NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baba AI, Câtoi C. Comparative Oncology. Bucharest (RO): The Publishing House of the Romanian Academy; 2007.
Endocrine gland neoplasms are the result of the action of successive events that lead to the disharmony or even the collapse of homeostatic control mechanisms, with the limitation of the number of differentiated cells, the appearance of cancerous cells and new functional properties, with exaggerated hormone secretion. Thus, there are spontaneous diseases secondary to primary endocrine gland defects (adrenal tumors) or these may originate from the feedback system, such as the hypophysis dependent Gushing disease [22]. The same author mentions the following possibilities of inducing endocrine tumors: endocrine gland transplantation; endocrine gland ablation; exocrine hormone administration; carcinogen administration; endocrine gland radiation. In addition to these possibilities of inducing endocrine neoplasms, more recent researches have amplified the spectrum of carcinogenic factors, especially with peculiarities for each endocrine gland. A more recent and special chapter is represented by tumors of the diffuse endocrine system (DES), also known as “amine precursor uptake and decarboxylation” (APUD).
In general, endocrine gland tumors clinically manifest by hormonal excesses, with signs and biochemical changes characteristic of the affected gland.
16.1. THYROID TUMORS
Most thyroid tumors are of follicular origin. Incidence is higher in dogs and cats, followed in decreasing order by horses [72], cattle, sheep and swine. In dogs, thyroid tumors occur in middle aged or old subjects, adenomas between 10 and 12 years, and carcinomas between 9 and 15 years [10]. Thyroid carcinomas are more frequent than adenomas in dogs, while in cats, the situation is reversed [30]. No sex predilection has been reported in dogs, unlike in humans, where females are more affected. A higher incidence has been found in Boxers and Brittany Spaniels, compared to other dog breeds [44, 45].
Histological, clinical and epidemiological researches on thyroid neoplasms in a Beagle dog population established a mean age of 16.2 years in subjects with benign tumors. The number of malignant tumors was higher than that of benign tumors at a younger age. It was found that 44% of thyroid tumors manifested hypothyroidism and no subject with thyroid tumors had hyperthyroidism [30].
Adult and old cats present a hyperthyroidism syndrome with thyroid hyperplasia, adenomas or adenocarcinomas, derived from follicular cells [11, 56].
The etiology of thyroid neoplasms as well as of other tumors is complex, sometimes difficult to establish accurately, but there are some well defined situations. Thus, exposure to ionizing radiation, iodine metabolic disorders, goitrogenic substances, the prolonged stimulation with thyroid hormones (TSH) etc., induce thyroid tumors.
Macroscopically, thyroid tumors are evidenced by the deformation and increase in volume of the anteroventral cervical region. Transcutaneously, on palpation, the thyroid appears as lobular, multinodular, firm, free or adherent to adjacent tissues. It should be emphasized that in general, thyroid adenomas move freely under the skin, while carcinomas are fixed by a local invasion extended to adjacent structures.
Clinical signs of hyperthyroidism in dogs with thyroid tumors predominantly manifest by polyuria, weight loss, polyphagia, fatigue, intolerance to heat and nervousness [10]. Laboratory studies on thyroid carcinomas monitor thyroglobulin and calcitonin levels, by immunoreactivity [1, 55]. Recent studies have approached the diagnosis of thyroid tumors by immunocytochemistry. A study performed on 32 follicular tumors in dogs showed that 100% reacted positively to thyroglobulin and 50% had a moderate positive reaction to neuron specific enolase. Diagnosis was: follicular adenomas; compact follicular carcinomas and C cell carcinomas [46]. The authors underscore the fact that all follicular cell tumors had a positive reaction for thyroglobulin, and C cell carcinomas showed 100% positive reaction for somatostatin. In conclusion, it is mentioned that thyroglobulin and calcitonin gene-related peptide (CGRP) are markers that can be used for the differentiation of follicular cell tumors or C cell tumors in dogs, while neuron specific enolase is not recommended as a marker for the diagnosis of C cell tumors. It may be concluded that positive diagnosis in thyroid carcinomas should benefit, in addition to histological examination, from cytochemical and immunocytochemical investigations [61].
The classification proposed by SANDERSLEBEN and HANICHEN (1974) includes: benign and malignant epithelial tumors; benign and malignant mesenchymal tumors; benign and malignant mesenchymal-epithelial tumors; secondary tumors; unclassified tumors; tumor-like lesions. The treatment of thyroid gland neoplasms will consider the mentioned classification, updated with the new data introduced by the WHO classification [33].
16.1.1. Follicular cell adenoma
Follicular adenomas macroscopically appear as well circumscribed nodules, in the thyroid parenchyma, being delimited by a connective capsule. Usually, tumor formations are single, more rarely multiple. Some adenomas are cystic, having a thin capsule that contains a yellowish or red-brown fluid. Cystic capsules are well vascularized, and towards the interior, the capsule is rough, with fine proliferations and a velvety aspect [10, 56].
Follicular adenomas and papillary adenomas can be morphologically distinguished.
Follicular cell adenomas may appear as microfollicular adenomas, in which cells are arranged as miniature follicles, with scant or no colloid, or macrofollicular adenomas, in which follicles are small, irregular, with abundant colloid and flat follicular cells.
Cystic adenoma can be usually formed by one or two cavities filled with protein fluid, with necrotic foci and erythrocytes. Tumor cells are arranged in nests, and at the periphery, a capsule of fibrous connective tissue is found. These adenomas gradually develop cystic degeneration.
Trabecular adenoma is the most poorly differentiated of all follicular types. Tumor cells are small and arranged in narrow columns separated by edematous fibrous stroma. Small follicular formations are evidenced.
Oxyphilic adenoma is formed by large cells the cytoplasm of which contains dense eosinophilic granulations in poorly evidenced follicles, with scant or no colloid. Oxyphilic cells are follicular cells with changed metabolism which have accumulated a high number of mitochondria in the cytoplasm.
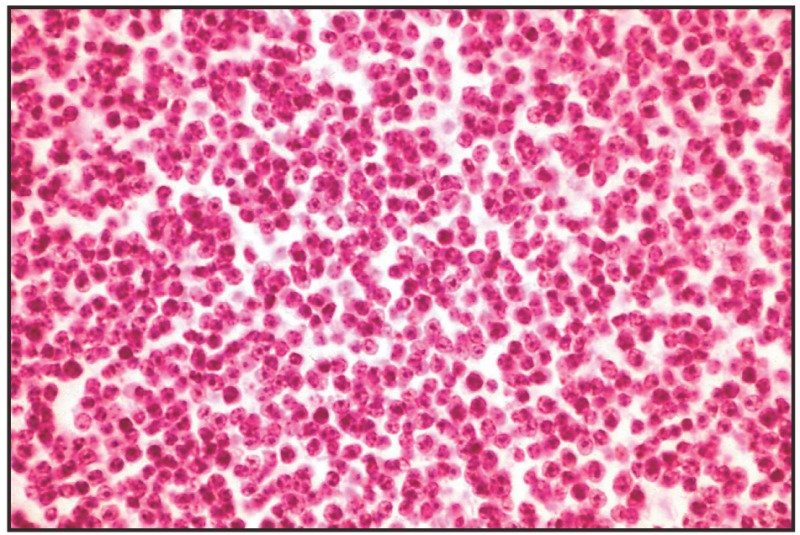
Papillary adenoma is characterized by the proliferation towards the cyst lumen of thin connective vascular peduncles, covered with columnar or cuboid cells disposed in one layer. Cysts contain desquamated tumor cells, colloid, erythrocytes and occasional mineralized foci [11]. Thyroid adenomas of papilliferous type have been diagnosed in old cats; thus, of 52 cats with enlarged thyroids, LEAV et al. (1976) [56] established in 17 subjects: multinodular adenomas, 22 adenomas, 8 atypical adenomas, 4 follicular carcinomas, and 1 papillary carcinoma (Fig. 16.1.).

Fig. 16.1
Papillary adenoma, thyroid gland.
16.1.2. Follicular cell carcinoma
Macroscopically, the neoplasm can be uni- or multinodular, being easy to identify due to its size. Carcinoma has local infiltrative growth, with penetration in the tracheal wall, cervical muscles, esophagus, larynx and structures from this area. Neoplastic cells penetrate the thyroid veins at an early stage, with the formation of emboli and lung metastases, even earlier than lymph node metastases [48]. Adenomas and especially carcinomas can develop at distance, from ectopic thyroid cells. Thus, thyroid tumors can develop in the anterior mediastinum, more frequently in dogs [70].
Histologically, thyroid follicular cell carcinoma is characterized by intense cellularization and marked pleomorphism. The microscopic structure determines differentiation into follicular carcinoma, papillary carcinoma and solid (compact) carcinoma, while intermediate forms may also exist.
In dogs, in which thyroid carcinomas are the most frequent, neoplasms have a mixed, follicular and compact aspect, while the papillary type is rare. In dogs, SULLIVAN et al. (1987), establish in 30 subjects examined clinically and postmortem a carcinoma diagnosis, 50% of cases having lung metastases.
Follicular adenocarcinoma is easy to diagnose microscopically, due to the follicular cell arrangement. Tumor cells are large, cuboid or columnar, forming follicles with various sizes, shapes and colloid content. Mitotic images are rare, and the colloid is under the form of clusters and frequently mineralized (Fig. 16.2.).

Fig. 16.2
Follicular carcinoma, thyroid gland.
Compact cellular carcinoma is characterized by the arrangement of cells in compact bands, compact cellular layers, separated by fine fibrous stroma, sometimes with a discrete follicular presence and scant colloid. Polyhedral follicular cells are compact, having a fine granular eosinophilic cytoplasm, with small vacuoles. Electron microscopically, these eosinophilic cells are mitochondria rich [64] (Fig. 16.3–16.5).

Fig. 16.3
Solid follicular carcinoma.

Fig. 16.4
Solid follicular carcinoma.
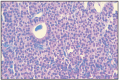
Fig. 16.5
Solid follicular carcinoma - thyroid gland
Mixed compact follicular-cellular carcinoma is characterized by a structure equally formed by compact and follicular tumor growth. This carcinoma type is more common in dogs. CAPEN (1993) remarks the following: follicles are smaller and contain less colloid compared to the purely follicular carcinoma type. Compact cells seem to be less morphologically and functionally differentiated than follicle forming and colloid secreting cells.
Papillary carcinoma is characterized by papillary proliferations that develop in the cyst lumen. This carcinoma type is more rarely found in animals. Fibrovascular proliferations are covered with cuboid cells arranged in single or multiple layers. Cells have pleomorphic and vesicular nuclei, with prominent nucleoli. Electron microscopy shows cytoplasmic invaginations in nuclear vacuoles. In dogs and cats, the papillary carcinoma type frequently presents an infiltrative growth of tumor cells in adjacent tissues. The early invasion of cells in veins induces lung metastases [10,11].
Microscopically undifferentiated thyroid carcinomas do not have a well differentiated structure and a pattern characteristic of tumor cells. In general, they are found accidentally (Fig. 16.5.).
Small cell carcinoma is an undifferentiated neoplasm, formed by highly malignant follicular cells, having a diffuse or compact growth. Cells are uniform, arranged in clusters, separated by fibrous stroma. Cells have a reduced cytoplasm, an oval hyperchromatic nucleus with numerous mitotic images.
Giant cell carcinoma has an undifferentiated character, with extremely high malignancy, deriving from undifferentiated follicular cells. Tumor cells are large, anaplastic, pleomorphic and fusiform, which makes difficult differentiation from fibrosarcoma. However, the presence of follicular remnants and transitional forms suggests the origin of giant cells from thyroid follicular cells [10, 11] (Fig. 16.6).

Fig. 16.6
Anaplastic carcinoma, with giant and pleomorphic cells.
Malignant mixed thyroid tumors have been described in dogs. Microscopy shows the presence of both malignant follicular cells and malignant mesenchymal structures of osteogenic or cartilaginous type.
Thyroid follicular cell hyperplasia. The increase in volume of the thyroid, without being of inflammatory or neoplastic nature, consequently hyperplasia, can be found in all species and requires differentiation from adenomas and adenocarcinomas.
The causes of this condition can be the deficiency of iodine, substances or plants that contain goitrogenic principles.
Thyroid hyperplasia may occur even in fetuses or newborns, in the case of imbalanced rations in gestating females. Such conditions have been described in bovine and swine abortions and newborns [4, 27].
The literature reports the sporadic presence of goiter in animals [10, 11], and areas in which the disease can be endemic are mentioned [4, 27]. Usually, goiter occurs on a dysmetabolic background, to which goitrogenic factors are added.
Macroscopically, thyroid hyperplasia manifests by increased volume, with a smooth or intensely bosselated surface and/or the presence of cysts. Color is red-brown, and when goiter has a cystic character, the gland is clayish. Consistency is dense, and adjacent tissues are infiltrated with gelatinous edema.
Nodular goiter is histologically characterized by multiple hyperplastic follicular cell foci, which are clearly delimited, without being encapsulated, from the adjacent thyroid structure. Hyperplastic cells are organized either as small follicles, with scant or no colloid, or larger follicles, of variable shapes and sizes. Columnar cells are disposed in single or multiple layers, sometimes with papillary projections towards the lumen. Sometimes, follicles overloaded with eosinophilic colloid form microcysts.
Diffuse hyperplastic colloid goiter has a uniform structure, the thyroid being uniformly and completely enlarged. In hyperplastic goiter, follicles have irregular shapes and sizes, with the presence of slightly eosinophilic vacuolated colloid. Follicular cells are columnar, with intensely eosinophilic cytoplasm, with small hyperchromatic nuclei, situated in the basal cell area. Follicular cells can form single or multiple layers, with papillary projections towards the lumen.
Colloid goiter is considered as a regression phase of diffuse thyroid hyperplasia. Follicles grow progressively, containing dense eosinophilic colloid. Due to compression exerted by a dense colloid without vacuoles, follicular cells become flat, with a compression atrophy aspect. A reduction of blood capillaries from perifollicular connective structures is found.
16.1.3. Thyroglossal duct remnant tumors
Thyroglossal duct remnant tumors appear extremely sporadically in animals, being mentioned in dogs. Due to the connection of the developing thyroid with the aortic sac, at least in dogs, accessory thyroid parenchyma is found in the mediastinum, which may undergo tumor changes. Thyroglossal duct remnants can become cystic, by the accumulation of protein material secreted by epithelial cells that line these cysts. The epithelial envelope can undergo neoplastic changes, with tumor proliferations under the form of papillary carcinomas.
Macroscopically, development manifests by the deformation of the lower cervical region. The tumor appears as multiple cysts, having a colloid content with whitish nodules in section.
Histologically, tumors have the appearance of well differentiated papilliferous carcinomas. Growths have a papilliferous character, covered by large cuboid cells in single or multiple layers. The cystic wall is formed by dense fibrous connective tissue with hemorrhagic foci and cholesterol deposits. Cells can be structured in follicles covered by cuboid epithelium, having variable colloid amounts.
These neoplasms are well differentiated, they have a slow growth, and postoperative recurrences are exceptional.
16.1.4. Thyroid parafollicular C cell tumors
Parafollicular C cell tumors have been diagnosed in adult and old bulls, in some laboratory rat lines, as well as in other domestic species. In sheep, OKADA et al. (1991) report two cases of C cell hyperplasia and a bilateral thyroid C cell carcinoma. The authors mention positive reactions to calcitonin and the identification of secretory granules in the C cell cytoplasms. An absolute priority is the communication of CRISSMAN et al. (1991), which describes ganglioneuromas in the thyroid gland of laboratory rats, in association with C cell tumors in the thyroid. By this scientific research, the authors argue for the origin of thyroid C cells in the neural crest. There are data mentioning the presence of these tumors in 30% of bulls [11], and 15–10% present C cell and ultimobranchial remnant hyperplasia. The literature only reports these neoplasms in bulls, never in cows [10]. In 1986, BUNDZA and STEAD reported C cell carcinomas in two cows.
The appearance of C cell tumors in both humans and bulls seems to be preceded by the formation of hyperplastic C cell nodules. In humans, these tumors seem to have a genetic component, being transmitted as an autosomal dominant character in some families [10].
Multiple endocrine tumors, especially bilateral pheochromocytoma, and occasionally hypophyseal adenoma, are present in bulls with C cell tumors. This type is explained by CAPEN (1978) as a simultaneous neoplastic transformation of multiple endocrine cell populations, which are derived from the neural crest. A diffuse or nodular hyperplasia of secretory cells from the medullary adrenal gland seems to precede the development of pheochromocytoma.
LEBLANC et al (1991) recommended for diagnostic purposes the use of immunocytochemistry techniques: follicular cell tumors were positive for thyroglobulin, but they were not immunoreactive for somatostatin and neurostatin.
C cell adenoma: macroscopically, dense excrescences, solitary or multiple gray-brown nodules 1–3 cm in diameter appear on the surface of the thyroid, in one or both thyroid lobes. In horses, nodules can be incorporated in the thyroid parenchyma.
Histologically, neoplastic C cells are disposed in small groups or nests, separated by fibrous connective tissue septa derived from the capsule. The adjacent thyroid parenchyma is compressed by the tumor nodule. Neoplastic cells are tall or columnar, forming small acinar or duct structures, having small amounts of colloid-like material. C cells are well differentiated, with abundant, slightly eosinophilic cytoplasm. Nuclei contain one or more nucleoli and uniformly distributed chromatin. Electron microscopically, the cytoplasm contains numerous secretory granules delimited by membranes, protein microfilaments, rough endoplasmic reticulum and a prominent Golgi apparatus. Adenoma C cells are well differentiated [11].
C cell carcinoma: macroscopically, it appears as multiple nodules situated in one or both thyroid lobes, sometimes the neoplastic proliferation involving the whole thyroid. Metastases are frequently located in cervical lymph nodes, in which necrotic and/or hemorrhagic foci appear in section. Lung metastases are rarer, under the form of small brown nodules, disseminated in all lung lobes.
Histology shows an intense cellularization of the tumor, cells being pleomorphic, of polyhedral or fusiform shapes, with slightly eosinophilic, fine granular, well delimited cytoplasm. Nuclei are vesicular, oval or elongated, with the presence of a moderate number of mitotic figures. Neoplastic C cell groups are delimited by fine vascular connective tissue, with the occasional presence of acinar or canalicular structures. Similar aspects are identified in lymph node and lung metastases (Fig. 16.7, 16.8).

Fig. 16.7
“C” cells carcinoma.

Fig. 16.8
“C” cells carcinoma.
Carcinomatous cells contain free ribosomes, a prominent Golgi apparatus, small vesicles, mitochondria and few mature secretory granules. When cells are grouped in acini and ducts, they form single or multiple layers, the plasma membranes of adjacent cells having an interdigital shape. These microvilli and cytoplasmic projections extend to the duct lumens. Amyloid deposits, fine fibers among collagen fiber bundles are frequently found.
Ultimobranchial tumors are found in bulls and are histologically similar to well differentiated C cells compared to thyroid follicular cells. The tumor mass is composed of differentiated C cells, accumulated under the form of foci, with abundant eosinophilic cytoplasm in the thyroid mass, and ultimobranchial follicles or large nodules with compact histological structures. These are accompanied by multifocal C cell hyperplasias in other parts of the thyroid lobules or hilum. Grouped neoplastic C cells are delimited by hyalinized stroma that contains amyloid. Portions of the thyroid neoplasm appear in bulls as derived from poorly differentiated ultimobranchial remnants with follicle-like structures, cysts and tubes formed by small, immature, basophilic cells. These formations resemble undifferentiated cells or normal stem cells from ultimobranchial structures in bulls or other species, from which both C cells and follicular cells may develop.
The chronic stimulation of C cells and ultimobranchial remnants with high calcium levels absorbed from the digestive tract can be associated with the pathogenesis of neoplasms developed from these structures [10].
In the thyroid gland, mesenchymal tumors, mixed epithelial-mesenchymal tumors, carcinosarcomas [14], as well as secondary tumors or unclassified tumors, may develop.
The literature reports primary thyroid tumors, such as: leiomyomas and leiomyosarcomas; hemagiomas and hemangiosarcomas; fibromas and fibrosarcomas, and other neoplasms, such as carcinosarcomas.
16.2. PARATHYROID TUMORS
In the parathyroid glands, adenomas and carcinomas have been diagnosed, in both cases the clinical manifestation being primary hyperparathyroidism. The increased sensitivity of the renal tube epithelium to parathormone excess is well known, which acts by stimulating phosphate excretion and calcium retention. Bone resorption is accelerated in hyperparathyroidism, which results in osteofibrosis.
In their studies, ROSOL and CAPEN (1992) [11] prove the involvement of primary parathyroid tumors in the general calcium and phosphorus metabolism, with special implications for bones. Parathyroid neoplasms associate with hypercalcemia associated with bone calcium resorption by osteoclasia and increased tubular calcium resorption.
The consequence will be the appearance of bone fractures, vertebral deformations and nerve root compressions, the appearance of pareses and paralyses. In dogs with primary hyperparathyroidism, facial hyperostosis occurs, determined by extensive osteoblast proliferation and poorly mineralized osteoid deposit, with tooth mobility and even tooth dislocation from the alveoli.
Parathyroid adenoma has been especially described in old dogs, without any breed or sex predisposition. In 1989, DOSTER et al. reported a parathyroid adenoma in association with a seminoma, in a 20-year-old leopard. The adenoma appeared as a whitish nodule 1 cm in diameter, being situated in the right thyroid, from which it was delimited by fine connective stroma.
CAPEN (1978; 1993) mentions the unilateral presence of parathyroid adenoma, of a light brown to red color, in the thoracic cavity, at the base of the heart. This location is explained by the presence of ectopic parathyroid tissue, displaced in the thorax with the expanding thymus, during the course of embryonic development. Adenomas are clearly delimited by connective tissue. The author mentions that the adenoma enlarges one of the parathyroid glands, while the rest of parathyroid glands are atrophied, becoming smaller than the normal size. In the case of the secondary hyperplasia of principal cells, all four parathyroid glands are 2–5-fold larger compared to the normal size. The presence of pseudohyperparathyroidism is mentioned in dogs and cats with disseminated lymphosarcoma.
Histologically, parathyroid adenoma is formed by principal cells disposed in tightly associated acini, and the vascular connective tissue delimits tumor tissue groups. Cells are cuboid or polyhedral, with slightly eosinophilic, occasionally vacuolated cytoplasm. Nuclei are basal, round, with fine chromatin and single nucleoli. Fat cells and mast cells are identified in the tumor mass.
Adenoma is delimited by a fine connective capsule, with obvious compression of the adjacent thyroid gland.
Electron microscopy evidences multiple large lamellar patterns of rough endoplasmic reticulum and groupings of free ribosomes, in the cytoplasm. Mature secretory granules are found, suggesting that the parathyroid hormone is secreted at a higher rate than synthesis and storage, which occurs in the principal autonomous cells. Large mitochondria and prominent Golgi apparatuses are present in the principal neoplastic cells. Annular lamellae are frequent in adenomatous cells, but they have not been reported in normal principal cells.
Parathyroid adenoma may contain mature oxyphilic cells and transitional forms of well developed organelles, involved in hormone synthesis and accumulation, as well as secretory granules. These images are in obvious contrast with the oxyphilic cells of normal glands, whose cytoplasm is full of densely associated mitochondria, with a poorly developed endoplasmic reticulum and Golgi apparatus [11, 20].
Parathyroid carcinoma is larger than adenoma, invading the connective capsule and adjacent structures, producing metastases in the regional lymph nodes and lungs. Incidence in domestic animals is low.
Histologically, the neoplasm is formed by principal cells, in the cytoplasm of which cell organelles with an extremely variable development are found. Malignant cell nuclei show changes in shape, size and chromatin arrangement. Atypical mitoses have been reported [10,11].
16.3. CHEMORECEPTOR TUMORS
Chemoreceptor organs, the carotid body (Glomus caroticum) and the aortic bodies (Corpora paraaortica or Glomera aortica) act as barometers sensitive to changes in blood carbon dioxide content, pH and oxygen pressure, regulating respiration and circulation.
Chemoreceptor tissues are widely distributed in the organism, and tumors develop in particular in the aortic and carotid bodies.
The etiology of these tumors, also termed chemodectomas, may include a genetic predisposition, especially in some dog breeds (Boxers and Boston Terriers). It is estimated that prolonged exposure to hypoxia, e.g. at high altitudes, predisposes to carotid body hyperplasia.
16.3.1. Aortic body tumors
Aortic body tumors are more frequent in dogs, and rarer in cats and cattle. Brachiochepalic dogs, Boxer and Boston Terrier breeds, are more predisposed to carotid and aortic body tumors; frequency is higher at ages over 8 years, and males are more frequently affected than females.
Aortic and carotid body tumors do not manifest by excessive hormone secretion, but by their sizes they can compress the atrium, the vena cava or both structures. The consequence of these compressions are evidenced by edematous conditions, transudates: hydrothorax, hydropericardium, ascites, subcutaneous edemas, as well as liver stasis.
In a 10-year-old, female, domestic shorthaired cat, PALTRINIERI et al. in 2004 [76] described aortic chemodectoma, presenting with dyspnea and pleural effusion because of the presence of a modified transudate. At necropsy, a locally extensive, firm, tan to red, multilobulated nodule surrounded the pulmonary vein, and was densely adherent to the ventral portion of the aorta, which was dorsally displaced. The pulmonary vein was embedded in the tumor and showed severe stenosis. On cut surface, the neoplasm had multiple necrotic foci. Histologically, tumor cells were organized in lobules defined by a thin fibrovascular stroma with numerous small nerves. Tumor cells were round to polygonal with indistinct cell borders; scant, slightly eosinophilic cytoplasm; and a central, round nucleus, with fine chromatin and one to two prominent nucleoli. Rare mitoses, rare multinucleated giant cells, macrophages, and peripheral lymphoid aggregates were visible. Tumor cells were immunohistochemically positive for chromogranin A, for synaptophysin and, faintly, for neuronspecific enolase and negative for vimentin, cytokeratin, α smooth muscle actin, glial fibrillary acidic protein, thyreoglobulin, and calcitonin.
Aortic body tumors can evolve as benign forms, adenomas, or malignant forms, carcinomas.
Aortic body adenoma is located in the adventitia of the pulmonary artery, of the ascending aorta, or it is fixed between the large vessel trunks. The tumor surface is smooth, and in section it is white, with red or brown spots. Large adenomas can invade the atrium or compress the trachea, sometimes forming true sheaths around the large vessels, without changing the vessel structure (Fig. 16.10, 16.11).

Fig. 16.10
Aortic body tumor; packets of cells separated by fine trabecula, giant nucleus.

Fig. 16.11
Aortic body tumor, calcified spheroids.
Aortic body carcinoma in dogs has a lower incidence than adenoma. This neoplasm can infiltrate the pulmonary artery wall, project papilliferous proliferations in the lumen, and cause thrombus formation, reaching the atrium. Tumor invasion in blood vessels exceptionally leads to the development of metastases in the lungs and liver (Fig. 16.12, 16.13).

Fig. 16.12
Aortic body tumor, invasion of the wall aorta.
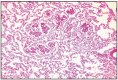
Fig. 16.13
Aortic body tumor, lung metastasis.
16.3.2. Carotid body tumors
Carotid body adenoma is located near the bifurcation of the carotid aorta in the cranial cervical region, in the proximity of the mandibular angle, usually bilaterally. The tumor is 1–4 cm in diameter, well encapsulated, with a smooth surface, of firm consistency, and in section it is whitish, with hemorrhagic spots, and extremely well vascularized. The aortic bifurcation can be incorporated in the tumor mass, with firm adherence to the vessel adventitia. A branch of the glossopharyngeal nerve can be incorporated in the tumor structure. The structure and vascularization of this tumor makes particularly difficult the complete removal of the neoplasm (Fig. 16.9).

Fig. 16.9
Carotid body tumor.
Carotid body carcinoma is larger than adenoma, reaching up to 12 cm in diameter; surface is rough, multinodular, and multiple hemorrhages and cystic formations are found in section. The neoplasm is delimited by a capsule, but tumor cells infiltrate the connective tissue, adjacent tissue, blood and lymphatic vessel walls. By extension, carcinoma can incorporate the jugular vein, the cranial nerves and the whole carotid bifurcation, and the trachea may be compressed and deviated. DEAN and STRAFUSS (1975) [10] found the presence of metastases in 30% of 22 dogs with carotid body carcinoma. Metastases were in the bronchial and mediastinal lymph nodes, lungs, liver, pancreas and kidneys. Approximately 65% of subjects with carotid body tumors also had aortic body tumors.
The histological characteristics of chemoreceptor tumors are essentially similar, with no differences between the aortic and carotid body. Neoplastic cells are organized in lobules by fibrous connective trabeculae, and fine collagen septa, reticulin fibers and small capillaries divide the lobe in small cell groups. Frequently, tumor cells are disposed along and around small capillaries, small lymphocyte and hemosiderin foci being identified.
Neoplastic cells have shapes that vary from cuboid to polyhedral, with a compact arrangement, a fine granular, slightly eosinophilic cytoplasm with vacuoles. Nuclei are round or oval, situated in the center of the cell, chromatin is fine granular, and mitoses are rare. Areas of large pleomorphic tumor cells can be identified in the tumor mass, and giant multinucleated cells, with bizarre multilobulated shapes and intensely basophilic nuclei may appear. This cell type can be more frequently found in carcinomatous forms, without being a characteristic of these neoplasms.
Aortic and carotid body tumors are extremely well vascularized, with the presence of muscle arterioles and large thin-wall veins and a network of septal capillaries, from which intratumoral hemorrhages are also produced.
Ultrastructure shows the presence of large cells, with bright or dark cytoplasm, depending on the intensity and presence of cell organelles. Mitochondria are small, spherical, the rough endoplasmic reticulum is arranged in parallel rows, and the Golgi apparatus has numerous prosecretory granules, dispersed in the cytoplasm. Neoplastic cells have cytoplasmic extensions around and among tumor cells, reaching the perivascular spaces.
Differential diagnosis in dogs is required between tumors from the base of the heart, which can be from chemoreceptor cells or ectopic thyroid follicular cells.
16.4. PITUITARY TUMORS
Pituitary gland tumors are less reported in animals compared to humans. In 1940, A. J. BRANDT reviewed the veterinary medical literature on this subject, mentioning some data on pituitary tumors in animals. In 1973, JOEST described a nut-sized tumor of the anterior pituitary lobe in an 8-year-old dog. Prior to death, the dog showed depression, inclined head, low appetite and weakness. The tumor was histologically formed by basophilic cells. VERMEULEN (1919, 1921) mentioned 2 cases of anterior lobe tumors, with eosinophilic cells, in two horses. The same author described a tumor of 4.8 g in a goat, formed by chromaffin and chromophobe cells. In 1932, PALLASKE mentioned a pituitary tumor of 26.5 g in a 20-year-old horse, located in the pars intermedia. TRAUTMANN (1934) described in a 7-year-old horse a pars intermedia tumor. VERSTRAETE and THOONEN (1939) mentioned in a 9-year-old Boxer dog a basophilic cell adenoma of the anterior lobe. During life, the subject presented a progressive atrophy of the genital organs, no sexual instinct, somnolence and hair loss.
BRANDT (1940) described 5 canine adenomas, of which 2 were mixed, 2 with principal cells and 1 with basophilic cells. The author described 5 adenomas in horses, located in the pars intermedia. In 2 dogs that presented principal cell adenoma, the author mentioned pulmonary and muscular calcifications, without establishing a causal relation between adenoma and calcifications.
In a study performed based on 53 literature cases and 12 personal cases in dogs, DÄMMRICH (1967) established the following histological types of pituitary tumors: 26 basophilic adenomas; 13 chromophobe adenomas; 6 mixed cell adenomas; 4 eosinophilic adenomas; 1 fetal adenoma, and 4 adenocarcinomas. In 8 dogs, the author mentioned that tumors caused no clinical symptoms. Nervous disorders were dominant in 10 dogs. In 12 dogs, symptoms secondary to diencephalic lesions were found, such as diabetes insipidus (3 dogs) and adiposogenital dystrophy (9 dogs). Hypopituitarism manifested in 3 cases.
According to NAGATANI et al. (1987), spontaneous pituitary tumors in old rats can be classified into 4 types, based on cellular morphology: granular cell tumors; small granular cell tumors; large polygonal granular cell tumors; and large thin elongated agranular cell tumors. Mixed tumors formed by two cell types were identified in 8 subjects. Following immunocytochemical reactions, all tumor types were found to be positive for prolactin, while all elongated agranular type tumors were positive for adrenocorticotropic hormone. Only agranular tumors with large polygonal cells had a non-defined or negative reaction for different pituitary hormones. The authors conclude that there is a relatively good correlation between cell morphology and the immunocytochemistry of spontaneous pituitary tumors in old rats.
16.4.1. Chromophobe (ACTH-secreting) adenoma
The functional tumors of the pituitary gland are formed by corticotropic (ACTH secreting) cells from the distal portion, being clinically associated with the excessive cortisol syndrome, similar to Cushing’s disease.
The highest incidence is in adult and old dogs, with a higher sensitivity of the Boxer and Boston Terrier breeds. The tumor has also been described in cats and horses, rarely in other animals.
ACTH-secreting pituitary adenomas cause hyperadrenocorticism. Clinical manifestations in dogs will be the subcutaneous storage of adipose tissue, exaggerated appetite and food consumption. Amyotrophy gradually occurs, as well as hepatomegaly by lipid and glycogen deposition. Cutaneous lesions constantly appear, manifesting by symmetrical hair loss, severe epidermal atrophy, and thickening of the horny layer, with hyper- and parakeratosis. Due to the increased susceptibility to bacterial infections, long-term cortisol excess leads to purulent inflammations in the skin, the urinary system, conjunctiva and lungs. Foci of purulent dermatitis and folliculitis, purulent, usually fatal bronchopneumonia, develop. Significant lymphopenia concomitantly occurs [10].
Similar symptoms have been described in horses [35]. The authors mention lymphopenia and eosinopenia, glycosuria and constant hyperglycemia.
Macroscopically, the pituitary gland is enlarged, without a direct correlation between the gland size and hyperfunctional clinical manifestations. In adjacent tissues, large adenomas compress the hypothalamus and even distort the sella turcica. Hemorrhage, necrosis, mineralization and liquefaction frequently occur in large tumors. Due to hormone hyposecretion, the cortical adrenal gland, in the first place its fasciculate area, is hypertrophied. Pancreatic islets are also hypertrophied [35].
Histologically, chromophobe adenomas consist of well differentiated secretory cells, with a fine connective tissue network. Immunohistochemically, neoplastic cells may demonstrate adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), luteinizing hormone (LH), β-endorphin, and β-lipoproteins. Based on cell architecture and patterns, sinusoid adenomas and diffuse adenomas are distinguished.
Sinusoid adenoma cells are divided into variable size groups, by well vascularized connective tissue, by sinusoid capillaries that come into direct contact with tumor cells, which can be arranged in a palisade pattern.
Diffuse adenomas do not have a characteristic architecture, cells being disposed in layers or under the form of cell masses with large chromophobe cells. The tumor is poorly vascularized, and connective tissue is reduced.
Chromophobe adenoma cells are small or large, polyhedral, with large vesicular nuclei, 1–2 prominent nucleoli, and the cytoplasm is abundant, eosinophilic, with well delimiting membranes. Small chromophobe cells are half the size of large chromophobe cells, they have small hyperchromatic nuclei, little visible nucleoli, and scant cytoplasm. In general, mitotic figures are rare. The delimitation of chromophobe adenomas from the rest of the gland is poor, and tumor cells are infiltrated in the posterior lobe and the infundibular stalk. The hypothalamus is compressed or invaded, replaced at some sites by large chromophobe adenomas. Hemorrhage, macrophages with hemosiderin, neuronal lysis and demyelination are found in the hypothalamus and thalamus. Electron microsopically, the organelles involved in protein and secretory product synthesis (endoplasmic reticulum, Golgi apparatus, respectively) are well developed in tumor cells. Functional adenoma cells contain numerous mature secretory granules, being delimited by a fine membrane. Secretory cells, especially those adjacent to the Golgi apparatus, are small, granular, intensely electron-dense and have a distinct submembranous space. Large secretory granules can be mixed with small secretory granulations, especially at the periphery of neoplastic cells. Large granules are less electron-dense, being granular and delimited by a well defined membrane. Neoplastic cells have an irregular outline, and cytoplasmic projections tend to extend to neighboring cells.
16.4.2. Inactive chromophobe adenoma
Non-functional chromophobe adenoma has been described in the pars distalis, being more common in dogs, cats and horses, and exceptional in other species. The consequence of these adenomas, which are endocrinologically inactive, can clinically manifest due to the compression and atrophy of the pituitary gland or the adjacent nervous system. Thus, clinical manifestations of non-coordination and lack of balance, depression, weight loss and behavioral changes have been found. In chronic evolutions, blindness, dehydration, high water consumption and frequent urination may occur. In general, there is a lack of specific clinical signs, which leads to confusion with numerous other diseases.
Macroscopically, non-functional chromophobe adenomas have particularly large sizes. Tumor cells incorporate adenohypophyseal tumors and the infundibular stalk. The tumor is attached to the sella turcica, which favors the development of a large base of extension in the brain. Sometimes, the tumor tissue replaces the whole pituitary gland, proliferates in the brain, extends over the thalamus, and penetrates the lateral ventricles. Optic nerves can be incorporated and compressed by the tumor mass, which explains the appearance of vision disorders. Subjects with large non-functional pituitary adenomas have small adrenal glands, with a cortex that is difficult to identify; the thyroid can have small sizes, and seminiferous tubes are also small, with low spermatogenic activity.
Histologically, tumor cells are cuboid to polyhedral, being disposed in layers or small groups delimited by fine connective tissue; the tumor is well vascularized. The tumor seems to develop from poorly differentiated pituitary cells, which are not involved in the synthesis and secretion of a specific trophic hormone [10, 11] (Fig. 16.18).

Fig. 16.18
Chromophobe pituitary adenoma.
16.4.3. Pars intermedia adenoma
Pars intermedia adenoma is most frequently found in horses, then in dogs, and more rarely in other species. Incidence is higher in old horses, generally betweeen 12 and 30 years of age, with a higher frequency in females [43].
Dogs with adenomas, which are usually endocrinologically inactive, manifest hypopituitarism and diabetes insipidus. ACTH secretion has been found in some subjects, resulting in bilateral adrenocortical hyperplasia [11].
In horses, clinical manifestations are polyuria, polydipsia, voracious appetite, muscle dystrophy, somnolence, intermittent hyperpyrexia, and all subjects manifest marked hirsuteness, due to the lack of cyclic seasonal hair loss. In addition to these, the following clinical signs may occur: diabetes insipidus, hydremia, lung lesions (thyroid adenoma; lung metastases of unknown origin) and other lesions [34, 62, 70].
Macroscopically, in the case of pars intermedia adenomas in dogs, a moderate increase in volume of the pituitary gland is found. Being clearly delimited, the tumor is easy to identify, it causes the compression and atrophy of the pituitary gland. The posterior lobe is incorporated in the tumor, without affecting the infundibular stalk.
In horses, adenoma induces a symmetrical increase in volume of the pituitary gland, and due to its extension, the tumor significantly compresses the hypothalamus and optic nerves. Adenomas are yellow to white, multinodular, and they have a capsule that delimits them from the rest of the pituitary gland. The tumor causes the compression and atrophy of the distal lobe [34]. The authors identify macroadenomas and microadenomas in the pars intermedia, which are delimited by a capsule, determining compressions and atrophies in the distal lobe.
Histologically, pars intermedia adenomas in dogs seem to be derived from the epithelial envelope of the residual pituitary lumen, which covers the infundibular process. The tumor is clearly delimited from the pars distalis, by an incomplete layer of condensed reticulum and a band of lymphocytes, without being encapsulated. Numerous follicles are identified in the structure of the tumor, which contain colloid, delimited by small cuboid chromophobe cells or columnar cells among which cup-shaped mucin-secreting cells are identified. Follicular formations and chromophobe cell nests are delimited by fibrous connective tissue. Cellular mitotic images are rare [10].
In horses, pars intermedia adenomas are partially encapsulated and clearly delimited from the compressed anterior lobular parenchyma. In addition, the tumor infiltrates and occasionally disorganizes the nervous lobe by compression. Pituitary adenoma is derived from pars intermedia and is traversed by fine connective septa, which delimit nodules formed by large columnar cells, fusiform cells and polygonal cells. Tumor cells are arranged in cords that make up palisades and pseudoacini, a fine connective vascular stroma also being present. Tumor cells can have a typically neuroendocrine architectural pattern, around the capillaries. The compressions exerted on the optic chiasm causes demyelination [43]. Sometimes, cuboid cells form follicular structures that may contain colloid. The tumor cell cytoplasm is weakly acidophilic. The nucleus is homogeneous, round and hyperchromatic, with prominent nucleoli. Hemorrhage, as well as mitotic figures, are rare [34].
The immunohistochemical evaluation in pars intermedia adenoma cells reveals a strong diffuse reaction in the cytoplasm for proopiomelanocortin (POMC), a moderate to intense reaction for the α-melanocyte-stimulating hormone (α-MSH) and β-endorphin (β-END), a weak reaction for ACTH and a negative reaction for prolactin, glial fibrally acid protein, neuron-specific enolase [34].
The clinical diagnosis of these tumors can be established by the measurement of plasma ACTH concentrations, associated with the ACTH stimulating test [43].
16.4.4. Acidophilic pars distalis adenoma
Acidophilic adenomas are rarely found in domestic animals. The tumor has been reported in dogs and cats, and in 1994, GONZALEZ et al. described an acidophilic pituitary adenoma in sheep, which in the authors’ opinion could be the first report.
The acidophilic tumors mentioned in the literature do not manifest by somatotropin and/or prolactin hypersecretion, and consequently have no specific clinical manifestations.
Macroscopically, acidophilic adenomas can have considerable sizes, compressing the pituitary gland, cranial nerves and/or the hypothalamus.
Microscopically, acidophilic adenoma is formed by cells arranged in irregular cords, among which sinusoid capillaries filled with blood are found. The fibrous stroma is discrete. Numerous secretory granules are identified in the cytoplasm of tumor cells. Among these cells, rare chromophobe cells are detected. The tumor cell nucleus is large, round and vesicular, and mitoses have a low incidence. Sometimes, tumor cells are organized in follicles that contain an intensely PAS-positive colloid [11]. The author makes some ultrastructural observations, mentioning the existence of small cells with numerous secretory granules. The plasma membranes of adjacent cells are relatively rectilineal, with few desmosomes. The Golgi apparatus is relatively little developed, with rare prosecretory granules. The endoplasmic reticulum is composed of small flat membranous vesicles, with attached ribosomes, and few randomly distributed mitochondria are detected in the cytoplasm, which corresponds to the storage phase of the secretory cell cycle (Fig. 16.19).

Fig. 16.19
Eosinophilic pituitary adenoma.
Large acidophilic neoplastic cells are fewer, having numerous cell organelles in their cytoplasm, but few secretory granules. The endoplasmic reticulum is extensive, formed by granular membranes, and the Golgi apparatus is prominent, being composed of agranular membranes associated with numerous small prosecretory granules. Mitochondria are in a much higher number. These cells are considered to be active secretory cells.
Acidophilic neoplastic cells contain numerous mature fine granular secretory granules, which are uniformly electron-dense, of spherical to oval shapes, and delimited by a fine membrane.
16.4.5. Chromophobe pituitary carcinoma
Chromophobe pituitary carcinomas are much rarer compared to adenomas, and are found in old dogs. These neoplasms are endocrinologically inactive, and clinical manifestations are due to the destruction of the pars distalis and the neuropituitary system, which may cause panhypopituitarism and diabetes insipidus.
Pituitary carcinomas are larger and invade both the brain and the sella turcica sphenoid bone. Metastases have been identified in the spleen and/or the liver. Microscopy shows an intense cellularization of the neoplasm, extensive hemorrhage and necrosis. Unlike adenomas, giant cells, nuclear pleomorphism and numerous mitoses appear.
16.4.6. Craniopharyngioma
Craniopharyngioma is a benign tumor, originating in the epithelial remnants of the Rathke’s pouch, with supra- or infrasellar location, which can manifest as panhypopituitarism or dwarfism syndrome, in young dogs. Cases of diabetes insipidus or adiposogenital syndrome have been mentioned in dogs with such tumors. Canine craniopharyngiomas are expansile and infiltrative neoplasms composed of polygonal-to-columnar cells arranged in solidly cellular areas with occasional cysts or tubules.
The tumor is formed by cysts that alternate with compact structures. Solid forms are composed of keratinized cells, with multiple mineralization foci. Cysts are lined by tall columnar epithelium, which is occasionally ciliated.
Pituitary cyst, derived from remnants of the craniopharyngeal duct. These are benign cysts lined by ciliated, cuboidal-to-columnar epithelium that contains mucin; they are common in the pars tuberalis and pars distalis of dogs [80].
16.4.7. Basophilic pars distalis adenoma
This adenoma is formed by granular basophilic cells, being a tumor with an extremely low incidence. In dogs, this tumor type is considered to be responsible for corticotropin secretion and consequently for hyperadrenocorticism. Corticotropic pituitary adenomas and pars intermedia tumors determine in most cases Cushing’s disease in dogs.
16.4.8. Metastatic tumors in the pituitary gland
Metastatic tumors in the pituitary gland may cause the partial or complete destruction of the glandular structure. Thus, metastases of malignant lymphoma have been described in cattle and dogs, malignant melanoma in horses and dogs, mammary gland adenocarcinoma in dogs. The pituitary gland can be destroyed by the infiltration produced by both sphenoid bone osteosarcoma and third ventricle infundibular recess ependymoma, as well as by malignant meningioma, in dogs and cats, and infundibular stalk glioma.
16.5. ADRENAL GLAND TUMORS
The classification of adrenal gland neoplasms can be approached from a functional and/or histological point of view. The difficulty of a complex classification consists in the presence of two glands with the possibility of erratic structures and the determining influence of pituitary secretions on adrenal glands.
Adrenal gland neoplasms will be treated taking into consideration their histological structure and to a certain extent, their secretory activity.
LABELLE et al., in 2004 [79] determined that histological criteria in combination with the immunohistochemical assessment of the Ki-67 proliferation index is helpful in the differentiation of adrenocortical adenomas and carcinomas in dogs. Morphologic criteria significantly associated with adrenocortical carcinoma included a size larger than 2 cm in diameter, peripheral fibrosis, capsular invasion, trabecular growth pattern, hemorrhage, necrosis, and single-cell necrosis, whereas hematopoiesis, fibrin thrombi, and cytoplasmic vacuolation were significantly associated with adrenocortical adenomas.
Tumors of the adrenal cortex account for 10–20% of the naturally occurring Cushing’s syndrome cases diagnosed in dogs.
16.5.1. Adrenocortical tumors
Myelolipoma is a benign tumor found in the adrenal glands of cattle, primates and less frequently in other animals. The tumor is formed by accumulations of well differentiated adipose cells and hematopoietic tissue, including myeloid and lymphoid elements. Mineralized or ossified elements are detected in the tumor mass. Due to the mentioned structures, these formations are not considered as true tumors [11].
Cortical adenoma has a higher incidence in old dogs and sporadically in horses, cattle and sheep; a higher incidence is found in castrated male goats.
Dogs with cortical adenomas clinically manifest cortisol excess signs, sometimes Cushing’s syndrome being described.
Macroscopically, adenoma appears as a clearly delimited structure from the rest of the cortex, although it may also occur in the medulla, with sizes that vary from discrete forms to prominent nodules or tumor masses on the gland surface. Tumors can be uni- or bilateral, yellow, partially or totally encapsulated, compressing the cortical parenchyma. Smaller cortical adenomas are light yellow, due to the high lipid content. Connective trabeculae are detected in section, which traverse larger tumors; cystic formations, hemorrhagic areas and occasional mineral deposits can be found [2].
Histologically, cortical adenomas are formed by well differentiated cells, similar to secretory cells of the fascicular or reticular area. Tumor cells are arranged in trabeculae or large nests, separated by small vascular spaces. The abundant cytoplasm is slightly eosinophilic, vacuolated, with lipid drops. Hematopoietic foci can be found, and in large tumors, necrosis and hemorrhage are present (Fig. 16.20, 16.21).
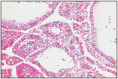
Fig. 16.20
Adenom with cystic spaces, adrenal gland.

Fig. 16.21
Adenoma, adrenal gland.
Cortical carcinoma is rarer than adenoma, being more frequently reported in cattle, sporadically in old dogs, and more rarely in other species [2, 10, 11]. Cortical carcinomas are sporadically described in horses [24], and polecats (Mmtela putorius furo) [54].
Macroscopically, cortical carcinoma is larger than adenoma and is more frequently bilateral. The neoplasm is 40/20 cm large or it can surround the whole adrenal gland. The tumor mass is friable, yellow to red-brown, multilobular, and it can invade the vena cava and the aorta, with the development of fibrous tissue adherent to adjacent tissues. Metastases can be found in the liver, spleen, lungs and regional lymph nodes and/or at distance. In section, hemorrhagic, necrotic and mineralized or ossified foci are detected in the neoplasm mass.
Histologically, the neoplasm structure is characterized by highly pleomorphic cells, architecturally grouped into trabeculae, lobules or nests. Tumor cells are large polyhedral, with eosinophilic vacuolated cytoplasm, the nucleus is large, vesicular, with prominent nucleoli. Fusiform cells with scant eosinophilic cytoplasm can also be found. Mitoses are generally rare. The neoplasm is well vascularized, with the presence of multiple hemorrhagic and necrotic foci [2,11, 24] (Fig. 16.22, 16.23).
Fig. 16.22
Carcinoma, adrenal gland.

Fig. 16.23
Carcinoma, adrenal gland.
Electron microscopy shows numerous desmosomes, tonofilaments, of adjacent neoplastic cells; some cells contain lysosomes and well developed endoplasmic reticulum [24, 54].
Adrenocortical neoplasms, adenomas or carcinomas, may be active secretory, producing negative feedback, inhibiting pituitary ACTH secretion, with increased blood cortisol levels.
Manifestations and lesions associated with hyperadrenocorticism, especially in dogs, are the result of high cortisol secretion with adrenocortical hyperactivity. This leads to the development of canine functional disorders and combined lesions of gluconeogenesis, protein catabolism and antiinflammatory effects of glucocorticoid hormones in numerous organs. Cortisol excess is a common endocrine disease in adult and old dogs, and rarer in other domestic animal species.
In general, functional adrenocortical neoplasms have an incidence between 10 and 15%. Hyperadrenocorticism occurs after a longer period of high dose corticoid administration, in the treatment of other diseases [11].
16.5.2. Adrenomedullary and paraganglionic tumors
Pheochromocytoma is the most common adrenomedullary tumor in domestic animals, being more frequently reported in cattle and dogs, more rarely in other species, such as horses [8, 66]. In bulls and humans, the presence of pheochromocytoma has been found in association with thyroid C cell tumors [11]. The age of dogs with pheochromocytoma is around 6 years or more. In horses, these neoplasms develop independently from age, breed or sex, and are usually benign and unilateral. In 1982, FROSHER and POWER described a pheochromocytoma in a 6-month-old foal [66].
Macroscopically, pheochromocytoma is large (over 10 cm in diameter), and it can be unilateral, more rarely bilateral, of a red-brown color, with hemorrhagic and necrotic foci. Smaller tumors are delimited, a denser compression area of the adrenal cortex being detected. Large neoplasms may incorporate the whole adrenal gland, having a multilobular structure in section. Extra-adrenal pheochromocytomas have been identified along the posterior aorta and the vena cava, being probably derived from chromaffin paraganglia. For a rapid diagnosing of pheochromocytoma, Zenker’s solution is applied on the freshly sectioned surface of the tumor, causing catecholamine oxidation, and a brown color appears after 20 minutes.
Histologically, pheochromocytoma is formed by cells that are more or less similar to adrenal medullary cells. Cells can be small cuboid or polyhedral, similarly to normal medullary cells, but they can be intensely pleomorphic, with multiple hyperchromatic nuclei. The cytoplasm is intensely eosinophilic, granular and, due to autolysis that occurs early after death, cellular limits are little distinct. Cells may be disposed in arched cords, in nests or straight cords, delimited by numerous blood capillaries. Pheochromocytoma with poorly differentiated cells and intense pleomorphism cannot be differentiated from carcinoma (Fig. 16.24).

Fig. 16.24
Phaeochromocytoma.
Electron microscopy differentiates two types of neoplastic cells, epinephrine-secreting and norepinephrine-secreting cells. Norepinephrine-secreting cell pheochromocytoma has granules with an eccentric electron-dense core, delimited by a large membranous space. In epinephrine-secreting cells, granules have a less dense macrogranular core and a narrow submembranous space. The Golgi apparatus is prominent, and the lamellar endoplasmic reticulum is present [11].
In 50% of cases, canine pheochromocytomas induce metastases in the liver, spleen, lungs and regional lymph nodes. Malignant forms can metastasize in the spinal cord, vertebral canal and other organs [66].
Adrenomedullary neoplasms may cause more or less characteristic manifestations, such as intermittent muscle contractures, sweating, mydriasis, tachycardia, cardiac arrhythmia, sometimes polypnea, polyuria, and polydipsia. Necropsy shows, in addition to the medullary tumor, myocardosis, myocardial infarction, hepatic necrosis, etc.
Neuroblastoma has been reported in young animals, under the form of large tumor masses in the abdominal cavity. Histologically, the neoplasm is composed of small cells with hyperchromatic nuclei and scant cytoplasm; they are arranged in pseudorosettes and may be confused with lymphocytes. Unmyelinated nerve fibers or neurofibrils are identified in the tumor mass. Neuroblastoma is considered as highly malignant for humans, while the behavior of this tumor in animals is unknown [2,11] (Fig. 16.16).

Fig. 16.16
Neuroblastoma.
Ganglioneuroma is a benign medullary tumor, of small sizes, formed by multipolar ganglionic cells and neurofibrils, with predominantly fibrous connective stroma. The tumor compresses the adrenocortical gland. Sometimes, medullary tumor cells can be differentiated into adjacent pheochromocytomas and ganglioneuromas, within the same gland [11] (Fig. 16.14, 16.15).

Fig. 16.14
Ganglioneuroblastoma.

Fig. 16.15
Ganglioneuroblastoma.
Metastatic tumors in the adrenal gland are relatively frequent, such as lymphosarcomas, mammary carcinomas, spindle cell carcinomas, etc.
16.6. DIFFUSE ENDOCRINE SYSTEM TUMORS or APUD-omas
The diffuse endocrine system (DES) or diffuse neuroendocrine system was termed in 1967 by PEARSE, the Amine Precursor Uptake and Decarboxylation (APUD) system.
The author includes in this APUD system: hypothalamic neurons, the epiphysis, the pituitary gland, the parotid gland, pancreatic islets, the placenta, sympathetic ganglia, the adrenal medulla, and melanoblasts. Subsequent researches have changed the concept proposed by PEARSE. The current tendency is to rehabilitate the notion of DES in relation to the APUD system.
At present, the DES concept is unanimously accepted, based on strictly morphological characteristics that ensure the morphofunctional and pathological understanding of this endocrine system.
The tumors of this group of endocrine cells, disseminated in the structures of some organs or tissues, were identified long before the clarification of their functions. These tumors were identified based on their histological characteristics alone, and histochemical, and electron microscopic investigations were subsequently performed.
The diagnosis of these neoplasms becomes more difficult in the case of DES cells in non-endocrine tumors or tissues that normally do not contain these cells. According to BOSMAN et al. (1984, 1985) [59], the appearance of several differentiation lines could be explained by the acceptance of some mechanisms, some of which purely theoretical, for the appearance of neuroendocrine cell components, in the structure of some tumors.
The authors advance the following possible mechanisms:
- The incorporation of benign neuroendocrine elements in the tumor, in the case of organs that normally contain neuroendocrine cells. The neoplastic character of these neuroendocrine cells is proved by their presence in metastases.
- The fusion of benign neuroendocrine cells with neoplastic cells. It is supposed that the cells of a benign tumor could fuse with malignant cells, resulting in a malignant hybrid cell. This hypothesis cannot explain the mechanism of appearance of a combined tumor in organs that do not normally have neuroendocrine cells. In addition, this mechanism has not been certainly demonstrated in vivo, but it can be easily demonstrated in vitro.
- Although most tumors have a unicellular origin, the polyclonal origin of some tumors cannot be excluded. This hypothesis cannot explain the formation of combined tumors in organs without neuroendocrine cells.
- The neoplastic transformation of a pluripotent stem cell seems to offer the most plausible explanation for the appearance of combined tumors. In support of this hypothesis, the authors cite the experiments of COX and PIERCE (1982), who transplanted single cells from a chemically induced rat colon adenocarcinoma that contained mucous, cylindrical and endocrine cells. The resulting tumors contained all three cell types. The hypothesis advanced was that, following the neoplastic transformation of “engaged” cells (mucous, cylindrical and endocrine cell precursors), the transformed cells express a single phenotype (e.g. typical adenocarcinoma or carcinoma). The transformation of an “unengaged” stem cell results in a tumor that expresses multiple phenotypes. This mechanism cannot explain the development of combined tumors in organs that lack neuroendocrine cells.
- The aberrant differentiation of neoplastic cells could explain the appearance of neuroendocrine cells in tumors of organs lacking these cells.
By synthesizing the literature data on the clinico-biological significance of neuroendocrine cell tumors, OLINICI and CALUSER (1988) expose two main aspects: inadequate hormone secretion and ectopic hormone secretion.
Inadequate hormone secretion has been found in some tumors that do not secrete hormones specific for cells from which the neoplasm has formed. Thus, pancreatic tumors may produce calcitonin, medullary thyroid tumors can elaborate ACTH, somatostatin, 5-HT, etc. Among possible explanations for “inadequate hormone secretion”, the following can be mentioned:
- - endocrine cell tumors could secrete the endocrine cell-specific protein precursor, but they either lack the necessary esterases for its transformation into a normal hormone, in which case an inactive precursor is secreted that is not normally detected, or some esterases that are usually repressed in the cell of origin will be derepressed, thus converting the precursor protein to one or more hormones that appear as “inadequate”.
- - during the neoplastic transformation, several cell types could be stimulated. In this case, precursor proteins or specific hormones, immunochemically detectable in separate cells, could be produced. This does not explain the secretion of inadequate hormones, but can explain the multihormone secretion in organs such as the pancreas, which contains different endocrine cells.
Ectopic hormone secretion occurs in tumors that do not arise from endocrine cells, but acquire the property to secrete hormones. Thus, pavimentous carcinomas and bronchopulmonary adenocarcinomas may secrete ACTH and ADH, and some bronchopulmonary carcinomas, hepatoblastomas and renal carcinomas, can produce chorionic gonadotropin. According to ODELL and WOLFSEN (1982) [59], all carcinomas release “ectopic” proteins. It has been demonstrated that the presence of neurosecretory granules in the cells of some tumors is not directly correlated with the appearance of obvious hormonal syndromes. This could be explained by the production of subliminal hormonal amounts. The incapacity of some neuroendocrine tumors to secrete stored material because of a deficiency in the intracytoplasmic transport system, or the production of “aberrant”, “immature” or fragmentary material unable to induce obvious responses in target organs, have been demonstrated.
According to the cited authors, the explanation of ectopic hormone secretion is probably similar to that of inadequate secretion. Neuroendocrine cells usually maintain their biosynthetic potential, but the genes involved are repressed. In tumors, these genes would be derepressed, allowing the synthesis of active hormones.
Diffuse endocrine system tumors or APUD-omas are termed after hormone secretion products: insulinomas, which secrete insulin; gastrinomas, which secrete gastrin, etc.
These tumors have been described especially in dogs and cats, with origin in the pancreas, e.g. insulinoma, gastrinoma, glucagonoma and pancreatic polypeptidoma. The characteristic of these tumors is that they frequently produce several types of peptides, but one hormone is predominant and responsible for the clinical syndrome [13, 47, 51, 68].
Diffuse endocrine system tumors have been initially termed APUDOMAS, and the term SEDOMAS has been subsequently proposed by MAGNOL. The study of diffuse endocrine system neoplasms can be performed depending on the location of these cells in the main systems, organs and tissues. In this sense, the function and morphology of SED cells will be considered. Tumor cells can functionally:
- – be maintained, increasing at the same time the secretion type of the element of origin, orthocrine;
- – cause the total loss of the secretory activity, anacrine;
- – secrete hormone products that they do not normally produce, metacrine.
SED cancer in animals is less studied and known, examples coming from human oncology. Thus, small cell (“oat cell”) bronchial cancer develops from endocrine cells, secreting a substance with ACTH-like effects, and is frequently associated with Cushing’s syndrome.
SED tumors in animals will be discussed by organ and tissue systems.
16.6.1. Digestive tract carcinoids
The term carcinoid was proposed by OBERNDORFFER in 1907, with express reference to intestinal tumors. Subsequently, the notion has been extended to pancreatic, pulmonary and other tumors. Regardless of their location, carcinoids have similar histological and histochemical characteristics, representing the “prototype” of endocrine tumors [18, 59].
Gastrointestinal carcinoids can be located in any segment of the gastrointestinal tract, being classified according to the different anatomical segments.
These neoplasms have a low incidence in animals, and they are reported especially in old dogs, more rarely in cattle and cats. In humans, it is estimated that 60–80% of these tumors are located in the middle intestine, appendix and ileum. In the anterior segment of the gastrointestinal tract, the stomach has the highest tumor incidence, followed by the duodenum and esophagus [18, 59].
In dogs, carcinoids are mainly located in the duodenum, colon and rectum. Clinical manifestations consist of intestinal obstruction and anemia secondary to hemorrhage caused by digestive mucosal ulcers. Rectal carcinomas occur as adenomatous polyps. Scarce literature data report evolution towards malignization, with local invasion and liver metastasis, which has also been reported in human oncology. In humans, the tumor size is relevant for the malignancy grade: neoplasms over 2 cm in diameter cause metastases in over 80% of cases. In dogs, the Zollinger-Ellison syndrome is a paraneoplastic manifestation, expressed by duodenal ulcers, as a result of excessive gastrin secretion by pancreatic islet tumors, or G-cell tumors in the stomach.
Some tumors produce high amounts of serotonin, gastrin, somatostatin or glucagon, clinically determining carcinoid syndrome. These tumors are called hormonally functional, while other neoplasms that do not manifest obvious clinical syndromes are called non-functional.
Immunocytochemical studies have shown that morphologically identical carcinoid tumors, located in the same anatomical segment, can secrete different regulating substances, some of which biologically inactive [59].
According to DAYAL and WOLF (1984) [59], gastrointestinal carcinomas can secrete: serotonin, somatostatin, substance P, pancreatic polypeptide, glucagon, calcitonin, ACTH, gastrin, insulin or they can be multisecretory.
Five types of tumors are histologically distinguished in humans: insular, trabecular, glandular, undifferentiated and mixed tumors (SOGA and TAZAWA, 1971; MARTIN and POTET, 1974 [59]).
The pure insular type is the most frequent gastrointestinal carcinoma, being characteristic of middle intestinal carcinoids. Tumors are formed by monomorphic cell islands, separated by connective vascular tissue trabeculae. Tumor cells are small, round or oval, with scant, pale granular cytoplasm. Usually, nuclei are uniform, sometimes presenting a remarkable polymorphism and bizarre shapes. Mitosis is reduced. Occasionally, tumor islets have cells arranged in a palisade pattern, with granular, intensely eosinophilic cytoplasm. Pseudoglandular formations may occur around the vessels. Cells are argentaffin, diazo-positive and fluorescent, which indicates the presence of 5-HT.
The trabecular type is characterized by a trabecular arrangement in cords, separated by collagenous stroma. Cells can be small and fusiform, with scant cytoplasm, or larger, with abundant cytoplasm. Nuclei are elongated and oriented with the long axis perpendicular to the cord axis. Usually, trabecular carcinoid cells are not argentaffin or diazo-positive, but some of them contain lead hematoxylin-positive granules or argyrophilic granules.
The glandular type is characterized by the presence of tubuloacinar or rosette structures. This type is found in pancreatic endocrine tumors; in has been noted that in gastrointestinal carcinoids, the glandular type is associated with the insular type, and pure forms are exceptional. The presence of argyrophilic granules has only been found in 50% of cases. Stroma is connective vascular and occasionally hyalinized.
The undifferentiated type is relatively rare, being characterized by small, medium or large undifferentiated cells that form large fields, with extremely poor stroma. Tumor cells can be small and fusiform, with elongated hyperchromatic nuclei and scant cytoplasm, with indistinct limits and few mitoses, or cells can be large, with abundant cytoplasm, large nuclei, remarkable polymorphism and mitotic activity. Cells may form acinar, insular or trabecular structures. Argyrophilia and argentaphilia can be histochemically found, which demonstrates the endocrine origin, and electron microscopy reveals the presence of intracytoplasmic secretory granules.
The mixed type is characterized by the presence of different cellular types, with a mixed, adjoining or mosaic-like structure.
Psammomatous carcinoids are characterized by the presence of numerous concentric calcified lamellar structures, being extremely rare, less than 1% of all tumors. They are preferentially located in the proximal duodenum, under the form of voluminous solitary tumors. Microscopically, structure is mixed, with the predominance of the glandular component, along with insular and trabecular structures. Acini are formed by a single row of cubic cells, with abundant, granular, weakly eosinophilic cytoplasm, and a weakly eosinophilic protein material appears in the lumen. The presence of concentric, lamellate, intensely PAS-positive psammomatous bodies, located in the glandular lumens, is characteristic. Immunocytochemistry has demonstrated the presence of a single immunoreactive peptide, somatostatin. The nature of these psammomatous bodies is supposed to be tumor cell secretion, not cell degeneration or necrosis (DAYAL et al., 1983 [59]).
Calciform cell carcinoids are located in their majority in the appendix, but they have also been reported in other segments, including the esophagus. Tumors are small, under 1 cm in diameter.
Microscopically, they are formed by short or small gland-like cords, with cubic, vacuolated calciform cells, with small eccentric nuclei. There is a cellular uniformity, nuclear atypias are minimal, and metastases are rare. Neutral and acid mucopolysaccharides, as well as cells with argentaffin and argyrophilic granules have been histochemically evidenced. Sometimes, differential diagnosis between carcinoids and adenocarcinomas is difficult.
In human oncology [59], neuroendocrine neoplasms are differentiated and diagnosed depending on location: in the endocrine glands, esophagus, stomach and duodenum. In the intermediate segment, middle and posterior intestinal tumors are mentioned. For comparative oncology, further investigations are required in order to confirm or not the resemblance to what is known about humans.
16.6.2. Hepatic neuroendocrine carcinoma
PATNAIK et al., in 2005 [77, 78] described these tumors in dogs and cats.
In dogs, clinical signs were anorexia, vomiting, polydipsia/polyuria, icterus, lethargy, paresis, ataxia, and weakness. Hematologic and biochemical findings included: anemia, leukocytosis, high liver enzyme activity (serum alkaline phosphatase, alanine transaminase, aspartate transaminase), and high total bilirubin. Grossly, the tumors were diffuse, involving all liver lobes or various-sized nodules in addition to diffuse involvement. Histologically, the tumors were divided into three groups on the basis of previously described patterns of neuroendocrine carcinoma in humans and dogs. Group A tumors consist of solid groups or nests of neoplastic cells separated by fibrovascular stroma, often with a peritheliomatous arrangement of peripheral cells. The cells are round to polygonal, with granular eosinophilic cytoplasm and vesiculated nuclei with prominent nucleoli. Group B tumors consist of cords of neoplastic cells separated by fibrovascular stroma. Group C tumors consist of groups of acinar or rosette-like structures, enclosed in similar stroma, and lined by cuboidal or columnar cells. Immunohistochemical studies were positive for at least one of the endocrine markers used: neuron-specific enolase, synaptophysin, and chromogranin-A. Electron microscopy confirmed the diagnosis by the presence of intracytoplasmic neurosecretory granules.
In cats, the most common clinical signs were: hepatomegaly, anorexia, weight loss, and vomiting. Histologically: acinar structures separated by vascular stroma; the extrahepatic neuroendocrine carcinomas and the gallbladder neuroendocrine carcinomas were characterized by solid sheets or groups of round to oval cells with vascular or fibrovascular stroma. Immunohistochemical studies: all the neoplasms were positive for NSE, and most of the bile duct and the gallbladder neoplasm were positive for synaptophysin.
The origin of hepatic neuroendocrine carcinoma is the early hepatic stem cells, which give rise to the bile ductules and hepatocytes. The neuroendocrine cells originate in the bile ductules. The bile duct and gallbladder neuroendocrine carcinomas arise from preexisting neuroendocrine cells in the epithelium. There is also growing evidence that bone marrow stem cells may contribute to the regeneration of hepatic cells [77, 78].
16.6.3. Pancreatic carcinoids
Diffuse endocrine system tumors or APUD-omas of the pancreas are formed by specialized neuroendocrine cells; in fact, these cells are also found in other organs or tissues (gastrointestinal tract, lungs, pituitary gland, thyroid, adrenal glands), which have common cytochemical, functional and ultrastructural features [75]. These neuroendocrine cells are able to absorb amine precursors (5-hydroxytryptophan, dopa, etc.) and form by their decarboxylation biogenic amines. APUD cells are able to synthesize and secrete hormone peptides such as insulin, gastrin and glucagon. Other secretion products, such as epinephrine, norepinephrine and vasoactive intestinal peptides show both hormonal properties and neurotransmitters [37].
Insulinoma. Pancreatic islet tumors are rare, being reported in middle aged and old dogs, in cats and swine. Pancreatic tumors have been sporadically reported in other species, such as donkeys [41]. FURUOKA et al. (1989) described the histogenesis of the neoformation of the endocrine pancreas in old horses, in association with the adenomatous hyperplasia of pancreatic ducts. The examination of the pancreas of 696 old cows with a mean age of 10 years, killed in slaughter houses, showed multiple nodules in 97% of cases, which histologically proved to be insulinomas, with cuboid or columnar cells [4].
Macroscopically, insulinomas appear as solitary or multiple nodules, 1–3 cm in diameter, yellowish to red, prominent on the surface of the pancreas. They have a firm consistency, with a fine connective stroma at the periphery, which delimits them from the rest of the pancreas [52]. Metastases are frequent in the pancreatic lymph nodes and liver, sporadically in the kidneys, peritoneal serosa and lungs [3].
Histologically, insulinomas are formed by β-cells and most of them have all the characteristics of a malignant tumor. Neoplastic cells are clearly separated from the pancreatic parenchyma by a fibrous connective tissue capsule. In the tumor mass, acinar epithelial cells form small nests, oriented especially towards the periphery of the neoplasm. Insulinoma is traversed by fine connective trabeculae, which make up small lobules. Neoplastic cells are well differentiated, being cuboid or columnar, with fine granular, discretely eosinophilic cytoplasm. The columnar epithelium has basal nuclei, associated with neoplastic cells; they appear in higher numbers than in preexisting pancreatic ducts and are considered part of neoplastic structures [10].
Pancreatic islet carcinomas have a marked cell pleomorphism, small tubular structures frequently appear, and neoplastic cells invade and infiltrate the connective capsule, adjacent tissues, blood and lymphatic vessels. Early metastases occur in the regional lymph nodes and liver [42].
Electron microscopically, β-cells have irregular, cuboid or polyhedral shapes, with a high number of cell organelles. Nuclei have peripheral chromatin condensations and an irregular appearance. Cytoplasmic membranes have interdigitations, desmosomes and connections with adjacent cells. β-cells are diffuse granular, which corresponds to the active synthesis phase of the secretory cycle, with an extensive network of the rough endoplasmic reticulum, with numerous free ribosomes and large mitochondria. Dense granular β-cells are in the storage phase of the secretory cycle, having reduced rough endoplasmic reticulum and few ribosomes. In neoplastic β-cells, secretory granules are obvious, with variable shapes, sizes and electron densities [10, 49].
The clinical picture has been almost exclusively described in dogs, and is dominated by hypoglycemia, weakness, ataxia, muscle tremor, seizures and collapse [13]. Insulinoma is suspected when high insulinemia associated with hyperglycemia is found. Diagnosis is confirmed by laparoscopy, the presence of pancreatic nodules, biopsy and microscopic examination.
Immunohistochemical investigations are mandatory for the identification of hormone and amyloid content [32, 57]. The authors mention positive glucagon and somatostatin immunoreactivity in most primary pancreatic islet tumors, as well as in liver and ganglionic metastases. The constant positive reaction for insulin and somatostatin should be emphasized. The peroxidase-antiperoxidase (PAP) technique is effective in formalin-fixed and paraffin-processed tissues, using guinea pig or rabbit antiserum as a primary antibody. The presence of amyloid deposit in endocrine tumor cells indicates its formation inside endocrine cells. Endocrine amyloid seems to be derived from a hormone variety or from endocrine peptides, associated with tumor cells, hypothesis reinforced by the fact that none of the necropsied dogs presents amyloid deposits in other tissues.
The treatment of insulinomas consists in the resection of the primary tumor and metastases. Due to high malignancy, surgery is a palliative treatment, which sometimes prolongs the patient’s life. Hypoglycemia will be symptomatically controlled, food will be administered in small and frequent rations with high hydrocarbon content, and prednisolone and diazoxide, which is an insulin inhibitor, will be administered. Streptozotocin will be used with caution, due to its nephrotoxicity in dogs [37].
Gastrinoma is a pancreatic islet tumor, whose neoplastic cells secrete gastrin, and from a clinical point of view, it manifests as a Zollinger-Ellison syndrome. Patients with Zollinger-Ellison syndrome show gastric acid hypersecretion and gastrointestinal ulcers, associated with pancreatic islet tumors. In reality, tumor cells secrete gastrin, hence the term gastrinoma.
Gastrinoma has been described in dogs and cats, at ages of over 6 years, as single or multiple tumors, 0.2–2 cm in diameter, which makes difficult the identification of these small nodules in the pancreas. The tumor grows slowly, but is malignant, metastases in the lymph nodes and liver being mentioned [31].
Clinical signs, constantly noted, are vomiting, anorexia, weight loss, and gastroduodenal ulcer. Chronic diarrhea refractory to any treatment may alert to the origin and presumption of gastrinoma. Hematemesis, melena and mucosal paleness concomitantly appear. Perforated ulcer determines collapse, shock and peritonitis.
Necropsy shows gastroduodenal ulcer, esophageal reflux, hypertrophic gastritis, enteritis, and the presence of pancreatic tumor nodules [6].
Microscopy reveals neoplasms formed by non-β-cells, and immuno-cytochemical examination is required for the identification of gastrin-secreting cells. Electron microscopically, gastrinoma cells have intracytoplasmic granules of variable densities [65].
Positive diagnosis can be sometimes made by the correlation of clinical data, laparoscopic examination, gastric endoscopy, radiological examination, immunocytobiochemical examination, etc.
Paraclinical examinations, such as the measurement of serum gastrin, the secretin test or the calcium infusion test, are generally difficult to interpret, since paradoxical reactions may also occur. Some dogs with clinical signs of gastrinoma show increased plasma ACTH concentrations, hypercortisolemia and moderate hyperglycemia [37].
In the case of affected animals, treatment involves some formulas used in humans. Chemotherapy using streptozotocin and 5-fluorouracil, with or without adriamycin, may result in improvements. Antisecretory medication favors the recovery of ulcers and prevents recurrences [38].
Glucagonoma, known by morphoclinical manifestations as diabetes mellitus-dermatitis syndrome or glucagonoma syndrome, is a neoplasm that develops from pancreatic islet α-cells, being associated with diabetes mellitus, and dermatitis, in humans.
Glucagonoma has been diagnosed by immunocytochemistry in dogs and cats. Subjects present erythematous, ulcerative and crusty dermatitis, with the involvement of the plantar pads, scrotum and perineal skin. Dogs manifest diabetes mellitus. Laparotomy identifies small pancreatic tumors, with positive reaction for glucagon by immunochemical tests, and insulin, somatostatin and pancreatic polypeptides are also identified [32, 57, 74].
Pancreatic peptidoma has been described in dogs, with chronic vomiting, anorexia and rapid weight loss. Necropsy detects duodenal ulcers and gastric mucosal hypertrophy. Serum polypeptide concentrations are markedly increased, and by immunocytochemistry, both in pancreatic tumors and in liver metastases, the reaction for pancreatic polypeptides is intensely positive. Hypoglycemia and hyperinsulinemia are concomitantly found. Immunocytochemically, reaction is positive for insulin, in approximately 25% of tumor cells [6, 73].
Veterinary medical pathology should consider the possible existence of syndromes induced by pancreatic tumors. Thus, in humans, the following entities have been diagnosed: watery diarrhea, hypokalemia, achlorhydria syndrome (VIP-oma), a syndrome induced by vasoactive intestinal peptide (VIP) secretion; somatostatinoma, a somatostatin-inducing tumor, by pancreatic delta cells, with glucose intolerance manifestations, cholelithiasis, maldigestion, diarrhea, steatorrhea, indigestion, hypochlorhydria, and weight loss; pancreatic tumors that may produce an excessive secretion of adrenocorticotropin, parathyroid hormone, serotonin or other amines [37].
16.6.4. Respiratory tract carcinoids
Endocrine cell tumors represent cell proliferations with different malignancy grades, derived from the primitive endoderm and resembling endocrine cells to different extents, as a consequence of differentiation in a certain direction [59].
Electron microscopic, immunocytochemical and gel electrophoretic investigations, performed by BLOBEL et al. (1985) [59], have demonstrated the presence of one or more neuroendocrine markers: bombesin, calcitonin, ACTH, leuenkephalin, gastrin, serotonin, somatostatin, α-melanocyte stimulating hormone (MSH), vasoactive intestinal peptide (VIP), glucagon, insulin, substance P, neuron specific enolase, in the neuroendocrine tumors of the bronchopulmonary tract.
Carcinoids, well differentiated neuroendocrine carcinomas, oat cell carcinomas and intermediate type carcinomas have been studied. Electron microscopic examination has demonstrated the presence of a variable number of neurosecretory granules, which are heterogeneous in size, shape and electron density; tonofilament and desmosome bundles have been concomitantly evidenced. Immunocytochemistry has demonstrated the presence of cytokeratins and neuroendocrine cellular substances. The investigations of the cited authors demonstrate the epithelial nature of these tumors.
A classification of bronchopulmonary neuroendocrine tumors according to OLINICI and CALUSER (1988) reveals the following types: carcinoids, well differentiated endocrine carcinomas, small cell carcinomas and atypical endocrine tumors. The authors also mention the tumor known as “tumorlet”, a name given by WHITWELL (1978), which can be considered a carcinoid from a structural and developmental point of view.
Carcinoids have been reported extremely rarely in animals [18], appearing as small cell anaplastic neoplasms. The tumor manifests by paraneoplastic syndromes, unlike in humans, who can present effects of secretion of ACTH (Cushing’s syndrome), ADH (Schwartz-Bartter syndrome), MSH (melanic hyperpigmentation), calcitonin or serotonin.
In humans, carcinoids are well delimited, but unencapsulated, of yellow-gray color, well vascularized. They are located in the main bronchi, but they have also been reported at the lung periphery. Tumors can also be multicentric, sometimes associated with tumors located in other organs. Tumor growth develops in the bronchial wall, but it can project in or obstruct the bronchial lumen.
Histologically, the tumor is formed by small, uniform, round or polygonal cells, with vesicular round or oval nuclei, and little obvious nucleoli. The cytoplasm is discretely eosinophilic, and mitotic figures are rare.
Diagnosis can be sometimes difficult, due to oncocytic changes occurring in some carcinoids, when large eosinophilic cells with numerous mitochondria appear in the cytoplasm. Clear cell or fusiform cell carcinoids are exceptional.
Electron microscopy confirms diagnosis, which is relatively easy and with a high degree of certainty, due to the presence of numerous neurosecretory granules, accumulated at the basal pole of the cells [59].
Well differentiated endocrine carcinomas, also considered as atypical carcinoids, are located in more than 50% of cases at the lung periphery, being frequently associated with Cushing’s syndrome. Well differentiated endocrine carcinomas have an intrabronchial growth, with a vegetating aspect, always infiltrating the pulmonary parenchyma, and 60% of cases present metastases that can be intrapulmonary, in the mediastinal lymph nodes, and/or systemic.
Histologically, tumors show a certain architectural similarity to carcinoids; cells are frequently fusiform, but they can also be polygonal. The cytoplasm is abundant, pale or slightly eosinophilic. Nuclei have a certain polymorphism, and mitoses are numerous. Cells may be arranged in a palisade or rosette pattern, and there is mucus in the lumen.
Electron microscopy shows the presence of neurosecretory granules, relatively fewer than in carcinoids; granules are small and of variable sizes; lumens and tonofilaments may be noted.
Immunohistochemically, cells contain neuron-specific enolase and different neuropeptides, more frequently ACTH, bombesin, leuenkephalin, serotonin, VIP, calcitonin, etc. There is an obvious similarity to carcinoids, but the presence of ectopic material, ACTH, respectively, is a difference from carcinoids [59].
Small cell carcinomas represent approximately 25% of lung neoplasms in humans. The incidence of these carcinomas is directly correlated with exposure to radiation (workers from uranium mines) and smoking.
The WHO classification (1981) differentiates three subtypes: oat cell carcinomas, intermediate cell carcinomas, and combined carcinomas.
Oat cell carcinomas are formed by round, oval or fusiform cells, they have reduced cytoplasm, nuclei have fine or abundant dense chromatin, nucleoli are little obvious, and mitoses are numerous.
Cells are arranged in trabeculae or islands, sometimes in pseudorosettes. A particular aspect, significant for diagnosis, is the presence of DNA in the blood vessel walls as a Feulgen-positive basophilic deposit, derived from the degradation of neoplastic cells.
Electron microscopy shows cytoplasmic extensions, small and rare neurosecretory granules, and some tumors contain cells rich in intermediate filaments. Immunocytochemical investigations have evidenced the presence of neuron-specific enolase, some neuropeptides, almost constantly bombesin and ACTH, and occasionally serotonin, leuenkephalin, calcitonin, somatostatin, and VIP.
Intermediate cell carcinomas are formed by large cells, with marked polymorphism, having polygonal or fusiform shapes, with abundant eosinophilic cytoplasm. Nuclei are vacuolated, with large nucleoli and numerous mitoses. Cells are arranged in trabeculae or islands with central necrosis, and peripheral palisade proliferations. Glandular or pavimentous differentiation is frequent.
Electron microscopy evidences cells with interconnected cytoplasmic extensions, neurosecretory granules are present, and intermediate filaments, as well as desmosomes, are obvious.
Immunocytochemistry shows the presence of neuron-specific enolase, serotonin, ACTH, bombesin, VIP, and leuenkephalin.
Combined carcinomas include, according to WHO classification, small cell carcinomas with areas of transition towards large carcinomas, adenocarcinomas or epidermoid carcinomas [59].
Atypical tumors represent a group of large cell pulmonary neuroendocrine neoplasms, epidermoid carcinomas or adenocarcinomas (MC DOWELL et al., 1981 [59]). The common characteristics of these neoplasms are: dense core granules, the presence of desmosomes and tonofilaments; serotonin has been immunocytochemically identified.
A practical aspect: the possibility of transformation of carcinoids into small cell anaplastic carcinomas, adenocarcinomas and epidermoid carcinomas should be considered [23].
In conclusion, OLINICI and CALUSER (1988) remark the absence of a single immunocytochemical marker for a diagnosis of certainty, in pulmonary neuroendocrine tumors. In contrast, the use of several possibilities of investigation, including electron microscopy, may lead to a classification and positive diagnosis for each lung neoplasm.
16.6.5. Ovarian carcinoids
Ovarian carcinoids are rare even in humans, while in animals further investigations are required in this field. Most ovarian carcinoids seem to have a teratoid origin.
Insular carcinomas are formed by polygonal cells, with abundant cytoplasm, cells being arranged as islands, nests or acini. Nuclei are central, round or oval, and have dense chromatin.
Trabecular carcinomas are characterized by the arrangement of cells in long, thin cords, separated by a lax or fibrous stroma. Nuclei are elongated, parallel to one another and perpendicular on the long axis of trabeculae.
Strumal carcinoids are characterized by the presence of thyroid follicles with colloid. The thyroid tissue may have a normal appearance, of macro- or microfollicular adenoma, more rarely of papilliferous follicular adenoma [59].
16.6.6. Cutaneous neuroendocrine carcinoids
According to OLINICI and CALUSER (1988), cutaneous neuroendocrine carcinoids in humans are relatively frequent, especially after the age of 60 years. The neoplasm is characterized by local recurrences, lymph node metastases and distant metastases (lungs, liver, bones, kidneys, pancreas, parathyroid glands, meninx, pleura, skin and adrenal glands).
Trabecular neuroendocrine carcinoids have been more rarely diagnosed, being located in the dermis and hair follicles. Tumor cells can be round or polygonal, occasionally elongated, with weakly eosinophilic cytoplasm, central nuclei, small nucleoli. Cells are arranged in cords or islands, with abundant stroma.
Intermediate neuroendocrine carcinoids have a dermal location, being formed by round cells with round nuclei and obvious nucleoli. The cytoplasm is reduced and amphophilic, and cells are arranged in large islands. Due to the cellular component, these neoplasms lend to confusions with lymphomas.
Small cell neuroendocrine carcinoids are histopathologically similar to oat cell lung carcinomas. Cells are small, with scant, poorly outlined cytoplasm, and nuclei are hyperchromatic, polymorphic, with numerous mitoses. Cells are arranged in islands, they form trabeculae or delimit rosettes or pseudoglandular spaces.
The electron microscopy of cutaneous neuroendocrine carcinoids shows secretory granules of round or cylindrical shape, with a dense center and a transparent peripheral area. The presence of intermediate filaments or perinuclear “fibrous bodies” is almost constantly found, which represents an essential diagnostic element.
Researches performed by OLINICI and PETRESCU (1987) demonstrate the practical value of Grimelius staining for the evidencing of paranuclear granules.
16.6.7. Endocrine tumors of other tissues
In humans, mammary gland carcinoids, with argyrophilic and electron dense granules, have been reported with a variable frequency. OLINICI and CALUSER (1988) mention that the term argyrophilic mammary carcinoid is preferable to carcinoid tumor.
Thymic carcinoids are macroscopically characterized by necrotic and/or hemorrhagic areas, with calcareous sandy deposits. Carcinoids have cells arranged in cords, rosettes or islands, and mitoses are constant. Electron microscopically, cells present characteristic dense, uniform neurosecretory granules, and tonofilaments, desmosomes and cytoplasmic extensions are exceptional.
Neuroendocrine carcinoids in animals have been described in dogs as small cellular masses, with marked nuclear pleomorphism and separation from the connective stroma. Electron microscopy shows neuroendocrine cytoplasmic granules, intermediate filament bundles, membranous interdigitations, rudimentary desmosomes. Tumors have a benign character, unlike those found in humans, which are generally malignant. Incidence is higher in old dogs. These tumors can be histologically confused with histiocytoma [18].
It may be concluded that DES neoplasms are still insufficiently known in animals, but analogy with human neoplasms may and should bring many clarifications in this field of comparative oncology.
BIBLIOGRAPHY
- 1.
- Anderson PG, Capen CC. Undifferentiated spindle cell carcinoma of the thyroid in a dog. Vet. Pathol. 1986;23:203–204. [PubMed: 3962088]
- 2.
- Appleby AC. Tumors of the adrenal gland and paraganglia. Bull. World Health Organ. 1976;53 (2–3):227–235. [PMC free article: PMC2366505] [PubMed: 1086153]
- 3.
- Asdrubali G, Muchetti L. Ulteriori ricerche sugli insulinomi del bovino. Nuova Vet. 1965:7–8.
- 4.
- Baba AI, Rotaru O, Aldea A. Proliferări nodulare cu aspect adenomatos în pancreas la bovine. Buletin IACN. ZMV. 1979;33:45–49.
- 5.
- Bălan M, Stancu M. Distrofia endemică tireotropă, in Endocrinologie clinică, sub red. S. Milcu, Ed. Med., Bucureşti. 1974
- 6.
- Boosinger TR, Zerbe CA, Grabau JH, Pletcher JM. Multihormonal Pancreatic Endocrine Tumor in a Dog with Doudenal Ulcers and Hypertrophic Gastropathy. Vet. Pathol. 1988;25:237–239. [PubMed: 2839924]
- 7.
- Boujon CE, Ritz U, Rossi GL, Bestetti GE. A clinico-pathological study of canine Cushing’s diseases caused by a pituitary carcinoma. J. Comp. Pathol. 1991;105 (3):353–365. [PubMed: 1662239]
- 8.
- Buckingham JDE. Pheochromocytoma in a mare. Can. Vet. J. 1970;11:205–208. [PMC free article: PMC1695117] [PubMed: 5530975]
- 9.
- Bundza A, Stead RH. Medullary thyroid carcinoma in two cows. Vet. Pathol. 1986;23:90–92. [PubMed: 3946060]
- 10.
- Capen CC. Tumors of the Endocrine Glands. In: MOULTON, editor. Tumors in domestic animals. 3 ed. Berkeley: University of California Press; 1978. pp. 372–429.
- 11.
- Capen CC. The Endocrine Glands. In: JUBB, KENNEDY, PALMER, editors. Pathology of Domestic Animals. ed 4. Vol. 3. Academic Press; New-York: 1993. pp. 267–347.
- 12.
- Caywood DD, Wilson KJW, Hardy MR, Shull RM. Pancreatic Islet Cell Adenocarcinoma: Clinical and Diagnostic Features of Six Cases. J. Am. Vet. Med. Assoc. 1979;174 (7):714–717. [PubMed: 218919]
- 13.
- Caywood DD, Klausner JS, O’leary TP. Pancreatic insulin-secreting neoplasms: Clinical, diagnostic and prognostic features in 73 dogs. J. Am. Anim. Hosp. Assoc. 1988;24:577–584.
- 14.
- Cella F. Tumore maligno misto (carcinno-sarcoma) della tiroide in un cane. Nuova Vet. 1968;1:1–7.
- 15.
- Chiba SK, Okada K, Numakunai S, Ohshima K. A case of Equine Thyroid Follicular Carcinoma Accompanied with Adenohypophysial, adenoma. Jpn. J. Vet. Sci. 1987;49 (3):551–554. [PubMed: 3613355]
- 16.
- Crissman JW, Valerio MG, Asiedu SA, Evangelista–Sobel I. Ganglioneuromas of the Thyroid Gland in a Colony of Sprague – dawley rats. Vet. Pathol. 1991;28:354–362. [PubMed: 1750160]
- 17.
- Dämmrich K. Die morphologische und funktionelle Pathologie der Geschwülste der Adenohypophyse bei Hunden. Zbl. Vet. Med., A. 1967;14:137–154. [PubMed: 4299713]
- 18.
- Delverdier M, Cabanie P, Van Haverbeke G. Le système endocrinien diffus. Rev. Méd. Vét. 1988;139 (12):1171–1179.
- 19.
- Doige CE, Mc Laughlin EG. Hyperplastic goitre in newborn foals in Western Canada. Can. Vet. 1981;22:42–45. [PMC free article: PMC1789868] [PubMed: 7225996]
- 20.
- Doster AR, Armstrong DL, Bargar TW. Seminoma and Parathyroid Adenoma in a Snow Leopard (Panthera unica). J. Comp. Pathol. 1989;100:475–480. [PubMed: 2760281]
- 21.
- Dow SK, Le Couteur RA, Gillette EL. Response of dogs with functional pituitary macroadenomas and macrocarcinomas to radiation. J. Sm. Anim. Pract. 1990;31(86):287–294.
- 22.
- Eigenmmann JE. Endocrine Tumours. In: Theilen, Madewell, editors. Veterinary Cancer Medicine. Lea & Fibiger; Philadelphia: 1987. pp. 619–633.
- 23.
- Eskenasy A. The carcinomatous transformation of bronchial carcinoids. Morphol. Embryol. 1981;27:231–238. [PubMed: 6460933]
- 24.
- Fix AS, Miller LD. Equine Adrenocortical carcinoma with Hypercalcemia. Vet. Pathol. 1987;24:190–192. [PubMed: 3576916]
- 25.
- Fukuoka H, Shirakawa T, Taniyama H, Ohishi H, Satoh H, Itakura C. Histogenesis of Neoformation in the Endocrine Pancreas of Aging Horses. Vet. Pathol. 1989;26:40–46. [PubMed: 2643841]
- 26.
- Gavrilă L, Constantinov M, Adomnicăi M, Cotrutz C. Amphicrine cells in the peritumoral gastric mucosa. Morphol. Embryol. 1985;31:275–278. [PubMed: 3001514]
- 27.
- Ghergariu S, Baba AI, Papuc I, Giurgiu G, Kadar L, Marieş V, Roca R, Avram E, Orha C. Struma netoxică simpla congenitală la viţei într-o zonă de guşă endemică Rev. Rom. Med. Vet. 1993;3 (1):36–54.
- 28.
- Gonzalez L, Balaguer L, Romano J, Idigoras I, Cuervo L. Prolactinoma in a sheep. J. Comp. Pathol. 1994;111 (3):321–326. [PubMed: 7836574]
- 29.
- Grigorescu A. Fiziopatologia tiroidă, în Sistemul endocrin, ed. I. Teodorescu. Exarcu, Ed. Med., Bucureşti. 1989:1198–1202.
- 30.
- Haley PJ, Hahn FF, Muggenburg BA. Thyroid neoplasms in a collony of Beagle dogs. Vet Pathol. 1989;26 (5):438–441. [PubMed: 2588438]
- 31.
- Happe RP, Van Der Gaag I, Lamers WGBH. Zollinger-Ellison syndrome in three dogs. Vet. Pathol. 1980;17:177–186. [PubMed: 6244688]
- 32.
- Hawkins KL, Summers BA, Kuhajda FP, Smith CA. Immunocytochemistry of Normal Pancreatic Islets and Spontaneous Islet Cell Tumors in Dogs. Vet. Pathol. 1987;24:170–179. [PubMed: 2883753]
- 33.
- Hedinger C, Williams D, Sobin LH. The WHO Histological Classification of Thyroid Tumors: A Commentary on the Second Edition. Cancer. 1989;63(5):908–911. [PubMed: 2914297]
- 34.
- Heinrichs M, Baumgärtner W, Capen CC. Immunocytochemical Demonstration of Proopiomelanocortin-derived Peptides in Pituitary Adenomas of the Pars Intermedia in Horses. Vet. Pathol. 1990;27:419–425. [PubMed: 2177580]
- 35.
- Horvath CJ, Ames TR, Metz AL, Larson VL. Adreno-corticotropin-containing neoplastic cells in a pars intermedia adenoma in a horse. J. Am. Vet. Med. Assoc. 1988;192:367–371. [PubMed: 2833479]
- 36.
- Hullin Marie. Contribution à l’étude du goitre chez le nouveau-né Thése Doc. Vet., Ecol Nat. Vet. Toulouse. 1969
- 37.
- Johnson SE. Pancreatic APUD-omas. Small Anim. 1989;4(3):202–211. [PubMed: 2697923]
- 38.
- Jones BR, Nicholls MR, Badman R. Peptic ulceration in a dog associated with an islet cell carcinoma of the pancreas and an elevated plasma gastrin level. J. Small. Anim. Pract. 1976;17:593–598. [PubMed: 185460]
- 39.
- Jubb KVF. The Pancreas. In: JUBB, KENNEDY, PALMER, editors. Pathology of domestic Animals. ed 4. Vol. 3. Acad. Press; New-York: 1993. pp. 423–424.
- 40.
- Keep CR. Cercetări asupra tiroidiilor la animalele domestice din regiunile endemice Sibiu şi Târnava Mare. Teză Doctoral, Fac. Med. Vet., Bucureşti. 1939
- 41.
- Kerr OM, Pearson GR, Rice DA. Pancreatic adenocarcinoma in a donkey. Equine vet. J. 1982;14:338–339. [PubMed: 7173147]
- 42.
- Kircher CH, W-Nielsen S. International Histological Classification of Tumours of Domestic Animals. Bull W.H.O; Genève: 1976. pp. 195–202.
- 43.
- Van Der Kolk JH, Kalsbeek HC, Van Garderen E, Wensing Th, Breukink HJ. Equine pituitary neoplasia: a clinical report of 21 cases (1990–1992). Vet. Rec. 1993;133:594–497. [PubMed: 8116170]
- 44.
- Lagadic M, Cohn-Bendit F. Tumeurs malignes de la thyroide chez le chien: étude retrospective de 137 cas. Prat. Medic. Chirurg. l’Anim. Comp. 1995;30 (1):85–90.
- 45.
- Leav I, Schiller AL, Rijnberk A, Legg MA, Der Kinderen P. Adenomas and carcinomas of the canine and feline thyroid. Am. J. Pathol. 1976;83:61–93. [PMC free article: PMC2032435] [PubMed: 776005]
- 46.
- Leblanc B, Parodi AL, Lagadic M, Hurtel M, Jobit C. Immunicytochemistry of canine thyroid tumors. Vet. Pathol. 1991;28 (5):370–380. [PubMed: 1750162]
- 47.
- Leifer C, Peterson E, Matus RE. Insulin-secreting tumor: Diagnosis and medical and surgical management in 55 dogs. J. Am. Vet. Med. Assoc. 1986;1:60–64. [PubMed: 3003016]
- 48.
- Long GG, Clemmons RM, Heathm H. Metastatic canine medullary thyroid carcinoma, A case report. Vet. Pathol. 1980;17:323–330. [PubMed: 6966103]
- 49.
- Manocchio I, Mughetti L, Ciorba A. Osservazioni di microscopia electtronica in un adenocarcinoma insulare a cellule B nel bovino. Nuova Vet. 1974;19:120–131.
- 50.
- Martens J, Rosenbruch M. Adenokarzinom der hypophyse bei einem Pferd. Tierärztl Prax. 1984;12:354–358. [PubMed: 6495317]
- 51.
- Mehlhaff CJ, Peterson ME, Patnaik AK. Insulin producing islet cell neoplasms: Surgical considerations and general management in 35 dogs. J. Am. Anim. Hosp. Assoc. 1985;21:607–612.
- 52.
- Midoro K, Nakayama H, Okada N, Ono K, Yasuda K, Sawa K, Takahashi R, Fujiwara K. Two Cases of Canine Insulinoma. Jpn. J. Vet. Sci. 1987;49 (6):1151–1153. [PubMed: 2828736]
- 53.
- Mogoş V. Hipotiroidismul, in Tratat de endocrinologie clinică, sub red. Milcu S, editor. Acad. Române; Bucureşti: 1982. pp. 247–281.
- 54.
- Mor N, Quaks CW Jr, Hoover JP. Concurrent mammary gland hyperplasia and adrenocortical carcinoma in a domestic ferret. J. Am. Vet. Med. Assoc. 1992;12:1911–1912. [PubMed: 1483915]
- 55.
- Moore FM, Kledzik GS, Wolfe HJ, De Lellis RA. Thyroglobulin and calcitonin immunireactivity in canine thyroid carcinomas. Vet. Pathol. 1984;21:168–173. [PubMed: 6375099]
- 56.
- O’Brien SE, Riley JH, Hagemoser WA. Unilateral thyroid neoplasm in a cat. Vet. Rec. 1980;30:199–200. [PubMed: 7445407]
- 57.
- O’Brien TD, Hayden DW, O’Leary TP, Caywood DD, Johnson KH. Canine Pancreatic Endocrine Tumors: Immunihistochemical Analysis of Hormone Content and Amyloid. Vet. Pathol. 1987;24:308–314. [PubMed: 2887054]
- 58.
- Okada H, Fujimoto Y, Ohshima K, Matsukawa K. Cell hyperplasia and carcinoma developing in sheep with experimentally-induced lymphosarco-ma. J. Comp. Pathol. 1991;105 (3):313–322. [PubMed: 1662237]
- 59.
- Olinici CD, Căluşer I. Sistemul neuroendocrin difuz. Med., Bucureşti. 1988
- 60.
- Patnaik AK, Lieberman PH, Erlandson RA, Acevedo WM, Liu SK. Canine medullary carcinoma of the thyroid. Vet. Pathol. 1978;15:590–599. [PubMed: 716155]
- 61.
- Patnaik AK, Lieberman PH. Gross, Histologic, Cytochemical and Immunocytochemical Study of Medullary Thyroid Carcinoma in Sixteen Dogs. Vet. Pathol. 1991;28:223–233. [PubMed: 1907046]
- 62.
- Pauli BU, Rossi GL, Straub R. Zwischenzelladenom der Hypophyse mit “Cushing-ähnlicher” Symptomatologie beim Pferd. Vet. Pathol. 1974;11:417–429. [PubMed: 4376879]
- 63.
- Perieteanu D, Lungu G. Hipotiroidismul, în Tratat de endocrinologie clinică, sub red. In: Milcu S, editor. Acad. Române, Bucureşti. 1992. pp. 285–350.
- 64.
- Pospischil A, Hänichen T, Von Bomhard D. Ultrastrukturelle untersuchungen an Schilddrüsentumoren Beim Hund. Schweiz. Arch. Tierheilk. 1980;122:233–246. [PubMed: 7403835]
- 65.
- Rousseaux CG. infrastructure of a canine gastrinoma. J. Comp. Pathol. 1987;97:605–607. [PubMed: 3680649]
- 66.
- Thierbart CRJ. Pathologie de la glande surrenale chez le cheval, Thèse. Ecole Nationale Vétérinaire de Toulouse; France: 1986. pp. 121–131.
- 67.
- Von Sandersleben J, Hänichen T. Tumors of the tyroid gland. Bull. Org. mond. Santé 1974;50:35–42. [PMC free article: PMC2481217] [PubMed: 4547654]
- 68.
- Schmechel D, Marangos PJ, Brightman M. Neuron-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978;276:834–836. [PubMed: 31568]
- 69.
- Slebodzinski A. Veterinärmedizinische Endokrinologie, Veb. Gustav Fischer Verlag; Jena: 1975.
- 70.
- Staempfli HR, Eigemmann EJ, Clarke LM. Insulin treatment and development of anti-insulin antibodies in a horse with diabetes mellitus asociated with a functional pituitary adenoma. Can. Vet. J. 1988;29(11):934–936. [PMC free article: PMC1680938] [PubMed: 17423172]
- 71.
- Sullivan M, Cox F, Pead MJ, MC Neil P. Thyroid tumours in the dog. J. Small Anim. Pract. 1987;28:505–512.
- 72.
- Velden Van, Der MA, Meulenar H. Medullary thyroid carcinoma in a horse. Vet. Pathol. 1986;23:622–624. [PubMed: 3535222]
- 73.
- Zerbe CA, Boosinger TR, Grabaum JH. Pancreatic polypeptide and insulin-secreting tumor in a dog with duodenal ulcers and hypertrophic gastritis. J. Vet. Intern med. 1989;3:178–182. [PubMed: 2674425]
- 74.
- Walton DK, Center SA, Scott DW. Ulcerative dermatosis associated with diabetes mellitus in the dog: A report of four cases. J. Am. Anim. Hosp Assoc. 1986;22:79–88.
- 75.
- Whitehead R. The APUD System – An Update. Pathology. 1980;12:333–335. [PubMed: 6107890]
- 76.
- Paltrinieri S, Riccaboni P, Rondena M, Giudice C. Pathologic and Immunohistochemical Findings in a Feline Aortic Body Tumor. Vet. Pathol. 2004;41:195–198. [PubMed: 15017037]
- 77.
- Patnaik AK, Newman SJ, Scase T, Erlandson RA, Antonescu C, Craft D, Bergman PJ. Canine Hepatic Neuroendocrine Carcinoma: An Immunohistochemical and Electron Microscopic Study. Vet. Pathol. 2005;42:140–146. [PubMed: 15753467]
- 78.
- Patnaik AK, Lieberman PH, Erlandson RA, Antonescu C. Hepatobiliary Neuroendocrine Carcinoma in Cats: A Clinicopathologic, Immunohistochemical, and Ultrastructural Study of 17 Cases. Vet. Pathol. 2005;42:231–237. [PubMed: 15872379]
- 79.
- Labelle P, Kyles AE, Farver TB, De Cock HEV. Indicators of Malignancy of Canine Adrenocortical Tumors: Histopathology and Proliferation Index. Vet. Pathol. 2004;41:490–497. [PubMed: 15347821]
- 80.
- Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ. Histological Classification of Tumors of the Nervous System of Domestic Animals. In: Schulman FY, editor. WHO International Histological Classification of Tumors of Domestic Animals. V. Armed Forces Institute of Pathology; Washington, D.C: 1999.
List of Figures 16.1-16.24
- Fig. 16.2 Follicular carcinoma, thyroid gland.
- Fig. 16.3 Solid follicular carcinoma.
- Fig. 16.5 Solid follicular carcinoma - thyroid glan.
- Fig. 16.10 Aortic body tumor; packets of cells separated by fine trabecula, giant nucleus.
- Fig. 16.11 Aortic body tumor, calcified spheroids. *)
- Fig. 16.12 Aortic body tumor, invasion of the wall aorta. *)
- Fig. 16.13 Aortic body tumor, lung metastasis. *)
- Fig. 16.14 Ganglioneuroblastoma. *)
- Fig. 16.15 Ganglioneuroblastoma. *)
- Fig. 16.16 Neuroblastoma. *)
- Fig. 16.17 Isomorphic pinealoma. *)
- Fig. 16.18 Chromophobe pituitary adenoma. *)
- Fig. 16.19 Eosinophilic pituitary adenoma. *)
- Fig. 16.20 Adenom with cystic spaces, adrenal gland.*)
- Fig. 16.21 Adenoma, adrenal gland.
- Fig. 16.22 Carcinoma, adrenal gland. *)
- Fig. 16.23 Carcinoma, adrenal gland.
- Fig. 16.24 Phaeochromocytoma. *)
Footnotes
- *)
Courtesy of W.H.O.
- ENDOCRINE TUMORS - Comparative OncologyENDOCRINE TUMORS - Comparative Oncology
Your browsing activity is empty.
Activity recording is turned off.
See more...