NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Donahue KE, Gartlehner G, Schulman ER, et al. Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Jul. (Comparative Effectiveness Review, No. 211.)

Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update [Internet].
Show detailsCondition
Rheumatoid arthritis (RA) is an autoimmune systemic inflammatory arthritis. RA affects 1 percent of the world’s population, including more than 1 million American adults.48 RA is characterized by synovial inflammation of joints, which can lead to progressive erosion of bone, irreversible damage to the joint, loss of function, and resultant disability. The average incidence of RA in the United States is approximately 70 per 100,000 adults annually.49 RA can develop at any age, but incidence increases with age, peaking in the fifth decade.50 The incidence of RA is 2 to 3 times higher in women.
Etiology
The etiology of RA is incompletely understood, but multiple environmental and genetic factors contribute to the development of the disease. Obesity, smoking, and nulliparity increase the risk.50 Other environmental risk factors associated with RA, although not well understood, include low socioeconomic status and viral and bacterial infections, including those caused by periodontal and lung pathogens.51–54 Additionally, researchers using animal models are investigating the contribution of the microbiome to the development of RA.55 Rates of RA development are higher in monozygotic twins, implicating genetics as a contributing factor.56 Genome-wide association studies have characterized more than 100 loci associated with RA risk; most involve immune mechanisms. The driving genetic force is the MHC (Major Histocompatibility Complex) Class II shared epitope.57 The confluence of both environmental and genetic factors in individuals (epigenetics) also contributes to the pathogenesis of RA.58
Burden of Disease
Disability associated with RA is significant. More than 35 percent of patients with RA have a work disability after 10 years.59 The lifespan of RA patients is 3 to 12 years shorter than that of the general population.60 Patients with RA, especially those with high disease activity, are at increased risk of cardiovascular disease, which contributes to higher mortality risk.
Definitions of Early RA and Challenges With the Definitions
Defining RA for the purposes of this systematic review proved to be most challenging, because no consensus exists on the definition of early RA. As our knowledge of the pathogenesis of RA has advanced, there is a broad understanding that RA begins well before the development of the well-characterized clinical signs and symptoms of joint stiffness, pain, and swelling. The disease exists on a continuum from this preclinical stage to established disease involving the typical inflammatory disease with damage to the joints.
Definitions, by expert groups, of the beginning of early RA include symptom onset to when a clinician diagnoses RA. Experts base their initial treatment recommendations on either time from diagnosis or, more stringently, time from initial symptoms. In terms of duration, the American College of Rheumatology (ACR) defines early RA as the first 6 months of symptoms,42 while other organizations advocate for up to 2 years after diagnosis.61
In theory, treating RA early, prior to joint damage, leads to better outcomes overall than treating disease later in the course. In addition, there is increasing interest in evaluating the effectiveness of therapy in patients thought to be at high risk of developing RA but who do not yet meet the ACR/European League Against Rheumatism (EULAR) classification criteria. This is a compelling idea that presses the question of whether we can prevent the development of full-blown RA in this subset of patients. In addition, it resets the notion of how we will define “early” disease going forward.
The course of RA is highly variable; this factor precludes using a specific biological or physical benchmark or marker to identify those with early RA. For example, some researchers have suggested that early RA should be defined as the time period before patients develop bone erosion, but some patients never develop erosions.
Given this variability, a European task force of experts in RA and clinical trial methodology recommended defining early RA as no more than 1 year of diagnosed disease duration.2 Defining early RA this way subsumes the ACR definition of duration as less than 6 months of disease symptoms, but it is consistent with early RA in clinical rheumatology practice.2 Given the above caveat and limitations of placing of boundaries on the continuum of early RA, this is the basic definition (no more than 1 year of diagnosed RA) we adopted for this systematic review update.
The goal of separating early disease from late disease, however one defines these stages, is not to assess whether, or imply that, response to certain therapeutics differs by stage of disease but to provide a framework to facilitate the discussion about the effects of treating RA earlier rather than later.
Current Practice and Treatment Strategies
In all patients with early RA, experts recommend early treatment with the goal of sustained remission or low disease activity. RA treatment aims to control pain and inflammation and, ultimately, slow the progression of joint destruction and disability. Disease activity, categorized as low, moderate, and high by validated scales, can guide the initial choice and subsequent adjustment of therapy including any disease-modifying antirheumatic drug (DMARD) adjustment.62 Disease activity, functional assessment, patient-reported outcomes, and structural damage observed on radiographs should be measured regularly. Based on these measurements, clinicians should assess drug therapy at regular intervals until patients reach the treatment target, which is ideally remission.
For symptomatic early RA, the ACR recommends a treat-to-target approach to achieving remission or low disease activity, rather than a nontargeted approach; this guidance is based on low strength of evidence.42 Treating to target includes regularly monitoring disease activity and adverse events and escalating treatment if patients do not reach a treatment target (ideally remission).62 DMARD monotherapy, methotrexate (MTX) preferred, is initially recommended instead of double or triple therapy in patients who have never taken a DMARD (low strength of evidence).42 If disease activity remains moderate or high, using double or triple combination DMARDs or adding a tumor necrosis factor (TNF) or non-TNF biologic DMARD is recommended (low strength of evidence). Low-dose glucocorticoids (≤10 mg/day prednisone or equivalent) are recommended in addition if disease activity is moderate or high despite DMARD use (low to moderate strength of evidence).42
The EULAR task force recommends starting treatment with DMARDs as soon as the RA diagnosis is made. It also recommends a treat-to-target approach to achieve remission or low disease activity. EULAR advocates using conventional synthetic DMARDs (csDMARDs) as monotherapy or combination therapy for the initial DMARD treatment strategy. The csDMARDs include hydroxychloroquine (HCQ), leflunomide (LEF), MTX, and sulfasalazine (SSZ). If patients who do not have poor prognostic factors such as high disease activity, early joint damage, or autoantibody positivity do not achieve the treatment target with the first DMARD, such as MTX, then clinicians should consider using a different csDMARD (e.g., LEF or SSZ). If patients do have poor prognostic factors, then adding a TNF or non-TNF biologic to the first DMARD is recommended.
The EULAR task force regards all currently approved biologic DMARDs as similarly effective and similarly safe after csDMARD failure.2 Anakinra is the exception, as it has not shown strong efficacy when compared with other DMARDs. The ACR guidelines also did not include anakinra because of its infrequent use in RA and the lack of new data on it since 2012.42
Drugs Approved by the U.S. Food and Drug Administration
Available therapies for RA include corticosteroids, csDMARDs, TNF and non-TNF biologics, targeted synthetic DMARDs (tsDMARDs), and biosimilars. Table 1 provides the names of specific pharmaceutical agents in these categories; it is ordered roughly from oldest to newest drugs in terms of approvals by the U.S. Food and Drug Administration (FDA).
Table 1
Corticosteroids and disease-modifying antirheumatic drugs (DMARDs) approved by the U.S. Food and Drug Administration.
Challenges in Treating Early Rheumatoid Arthritis
Challenges and controversies related to early RA include several main issues. The first issue surrounds the role of newly approved drugs in the treatment strategies in the context of older medications. The number of drugs for treating early RA continues to increase with the addition of tsDMARDs, newer biologics, and biosimilars. It is important to examine whether additional improvement in patient outcomes is gained and if improvements are tempered by potential harms. A second issue is the appropriate use and order or combination of different therapeutic options. There is a dizzying array of RA medications, and combining them and designing treatment strategies demand additional choices from clinicians. Finally, identifying the optimal approach to managing RA therapy in the context of coexisting conditions (e.g., malignancy, infections like hepatitis C, congestive heart failure, diabetes) is a third challenge; pregnancy can also be an issue. Careful consideration of RA treatment drug choice is essential in these populations.
Clinicians face the challenge of identifying which DMARD to initiate for patients with early RA. Traditionally, biologics are not approved as first-line treatment. Nevertheless, clinicians still must decide whether to institute csDMARDs, tsDMARDs, or biologics earlier in the disease course.
The overarching principle should be to treat to target using disease activity remission criteria.62 Among the questions clinicians have are whether they should adopt one of the following approaches:
- Apply step-up treatment (i.e., progress from single therapy to combination therapy) or
- Apply step-down therapy (i.e., begin with combination therapy and back down treatment when symptoms are under control).
Treatment tapering or stopping strategies are also debated. When patients respond (e.g., reach low disease activity) or reach remission, the main question is whether DMARDs can be tapered or stopped. This quandary raises questions about other issues, such as how to define remission or set the appropriate taper. Also, patients may want to taper off DMARDs when their symptoms have improved; however, clinically, inflammation may be ongoing, rendering tapering off potentially inappropriate.
Scope and Key Questions
Scope of the Review
This systematic review and meta-analysis updates the 2012 report, Drug Therapy for Rheumatoid Arthritis in Adults: An Update.1 However, the targeted scope for this review focuses solely on patients with early RA.
Evidence Gaps From Prior Review
In the 2012 review, the existing evidence was insufficient to draw conclusions on the best treatment regimen for patients with early RA. Mainly, studies were of limited duration. This factor did not allow comparisons of whether early initiation of a biologic in addition to a csDMARD improved disease activity, radiographic findings, functional capacity, or quality of life compared with csDMARDs (HCQ, LEF, MTX, SSZ).1 No studies investigated efficacy, effectiveness, and harms among subgroup populations.
New Therapies
Since the 2012 review, information from additional clinical trials of four biosimilar drugs, a tsDMARD (an oral synthetic Janus kinase inhibitor), and one non-TNF biologic have become available. In addition, studies continue to be published on established therapies.
Newly approved drugs are marked with a footnote in Table 1 above. Few data are available on the efficacy of these drugs; even less is known about the effectiveness and harms compared with those of the previously existing drugs. Only a few large head-to-head trials have been conducted on any of the existing medications or new therapies. Consequently, examining the current literature as to whether all drugs examined have longer follow-up periods or include subgroups would be important knowledge gained in this review.
What This Review Aims To Do
This review focuses on patients with early RA as defined earlier. It updates the 2012 review on the comparative effectiveness of drug therapies with respect to disease activity, joint damage, patient-reported symptoms, functional capacity, and quality of life. We also examine comparative harms of drug therapies in terms of tolerability, adherence, and adverse effects. Finally, we examine comparative effectiveness and harms of drug therapies in patient subgroups. We address four Key Questions (KQ) and two Contextual Questions (CQs).
Key Questions
- KQ 1:
For patients with early RA, do drug therapies differ in their ability to reduce disease activity, slow or limit the progression of radiographic joint damage, or induce remission?
- KQ 2:
For patients with early RA, do drug therapies differ in their ability to improve patient-reported symptoms, functional capacity, or quality of life?
- KQ 3:
For patients with early RA, do drug therapies differ in harms, tolerability, patient adherence, or adverse effects?
- KQ 4:
What are the comparative benefits and harms of drug therapies for early RA in subgroups of patients based on disease activity, prior therapy, demographics (e.g., women in their childbearing years), concomitant therapies, and presence of other serious conditions?
Contextual Questions
CQs are not systematically reviewed. Rather, we use evidence readily available to us from our literature searches for the KQs and additional searches as needed.
- CQ 1:
Does treatment of early RA improve disease trajectory and disease outcomes compared with the trajectory or outcomes of treatment of established RA?
- CQ 2:
What barriers prevent individuals with early RA from obtaining access to indicated drug therapies?
Analytic Framework
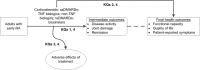
Figure 1 visually depicts the KQs within the context of the PICOTS (Populations, Interventions, Comparators, Outcomes, Timing, Setting, Study design) or eligibility criteria described in detail in the Methods section below. In general, the figure illustrates the potential outcomes that adults with early rheumatoid arthritis may experience following treatment with corticosteroids, csDMARDs, TNF biologics, non-TNF biologics, or biosimilars versus any of these same treatments. KQ 1 considers whether patients may experience benefits in intermediate outcomes such as disease activity, joint damage, and remission. KQ 2 asks the same question, but regarding benefits in final health outcomes such as functional capacity, quality of life, and patient-reported symptoms. KQ 3 considers the potential adverse effects of the medications described previously. KQ 4 addresses the comparative benefits and harms of medications for subgroups of patients with early rheumatoid arthritis.
Organization of This Report
We describe our methods next and then present our key findings in the Results chapter. In the Discussion chapter, we explore the implications of our findings and examine the limitations of the evidence base in this review, clarify gaps in the knowledge base, and offer recommendations for future research. References follow the appendixes. The main report has several appendixes, as follows: Appendix A, Search Strings; Appendix B, Excluded Articles; Appendix C, Detailed Evidence Table; Appendix D, Risk of Bias Ratings and Rationales for Included Studies; Appendix E, Strength of Evidence for Key Question 1–4 Outcomes; Appendix F, Eligible Clinical and Self-Reported Scales and Instruments Commonly Used in Eligible Studies of Drug Therapy for Rheumatoid Arthritis; Appendix G, Tests of Consistency for Main Network Meta-Analyses; Appendix H, Supplementary Primary Network Meta-Analyses; Appendix I, Sensitivity Analyses for Network Meta-Analyses; Appendix J, Expert Guidance and Review; and Appendix K, PCORI Methodology Standards Checklist.
Footnotes
Note: The reference list follows the appendixes.
- Introduction - Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review ...Introduction - Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update
Your browsing activity is empty.
Activity recording is turned off.
See more...