NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Fink HA, Hemmy LS, Linskens EJ, et al. Diagnosis and Treatment of Clinical Alzheimer’s-Type Dementia: A Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Apr. (Comparative Effectiveness Review, No. 223.)

Diagnosis and Treatment of Clinical Alzheimer’s-Type Dementia: A Systematic Review [Internet].
Show detailsBackground
Dementia is a clinical syndrome in which an acquired cognitive deficit interferes with independence in daily activities.5 It affects about 10 percent of older adults in the United States.6, 7 Dementia lowers patients’ quality of life (QOL), burdens caregivers, increases institutionalization, and is costly to families and society.8 Agitation, aggression, and other behavioral and psychological symptoms in dementia (BPSD) are common,9 especially late in the disease course. These symptoms may threaten the safety of patients and others and are often highly distressing to caregivers.
The ultimate reason for accurately diagnosing clinical Alzheimer’s-type dementia (CATD) and for determining whether Alzheimer’s disease (AD) is the underlying neuropathological etiology is to inform decision making about drug and nondrug treatments to improve patient and caregiver outcomes. In most individuals with a clinical dementia syndrome, AD is the primary underlying cause or at least is a contributing factor.10, 11 Historically, patients with suspected AD have been diagnosed clinically, including by criteria set by the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA),5 the Diagnostic and Statistical Manual of Mental Disorders (DSM),12–14 and the National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroup.15 NINCDS-ADRDA and DSM criteria prior to DSM-V require acquired, persistent impairment in memory and at least one other cognitive domain, along with associated functional disability not attributable to another disorder. Though DSM-V and NIA-AA criteria for major neurocognitive disorder and probable AD dementia, respectively, also require acquired deficits in at least two cognitive domains and functional disability not attributable to another disorder, memory need not be the initial or most prominently impaired cognitive domain. According to NIA-AA criteria, a diagnosis of possible AD dementia applies when either the clinical course has been atypical for AD or the clinical presentation suggests contribution from a non-AD etiology. In this report, we refer to patients with a dementia syndrome clinically suggestive of AD as having CATD.
Whereas these clinical criteria dichotomously frame AD as present or absent, AD is an insidious disease in which neuropathologic changes begin and progress for many years before symptoms are detectable and multiple neuropathologies may contribute. In clinical settings, even when the etiology of CATD is thought to be AD, it may be impossible to differentiate between CATD attributable to isolated AD, to a combination of AD plus another etiology (e.g., cerebrovascular disease), or to a non-AD neurodegenerative disease. Many individuals with CATD do not meet neuropathological AD criteria at autopsy. In patients followed in research centers, sometimes for extended durations, sensitivity and specificity of a clinical diagnosis of probable AD for autopsy-confirmed AD are approximately 80 percent and 70 percent, respectively.16 Accuracy of clinical diagnoses earlier in the disease course and in primary care settings are likely to be lower.17 Many patients with clinical AD and neuropathological AD changes also have other neuropathologic changes (e.g., microinfarcts or Lewy bodies).18
Neuropsychological testing may quantify the severity of cognitive impairment and the pattern of cognitive performance across multiple domains, helping to diagnose dementia and distinguish between different dementia subtypes. However, comprehensive neuropsychological testing is time consuming and access is limited in some healthcare settings. This has heightened interest in identifying which brief cognitive tests or their combinations are most accurate for distinguishing CATD from mild cognitive impairment (MCI) or normal cognition in patients in whom CATD is suspected (case finding) (Appendix Table K.1). Currently, no evidence-based guidelines address the merits of brief cognitive testing in patients suspected to have CATD. Though brief cognitive tests are not by themselves sufficient for diagnosing CATD, identifying brief tests that are sensitive and specific for distinguishing CATD from MCI and normal cognition in patients with suspected cognitive impairment could increase the efficiency of recognizing CATD in clinical settings, especially in primary care.
Limitations in the accuracy of clinical diagnosis of AD as the underlying cause of CATD, even after a full clinical evaluation with or without neuropsychological testing, have spurred efforts to identify specific biological markers of AD (i.e., biomarkers) that may be present across preclinical, MCI and dementia clinical stages. Existing brain imaging and cerebrospinal fluid (CSF) biomarkers may reflect specific manifestations of AD pathology, including localized neuronal hypometabolism, localized neuronal loss, abnormal ß-amyloid metabolism, cortical amyloid deposition, and accumulation of tau pathology.19 Blood tests are earlier in development.20, 21 Because of the promise of these biomarkers, NIA-AA proposed research diagnostic criteria that combine clinical and biomarker information—probable or possible AD dementia with evidence of the AD pathophysiological process.15 More recently, NIA-AA proposed a research framework classifying individuals as cognitively unimpaired, or having either MCI or dementia, based only on cognitive symptom severity and without inference about an underlying etiology.22 This framework then defines and stages AD in living persons based only on biomarkers that reflect ß amyloid deposition, pathologic tau, and neurodegeneration (AT[N]), but suggests using variable cut points to categorize biomarker levels as abnormal versus normal based on the particular research question being addressed. No cut points for specific biomarker levels or pattern of biomarker abnormalities have been proposed for clinical decision making.18
Many interventions are used to treat CATD or have been proposed for treatment, with the goal of improving, stabilizing or slowing decline in cognition, function, quality of life, and BPSD. These include nondrug interventions (e.g., exercise and cognitive training for patients, social support for caregivers), prescription drug interventions, and nonprescription drug interventions (e.g., over-the-counter drugs, supplements).
A recent Agency for Healthcare Research and Quality (AHRQ) report examined the effects of nondrug interventions for preventing or slowing cognitive decline in adults with normal cognition or MCI.23 Moderate-strength evidence showed that in patients with normal cognition, cognitive training could improve the cognitive domain trained, but did not improve untrained cognitive domains. In contrast, effects of cognitive training in patients with MCI appeared mixed and there was minimal data about whether cognitive training could delay clinical progression to MCI or CATD. This report also found no cognitive benefit from most physical activity interventions in individuals with normal cognition or MCI and that evidence was insufficient to draw conclusions about the benefits and harms of dietary interventions. We are not aware of a recent review on the effect of nondrug interventions for treating cognition, function, and QOL in patients with established CATD. Though nondrug interventions are recommended as first line treatments for BPSD,24 a recent AHRQ report found that patient-level and care delivery-level interventions were not superior to usual care for managing agitation and aggression, and that evidence was insufficient to draw conclusions about the efficacy of most caregiver-level interventions.25 While these interventions generally are presumed safe, very few trials have reported information about harms.
A 2008 American Academy of Family Physicians (AAFP)/American College of Physicians (ACP) guideline focused on drug treatment of CATD (Appendix Table K.2).26 It reported that evidence from mostly short-term randomized controlled trials (RCTs) showed that cholinesterase inhibitors and memantine statistically significantly improve cognition, but that the mean differences in cognitive scores between active treatment and control groups were not clinically important. Some studies reported that more patients assigned cholinesterase inhibitors than placebo had clinically important improvements in cognition, suggesting a possible subpopulation benefit. However, these studies did not report formal test results for whether the proportions with clinically important improvements significantly differed between treatment groups. Data reported on function was limited, and these treatments did not improve behavioral symptoms. The guideline stated that evidence was insufficient to compare the effectiveness of different drugs for treatment of dementia. The guideline recommended that decisions to initiate one of these therapies should be individualized to the patient and should consider issues of adverse effects, ease of use, and cost, and that further research on the clinical effectiveness of drug treatments for dementia was urgently needed. Supplements were included in the evidence review but not addressed in the guideline. Though no new medications have been approved for treatment of CATD by the U.S. Food and Drug Administration (FDA) since before the 2008 AAFP/ACP guideline, many new trials of existing agents have been published. A recent nonsystematic review reported that antipsychotics and mood stabilizers for treating BPSD in patients with dementia did not improve behavioral symptoms more than placebo, but had a substantially increased risk of harms.27 Results for selective serotonin reuptake inhibitor (SSRI) antidepressants were mixed. Treatment with supplements was not addressed.
Notably, claims abound on the internet and elsewhere about the benefits of various supplements for cognition, function, and BPSD in patients with CATD. In addition, 13 states currently include AD as a qualifying condition for their medical marijuana program, with use specified for severe or end-stage AD in one of the states and for individuals with AD-related BPSD in eight of the States.28 Anecdotally, patient and caregiver questions to primary care providers about the potential benefits of supplements for CATD are common. The efficacy of some older supplements has been evaluated in RCTs and systematic reviews. For many of these, it is not likely that new trials have been conducted, and thus fresh reviews are not warranted. However, for old agents with new trials, new agents, or agents with increased public interest (e.g., cannabinoids, ginseng, omega 3, gingko, huperzine A), a new comprehensive systematic review examining the effects of these agents on cognition, function, QOL, BPSD, and harms is needed.
Primary care providers routinely provide dementia care and need current, evidence-based guidance to optimize diagnosis and treatment. To address this need, the AAFP nominated this topic to update their 2008 AAFP/ACP guideline on prescription drugs and supplements for treatment of CATD.26 The AAFP also sought to broaden the guideline by adding questions about the efficacy and harms of nondrug CATD treatment, and the accuracy and harms of cognitive and biomarker testing of adults with suspected cognitive impairment. Separate ongoing AHRQ reviews are focused on screening asymptomatic older adults for dementia (https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cognitive-impairment-in-older-adults-screening?ds=1&s=cognitive)29 and on the efficacy and harms of care interventions (including nondrug treatments) for patients with CATD and care interventions for their caregivers (https://effectivehealthcare.ahrq.gov/products/care-interventions-pwd/protocol).30 These topics are not addressed by the present review except when a nondrug treatment is included as the control group for drug treatment. Therefore, the scope of the present review is limited to brief cognitive testing for CATD in patients in whom there is suspicion for CATD, biomarker testing for AD in patients with dementia, and prescription drug and supplement treatment of CATD.
Because primary care providers must make clinical decisions for individual patients, group-level results for the accuracy of brief cognitive and biomarker tests and treatment efficacy and harms may have limited utility. Identification of the patient characteristics associated with cognitive and biomarker test accuracy and harms, and with drug treatment efficacy and harms, may help physicians, patients, and caregivers make more individualized decisions about how to test, whether to treat, with what to treat and when, and when to stop treatment. Therefore, this review also examines whether factors such as age, sex, race/ethnicity, education, depression, pre-treatment cognitive or functional level/CATD stage, pre-treatment BPSD severity, and living setting modify the accuracy and comparative accuracy of cognitive and biomarker tests or the efficacy and comparative effectiveness of drug treatments.
The main target audiences of this report are primary care clinicians who diagnose and treat the vast majority of older patients with cognitive disorders, psychologists who may perform diagnostic cognitive testing in primary care settings, and dementia specialists who may be most likely to consider biomarker testing for further disease classification.
Scope and Key Questions
Key Questions
- Key Question (KQ) 1:
In adults with suspected cognitive impairment, what are the accuracy, comparative accuracy, and harms of brief cognitive tests and their combinations for identifying CATD as defined by full clinical evaluation or neuropsychological testing with explicit diagnostic criteria?
- KQ 1a:
Do the accuracy and comparative accuracy of brief cognitive tests for identifying CATD vary as a function of patient characteristics (i.e., age, sex, race/ethnicity, depression, education, pre-testing cognitive or functional level/CATD stage)?
- KQ 2:
In adults with a clinical diagnosis of CATD, what are the accuracy, comparative accuracy, and harms of brain imaging, CSF, and blood tests for identifying pathologically confirmed AD as the underlying etiology?
- KQ 2a:
Do the accuracy and comparative accuracy of brain imaging, CSF, and blood tests for identifying pathologically confirmed AD vary as a function of patient characteristics (i.e., age, sex, race/ethnicity, depression, education, pre-testing cognitive or functional level/CATD stage)?
- KQ 3:
In adults with CATD, what are the efficacy and harms of prescription drug interventions versus placebo/inactive control for treatment of cognition, function, and quality of life?
- KQ 3a:
Does the efficacy of prescription drug interventions versus placebo/inactive control vary as a function of patient characteristics (i.e., age, sex, race/ethnicity, depression, pre-treatment cognitive or functional level/CATD stage, living setting)?
- KQ 4:
In adults with CATD, what are the efficacy and harms of supplements versus placebo/inactive control for treatment of cognition, function, and quality of life?
- KQ 4a:
Does the efficacy of supplements versus placebo/inactive control vary as a function of patient characteristics (i.e., age, sex, race/ethnicity, depression, pre-treatment cognitive or functional level/CATD stage, living setting)?
- KQ 5:
In adults with CATD, what is the comparative effectiveness for cognition, function, and quality of life, and what are the comparative harms for the following interventions:
- KQ 5a:
Prescription drugs versus other prescription drugs?
- KQ 5b:
Prescription drugs versus supplements?
- KQ 5c:
Prescription drugs versus nondrug interventions (e.g., exercise, cognitive training, caregiver social support)?
- KQ 5d:
Does comparative effectiveness of prescription drugs versus other prescription drugs, supplements, or nondrug interventions for cognition, function, and quality of life vary by patient characteristics (i.e., age, sex, race/ethnicity, depression, pre-treatment cognitive or functional level/CATD stage, living setting)?
- KQ 6:
In adults with CATD and behavioral and psychological symptoms of dementia (BPSD), what are the efficacy and harms of prescription drug interventions versus placebo/inactive control for:
- KQ 6a:
Acute treatment of BPSD?
- KQ 6b:
Reducing frequency and severity of future BPSD?
- KQ 6c:
Does efficacy of prescription drugs versus placebo/inactive control for acute treatment and reducing frequency and severity of future BPSD vary by patient characteristics (i.e., age, sex, race/ethnicity, depression, pre-treatment cognitive or functional level/CATD stage, pre-treatment BPSD severity, living setting)?
- KQ 7:
In adults with CATD and BPSD, what are the efficacy and harms of supplements versus placebo/inactive control for:
- KQ 7a:
Acute treatment of BPSD?
- KQ 7b:
Reducing frequency and severity of future BPSD?
- KQ 7c:
Does efficacy of supplements versus placebo/inactive control for acute treatment and reducing frequency and severity of future BPSD vary by patient characteristics (i.e., age, sex, race/ethnicity, depression, pre-treatment cognitive or functional level/CATD stage, pre-treatment BPSD severity, living setting)?
- KQ 8:
In adults with CATD and BPSD, what is the comparative effectiveness for BPSD, and what are the comparative harms for the following interventions:
- KQ 8a:
Prescription drugs versus other prescription drugs?
- KQ 8b:
Prescription drugs versus supplements?
- KQ 8c:
Prescription drugs versus nondrug interventions (e.g., exercise, cognitive training, caregiver social support)?
- KQ 8d:
Does comparative effectiveness of prescription drugs versus other prescription drugs, supplements, or nondrug interventions for BPSD vary by patient characteristics (i.e., age, sex, race/ethnicity, depression, pre-treatment cognitive or functional level/CATD stage, living setting)?
PICOTS
Table 1.1 outlines the populations, interventions, comparisons, outcomes, timing, and settings (PICOTS) eligible for the present review.
Table 1.1
PICOTS.
Analytic Framework
The analytic framework for this review is illustrated in Figure 1.1 (KQs 1–2), Figure 1.2 (KQs 3–5), and Figure 1.3 (KQs 6–8).

Figure 1.1
Analytic framework for Key Questions 1–2.
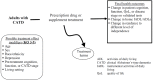
Figure 1.2
Analytic framework for Key Questions 3–5.

Figure 1.3
Analytic framework for Key Questions 6–8.
- Introduction - Diagnosis and Treatment of Clinical Alzheimer’s-Type Dementia: A ...Introduction - Diagnosis and Treatment of Clinical Alzheimer’s-Type Dementia: A Systematic Review
Your browsing activity is empty.
Activity recording is turned off.
See more...