Corresponding author: Dorairaj Prabhakaran, Centre for Chronic Disease Control, New Delhi; and Centre for Control of Chronic Conditions, Public Health Foundation of India, Haryana, India; gro.aidnicdcc@narakahbarpd.
Introduction
Cardiovascular, respiratory, and related disorders (CVRDs) are a subset of noncommunicable diseases (NCDs) that are an important and increasing cause of morbidity and mortality in low- and middle-income countries (LMICs). CVRDs share common risk factors such as smoking, poor diet, and physical inactivity. They also share common interventions at the clinical, public health, and policy levels. Public health professionals and decision makers share a widespread notion that CVRDs are diseases of the affluent (WHO 2010b). Yet recent cross-national studies have demonstrated that the burden of CVRDs falls disproportionately on lower-income countries and disadvantaged groups within countries. Prevention and control of CVRDs, then, have important equity implications. Addressing CVRDs also fits in with the Sustainable Development Goals that focus on reducing poverty and improving health, particularly through mechanisms (such as universal health coverage) that can address the rise in CVRD risk factors and the potential impoverishing effects of chronic illness.
The concept that current or past exposure to specific factors increases the risk of future ischemic heart disease (IHD) was first established in the Framingham Heart study in the United States (Kannel and others 1961), but it has been validated extensively in LMICs (O’Donnell and others 2010; Yusuf and others 2004). These risk factors are now well established globally, not only for IHD (Pearson and others 2003; Perk and others 2013; Yusuf and others 2001; Yusuf and others 2004), but also for stroke (Colditz and others 1988; Markus 2011; O’Donnell and others 2010), other cardiovascular diseases (CVDs) (Greenland and others 2010; Khatibzadeh and others 2013; Mosca and others 2004; Smith and others 2011), diabetes (Caballero 2003; Singh and others 2010; Weber and others 2012; Zimmet and others 1999), chronic lung disease (Madison, Zelman, and Mittman 1980; Palta and others 1991; Salvi and Barnes 2009; Strope and Stempel 1984), and other chronic NCDs (Allender and others 2011; Ezzati and Riboli 2013; Hallal and others 2012). Exposure to these risk factors may occur early in life, including in utero, and continue throughout life or may be limited to only certain phases of the life span. These risk factors may be strongly influenced by socioeconomic and environmental determinants, policy and legislative interventions, lifestyle and behavioral choices, and familial and genetic predisposition. Among modifiable risk factors, reducing the level of individual or population risk or discontinuing the exposure leads to corresponding reductions in the magnitude of disease burden and preventable deaths. In-depth knowledge of these relationships as well as the distribution of risk factors in the population provides a sound basis for developing prevention strategies at the individual and population levels.
This chapter describes recent trends in mortality and morbidity from CVRDs in LMICs and the specific conditions (including IHD, structural heart disease, heart failure, stroke, peripheral arterial disease, diabetes, kidney disease, and chronic lung disease) and risk factors covered in this volume. It then reviews the evidence regarding the complex interrelationships between specific risk factors, their early- and late-life determinants, and their corresponding influence on CVRD risk later in life. Finally, it presents steps for addressing CVRD risk factors and for reducing preventable deaths within a socioecological framework.
Trends in CVRD Mortality and Morbidity
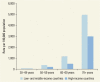
Of the 55.9 million deaths globally in 2012, 37.9 million were from NCDs, including 23.9 million deaths from CVRDs. A plurality of deaths (17.5 million) were from CVDs, with respiratory diseases (4.0 million) being the second-most frequent cause of death; diabetes mellitus and kidney diseases followed, with 1.5 million and 864,000 deaths, respectively. NCDs not covered in this volume—including mental and neurological disorders and cancers—constituted 37 percent of total NCD deaths. presents total deaths by cause and country income group.
CVRD Deaths, by Cause and Country Income Group, for All Ages and Both Genders, 2012. Thousands, unless otherwise noted
Because of a convergence of population size and the epidemiological transition, the vast majority of CVRD deaths occur in LMICs, including 74 percent of cardiovascular deaths, 83 percent of diabetes deaths, 84 percent of respiratory disease deaths, and 76 percent of kidney disease deaths. illustrates the proportion of total deaths caused by each group: in low-income countries, 21 percent of deaths were from CVRDs, compared with 30 percent and 54 percent of deaths, respectively, in lower-middle-income and upper-middle-income countries.
Proportion of Total Deaths Caused by Cardiovascular, Respiratory, and Related Disorders and Other Noncommunicable Diseases, by Country Income Group, for All Ages and Both Genders, 2012.
Most CVRD deaths occur in LMICs because of disparities in age-specific mortality rates and demographic changes. First, age-specific mortality rates from CVRDs are substantially higher in LMICs than in high-income countries (HICs) (). Although the prevalence of various risk factors is generally lower in LMICs (particularly in low-income countries), case fatality rates are much higher, probably because of the low use of evidence-based prevention and treatment interventions (Yusuf and others 2011; Yusuf and others 2014).
Age-Specific Mortality Rates from Cardiovascular, Respiratory, and Related Disorders, by Country Income Group, for Both Genders, 2012.
Second, rapid demographic changes are driving the overall increase in observed deaths. Between 2000 and 2012, the total population increased 8 percent in HICs and 17 percent in LMICs. The adult population grew even faster (14 percent and 32 percent, respectively). These trends are probably being driven by persistently high fertility rates in many LMICs as well as lower under-five mortality rates, which together contribute to overall population growth and aging. In 1960 less than 5 percent of the global population was older than age 65 years, and 15 percent was younger than age 5 years. By 2015, these proportions had converged and were projected to reverse by 2040. The result of this demographic change is that a larger total number of persons in LMICs are living to older ages and are more exposed to CVRD risk determinants and CVRDs themselves (WHO and U.S. National Institute on Aging 2011).
Age-standardized mortality rates are a useful metric for disentangling epidemiological and demographic changes in CVRD mortality. We calculated age-standardized mortality rates for CVRDs using the 2012 global population structure as a reference for region-specific rates in 2000 and 2012. For CVDs, deaths are rising in all regions because of demographic changes; however, age-standardized mortality rates are declining in HICs much faster than in LMICs, probably because of reductions in risk factors and a decline in case fatality rates ().
Percentage Changes in Total Deaths and Age-Standardized Mortality Rates from Cardiovascular, Respiratory, and Related Disorders, by Country Income Group, for Both Genders, 2000–12. Percentage change
Age-standardized mortality rates in LMICs are rising for diabetes but declining for respiratory and kidney diseases. The substantial decline in respiratory disease rates is probably related to progress in reducing household air pollution, although this reduction has been offset somewhat by the rise in outdoor air pollution, a subject discussed in volume 7 of DCP3 (Watkins, Dabestani, and others 2017).
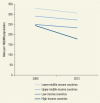
CVDs—the predominant cause of CVRD mortality—demonstrate marked and increasingly disparate trends in age-standardized mortality rates. Rates in high- and low-income countries were similar in 2000, but much more rapid progress had been made in HICs by 2012. Lower-middle-income and upper-middle-income countries had higher rates than HICs in 2000, and their rates have continued to decline modestly, as they have in low-income countries (). Again, this growing inequality in cardiovascular health requires further exploration but, in general, reflects rapid epidemiological change in LMICs and the absence of a health system response to address cardiovascular mortality. Unfortunately, if current trends continue, LMICs are unlikely to meet the Sustainable Development Goal target of achieving a one-third reduction in premature mortality from NCDs.1
Age-Standardized Mortality Rates from Cardiovascular Disease, by Country Income Group, for Both Genders, 2000–12.
Finally, the summary metric of disability-adjusted life-years (DALYs) provides insight into the relative impact of CVRD morbidity and mortality. Generally, premature mortality (years of life lost) is the major contributor to total DALYs from CVRDs, but morbidity (years lived with disability) is a particularly important aspect of diabetes and respiratory and kidney diseases. CVDs are responsible for 74 percent of deaths, but only 64 percent of DALYs. Diabetes is responsible for 6 percent of deaths, but 10 percent of DALYs; respiratory diseases are responsible for 17 percent of deaths, but 22 percent of DALYs; and kidney diseases are responsible for 4 percent of deaths, but 5 percent of DALYs.
Specific CVRDS
Ischemic Heart Disease
IHD is caused by gradual narrowing of the blood vessels that supply the heart muscles as a result of the buildup of fatty plaques. This narrowing reduces the supply of blood and starves the heart muscles of oxygen, culminating in heart attack. The underlying causes of IHD are predominantly of lifestyle origin, including tobacco use, unhealthy diet, low consumption of fruits and green leafy vegetables, physical inactivity, and harmful use of alcohol.
IHD is a leading cause of death and disability worldwide, accounting for 13 percent of all deaths in 2012. IHD rates vary across populations. The onset of IHD is found to be at least a decade earlier among South Asian populations than among Western populations (McKeigue and others 1993). IHD-related mortality rates are also higher among South Asians. Indeed, in the past few years, IHD rates have been declining in many HICs but rising in LMICs as result of increasing longevity, urbanization, and lifestyle changes.
Stroke
Stroke continues to be a leading cause of death in Africa and major parts of South-East Asia. The causative reasons for stroke in these regions include ethnicity as well as the high prevalence of hypertension among Africans and the high consumption of salt (a precursor of hypertension) among South-East Asians. Stroke can be fatal, but it is more frequently debilitating and has been described as worse than death in some communities. However, stroke is eminently preventable: the single most important modifiable factor for preventing stroke is hypertension. A third of stroke events are attributable to hypertension alone, and hypertension is the entry point for all stroke prevention interventions.
Other Cardiovascular Diseases
Peripheral vascular disease is an important cause of morbidity that shares many risk factors and treatments with IHD and stroke. Hypertensive heart disease, which leads to heart failure without overt ischemia, is particularly relevant in African countries early in the epidemiological transition. Congenital heart disease affects about 1 percent of the world’s population, and its frequency is similar in LMICs. It is discussed along with two other structural heart diseases that are endemic in LMICs (rheumatic heart disease and Chagas disease) in chapter 11 of this volume (Watkins, Hasan, and others 2017). Although all of these conditions are relatively neglected compared with IHD and stroke, they accounted for 18 percent of all cardiovascular deaths in LMICs in 2012 ().
Specific Causes of Cardiovascular Death in Low- and Middle-Income Countries, 2012.
Diabetes
Diabetes and metabolic syndrome have emerged as a major problem in many parts of the world, especially LMICs. China and India are home to a large number of people with diabetes. These two countries have an estimated 69 million and 109 million diabetics, respectively, numbers that are projected to increase to 123 million and 150 million, respectively, by 2040 (IDF 2015). Diabetes, in the long term, results in both microvascular and macrovascular complications. Microvascular complications include retinopathy, nephropathy, and neuropathy, and are highly disabling. Macrovascular complications include IHD, ischemic stroke, and amputations as a result of foot infections. Many individuals with diabetes die from IHD or stroke, with the cause of death often being registered as IHD or stroke, not diabetes; therefore, existing estimates likely underestimate the true impact of diabetes on population health.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. Tobacco smoking is established as a major risk factor, but emerging evidence suggests that other risk factors are also important, especially in LMICs. An estimated 25 percent to 45 percent of patients with COPD have never smoked, so the burden of nonsmoking COPD is much higher than previously believed. Half of the world’s population—about 3 billion people—are exposed to smoke from biomass fuel, compared with 1.01 billion people who smoke tobacco, suggesting that exposure to biomass smoke might be the biggest risk factor for COPD globally. At the same time, age-standardized mortality rates from air pollution are declining in areas where indoor biomass fuels are being replaced with cleaner fuels. Other factors associated with COPD are occupational exposure to dusts and gases, history of pulmonary tuberculosis, chronic asthma, respiratory tract infections during childhood, outdoor air pollution, and poor socioeconomic status.
Asthma
Asthma, a disease of the airways, occurs in people of all ages. Globally, 334 million people have asthma, and the burden of disability is as high as 222 million DALYs; the most affected people are in LMICs (Global Asthma Network 2014; Institute of Health Metrics and Evaluation 2013). Asthma burden is found to have an age-specific gradient. Children ages 10–14 years and elderly persons ages 75–79 years have higher rates of asthma (Global Asthma Network 2014). Asthma symptoms became more common in children in many LMICs between 1993 and 2003; globally, 14 percent of children experience asthma symptoms (Global Asthma Network 2014). Factors responsible for increasing asthma rates are not fully understood, but environmental and lifestyle changes play the key roles.
Relationships Between Risk Exposure and Disease Burden
Nonmodifiable Risk Factors
Risk factors for CVD, diabetes, chronic lung disease, and other chronic NCDs are considered nonmodifiable if their values, once established at birth, cannot be modified. Examples of these nonmodifiable risk factors include age, gender, race, ethnicity, birthweight, prematurity at the time of birth, family medical history, and genetic makeup. Although their actual values cannot be changed, their physiological implications and overall impact on morbidity and mortality can be modified, especially when knowledge of these risk factors appropriately informs strategies for screening for, evaluating, preventing, treating, and controlling NCDs and their modifiable risk factors.
In addition to their individual roles as nonmodifiable risk factors, they have important interactions. Notable examples are the interactions between age, gender, and race or between genes and the environment. These interactions could be taken into account in the prevention and control of disease and risk factors (Ahmad and others 2013; Giolo and others 2010; Hui and others 2013; Lin and others 2009; Schumacher, Hunt, and Williams 1990).
Age is the most important nonmodifiable risk factor for CVRDs. Advancing age is the most powerful independent predictor of death and disability because it effectively integrates the cumulative impact of all modifiable and nonmodifiable risk factor exposures over time. Advancing age is a key factor in the global rise in CVRD mortality (Lozano and others 2012). As a result, interventions designed to address modifiable risk factors early in life and through youth and middle age have the greatest potential to reduce preventable death and disability from CVRDs (WHO 2005). Nonetheless, effective primary and secondary prevention and control of modifiable risk factors in older adults remain important, because these risk factors can still be modified as individuals age, resulting in moderate to large reductions in mortality.
CVDs, diabetes, and chronic lung disease are just as important in women as they are in men. However, at equivalent chronological ages, IHD is two to five times more common in men than in women, depending on the population studied (Jousilahti and others 1999). This gender difference in IHD is particularly prominent before the fifth decade of life; however, the difference reverses and IHD becomes more prominent in women in later decades. More than half of the observed gender-related difference in susceptibility to IHD can be explained by gender-related differences in measured risk factors (Jousilahti and others 1999).
Modifiable Risk Factors
The INTERHEART study showed that high blood pressure, smoking, abnormal lipids, diabetes, abdominal obesity, psychosocial factors, low consumption of fruits and vegetables, physical inactivity, and harmful use of alcohol account for most of the risk of IHD worldwide in both men and women at all ages and in all regions (Yusuf and others 2004). Similarly, the INTERSTROKE study demonstrated that 10 risk factors are associated with 90 percent of the risk of stroke and that targeted interventions that reduce blood pressure and smoking and promote physical activity and a healthy diet could substantially reduce the burden of stroke (O’Donnell and others 2010). Many studies have replicated these findings, and these risk factors are now routinely measured in cardiovascular epidemiology and are tracked in comparative risk assessments conducted for burden of disease studies (GBD 2013
Risk Factors Collaborators 2015).
The prevalence of modifiable risk factors for CVRDs is increasing in many LMICs, particularly middle-income countries, as Western dietary and lifestyle patterns become global dietary and lifestyle patterns ().
Prevalence of Selected Cardiovascular, Respiratory, and Related Disorders Risk Factors, by Country Income Group, Various Years. Percent
Tobacco Use
Tobacco use is an important modifiable risk factor for NCD and total mortality. Tobacco use accounted for more than 6.1 million deaths worldwide in 2013 (GBD 2013 Risk Factors Collaborators 2015). This estimate includes exposure to passive smoking (secondhand smoke), which increases the risk of development and progression of atherosclerosis (Law, Morris, and Wald 1997). Cigarettes are the most common smoked form of tobacco, although several other forms are in use. Bidis, small hand-rolled cigarettes widely used in South Asian countries, have levels of nicotine equal to or higher than those in traditional cigarettes and are associated with higher risk for CVRD and cancers (Gupta and Asma 2008). Nearly 80 percent of the world’s 1 billion smokers live in LMICs, and that number is projected to rise during the next decade if trends continue (Bilano and others 2015). Most smokers in LMICs are male, which is in contrast to HICs. Tobacco smokers have 2–3 times higher relative risk of IHD, 1.5 times higher risk of stroke, 1.4 times higher risk of COPD, and 12 times higher risk of lung cancer. These risks have an age gradient, with higher relative risk (5–6 times) in younger age groups, although they are similar for men and women (Parish and others 1995; Peto and others 1994). Most of these risks decline within 10 years of quitting smoking (Rosenberg and others 1985). Furthermore, tobacco smoke interacts with other risk factors, and chewable forms of tobacco are as dangerous as cigarettes, causing a range of cancers.
Dyslipidemia (Abnormal Pattern of Blood Fat Level)
Cholesterol in excess, particularly bad cholesterol (low-density lipoprotein), increases the risk of heart disease, stroke, and other vascular diseases. Cholesterol is the key component in the development of atherosclerosis (accumulation of fatty deposits on the inner lining of arteries). The prevalence of high cholesterol among adults in LMICs ranges from 24.3 percent in low-income countries to 45.6 percent in upper-middle-income countries (). High cholesterol is estimated to cause 2.6 million deaths and 30 million DALYs annually.
High Blood Pressure
Globally, high blood pressure (hypertension) is implicated in about 7.5 million deaths (about 13 percent of all deaths) and 57 million DALYs (WHO 2010b). High blood pressure is often without symptoms, silently damaging the arteries that supply blood to the heart, brain, kidneys, and elsewhere and producing a variety of structural changes. High blood pressure increases the risk of stroke, heart attack, kidney failure, and congestive heart failure. When high blood pressure exists with obesity, smoking, high blood cholesterol, or diabetes, the risk of IHD and stroke increases several fold. The relationship of blood pressure to coronary heart disease is linear, positive, and graded, with no discernible lower threshold. Each difference of 20 millimeters of mercury (mmHg, a measure of pressure) in systolic blood pressure is associated with a twofold increase in the relative risk of coronary heart disease, while a 5 mmHg reduction in systolic blood pressure in the population is associated with a 14 percent overall reduction in mortality attributable to stroke, a 9 percent reduction in mortality attributable to coronary heart disease, and a 7 percent decrease in all-cause mortality (Chobanian and others 2003). The prevalence of hypertension among adults in LMICs ranges from 20.3 percent in upper-middle-income countries to 27.6 percent in low-income countries ().
Obesity and Overweight
In 2014, more than 1.9 billion adults were estimated to be overweight; of these, more than 600 million were obese (WHO 2016a). The prevalence of overweight and obesity among adults ranges from 21.0 and 4.7 percent, respectively, in low-income countries to 42.7 and 13.0 percent, respectively, in upper-middle-income countries (). Obesity is often defined as the accumulation of abnormal or excessive fat in adipose tissue to the extent that health may be impaired (WHO 2000b). People who develop excess body fat, especially at the waist, are more likely to develop heart disease and stroke even if they have no other risk factors. This condition is termed abdominal obesity or central obesity. The presence of abdominal obesity along with high glucose and high blood pressure and low levels of good cholesterol (high-density lipoprotein) leads to an insulin-resistant state called metabolic syndrome. There is strong evidence that weight loss in overweight and obese individuals reduces the risk of diabetes and CVD. Weight loss lowers blood pressure in overweight individuals with and without hypertension (Appel and others 2006), reduces serum triglycerides, and increases high-density lipoprotein (Miller and others 2011). More important, reductions in blood pressure occur even before attaining a desirable body weight (Appel and others 2006).
Physical Inactivity
According to the World Health Organization, globally, one in four adults is not active enough, and the problem of insufficient physical activity increases as country income rises (WHO 2016b). A concerning counter to this trend is the recent rise in insufficient physical activity among adolescents (), a problem that is just as large in low-income countries as in HICs (WHO 2016b). An inactive lifestyle is a risk factor for later overweight and obesity and all of the attendant health consequences. Regular, moderate-to-vigorous physical activity helps prevent CVD over the long term. Specifically, exercise can help control blood pressure, cholesterol, and diabetes as well as lead to weight loss. Even among persons who cannot achieve a normal weight, regular exercise can decrease the number and dosages of drugs to treat these other risk factors, thereby reducing costs and the risk of side effects.
Alcohol
The relationship of alcohol to overall mortality and cardiovascular mortality is thought to be J-shaped. A consistent cardio-protective effect has been observed for moderate consumption of one to two standard drinks per day, but heavy drinkers (including binge drinkers) have higher total mortality than moderate drinkers or abstainers (Srinath Reddy and Katan 2004). Moderate alcohol consumption has also been associated with a lower risk of ischemic stroke in men and women, with exceptions in populations such as South Asians (Sacco and others 1999). In contrast, long-term heavy alcohol consumption increases an individual’s risk for all subtypes of stroke (O’Donnell and others 2010). The possible beneficial effects of moderate alcohol consumption must be weighed against the deleterious effects of high intake, including increased risk of hypertension, alcoholic cardiomyopathy, atrial arrhythmias, and hemorrhagic stroke (Klatsky 2015). Alcohol consumption in excess of three drinks per day is associated with a rise in blood pressure and plasma triglyceride levels. Many international guidelines recommend reducing or stopping alcohol consumption for nonpharmacologic therapy of hypertension (Srinath Reddy and Katan 2004).
Social and Ecological Determinants of Risk
During the second half of the twentieth century, health indicators improved in almost all regions of the world. However, striking variations in cause-specific mortality rates, particularly related to NCDs, were observed within and between HICs and LMICs. These differentials suggested that social and ecological determinants of NCD risk were at work across populations.
Studies have found that the rapid rise in CVRD risk in LMICs is shaped largely by globalization and urbanization, which have profound impacts on dietary habits, tobacco consumption, and physical activity. The pathway is further modified by poverty, education, and stress levels. These upstream determinants decisively influence CVRD risk operating through behavioral factors (for example, tobacco use, diet, physical activity), biological factors (for example, blood pressure, cholesterol, blood glucose levels), psychosocial factors (for example, depression, anxiety, acute and chronic life stressors, lack of social support), and health system factors (for example, access to care, screening, diagnosis, quality of care) (Fuster and Kelly 2010).
The biosocial pathway alone cannot explain the cumulative CVRD risk that operates across the life course of individuals and populations. Socioeconomic conditions throughout the life course shape adult health and disease risk. For example, persons living in adverse childhood social circumstances are more likely to be of low birth weight and to be exposed to poor diet, childhood infections, and passive smoking. These exposures may increase their CVRD risk in adult life, perhaps through chains of risk or pathways over time where one adverse (or protective) experience tends to lead to another adverse (or protective) experience in a cumulative way (WHO 2010a). Thus, physical and social exposures during gestation, childhood, adolescence, young adulthood, and later adult life have long-term effects on the risk of NCDs.
Early-Life Determinants
Considerable work in recent years has explored how chronic disease risk begins in fetal life and continues into old age. Multiple studies have shown that adult onset of CVRDs is determined by risks that accumulate throughout life. Such risks are determined largely by what happens in early life and socioeconomic position throughout the life course. There are two major drivers for CVRD risk in adult life: (1) exposures during critical periods in life and (2) accumulation of risks throughout the life course.
Exposures during critical periods such as in the womb, infancy, and childhood have lasting or lifelong adverse effects on body systems that are not modified by any subsequent exposure. The concept of fetal programming explains how and why critical periods are important in the life of an individual, a subject discussed in detail in chapter 3 of this volume (Kumaran, Osmond, and Fall 2017). Furthermore, poverty and chronic undernutrition of women during pregnancy are associated with low birth weight of babies. Given these findings, the importance of the first 1,000 days of life for health and human development has gained greater recognition. The 1,000 days between conception and a child’s second birthday offer a unique window of opportunity to shape a healthier and more prosperous future. The right nutrition during this 1,000-day window can have a profound impact on a child’s ability to grow, learn, and rise out of poverty.
Risk during Childhood and Adolescence
Growing evidence suggests that environmental exposures do more damage to health and potential long-term health during critical periods of growth and development in childhood and adolescence than they would at other times. Children with low birth weight continue to be underweight up to two years, after which growth catches up and they become prone to childhood obesity (Sachdev and others 2005). Higher rates of obesity in these children increase the risk of adult overweight and obesity and CVRDs. Cohort studies have also found that many children with high blood pressure or obesity become adults with high blood pressure or obesity. The stability of these risk factors over time or the predictability of their future levels through measurements early in life is called tracking (Singh and others 2008).
In light of these findings, the impact of socioeconomic status is clearly evident in shaping the environment for CVRD risk of populations. Socioeconomic status bears heavily on household income and nutritional status; it can influence nutrition among pregnant women and their fetuses and affect future cognition, intelligence, schooling, and attainment of optimal height and weight and thus risk of CVRD in the next generation. As a result, households in lower socioeconomic positions face a vicious intergenerational cycle of poor nutrition, ill health, and poverty. Furthermore, children and adolescents from low socioeconomic backgrounds more frequently experiment with risky behaviors such as smoking and alcohol use and continue to maintain such risky behaviors into adulthood. Poor diet, another risk factor for CVRD, has also been documented among such children. Finally, girls who grow poorly become stunted as adults and are more likely to give birth to low-birth-weight babies who are likely to continue the cycle by being stunted in adulthood.
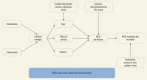
The relatively larger role played by social, economic, and environmental factors in the epidemic of chronic diseases is explained by several theoretical models that highlight the importance of policy-based approaches for protecting the health of populations. A life course perspective in policies and programs helps identify chains of risk that can be broken and times when intervention may be especially effective. Particularly during key life transitions—for example, late adolescence to early adulthood—not just “safety nets” but “springboards” must be provided that can alter life course trajectories and improve the health of subsequent generations (WHO 2000a). outlines the relationships between specific risk factors, their early- and late-life determinants, and their contributions to NCD risk.
Complex Interplay of Determinants and Risk Factors in the Pathway of Noncommunicable Diseases in the Population.
A Framework for Addressing the Challenge of CVRDS
The growing burden of NCDs in the past few decades clearly indicates that the health systems in LMICs need to focus more on preventing and controlling CVRDs, given that these nations face the major brunt of this epidemic. An integrated life course approach to prevention and control will be needed. The life course approach is an integrated continuum that affects all stages of life, including (but not limited to) the following:
Addressing nutrition during fetal and early life
Tackling childhood obesity through policy-level interventions such as banning certain advertisements aimed at schoolchildren, particularly in relation to food products
Vigorously controlling tobacco, particularly to prevent children from experimenting with and getting habituated to smoking
Effectively implementing the World Health Organization’s Framework Convention on Tobacco Control
Controlling risk factors in persons with single or multiple risk factors for primary prevention
Managing diseases for secondary prevention
Rehabilitating people with impairments and disabilities
Financially protecting people with acute illness
Focusing on neglected CVRDs.
It is also important to create an environment that is conducive for individuals to adopt and maintain a healthy life through macro-level policies, including agricultural policies promoting the consumption of fruits and vegetables, an enabling environment for physical activity, public transportation, and access to and affordability of essential drugs and diagnostics. This section describes the socioecological model as a basis for designing interventions for CVRD prevention and control.
Reducing Risk
Intervening in the causal pathway of CVRDs is complex. An ideal but comprehensive approach would follow a socioecological model for intervening that enables identification of interactions between an individual and his or her environment. The socioecological model integrates theories of individual behavior change with an understanding of the role of environmental enhancement in the interactions between an individual and his or her social and physical environments (McLeroy and others 1988). According to this socioecological model, human behaviors are fundamentally determined by five broad categories of factors: intrapersonal factors, interpersonal factors, organizational factors, community factors, and public policy (McLeroy and others 1988).
Multiple pathways and factors are involved in the causal pathway of CVRD risk, and a model that focuses exclusively on individuals is likely to fail. Therefore, efforts to improve individuals’ health must be directed simultaneously at multiple levels. The ideal approach would be to take advantage of opportunities for intervention at all stages of the life course by preventing the acquisition and augmentation of risk, detecting and reducing risk, managing NCD events, and preventing the progression of disease and recurrence of NCD events (Fuster and Kelly 2010). Relying on these concepts, the Institute of Medicine has provided a framework for NCD interventions ().
Framework for a Comprehensive Strategy to Address Noncommunicable Diseases.
An ideal approach would take advantage of multiple strategies that coordinate across multiple sectors with a mix of interventions that take into account context and locale:
An individual-level (high-risk) approach that focuses on clinical identification of individuals in the population at highest risk of CVRD and intensive treatment through behavior change interventions, pharmacological measures, or both
A population-level approach that focuses on shifting the distribution of risk factors in the population by implementing evidence-based policies, laws, and regulations that favorably affect the consumption of healthier foods, the built environment, and tobacco and alcohol use.
A comprehensive approach takes into account the full range of complex determinants of CVRDs to produce synergies among approaches at the individual and population levels. Concurrent modalities could include health promotion campaigns (for example, in communities, schools, and worksites) and reorientation and strengthening of health systems, with greater use of innovative applications of communications technologies, efficient use of medical therapies and technologies, and integrated clinical programs (Fuster and Kelly 2010).
Addressing Social Determinants to Prevent the Poor from Acquiring Unhealthy Habits
CVRDs and poverty form a vicious cycle. The poor are more vulnerable to CVRDs and are more likely to use tobacco products, to consume energy-dense food, and to consume fewer fruits and vegetables. These risky behaviors arise from social and economic inequalities such as lack of opportunity, lack of education, psychosocial stress, limited choice of consumption patterns, and inadequate access to health care (WHO 2005). They are exacerbated by aggressive marketing of tobacco products and greater access to cheaper energy-dense fried or processed foods, particularly in middle-income countries. As a result, the poor tend to be more obese than the wealthy and at greater risk of dying prematurely from CVRDs (WHO 2005). Ill health has a direct bearing on the livelihoods of poor individuals and families because the resulting loss of jobs and wages can exacerbate the poverty trap. Policies for inclusive growth and social protection for poor and marginalized groups are essential to protecting and promoting public health.
Conclusions
CVRDs are an increasing contributor to poor health in LMICs. The epidemic of CVRDs is being driven by population growth and aging in combination with increasing prevalence of risk factors and inadequate clinical management. Health systems in LMICs, which account for most of the CVRD burden globally, are ill equipped to address this challenge and will require additional investments to strengthen access to evidence-based prevention and treatment. At the same time, public policy could be reoriented with a life course focus so that CVRD prevention and control are not limited to clinical settings but also harness the power of specific policies to improve population health and well-being.
Notes
World Bank Income Classifications as of July 2014 are as follows, based on estimates of gross national income (GNI) per capita for 2013:
Low-income countries (LICs) = US$1,045 or less
Middle-income countries (MICs) are subdivided:
lower-middle-income = US$1,046 to US$4,125
upper-middle-income (UMICs) = US$4,126 to US$12,745
High-income countries (HICs) = US$12,746 or more.
- 1
References
Ahmad S, Rukh G, Varga T V, Ali A, Kurbasic A., and others. 2013. “Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry.” PLoS Genetics
9 (7): e1003607. [
PMC free article: PMC3723486] [
PubMed: 23935507]
Allender S, Wickramasinghe K, Goldacre M, Matthews D, Katulanda P. 2011. “Quantifying Urbanization as a Risk Factor for Noncommunicable Disease.” Journal of Urban Health: Bulletin of the New York Academy of Medicine
88 (5): 906–18. [
PMC free article: PMC3191205] [
PubMed: 21638117]
Appel L J, Brands M W, Daniels S R, Karanja N, Elmer P J., and others. 2006. “Dietary Approaches to Prevent and Treat Hypertension: A Scientific Statement from the American Heart Association.” Hypertension
47 (2): 296–308. [
PubMed: 16434724]
Bilano V, Gilmour S, Moffiet T, D’Espaignet E T, Stevens G A., and others. 2015. “Global Trends and Projections for Tobacco Use, 1990–2025: An Analysis of Smoking Indicators from the WHO Comprehensive Information Systems for Tobacco Control.” The Lancet
385 (9972): 966–76. [
PubMed: 25784347]
Caballero A E.
2003. “Endothelial Dysfunction in Obesity and Insulin Resistance: A Road to Diabetes and Heart Disease.” Obesity Research
11 (11): 1278–89. [
PubMed: 14627747]
Chobanian A V, Bakris G L, Black H R, Cushman W C, Green L A., and others. 2003. “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.” Journal of the American Heart Association
42 (6): 1206–52. [
PubMed: 14656957]
Colditz G A, Bonita R, Stampfer M J, Willett W C, Rosner B., and others. 1988. “Cigarette Smoking and Risk of Stroke in Middle-Aged Women.” New England Journal of Medicine
318 (15): 937–41. [
PubMed: 3352685]
Ezzati M, Riboli E. 2013. “Behavioral and Dietary Risk Factors for Noncommunicable Diseases.” New England Journal of Medicine
369 (10): 954–64. [
PubMed: 24004122]
Fuster V, Kelly B B. eds. 2010. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington, DC: National Academies Press. [
PubMed: 20945571]
GBD (Global Burden of Disease) 2013 Risk Factors Collaborators. 2015. “Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013.” The Lancet
386 (10010): 2287–323. [
PMC free article: PMC4685753] [
PubMed: 26364544]
Giolo S R, Pereira A C, Andrade M de, Krieger J E, Soler J P. 2010. “Evaluating Gene by Sex and Age Interactions on Cardiovascular Risk Factors in Brazilian Families.” BMC Medical Genetics
11 (January): 132. [
PMC free article: PMC2955567] [
PubMed: 20854676]
Global Asthma Network. 2014. The Global Asthma Report 2014. Auckland, New Zealand: Global Asthma Network.
Greenland P, Alpert J S, Beller G A, Benjamin E J, Budoff M J., and others. 2010. “2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.” Circulation
122 (25): e584–636. [
PubMed: 21098428]
Gupta P C, Asma S. eds. 2008. Bidi Smoking and Public Health. New Delhi: Ministry of Health and Family Welfare, Government of India.
Hallal P C, Clark V L, Assunção M C, Araújo C L P, Gonçalves H., and others. 2012. “Socioeconomic Trajectories from Birth to Adolescence and Risk Factors for Noncommunicable Disease: Prospective Analyses.” Journal of Adolescent Health
51 (Suppl 6): S32–37. [
PMC free article: PMC3508416] [
PubMed: 23283158]
Hui X, Matsushita K, Sang Y, Ballew S H, Fülöp T., and others. 2013. “CKD and Cardiovascular Disease in the Atherosclerosis Risk in Communities (ARIC) Study: Interactions with Age, Sex, and Race.” American Journal of Kidney Diseases
62 (4): 691–702. [
PMC free article: PMC3783539] [
PubMed: 23769137]
IDF (International Diabetes Federation). 2015. IDF Diabetes Atlas 2015. 7th ed. Brussels: IDF.
Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. 1999. “Sex, Age, Cardiovascular Risk Factors, and Coronary Heart Disease: A Prospective Follow-Up Study of 14,786 Middle-Aged Men and Women in Finland.” Circulation
99 (9): 1165–72. [
PubMed: 10069784]
Kannel W B, Dawber T R, Kagan A, Revotskie N, Stokes J. 1961. “Factors of Risk in the Development of Coronary Heart Disease—Six-Year Follow-Up Experience. The Framingham Study.” Annals of Internal Medicine
55
33–50. [
PubMed: 13751193]
Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. 2013. “Worldwide Risk Factors for Heart Failure: A Systematic Review and Pooled Analysis.” International Journal of Cardiology
168 (2): 1186–94. [
PMC free article: PMC3594565] [
PubMed: 23201083]
Klatsky A L.
2015. “Alcohol and Cardiovascular Diseases: Where Do We Stand Today?” Journal of Internal Medicine
278 (3): 238–50. [
PubMed: 26158548]
Kumaran K, Osmond C, Fall C H D. 2017. “Early Origins of Cardiometabolic Disease.” In Disease Control Priorities (third edition): Volume 5, Cardiovascular, Respiratory, and Related Diseases, edited by Prabhakaran D, Anand S, Gaziano T A, Mbanya J C, Wu Y, Nugent R, editors. . Washington, DC: World Bank.
Law M R, Morris J K, Wald N J. 1997. “Environmental Tobacco Smoke Exposure and Ischaemic Heart Disease: An Evaluation of the Evidence.” British Medical Journal
315 (7114): 973–80. [
PMC free article: PMC2127675] [
PubMed: 9365294]
Lin E, Pei D, Huang Y J, Hsieh C H, Wu L S H. 2009. “Gene-Gene Interactions among Genetic Variants from Obesity Candidate Genes for Nonobese and Obese Populations in Type 2 Diabetes.” Genetic Testing and Molecular Biomarkers
13 (4): 485–93. [
PubMed: 19594364]
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K., and others. 2012. “Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010.” The Lancet
380 (9859): 2095–128. [
PubMed: 23245604]
Madison R, Zelman R, Mittman C. 1980. “Inherited Risk Factors for Chronic Lung Disease.” Chest
77 (Suppl 2): 255–57. [
PubMed: 6965632]
Markus H S.
2011. “Stroke Genetics.” Human Molecular Genetics
20 (R2): R124–31. [
PubMed: 21831884]
McKeigue P M, Ferrie J E, Pierpoint T, Marmot M G. 1993. “Association of Early-Onset Coronary Heart Disease in South Asian Men with Glucose Intolerance and Hyperinsulinemia.” Circulation
87 (1): 152–61. [
PubMed: 8419002]
McLeroy K R, Bibeau D, Steckler A, Glanz K. 1988. “An Ecological Perspective on Health Promotion Programs.” Health Education Quarterly
15 (4): 351–77. [
PubMed: 3068205]
Miller M, Stone N J, Ballantyne C, Bittner V, Criqui M H., and others. 2011. “Triglycerides and Cardiovascular Disease: A Scientific Statement from the American Heart Association.” Circulation
123 (20): 2292–333. [
PubMed: 21502576]
Mosca L, Appel L J, Benjamin E J, Berra K, Chandra-Strobos N., and others. 2004. “Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women.” Journal of the American College of Cardiology
43 (5): 900–21. [
PubMed: 14998635]
O’Donnell M J, Xavier D, Liu L, Zhang H, Chin S L., and others. 2010. “Risk Factors for Ischaemic and Intracerebral Haemorrhagic Stroke in 22 Countries (the INTERSTROKE Study): A Case-Control Study.” The Lancet
376 (9735): 112–23. [
PubMed: 20561675]
Palta M, Gabbert D, Weinstein M R, Peters M E. 1991. “Multivariate Assessment of Traditional Risk Factors for Chronic Lung Disease in Very Low Birth Weight Neonates: The Newborn Lung Project.” Journal of Pediatrics
119 (2): 285–92. [
PubMed: 1861218]
Parish S, Collins R, Peto R, Youngman L, Barton J., and others. 1995. “Cigarette-Smoking, Tar Yields, and Nonfatal Myocardial-Infarction: 14,000 Cases and 32,000 Controls in the United Kingdom.” British Medical Journal
311 (7003): 471–77. [
PMC free article: PMC2550542] [
PubMed: 7647641]
Pearson T A, Bazzarre T L, Daniels S R, Fair J M, Fortmann S P., and others. 2003. “American Heart Association Guide for Improving Cardiovascular Health at the Community Level: A Statement for Public Health Practitioners, Healthcare Providers, and Health Policy Makers from the American Heart Association Expert Panel on Population and Prevention Science.” Circulation
107 (4): 645–51. [
PubMed: 12566381]
Perk J, Backer G De, Gohlke H, Graham I, Reiner Z., and others. 2013. “European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (Version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice.” Giornale Italiano di Cardiologia
14 (5): 328–92. [
PubMed: 23612326]
Peto R, Lopez A D, Boreham J, Thun M, Heath Jr C. . 1994. Mortality from Smoking in Developed Countries 1950–2000: Indirect Estimates from National Vital Statistics. Oxford U.K.: Oxford University Press.
Rosenberg L, Kaufman D W, Helmrich S P, Shapiro S. 1985. “The Risk of Myocardial Infarction after Quitting Smoking in Men under 55 Years of Age.” New England Journal of Medicine
313 (24): 1511–14. [
PubMed: 4069159]
Sacco R L, Elkind M, Boden-Albala B, Lin I F, Kargman D E. and others. 1999. “The Protective Effect of Moderate Alcohol Consumption on Ischemic Stroke.” JAMA
281 (1): 53–60. [
PubMed: 9892451]
Sachdev H S, Fall C H D, Osmond C, Lakshmy R, Biswas S K Dey., and others. 2005. “Anthropometric Indicators of Body Composition in Young Adults: Relation to Size at Birth and Serial Measurements of Body Mass Index in Childhood in the New Delhi Birth Cohort.” American Journal of Clinical Nutrition
82 (2): 456–66. [
PubMed: 16087993]
Salvi S S, Barnes P J. 2009. “Chronic Obstructive Pulmonary Disease in Non-Smokers.” The Lancet
374 (9691): 733–43. [
PubMed: 19716966]
Schumacher M C, Hunt S C, Williams R R. 1990. “Interactions between Diabetes and Family History of Coronary Heart Disease and Other Risk Factors for Coronary Heart Disease among Adults with Diabetes in Utah.” Epidemiology
1 (4): 298–304. [
PubMed: 2083307]
Singh A S, Mulder C, Twisk J W R, Mechelen W Van, Chinapaw M J M. 2008. “Tracking of Childhood Overweight into Adulthood: A Systematic Review of the Literature.” Obesity Reviews
9 (5): 474–88. [
PubMed: 18331423]
Singh S, Dhingra S, Ramdath D D, Vasdev S, Gill V., and others. 2010. “Risk Factors Preceding Type 2 Diabetes and Cardiomyopathy.” Journal of Cardiovascular Translational Research
3 (5): 580–96. [
PubMed: 20593256]
Smith S C, Benjamin E J, Bonow R O, Braun L T, Creager M A., and others. 2011. “AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and Other Atherosclerotic Vascular Disease: 2011 Update: A Guideline from the American Heart Association and American College of Cardiology Foundation.” Journal of the American College of Cardiology
58 (23): 2432–46. [
PubMed: 22055990]
Srinath Reddy K, Katan M B. 2004. “Diet, Nutrition, and the Prevention of Hypertension and Cardiovascular Diseases.” Public Health Nutrition
7 (1A): 167–86. [
PubMed: 14972059]
Strope G L, Stempel D A. 1984. “Risk Factors Associated with the Development of Chronic Lung Disease in Children.” Pediatric Clinics of North America
31 (4): 757–71. [
PubMed: 6462798]
Watkins D A, Dabestani N, Mock C N, Cullen M, Smith K R., and others. 2017. “Trends in Morbidity and Mortality Attributable to Injuries and Selected Environmental Hazards.” In Disease Control Priorities (third edition): Volume 7, Injury Prevention and Environmental Health, edited by Mock C N, Kobusingye O, Nugent R, Smith K, editors. . Washington, DC: World Bank. [
PubMed: 30212111]
Watkins D A, Hasan B, Mayosi B, Buhkman G, Marin-Neto J A., and others. 2017. “Structural Heart Diseases.” In Disease Control Priorities (third edition): Volume 5, Cardiovascular, Respiratory, and Related Conditions, edited by Prabhakaran D, Anand S, Gaziano T A, Mbanya J C, Wu Y, Nugent R, editors. . Washington, DC: World Bank.
Weber M B, Oza-Frank R, Staimez L R, Ali M K, Venkat K M Narayan. 2012. “Type 2 Diabetes in Asians: Prevalence, Risk Factors, and Effectiveness of Behavioral Intervention at Individual and Population Levels.” Annual Review of Nutrition
32: (August): 417–39. [
PubMed: 22524185]
WHO (World Health Organization). 2000a. The Implications for Training of Embracing a Life Course Approach to Health. Geneva: WHO.
WHO (World Health Organization). 2000b. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation. Technical Report Series 894. Geneva: WHO. [
PubMed: 11234459]
WHO (World Health Organization). 2005. Preventing Chronic Diseases: A Vital Investment. Geneva: WHO.
WHO (World Health Organization). 2010a. A Conceptual Framework for Action on the Social Determinants of Health. Geneva: WHO.
WHO (World Health Organization). 2014. “Global Health Estimates.” WHO, Geneva.
WHO (World Health Organization). Various years. “Global Health Observatory.” WHO, Geneva.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A., and others. 2004. “Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study.” The Lancet
364 (9438): 937–52. [
PubMed: 15364185]
Yusuf S, Islam S, Chow C K, Rangarajan S, Dagenais G., and others. 2011. “Use of Secondary Prevention Drugs for Cardiovascular Disease in the Community in High-Income, Middle-Income, and Low-Income Countries (the PURE Study): A Prospective Epidemiological Survey.” The Lancet
378 (9798): 1231–43. [
PubMed: 21872920]
Yusuf S, Rangarajan S, Teo K, Islam S, Li W., and others. 2014. “Cardiovascular Risk and Events in 17 Low-, Middle-, and High-Income Countries.” New England Journal of Medicine
371 (9): 818–27. [
PubMed: 25162888]
Yusuf S, Reddy S, Ounpuu S, Anand S. 2001. “Global Burden of Cardiovascular Diseases: Part I: General Considerations, the Epidemiologic Transition, Risk Factors, and Impact of Urbanization.” Circulation
104 (22): 2746–53. [
PubMed: 11723030]
Zimmet P, Boyko E J, Collier G R, Courten M de. 1999. “Etiology of the Metabolic Syndrome: Potential Role of Insulin Resistance, Leptin Resistance, and Other Players.” Annals of the New York Academy of Sciences
892 (November): 25–44. [
PubMed: 10842650]