cardiology
26 (5): 1287–92. [
PubMed: 7594045]
Petrick L M, Dubowski Y. 2011. “Thirdhand Smoke: Heterogeneous Oxidation of Nicotine and Secondary Aerosol Formation in the Indoor Environment.” Environmental Science and Technology
45 (1): 328–33. [
PubMed: 21141815]
Piano M R, Benowitz N L, Fitzgerald G A, Corbridge S, Heath J., and others. 2010. “Impact of Smokeless Tobacco Products on Cardiovascular Disease: Implications for Policy, Prevention, and Treatment: A Policy Statement from the American Heart Association.” Circulation
122 (15): 1520–44. [
PubMed: 20837898]
Pinto-Sietsma S J, Mulder J, Janssen W M, Hillege H L, Zeeuw D de., and others. 2000. “Smoking Is Related to Albuminuria and Abnormal Renal Function in Nondiabetic Persons.” Annals of Internal Medicine
133 (8): 585–91. [
PubMed: 11033585]
Powell J T.
1998. “Vascular Damage from Smoking: Disease Mechanisms at the Arterial Wall.” Vascular Medicine
3 (1): 21–28. [
PubMed: 9666528]
Prescott E, Hippe M, Schnohr P, Hein H O, Vestbo J. 1998. “Smoking and Risk of Myocardial Infarction in Women and Men: Longitudinal Population Study.” BMJ
316 (7137): 1043–47. [
PMC free article: PMC28505] [
PubMed: 9552903]
Primatesta P, Falaschetti E, Gupta S, Marmot M G, Poulter N R. 2001. “Association between Smoking and Blood Pressure: Evidence from the Health Survey for England.” Hypertension
37 (2): 187–93. [
PubMed: 11230269]
Pujades-Rodriguez M, George J, Shah A D, Rapsomaniki E, Denaxas S., and others. 2015. “Heterogeneous Associations between Smoking and a Wide Range of Initial Presentations of Cardiovascular Disease in 1 937 360 People in England: Lifetime Risks and Implications for Risk Prediction.” International Journal of Epidemiology
44 (1): 129–41. [
PMC free article: PMC4339760] [
PubMed: 25416721]
Qiao Q, Tervahauta M, Nissinen A, Tuomilehto J. 2000. “Mortality from All Causes and from Coronary Heart Disease Related to Smoking and Changes in Smoking during a 35-Year Follow-Up of Middle-Aged Finnish Men.” European Heart Journal
21 (19): 1621–26. [
PubMed: 10988015]
Rastogi T, Jha P, Reddy K S, Prabhakaran D, Spiegelman D., and others. 2005. “Bidi and Cigarette Smoking and Risk of Acute Myocardial Infarction among Males in Urban India.” Tobacco Control
14 (5): 356–58. [
PMC free article: PMC1748103] [
PubMed: 16183987]
Regan A K, Promoff G, Dube S R, Arrazola R. 2013. “Electronic Nicotine Delivery Systems: Adult Use and Awareness of the ‘E-Cigarette’ in the USA.” Tobacco Control
22 (1): 19–23. [
PubMed: 22034071]
Rigotti N A, Clair C. 2013. “Managing Tobacco Use: The Neglected Cardiovascular Disease Risk Factor.” European Heart Journal
34 (42): 3259–67. [
PubMed: 24014389]
Rosenthal L, Carroll-Scott A, Earnshaw V A, Sackey N, O’Malley S S., and others. 2013. “Targeting Cessation: Understanding Barriers and Motivations to Quitting among Urban Adult Daily Tobacco Smokers.” Addictive Behaviors
38 (3): 1639–42. [
PMC free article: PMC3575130] [
PubMed: 23254211]
Roth G A, Forouzanfar M H, Moran A E, Barber R, Nguyen G., and others. 2015. “Demographic and Epidemiologic Drivers of Global Cardiovascular Mortality.” New England Journal of Medicine
372 (14): 1333–41. [
PMC free article: PMC4482354] [
PubMed: 25830423]
Roth G A, Nguyen G, Forouzanfar M H, Mokdad A H, Naghavi M., and others. 2015. “Estimates of Global and Regional Premature Cardiovascular Mortality in 2025.” Circulation
132 (13): 1270–82. [
PubMed: 26408271]
Salahuddin S, Prabhakaran D, Roy A. 2012. “Pathophysiological Mechanisms of Tobacco-Related CVD.” Global Heart
7 (2): 113–20. [
PubMed: 25691307]
Saleheen D, Zhao W, Rasheed A. 2014. “Epidemiology and Public Health Policy of Tobacco Use and Cardiovascular Disorders in Low- and Middle-Income Countries.” Arteriosclerosis, Thrombosis, and Vascular Biology
34 (9): 1811–19. [
PubMed: 25035346]
Seo D C, Torabi M R. 2007. “Reduced Admissions for Acute Myocardial Infarction Associated with a Public Smoking Ban: Matched Controlled Study.” Journal of Drug Education
37 (3): 217–26. [
PubMed: 18047180]
Shao B, O’Brien K D, McDonald T O, Fu X, Oram J F., and others. 2005. “Acrolein Modifies Apolipoprotein A-I in the Human Artery Wall.” Annals of the New York Academy of Sciences
1043 (June): 396–403. [
PubMed: 16037261]
Shaper A., 2003. “Pipe and Cigar Smoking and Major Cardiovascular Events, Cancer Incidence, and All-Cause Mortality in Middle-Aged British Men.” International Journal of Epidemiology
32 (5): 802–8. [
PubMed: 14559754]
Shihadeh A., 2003. “Investigation of Mainstream Smoke Aerosol of the Argileh Water Pipe.” Food and Chemical Toxicology
41 (1): 143–52. [
PubMed: 12453738]
Shlipak M G, Fried L F, Cushman M, Manolio T A, Peterson D., and others. 2005. “Cardiovascular Mortality Risk in Chronic Kidney Disease.” JAMA
293 (14): 1737. [
PubMed: 15827312]
Sibai A M, Tohme R A, Almedawar M M, Itani T, Yassine S I., and others. 2014. “Lifetime Cumulative Exposure to Waterpipe Smoking Is Associated with Coronary Artery Disease.” Atherosclerosis
234 (2): 454–60. [
PubMed: 24814409]
Simpson C R, Buckley B S, McLernon D J, Sheikh A, Murphy A., and others. 2011. “Five-Year Prognosis in an Incident Cohort of People Presenting with Acute Myocardial Infarction.” PLoS One
6 (1): e26573. [
PMC free article: PMC3197664] [
PubMed: 22028911]
Sleiman M, Gundel L A, Pankow J F, Jacob P, Singer B C., and others. 2010. “Formation of Carcinogens Indoors by Surface-Mediated Reactions of Nicotine with Nitrous Acid, Leading to Potential Thirdhand Smoke Hazards.” Proceedings of the National Academy of Sciences of the United States of America
107 (15): 6576–81. [
PMC free article: PMC2872399] [
PubMed: 20142504]
Smith C J, Fischer T H.
2001. “Particulate and Vapor Phase Constituents of Cigarette Mainstream Smoke and Risk of Myocardial Infarction.” Atherosclerosis
158 (2): 257–67. [
PubMed: 11583703]
Smith F B, Lee A J, Fowkes F G, Price J F, Rumley A., and others. 1997. “Hemostatic Factors as Predictors of Ischemic Heart Disease and Stroke in the Edinburgh Artery Study.” Arteriosclerosis, Thrombosis, and Vascular Biology
17 (11): 3321–25. [
PubMed: 9409328]
Stadler M, Tomann L, Storka A, Wolzt M, Peric S., and others. 2014. “Effects of Smoking Cessation on B-Cell Function, Insulin Sensitivity, Body Weight, and Appetite.” European Journal of Endocrinology
170 (2): 219–27. [
PubMed: 24179100]
Stefanadis C, Fischer E, Vlachopoulos C, Stratos C, Toutouzas K. 1997. “Unfavorable Effect of Smoking on the Elastic Properties of the Human Aorta.” Circulation
95 (1): 31–38. [
PubMed: 8994413]
Stoekenbroek R M, Boekholdt S M, Luben R, Hovingh G K, Zwinderman A H., and others. 2015. “Heterogeneous Impact of Classic Atherosclerotic Risk Factors on Different Arterial Territories: The EPIC-Norfolk Prospective Population Study.” European Heart Journal
37 (11): 880–90. [
PubMed: 26681771]
Svendsen K H, Kuller L H, Martin M J, Ockene J K. 1987. “Effects of Passive Smoking in the Multiple Risk Factor Intervention Trial.” American Journal of Epidemiology
126 (5): 783–95. [
PubMed: 3661526]
Sweeting M J, Thompson S G, Brown L C, Powell J T, RESCAN Collaborators. 2012. “Meta-Analysis of Individual Patient Data to Examine Factors Affecting Growth and Rupture of Small Abdominal Aortic Aneurysms.” British Journal of Surgery
99 (5): 655–65. [
PubMed: 22389113]
Tachmes L, Fernandez R J, Sackner M A. 1978. “Hemodynamic Effects of Smoking Cigarettes of High and Low Nicotine Content.” Chest
74 (3): 243–46. [
PubMed: 688779]
Teo K K, Ounpuu S, Hawken S, Pandey M R, Valentin V., and others. 2006. “Tobacco Use and Risk of Myocardial Infarction in 52 Countries in the INTERHEART Study: A Case-Control Study.” The Lancet
368 (9536): 647–58. [
PubMed: 16920470]
Tracy R P, Psaty B M, Macy E, Bovill E G, Cushman M., and others. 1997. “Lifetime Smoking Exposure Affects the Association of C-reactive Protein with Cardiovascular Disease Risk Factors and Subclinical Disease in Healthy Elderly Subjects.” Arteriosclerosis, Thrombosis, and Vascular Biology
17 (10): 2167–76. [
PubMed: 9351386]
Twyman L, Bonevski B, Paul C, Bryant J. 2014. “Perceived Barriers to Smoking Cessation in Selected Vulnerable Groups: A Systematic Review of the Qualitative and Quantitative Literature.” BMJ Open
4 (12): e006414. [
PMC free article: PMC4275698] [
PubMed: 25534212]
U.S. Department of Health and Human Services. 1983. The Health Consequences of Smoking: Cardiovascular Disease. A Report of the Surgeon General. Washington, DC: U.S. Department of Health and Human Services.
U.S. Department of Health and Human Services. 1988. The Health Consequences of Smoking: Nicotine Addiction. A Report of the Surgeon General. Washington, DC: U.S. Department of Health and Human Services.
U.S. Department of Health and Human Services. 2001. Women and Smoking. A Report of the Surgeon General. Atlanta, GA: U.S. Surgeon General.
U.S. Department of Health and Human Services. 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General.
Atlanta, GA: U.S. Surgeon General. [
PubMed: 21452462]
U.S. Department of Health and Human Services. 2014. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General: Executive Summary. Washington, DC: U.S. Department of Health and Human Services. [
PubMed: 24455788]
Varsano S, Ganz I, Eldor N, Garenkin M. 2003. “Water-Pipe Tobacco Smoking among School Children in Israel: Frequencies, Habits, and Attitudes.” Harefuah
142 (11): 736–41, 807. [
PubMed: 14631902]
Venn A, Britton J. 2007. “Exposure to Secondhand Smoke and Biomarkers of Cardiovascular Disease Risk in Never-Smoking Adults.” Circulation
115 (8): 990–95. [
PubMed: 17296856]
Vinci M C, Polvani G, Pesce M. 2013. “Epigenetic Programming and Risk: The Birthplace of Cardiovascular Disease?” Stem Cell Reviews and Reports
9 (3): 241–53. [
PubMed: 22773406]
Vlietstra R E, Kronmal R A, Oberman A, Frye R L, Killip T. 1986. “Effect of Cigarette Smoking on Survival of Patients with Angiographically Documented Coronary Artery Disease. Report from the CASS Registry.” JAMA
255 (8): 1023–27. [
PubMed: 3945013]
WHO (World Health Organization). 2012. WHO Global Report: Mortality Attributable to Tobacco. Geneva: WHO.
WHO (World Health Organization). 2013. WHO Report on the Global Tobacco Epidemic: Enforcing Bans on Advertising, Promotion, and Sponsorship. Geneva: WHO.
WHO (World Health Organization). 2015a. “Cardiovascular Diseases: Fact Sheet.” WHO, Geneva.
Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. 2008. “Heart Failure in the General Population of Men: Morbidity, Risk Factors, and Prognosis.” Journal of Internal Medicine
249 (3): 253–61. [
PubMed: 11285045]
Will J C, Galuska D A, Ford E S, Mokdad A, Calle E E. 2001. “Cigarette Smoking and Diabetes Mellitus: Evidence of a Positive Association from a Large Prospective Cohort Study.” International Journal of Epidemiology
30 (3): 540–46. [
PubMed: 11416080]
Willigendael E M, Teijink J A W, Bartelink M L, Kuiken B W, Boiten J., and others. 2004. “Influence of Smoking on Incidence and Prevalence of Peripheral Arterial Disease.” Journal of Vascular Surgery
40 (6): 1158–65. [
PubMed: 15622370]
Wilson K, Gibson N, Willan A, Cook D. 2000. “Effect of Smoking Cessation on Mortality after Myocardial Infarction: Meta-Analysis of Cohort Studies.” Archives of Internal Medicine
160 (7): 939–44. [
PubMed: 10761958]
Winickoff J P, Friebely J, Tanski S E, Sherrod C, Matt G E., and others. 2009. “Beliefs about the Health Effects of ‘Thirdhand’ Smoke and Home Smoking Bans.” Pediatrics
123 (1): e74–79. [
PMC free article: PMC3784302] [
PubMed: 19117850]
Winkelmann B R, Holt K von, Unverdorben M. 2009. “Smoking and Atherosclerotic Cardiovascular Disease: Part I: Atherosclerotic Disease Process.” Biomarkers in Medicine
3 (4): 411–28. [
PubMed: 20477486]
Wolf M B, Baynes J W. 2007. “Cadmium and Mercury Cause an Oxidative Stress-Induced Endothelial Dysfunction.” Biometals
20 (1): 73–81. [
PubMed: 16752219]
Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S., and others. 2007. “Risk Factors for Chronic Kidney Disease in a Community-Based Population: A 10-Year Follow-Up Study.” Kidney International
71 (2): 159–66. [
PubMed: 17136030]
Yamaguchi Y, Kagota S, Haginaka J, Kunitomo M. 2000. “Evidence of Modified LDL in the Plasma of Hypercholesterolemic WHHL Rabbits Injected with Aqueous Extracts of Cigarette Smoke.” Environmental Toxicology and Pharmacology
8 (4): 255–60. [
PubMed: 10996545]
Yoshikawa H, Hellström-Lindahl E, Grill V. 2005. “Evidence for Functional Nicotinic Receptors on Pancreatic Beta Cells.” Metabolism: Clinical and Experimental
54 (2): 247–54. [
PubMed: 15690320]
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A., and others. 2004. “Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study.” The Lancet
364 (9438): 937–52. [
PubMed: 15364185]
Zhu Y, Zhang M, Hou X, Lu J, Peng L., and others. 2011. “Cigarette Smoking Increases Risk for Incident Metabolic Syndrome in Chinese Men—Shanghai Diabetes Study.” Biomedical and Environmental Sciences
24 (5): 475–82. [
PubMed: 22108412]