NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chinnadurai S, Snyder K, Sathe N, et al. Diagnosis and Management of Infantile Hemangioma [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jan. (Comparative Effectiveness Reviews, No. 168.)
We present results for Contextual Questions (CQ) followed by those for our network meta-analysis, which includes studies of beta-blockers and steroids. We then present results for each Key Question (comparative effectiveness questions).
We identified 966 publications potentially relevant to the CQ in our database searches. We also flagged studies for potential relevance to CQ in our screening of studies for Key Questions. We included 68 studies in the narrative summary of information addressing CQ.
CQ1. Natural History of Untreated IH and Adverse Outcomes of Untreated IH
Natural History of IH
IH have been estimated to occur in around 5 percent of neonates and infants.2 IH may be classified into subtypes including localized, segmental, indeterminate, and multifocal. Several studies have shown most IH to be of the localized type, and regardless of type, most IH involute with time;30–33 however, the presentation and course of IH in individual children are heterogeneous.34 IH usually present within the first month of life and undergo rapid proliferation over the first several months of life.35,36 One study found that IH reached 80 percent of their final size by 5 months of age.9 Many experts recommend referral at an early age (as early as 4 to 8 weeks of life) to subspecialists given this rapid proliferation.9
Segmental IH are more likely to have more prolonged growth, defined as after 9 months of age.9,37 Involution typically starts by 1 year of age, but the timing of involution varies markedly.38,39 In one large retrospective review of 1109 referred patients (median age=8 months) conducted in the pre-propranolol era, 769 were returned to the care of their primary provider without subspecialty followup, and only 102 (9%) required intervention.40
Most lesions involute by age 5 to 7,41,42 though timing varies, and disfigurement may remain.22,38,43,44 The majority (80%) of lesions involuting after age 6 years in one series resulted in residual scarring or telangiectasia, compared with 38 percent involuting before age 6.35 In studies of referred populations, residual lesions (e.g., telangiectasias, atrophy, fibrofatty tissue, hypopigmentation) were reported in 25 to 69 percent of untreated IH.38,45 Lesions affecting visual cortex development may result in lasting deficits in vision even after resolution of the IH.46
Indications for Treatment
The major indications for treatment of IH include risks of ulceration, disfigurement, and functional impact.39,47–52 While psychological impact on the child also plays a role in treatment decisions, data on the effects of IH on quality of life for the child suggest minimal impact. Such data are often limited by the necessity to parent-report in this young population.22,53,54 Estimates of complications from IH vary but are generally noted to occur in approximately 30 percent of the studied population.31,32,55 One study found higher initial complication rates for patients referred to a surgical center, potentially due to the higher likelihood of more advanced lesions being referred.56 Given that the literature typically includes children treated at referral centers, it is likely that the overall complication rate may be higher in study populations than in the general population.
Risk of complication is generally related to the size of lesions, location of lesions, and/or subtype.31,57–59 Larger lesions are more likely to have complications. One study found a 5 percent increase in the likelihood of experiencing complications for every 10 cm2 increase in size (OR 1.051, p<0.05).31 Lesions on the face and perineal regions have the highest rates of complications.30,31 Segmental lesions typically have a higher overall complication risk,30 though at least one study reported a lack of association with complications.60 Even after controlling for lesion size, segmental subtype lesions were eleven times more likely to have an associated complication and required treatment eight times more often than other subtypes in one study.31
Ulceration is the most common complication leading to intervention, with incidence estimated to range from 7 to 25 percent.12,31,32,40,61,62 Large size is related to increased risk of ulceration, while white discoloration to the lesion was premonitory of ulceration in one study.56,61,63 Ulceration may occur due to mechanical breakdown. Ulceration can occur throughout the proliferation phase, and segmental lesions and those in the anogenital, neck, or oral areas are among those at increased risk.47,61
Location of IH may also influence the decision to treat. Due to the delicate nature of the nasal framework, nasal tip IH can lead to structural complications even after complete resolution, including bulbous tip, tip ptosis, alar notching, splayed alar cartilage and asymmetry. Facial IH are at risk for increased residual skin changes even after involution and concern for long-term poor cosmesis is an indication for treatment. Visual complications such as amblyopia or vision loss have been noted in roughly 7 to 40 percent of periorbital lesions.31,32,64 Size of periorbital lesion is predictive of amblyopia risk, and nasal location increases the disturbance risk compared to other periorbital locations.64,65
Airway compromise is another functional disturbance that creates a need for intervention. For patients with airway IH, the degree of obstruction is the best predictor of need for intervention. Unilateral subglottic IH had a lower risk for intervention when compared to circumferential lesions in one report.66 For patients with cutaneous IH in the beard region, the finding of a subglottic hemangioma has been shown to increase with bilateral involvement.47,67
Half of the infants with cutaneous lumbosacral IH were found to have intraspinal involvement, including occult spinal dysraphism (OSD), found on MRI screening in one recent study.68 In 17 percent of patients with known OSD, a midline lumbosacral cutaneous hemangioma was observed in another study.69 Hepatic IH are associated with arteriovenous shunting and high output cardiac failure (0.4%) and were more likely to undergo treatment if signs of congestive heart failure were present in two studies.31,70 Extensive liver involvement is also associated with hypothyroidism, but the need for treatment of asymptomatic liver IH varies; in one study, for example, 8 percent of children required treatment71 while 50 percent of children in another had treatment for the IH or associated complications including hypothyroidism or cardiac failure.72
PHACE syndrome (Posterior fossa malformations, arterial anomalies, cardiac defects, eye abnormalities, sternal cleft, and supraumbilical raphe) has been identified in 19.7 percent to 30 percent of infants with large segmental facial IH.73,74 Larger lesions and involvement of more than one facial segment were found to be increased risk factors for PHACE.74 Children with PHACE are at greater risk for IH-related complications such as ulceration or visual impairment and generally require treatment for IH. Propranolol has been used in this population, and investigators developed methods for risk stratification to determine the appropriateness of beta-blockers.15,75 Other, potentially related syndromes may also be associated with segmental IH in the perineal or lumbosacral regions (LUMBAR [lower body hemangioma and other cutaneous defects, urogenital anomalies, ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies, and renal anomalies], PELVIS [perineal hemangioma, external genitalia malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus, and skin tag] and SACRAL [spinal dysraphism, anogenital, cutaneous, renal, and urologic anomalies, associated with an angioma of lumbosacral localization]) and may require treatment of the IH to avoid functional or disfiguring sequelae.76,77
CQ2. Evidence for Association of Cutaneous IH and Occult IH
Overall, limited literature addresses the association of a higher number of cutaneous IH and extracutaneous IH. Some data from case series suggest support for a higher index of suspicion in children with multiple lesions or with facial lesions in a beard distribution. Studies have primarily assessed associations between cutaneous IH and hepatic IH and cutaneous facial IH and airway IH. One study addressed associations with IH in the spinal area and reported that nine of 48 children with cutaneous IH on the lumbosacral skin had intraspinal lesions (19%), though the study did not report the number of cutaneous lesions. We summarize studies addressing hepatic and airway IH below.
The basis for the association of a greater number of cutaneous IH with hepatic IH comes primarily from case series including 453 infants with IH.71,72,78–82 In one retrospective series, investigators analyzed data from 26 children with hepatic IH (presentation of IH at birth or up to 4 months of age).72 Among the 26, 18 also had multiple or diffuse cutaneous lesions (69%) and underwent imaging, and 15 of 18 had multiple or diffuse liver IH. Investigators classified the liver IH as focal (n=8 children), multifocal (n=12 children), or diffuse (n=6 children). Among children with focal lesions, three had multiple cutaneous IH, two had a single cutaneous IH, and three had no cutaneous IH. In the multifocal group, 11 of 12 children had multiple cutaneous lesions (mean 14.25 ± 12.50 lesions) and liver IH. All but one of the 6 children with diffuse hepatic IH had multiple cutaneous lesions. Across lesion types, cutaneous lesions generally resolved before hepatic lesions.
Another series included 37 children, 16 percent of whom had three to five small cutaneous lesions; 43 percent had six or more small cutaneous lesions; 16 percent had cutaneous miliary (30–100 pinpoint lesions) lesions; 11 percent had a single large IH; and 14 percent had a combination of a large and one or more small cutaneous IH.78 Eight of 37 (22%) children had concurrent hepatic IH. Children with cutaneous miliary IH had a greater number of hepatic IH (n=7 to 35) than did infants with other cutaneous patterns. Another retrospective series reported that 17 of 23 infants (53%) with hepatic IH had multiple (≥5) cutaneous IH.80
In another retrospective series of children seen at referral centers, 62 children had six or more cutaneous IH or one large (≥5 cm) cutaneous IH and seven had three to five small (<5 cm) cutaneous IH. Fifteen of the 69 children (22%) had liver IH (14/62 with 6 or more or 1 large cutaneous and 1/7 with 3–5 small lesions).79 Forty-five percent of children with miliary cutaneous IH (n=5/11) had hepatic IH, and all five had multiple, small, widespread hepatic IH on ultrasound. Six of 69 children also had other visceral involvement: five with cervicofacial IH had airway IH (2 of these had concurrent hepatic IH) and one had concurrent hepatic IH and bladder IH. Hepatic lesions regressed earlier than cutaneous in four out of nine children with followup hepatic ultrasounds (not clear if children were treated/untreated); lesions regressed concurrently in three children, and in two children, cutaneous lesions regressed earlier than hepatic.
In another retrospective series including 39 infants (2 weeks–6 months old at presentation), 16 had solitary hepatic IH, 23 had multiple hepatic IH, and 17 of these 23 had cutaneous IH.81 In a series of 43 infants with IH, 27 had at least 10 cutaneous IH (median=16) and 16 had between five and nine cutaneous lesions (median=6.5).82 Among the nine children treated for their IH, 9 had internal IH (8 hepatic, 1 splenic), and five of the nine had more than one internal lesion. All of these children had ≥10 cutaneous IH and had no symptoms of internal IH. The study does not clearly report if any internal IH were reported among the children who did not receive treatment for cutaneous IH.
In a prospective case series including 201 infants between 0 and 6 months of age with IH seen at specialty pediatric dermatology clinics, 24 of the 151 (16%) infants with at least five cutaneous IH had hepatic IH, while none of the children with one to four IH had hepatic involvement (p=0.003).71 Preterm birth (< 37 weeks gestation) and lower birth weight were associated with having five or more cutaneous IH (p values <.05, OR for 5 or more cutaneous IH after preterm birth=4.5, 95% CI: 1.45 to 14.25). There was no significant association between the number of cutaneous IH and the number of hepatic IH, and two children with 5 or more cutaneous IH but without hepatic IH had airway or gastrointestinal IH. Other reports have also noted liver IH occurring in conjunction with multiple cutaneous IH6,83–86 and reviews have also reported an association between multiple cutaneous IH and potential parenchymal IH.87
Case series have also described an association between cutaneous IH, particularly on the face, and airway IH. The finding of a subglottic IH has been shown to increase with increasing cutaneous involvement in the beard distribution. In one report including 187 children with IH on the face and neck, 16 (8.5%) had lesions with a beard distribution.67 Ten of these 16 (63%) had symptomatic airway IH, and four of these required tracheostomy. In another case series of 25 children, seven had bilateral cutaneous IH of the head and neck, and three of these (43%) had airway IH.46 In one large series including 1226 children with cutaneous IH, 108 had segmental lesions and 56 of these had lesions in a beard distribution pattern on at least one side of the face.88 Sixteen of these 56 (29%) had concurrent upper airway IH, also with a segmental distribution. Approximately 39 of 116 children with airway IH in another series had cutaneous IH of the head and neck, and presence of cutaneous IH was significantly associated with treatment outcomes.66
Another retrospective case series assessed 342 children with IH on the upper or lower lips.89 Two-hundred thirteen children had focal lesions, and 129 had segmental, nearly 50 percent of these had unilateral or bilateral mandibular lesions. Thirty children (24 with V3 distribution) had concomitant airway IH, also in a segmental distribution. One child had PHACES syndrome. No children with focal lesions had airway IH.
In another series of 31 infants with subglottic IH, 20 had concomitant cutaneous IH, but the study did not assess the association with specific numbers of lesions or anatomic region.90 Over half of cutaneous lesions were on the head or neck. Children with cutaneous IH had more accurate diagnosis of airway IH (correct in 14/20 cases compared with 1/10 cases of airway IH without cutaneous IH, p=0.03) and with longer duration of tracheostomy (575 vs. 295 days, p=0.05). One recent meta-analysis reported that, among 61 children with IH, nine had co-existing cutaneous lesions (number of cutaneous IH not reported).91
Results of Literature Searches for Key Questions
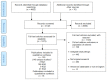
We identified 4132 nonduplicative titles or abstracts with potential relevance, with 1273 proceeding to full text review (Figure 4). We excluded 1120 studies at full text review. We included 148 unique studies (153 publications) in the review. These 148 studies included 42 comparative studies, 38 addressing effectiveness and harms of therapies four assessing effectiveness only, and 106 case series providing data on harms only. We present findings by intervention under each Key Question.
Description of Included Studies
The 148 unique studies addressing Key Questions comprise 15 randomized controlled trials (RCTs), five prospective and 19 retrospective cohort studies, two diagnostic accuracy studies (defined as studies that compared the accuracy of imaging modalities in identifying or characterizing infantile hemangioma [IH]), one prospective comparative study that used an untreated IH as a control, and 106 case series (used for harms data only). Most studies were conducted in Europe (n = 51) or Asia (n = 44). Forty-one were conducted in the United States or Canada and 12 in other countries including Australia, Egypt, Argentina, and Chile (Table 3). Forty-two comparative studies reported effectiveness outcomes. We considered six of these studies to be good quality, 22 fair quality, and 14 poor quality. One-hundred and forty-four studies (comparative studies and case series) reported harms/adverse events data. We considered 14 of these as good quality for harms reporting, three as fair quality for harms reporting, and the remainder (n = 127) as poor quality for harms reporting. Most studies addressed beta-blockers (n = 81, 13 of which compared a beta-blocker to another category of intervention such as corticosteroids or laser); 26 addressed lasers; 24 addressed steroids; 15 addressed surgical approaches; and two addressed diagnostic modalities.
Table 3
Characteristics of included studies addressing effectiveness and harms.
We included 18 studies in a network meta-analysis. All studies addressed pharmacologic agents and included five RCTs and four cohort studies evaluating oral propranolol and placebo or observation or another active agent,92–100 including steroids; 96–98,100 one RCT and one cohort study comparing propranolol and other beta-blockers;101,102 three cohort studies and two RCTs assessing topical timolol compared with placebo or observation or another agent;14,103–106 and one RCT and one cohort study comparing different steroids, including oral prednisone and intralesional triamcinolone.107,108 Four studies were good quality92,98,104,107; nine were fair quality14,93,94,96,97,99,100,102,105; and five were poor quality.95,101,103,106,108 Studies in the meta-analysis included a total of 1265 children with IH.
Grey Literature
In response to 21 requests for Scientific Information Packets, we received four documents, all of which addressed medications (becaplermin gel, recombinant interferon alfa-2b) that were not evaluated in studies meeting our criteria. The documents yielded no citations of relevance for this review, and the documents themselves did not meet criteria for inclusion in the review (one case series of 8 individuals, one addendum to an article, two files of prescribing information).
Our search of ClinicalTrials.gov did not yield any results not identified in our other searches, and our searches of the web sites of relevant organizations yielded background information for informing our contextual questions.
Key Question 1. Effectiveness and Harms of Imaging Modalities
Key Points
- Strength of the evidence (SOE) for the effectiveness of imaging for IH was insufficient given few studies assessing varied outcomes.
- Studies assessed IH in different anatomic locations and reported differing findings for the sensitivity of ultrasound and effectiveness of imaging modalities depending on location or subtype.
Overview of the Literature
Two poor quality diagnostic accuracy studies—one prospective68 and one retrospective70—addressed imaging modalities. Both studies were conducted in tertiary care settings with care settings in the United States, Canada, and Spain. One study enrolled patients from nine centers and included patients less than18 years old with IH in the lumbosacral area measuring greater than 2.5 cm.68 The retrospective cohort study reported chart review data from two tertiary care centers and included 55 patients (mean age of 30 days) with liver IH.70
Overall, studies were limited by the size of cohorts, lack of standard processes, and lack of direct comparison at the same time point using the various imaging modalities. We considered the SOE for all imaging modalities to be insufficient given single, small studies addressing different approaches, using weaker study designs and precluding a meta-analysis. The studies did not address harms.
Detailed Analysis
In one prospective cohort study, seven out of 26 (26.9%) children who underwent ultrasound had an abnormality compared with 21 of the 41 (51.2%) patients who received MRI and were noted to have a spinal abnormality.68 Nineteen of these patients underwent both ultrasound and MRI. In five cases ultrasound did not reveal an abnormality later found on MRI. Agreement between ultrasound and MRI was 0.27 (95% CI: −0.15 to 0.7, p=0.21), which was consistent with chance. Ultrasound had a sensitivity of 50 percent (95% CI: 18.7% to 81.3%) and specificity of 77.8 percent (95% CI: 40% to 97.2%) for identifying anomalies including tethered cords and intraspinal IH. We calculated the sensitivity of both modalities for identifying intraspinal IH specifically: assuming a false positive value of 0, ultrasound, which missed 4 intraspinal IH in 26 scans, had a sensitivity of 20 percent (95% CI: 3.30% to 71.19%), and the sensitivity of MRI was 100 percent (95% CI: 66.21% to 100%).
In a retrospective cohort study,70 ultrasound was commonly used as the first imaging technique and identified lesions in 42 of 44 patients (sensitivity of 95%). Ultrasound identified direct shunts in 9 of 10 patients with shunts identified by angiography. Children with findings of congestive heart failure or aortic tapering on imaging were more likely to require intervention for their hepatic lesion. Given the small number of studies and heterogeneity of interventions and outcomes, we considered SOE to be insufficient for all outcomes.
Key Question 2. Effectiveness and Harms of Corticosteroids or Beta-Blockers
Network Meta-Analysis of the Effectiveness of Pharmacologic Agents
Full and detailed methods and results of the network meta-analysis are available in Appendix D. Effect measures (Table 4) reflect effects on the logit scale and are not immediately clinically interpretable, but they demonstrate the nominal superiority of beta-blockers. Specifically, oral propranolol had the highest estimated effect size, though there is overlap among the credible intervals of the estimates. The estimated additive effect of intralesional delivery for propranolol was −6.9 (95% Bayesian credible interval [BCI]: −11.9 to −2.5).
Table 4
Posterior estimates of effect size.
More clinically interpretable are the clearance rates, presented in Figure 5, which presents mean expected clearance rates and our confidence bounds around the estimates. The expected efficacy of control arms was estimated to be 6 percent (95% BCI: 1% to 11%), i.e., we would expect to see, on average, 6 percent clearance of IH in children who receive placebo or no treatment during the study period. All non-control treatments were estimated to have a larger expected clearance than control.
The largest mean estimate of clearance was for oral propranolol (95%, 95% BCI: 88% to 99%). Clearance associated with the use of oral steroids was 43% (95% BCI: 21% to 66%), thus providing a clearance rate intermediate to control and use of beta-blockers. Triamcinolone, an intralesional injectable steroid, had a higher clearance rate than oral steroids, with wide BCI (58%; 95% BCI: 22% to 99%). Few data were available for intralesional propranolol, which is reflected in its larger credible interval (estimated clearance: 9%, 95% BCI: 0 to 45%).
With fairly wide confidence bounds and limited data in some areas, the relative differences among estimates are of greater importance than absolute effects in interpreting these results. The estimates provide a relative ranking of anticipated rates of lesion clearance among treatment options. Families and clinicians making treatment decisions should also factor in elements such as lesion size, location, type, and number, which may affect choice of treatment modality, as well as patient/family preferences.
Figure 6 represents the variability in effects seen across the patient populations in terms of percent clearance. Oral propranolol was estimated to have the largest variability in clearance rate with some patients experiencing much greater clearance than others (σ=2.5, 95% BCI: 2.1 to 2.9) with timolol (σ=1.5, 95% BCI: 1.4 to 1.6), intralesional triamcinolone (σ=1.8, 95% BCI: 1.3 to 2.3), and oral steroids (σ=1.3, 95% BCI: 1.1 to 1.6) yielding similar, lower estimates. All of the estimates of effect standard deviation were at least nominally higher than the control standard deviation, which may be a reflection of the heterogeneity of the study population in terms of response of IH to treatment.

Figure 6
Estimates of the variation of each treatment. Note: Estimates of the variation of each treatment are expressed as standard deviation, along with associated posterior interquartile range (thick lines) and 95% credible interval (thin lines).
Because of relatively sparse information from several treatment agents, we were unable to separately estimate variance parameters for all of the interventions, and instead fit a simplified model that assumed variances were equal. To check the validity of this assumption, we also fit a model on the subset of interventions with sufficient numbers of studies (>3) to estimate variance parameters, and noted that the variance estimates ranged from 1.3 (1.1 to 1.6) to 2.6 (2.2 to 2.9) on the logit scale. This was reasonably close to the 1.8 (1.1 to 2.6) estimated as the pooled variance.
To assess for methodologic heterogeneity, we ran additional models with only RCTs and with only good and fair quality studies. Estimates did not differ markedly when poor quality studies were removed, though BCI typically widened; thus, we report the model with poor quality studies included. To examine the possible effect of bias due to the inclusion of cohort studies, we fit the same model to RCT studies only. The resulting estimates were similar to those of the model fit to all studies, but with much wider posterior credible intervals. Since there was no obvious systematic bias due to study design, we reported the model estimates based on the entire body of evidence.
Effectiveness and Harms of Corticosteroids
Key Points
- In our network meta-analysis, oral steroids had a clearance rate of 43 percent (95% Bayesian credible interval [BCI]: 21% to 66%), and the rate for intralesional triamcinolone was 58 percent (95% BCI: 22% to 93%) compared with 6 percent (95% BCI: 1% to 11%) for placebo or observation (moderate SOE for improvement in IH with oral steroids vs. observation or placebo; low SOE for greater effectiveness of intralesional steroids vs. observation or placebo). This means that we would expect to see, on average, 43 percent clearance of IH in children receiving oral steroids relative to 6 percent with placebo or no treatment.
- Steroids studied varied in dose, type, and route of administration.
- Children in treatment arms typically experienced reductions in lesion size, but outcomes across studies are difficult to compare given differences in scales.
- Harms were varied and frequently included Cushingoid facies, irritability/mood changes, growth retardation, and skin atrophy or depigmentation. Ulceration was frequently reported in studies of intralesional steroids. SOE was moderate for the association of steroids with clinically important harms.
Overview of the Literature
We identified 24 studies (three RCTs, one cohort study, and 20 case series) reporting outcomes and/or harms following corticosteroid use in children with IH.40,107–129 One RCT and one case series120,122 likely report on a subset of the same children; however, the extent of overlap is not clear. Three RCTs107,108,122 and one retrospective cohort study40 addressed corticosteroids and included a total of 239 children (age range 1–72 months) with IH in multiple anatomic sites. Studies were conducted in India,122 Canada,107 Pakistan,108 and Turkey.40 Two studies included children with cutaneous IH, and IH types across all studies included superficial, deep, and mixed.
Comparative studies and case series assessed oral methylprednisolone, oral prednisolone, intravenous methylprednisolone, topical mometasone furoate, topical betamethasone, topical clobetasol, topical halobetasol, intralesional betamethasone, and intralesional triamcinolone acetonide and compared one agent to another or various doses of agents. One RCT included an observational/conservative control group.108 Only one RCT explicitly noted that assessors were blinded to treatment status.107 Treatment duration (where clearly reported) in comparative studies ranged from 3 weeks to 12 months. We rated one RCT as good,107 one as fair,122 and one as poor108 quality and the cohort study40 as fair quality for effectiveness outcomes. We considered the cohort study and one RCT40,122 as poor quality for harms reporting and two RCTs as good quality for harms reporting.107,108
In our network meta-analysis, oral steroids had a mean estimated expected clearance rate of 43 percent (95% BCI: 21% to 66%). Intralesional triamcinolone had a rate of 58 percent but with wide confidence bounds (95% BCI: 22% to 93%). Thus, there is adequate evidence to support a moderate strength of evidence for oral steroids to have a modest effect on clearance rates and low SOE for intralesional steroids to have a modest (albeit larger) effect relative to control with wide confidence bounds.
We also report harms from two RCTs98,100 and five cohort studies96,97,130–133 that compared steroids with propranolol (effectiveness outcomes reported in Effectiveness and Harms of Beta-Blockers Compared With Other Active Modalities section below). These studies were conducted in the U.S.,97,98 Canada,96 India,100, the Netherlands,131 Germany,132,133 and Egypt130 and included 308 children with IH (age range=1 to more than 9 months). We rated these studies as good98 and poor96,97,100,130–133 quality for harms reporting.
Twenty case series provided harms data on corticosteroids.109–121,123–129 Children in case series (n=3508) ranged in age from 0 to 19 years and typically had IH in multiple anatomic sites. Nine case series were conducted in the United States, three in India, two in the U.K., two in China, and one each in Qatar, Israel, Thailand, and the Netherlands. Four studies reported on only orbital or periocular IH.111,121,127,128 Treatment duration was frequently not reported. We rated all case series as poor quality for harms reporting.
Steroids were consistently associated with clinically important harms including Cushingoid appearance, infection, growth retardation, hypertension, and mood changes that may be important in making treatment decisions. The SOE is moderate for the association of steroids with these clinically important harms.
Detailed Analysis
Effectiveness of Steroids
Intravenous or Intralesional Versus Oral Steroids
One good quality RCT conducted at a Canadian tertiary care hospital randomized 20 children with problematic facial IH (defined as causing visual impairment or disfigurement) to oral prednisolone (2 mg/kg/day tapered over 9–12 months, n=10, mean age=11±4 weeks) or monthly IV methylprednisolone (30 mg/kg infused over 1 hour for 3 days for 3 months, n=10, mean age=12±3 weeks).107 Children in the oral steroid group had greater improvement in size at both the 3-month post-treatment and first birthday followup timepoints (median VAS of 70 in oral group compared with 12 in IV group, p=0.002 and median VAS of 50 in oral group vs. −1.5 in IV group, p=0.005). Vision improved in six of the eight children with eye involvement (2 in oral group and 4 in IV group), and seven children in the oral group and six in the IV group required additional steroids due to rebound growth or lack or response. In combined group analyses, children with periorbital involvement had less improvement at both time points (median VAS of 4 vs. 48, p=0.049 at 1 year).
A poor quality RCT conducted in Pakistan compared oral prednisolone (n=25) at a low dose (2 mg/kg/day on alternate days) and intralesional triamcinolone (n=25) and observation (n=25) in children (mean age=5.0±2.9 months) with superficial (73.3%), mixed (20%), and deep (6.6%) cutaneous IH.108 Lesion sites varied significantly among groups at baseline (p<0.015). Lesion size decreased significantly (p<0.001) in all three groups, though baseline size measures are not reported. Overall, 19 children had at least 50 percent reduction (8 in prednisolone arm and 11 in triamcinolone arm). Thirty-one children had little or no change (19 in observation arm, 6 in each treatment arm). Morphology changed in 88 percent of the prednisolone group, 92 percent of the triamcinolone group, and 16 percent of the observation group. Differences in morphology were significant between the conservative management group and both treatment groups combined (p<0.005). Proliferation time did not decrease in 88 percent of the observation group (statistically significant vs. the triamcinolone arm, p<0.001). One child in the prednisolone arm had rebound growth. In these two small studies, oral and intralesional steroids were associated with decreases in lesion size. Table 5 outlines outcomes in these studies.
Table 5
Key resolution outcomes in studies comparing intravenous or intralesional and oral corticosteroids.
Intralesional Versus Topical Steroids
One fair quality RCT conducted in India randomized children (age range=NR) with less than or equal to two superficial IH of less than 5 cm to daily topical mometasone furoate (n=52) or monthly intralesional triamcinolone (n=47) for 6 to 8 months (Table 6).122 Patients in this study likely overlap with those described in a retrospective case series,120 but the extent of overlap is not clear. Forty-five children in each group responded to treatment (mometasone: 50% excellent, 36.5% good, 13.4% poor response; triamcinolone: 63.8% excellent, 31.9% good, 4.2% poor response). Response to steroids did not differ by age or sex.
Table 6
Key resolution outcomes in studies comparing intralesional and topical corticosteroids.
Methylprednisolone Versus Prednisolone
In one fair quality Turkish retrospective cohort study, 283 of 1,109 children with superficial (53.7%), deep (18.8%), or mixed (16%) IH seen over 23 years at one hospital received either observation (n=238), 2 mg/kg/day prednisolone (n=26, median age at initiation=5 months), 10mg/kg/day methylprednisolone (n=11, median age at initiation=6 months), or methylprednisolone tapered from 30 mg/kg/day to 10 mg/kg/day for 7 days (n=8, median age at initiation=7 months).40 Among the children in the observation group at a median of 2 years of followup, 92 had complete or near complete (75–100%) regression, 37 had 50 to 75 percent regression, 20 had 25 to 50 percent regression, and 89 had less than 25 percent regression. By age 5, 68 percent out of an unstated number of children followed had complete regression, and 90 percent of 92 children followed had complete regression by age 9. Overall, 16 children (36%) had a good or excellent response to steroids; 15 (33%) had a fair response; and 14 (31%) had poor response. Response did not differ significantly among or between the three groups, but rebound growth was significantly higher (p=0.045) among those receiving methylprednisolone (dose not clearly reported, n=8 with rebound growth) compared with prednisolone (n=4 with rebound growth). Table 7 outlines resolution outcomes.
Table 7
Key resolution outcomes in studies comparing methylprednisolone and prednisolone.
Harms of Steroids
Harms Reported in Studies Included in This Review
Two comparative studies that addressed steroids explicitly defined harms and were considered good quality for harms reporting.107,108 Another RCT (good quality for harms reporting) that compared prednisolone and propranolol also predefined harms.98 Studies included a limited number of participants and may not have been adequately powered to detect harms. One RCT that compared harms reported in the prednisolone arm with those reported in the methylprednisolone arm noted no significant differences in harms between groups,107 as did an RCT comparing prednisolone, triamcinolone, and conservative management.108 One child receiving oral prednisolone discontinued the study due to persistent vomiting.107 Another RCT comparing oral propranolol alone, prednisolone alone, and propranolol plus prednisolone noted significantly more complications in the steroid arms compared with propranolol alone (p values not clearly reported).100 Complications in the combination arm and prednisolone only arm included Cushingoid appearance (n=6/10 in combination, 5/10 in prednisolone arms) and gastrointestinal upset (n=4/10 in combination arm and 3/10 in prednisolone). One child in the prednisolone arm discontinued the study due to ulceration and infection.100 A final RCT reported harms using a general classification.98 The frequency of harms between the prednisolone and propranolol groups did not differ significantly (44 vs. 32, respectively), and harms associated with prednisolone included endocrine (n=0.18% of lesions), gastrointestinal (n=0.14% of lesions), growth and development (n=0.23% of lesions), infection (n=0.09% of lesions), metabolic (n=0.02% of lesions), and pulmonary/respiratory (n=0.11% of lesions). Severe adverse events occurred more frequently in the prednisolone arm (11 vs. 1 in propranolol arm, p=0.01). Nine of the 11 severe events were related to growth restriction. Fewer children in the prednisolone arm had pulmonary events (typically upper respiratory tract infection) compared with children in the propranolol group (5 vs. 14, p<0.001). Five of eight participants receiving prednisolone discontinued due to adverse events, and study enrollment was stopped due to adverse events.98
One cohort study (poor quality for harms reporting) did not report precise harms data but noted that 20 of 45 children receiving either prednisolone or moderate or high dose methylprednisolone developed Cushingoid facies, and 16 of 45 developed irritability, both of which resolved upon cessation of the drug.40 Three cohort studies (effectiveness outcomes reported in Effectiveness and Harms of Beta-Blockers Compared With Other Active Modalities section below) comparing oral or intralesional steroids with oral or intralesional propranolol reported harms including irritability, Cushingoid features, and hypertension.96,97 One study of intralesional triamcinolone reported that no adverse events occurred.130 Harms frequently reported across all comparative studies addressing steroids included irritability, crying, pain, Cushingoid appearance, and skin depigmentation (Table 8).
Table 8
Harms/adverse effects in comparative studies of steroids to treat IH.
Serious harms included two cases of respiratory distress requiring hospitalization in children receiving either prednisolone or methylprednisolone.107 A child receiving prednisolone also developed uncomplicated chickenpox, and some children (exact number not reported) in the prednisolone arm in this RCT evidenced growth (height and weight) retardation at 1 year of age compared with children in the methylprednisolone arm (p values ≤0.003). Children (>70%) in both arms in this study also experienced blood pressures ≥ the 90th percentile (>15% in either arm were ≥ the 95th percentile) though only one required antihypertensive medication for persistent elevation, and 52 of 73 cortisol tests were abnormal (31 in prednisolone arm and 21 in methylprednisolone). Twelve cortisol levels in the prednisolone arm and one in the methylprednisolone arm were in the undetectable range, and blood glucose was transiently elevated in 5 of 70 tests.107 In total, seven of 330 participants receiving steroids in comparative studies discontinued treatment due to adverse events.
Case series included 3508 children receiving intralesional, oral, or topical steroids or combinations of agents, with doses of oral steroids ranging from 1 to 5 mg/kg/day and intralesional doses (where reported) ranged from 0.5 to 6 ml (Table 9). We considered all studies as poor quality for harms reporting. No studies explicitly reported harms sought, and the lack of a comparison group and typically small sample sizes limit our understanding of the significance of these harms.
Table 9
Adverse effects in case series of steroids to treat IH.
Frequently reported harms across agents were Cushingoid facies (reported in 0.45%–100% of children in 12 studies), diminished height or weight gain or growth retardation (0.45%–47% of children in 8 studies), skin atrophy (0.95%–17% of children in five studies), hypopigmentation (1.4% to 16% of children in 6 studies), hypertension (0.11% to 5% of children in five studies), infection (2% to 15% of children in 5 studies), and behavioral changes (25% to 100% of children in four studies). Cushingoid appearance and growth retardation occurred regardless of dosage form (i.e., intralesional, oral).
One study reported on several “ultrapotent” topical steroids (betamethasone dipropionate, clobetasol propionate, halobetasol propionate, 0.05%) in children with primarily superficial IH and noted that 2 of 34 children (agents received not specified) experienced hypopigmentation.127 Another reporting on several corticosteroids including oral prednisolone, clobetasol propionate, and intralesional triamcinolone plus betamethasone in 30 children with complicated IH reported adverse effects in the aggregate rather than by agent.129 Most children received prednisolone, and harms included decreased rate of linear growth (n=14), decreased weight gain (n=9), Cushingoid facies (n=7), increased weight gain (n=5), decreased head growth (n=4), hirsutism (n=4), delayed motor milestones (n=3), thrush (n=3), premature thelarche (n=2), increased rate of linear growth (n=1), sterid acne (n=1), gastritis (n=1), and varicella infection (n=1).129 Three case series evaluating intralesional steroids reported that no adverse events occurred,123,126,128 and none explicitly reported discontinuation of treatment due to adverse events.
Harms Reported in Package Insert Data
The safety and efficacy of pediatric use of corticosteroids has been studied in the literature for the treatment of nephrotic syndrome (>2 years of age), and aggressive lymphomas and leukemias (>1 month of age).134–139 It has been reported that the adverse events identified in pediatric patients were similar to the events experienced in adults. Monitoring pediatric patients for blood pressure, weight, height, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis is recommended. Specifically, pediatric patients may have a decrease in growth velocity after taking corticosteroids by any route of administration. Therefore, children should be titrated to the lowest effective dose.
Common adverse events of corticosteroids include: fluid retention, alteration in glucose tolerance, elevation in blood pressure, behavioral and mood changes, increased appetite and weight gain.134–142 Additional adverse events include: anaphylactoid reaction, anaphylaxis, angioedema, bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis, acne, allergic dermatitis, cutaneous and subcutaneous atrophy, dry scalp, edema, facial erythema, hyper or hypopigmentation, impaired wound healing, increased sweating, petechiae and ecchymoses, rash, sterile abscess, striae, suppressed reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria, abnormal fat deposits, decreased carbohydrate tolerance, development of Cushingoid state, hirsutism, manifestations of latent diabetes mellitus and increased requirements for insulin or oral hypoglycemic agents in diabetics, menstrual irregularities, moon faces, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery or illness), suppression of growth in children, potassium loss, hypokalemic alkalosis, sodium retention, abdominal distention, elevation in serum liver enzymes levels (usually reversible upon discontinuation), hepatomegaly, hiccups, malaise, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, ulcerative esophagitis, osteonecrosis of femoral and humeral heads, Charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, steroid myopathy, tendon rupture, vertebral compression fractures, arachnoiditis, convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually following discontinuation of treatment, insomnia, meningitis, mood swings, neuritis, neuropathy, paraparesis/paraplegia, paresthesia, personality changes, sensory disturbances, vertigo, exophthalmos, glaucoma, increased intraocular pressure, posterior subcapsular cataracts, alteration in motility and number of spermatozoa.
We also identified safety data for another steroid evaluated in studies in this review, mometasone furoate. The use of this medication in pediatric patients (≥2 years) is not recommended for more than 3 weeks.143 This medication is administered topically, and pediatric patients will have an increase in the skin surface area to body mass ratio. As a result, adverse events such as hypothalamic-pituitary-adrenal axis suppression, Cushing’s syndrome, adrenal insufficiency upon cessation, skin atrophy, striae, linear growth retardation, delayed weight gain, and intracranial hypertension are more likely to occur in pediatric patients. We report additional harms data form package inserts and U.S. Food and Drug Administration (FDA) approval documents in Appendix H.
Effectiveness and Harms of Beta-Blockers
Key Points
Propranolol Versus Observation or Placebo
- In our network meta-analysis, oral propranolol was associated with a mean estimate of expected clearance of IH of 95% (95% BCI: 88% to 99%) compared with 6 percent (95% BCI: 1% to 11%) for placebo or observation arms (high SOE for greater effectiveness of propranolol versus placebo or observation).
- Oral propranolol at doses of 2–3 mg/kg/day divided two to three times daily and given for up to 6 months promoted resolution or near resolution of IH in children under the age of 12 months with superficial, deep, mixed, or ulcerated IH in most studies.
- Adverse events, measured in the short-term only, associated with these doses of propranolol in this same population were limited in frequency and severity (moderate SOE association of propranolol with clinically important and minor harms).
Propranolol Versus Other Active Modalities
- In network meta-analysis, oral propranolol was associated with a mean estimate of expected clearance of IH of 95% (95% BCI: 88% to 99%) compared with a lower rate for oral steroids (43% [95% BCI: 21% to 66%]), while in head-to-head comparisons three small studies found propranolol was more effective than corticosteroids, and two did not find a significant difference in effectiveness between the two therapies. Combined effects from individual studies and network meta-analysis conferred moderate SOE for the superiority of propranolol over steroids at achieving IH clearance.
- In one cohort study comparing the effects of intralesional steroids and oral propranolol on vision outcomes, improvement in amblyopia did not differ between agents, but fewer children receiving propranolol required additional treatments or had side effects than those receiving steroids.
- Propranolol combined with pulsed dye laser (PDL), either concurrently or sequentially, was more effective than propranolol alone in one study.
- In a study comparing oral propranolol with intralesional bleomycin, 6 of 10 children in each arm had at least 75 percent clearance of IH.
- One study found that patients who received propranolol had a lower likelihood of subsequent laser treatment than those who received other interventions.
- Propranolol was associated with faster healing of ulceration versus historical treatments including laser and antibiotics.
Oral Propranolol Versus Other Beta-Blockers or Dosage Forms
- Other oral beta-blockers (atenolol, nadolol) investigated in three studies were reported to be effective in promoting IH resolution and potentially associated with fewer adverse events than propranolol (low SOE for no difference in response of IH to propranolol, nadolol, or atenolol).
Timolol Versus Placebo/Observation or Other Active Modalities
- In our network meta-analysis, topical timolol had a mean expected clearance rate of 62 percent (95% BCI: 39% to 83%) compared with 6 percent (95% BCI: 1% to 11%) for placebo or observation (low SOE for effectiveness of timolol versus placebo or observation).
- Topical timolol 0.5 percent maleate gel promoted improvement of superficial IH without reported adverse effects in four comparative studies (low SOE for lack of association with harms). Studies reported effectiveness at 24 weeks with the noticeable change in IH lesions occurring approximately 12 to 16 weeks after initiation of treatment.
Overview of the Literature
We identified a total of 81 studies (nine RCTs,14,17,92,93,98–100,102,104 16 cohort studies,95–97,101,103,105,106,130–133,144–150 and 56 case series16,18,151–205) addressing beta-blockers including propranolol, atenolol, nadolol, and timolol. Comparative studies addressed the following interventions and comparators: propranolol compared with observation or placebo arms, propranolol compared with other active modalities (e.g., steroids), oral propranolol compared with other beta-blockers or dosage forms, and timolol compared with observation/placebo or another modality (e.g., laser). Comparative studies included a total of 1539 children between the ages of less than one month to 9 years. We considered four RCTs to be good quality and five as fair quality for effectiveness outcomes and 11 cohort studies as fair quality and five as poor quality for effectiveness outcomes.
Propranolol Versus Observation or Placebo
We identified four studies (two good17,92 and one fair99 quality RCTs and one fair quality cohort study94) evaluating propranolol versus placebo or observation. Propranolol was associated with significantly greater clearance of IH compared with the control arm in all four studies. In the largest RCT, which included 456 children without problematic IH receiving up to 3 mg/kg/day of propranolol, 60 percent of children in the propranolol group had complete or near complete resolution of IH after 24 weeks of treatment compared with 4 percent in the placebo group.92 The recommended dose of propranolol in this IH population remains to be determined, but the majority of studies to date have investigated the 2 mg/kg/day dosing regimen. Despite changes in lesion size in many children receiving propranolol, some children do not appear to respond to propranolol, but these children are not well-characterized to date.
In network meta-analysis, the mean expected clearance rate for oral propranolol was 95 percent (95% BCI: 88% to 99%) relative to 6 percent (95% BCI: 1% to 11%) for placebo/observation arms; IH size reductions were greater in propranolol arms versus control in all individual studies, thus we considered the SOE as high for greater effectiveness of propranolol compared with placebo or observation based on individual comparisons and the meta-analysis.
Propranolol Versus Other Active Modalities
Ten studies compared propranolol to another modality including steroids, pulse dye laser (PDL), bleomycin, or historical treatments.95–98,130–133,145,149,150 Studies comparing propranolol and steroids to reduce IH size had conflicting findings. Propranolol was more effective than steroids in three studies,96,97,132,133 while two others studies did not find effectiveness differed significantly between these treatments.98,130 In network meta-analysis, pooling data from multiple studies, propranolol was superior to oral steroids (95% clearance [95% BCI: 88% to 99%]) versus 43% clearance (95% BCI: 22% to 66%). These combined effects from individual studies and meta-analysis conferred moderate SOE for superiority of propranolol over steroids at achieving clearance.
One additional retrospective cohort study assessing only vision outcomes reported no significant differences between oral propranolol and intralesional steroids in improving amblyopia, but children in the propranolol arm had a significantly shorter duration of therapy (p<0.001) and required fewer additional treatments than those receiving steroids (p=NS).131
Another retrospective study found that PDL therapy either in conjunction with or subsequent to propranolol therapy is more effective than propranolol alone.150 Another study found the likelihood of laser treatment was lower in participants treated with propranolol than participants who did not receive the medication.149 The study that compared propranolol with bleomycin95 did not demonstrate that one intervention was more effective than the other. In a final study, ulcerated lesions healed more quickly with propranolol than with other treatments including laser.145
Oral Propranolol Versus Other Beta-blockers or Dosage Forms
Three small studies compared propranolol with nadolol101 or atenolol,102,146,147 and one study evaluated oral, intralesional, and topical propranolol.93 Atenolol and nadolol demonstrated promising effects on lesion size (no significant differences in effectiveness of propranolol and atenolol and greater effectiveness in a small study comparing nadolol and propranolol) and low levels of adverse effects, which may suggest that improvements can be achieved in the propranolol safety profile. More children receiving oral propranolol had an excellent or good level of resolution than those receiving topical or intralesional (n=11/15, 8/15, 5/15, respectively), but the difference among groups was not significant.93
In head-to-head comparisons, there were no significant differences in response between propranolol and atenolol in two studies and better response to nadolol versus propranolol in one small study. We considered the SOE as low for no difference in response with propranolol, nadolol, or atenolol (systemic beta-blockers).
Timolol Versus Placebo/Observation or Other Active Modality
Six comparative studies addressed timolol (two RCTs14,104 and four cohort studies103,105,106,144). All studies included children with superficial IH, and two (one comparing timolol with observation and one comparing timolol and laser) also included children with mixed (superficial and deep) IH.14,144 Timolol was significantly more effective than observation or placebo in three studies,103,104,144 and one study comparing imiquimod with timolol did not demonstrate that one intervention was more effective than the other.105 In one study comparing timolol and PDL+Nd:YAG laser, timolol was associated with greater improvements in superficial lesions, while laser was associated with greater improvements in mixed (superficial and deep) lesions.106 In another comparing timolol alone with timolol plus PDL, mean global assessment scores were more improved in the combination arm than in the timolol arm, though IH in 97 percent of children in both arms improved from baseline.14 No harms of timolol were observed in any study.
In network meta-analysis, the mean expected clearance rate for topical timolol was 62 percent (95% BCI: 39% to 83%) relative to 6 percent (95% BCI: 1% to 11%) for placebo or observation arms. We considered SOE as low for the effectiveness of timolol compared with placebo or observation.
Harms of Beta-blockers
In addition to these comparative studies, a total of 56 case series addressed harms of beta-blockers for IH.16,18,151–205 We assessed four case series as good quality for harms reporting,168,171,181,186 one as fair quality,182 and 51 as poor quality.16,18,151–167,169,170,172–180,183–185,187–205 Twenty-four comparative studies also reported harms data, and we assessed four as good quality for harms reporting92,98,104,105 and the remainder as poor quality for harms reporting.14,17,93–97,99–103,106,130–133,144–147,150 Harms most frequently reported with use of oral beta-blockers (propranolol, atenolol, nadolol) included sleep disturbances, cold extremities, gastrointestinal symptoms, bronchial irritation (classified as hyperreactivity, bronchospasm, bronchiolitis, cold induced wheezing), and decreases in blood pressure or heart rate. Rates of significant clinically important harms ranged from 0 to 100 percent across studies of propranolol and from 1 percent to 50 percent for minor harms. We considered SOE as moderate for the association of propranolol with these harms. Data were insufficient to comment on harms in studies of nadolol and atenolol. No harms were observed in four small studies of timolol. We considered SOE to be low for lack of association of timolol with harms.
Detailed Analysis
Propranolol Versus Placebo or Observation
One good quality RCT conducted in 56 centers in 16 countries randomized 460 infants with a proliferating IH measuring at least 1.5 cm in diameter to treatment with either placebo twice daily for 6 months (n=55) or one of four oral propranolol treatment regimens (1 mg/kg/day of propranolol divided twice daily for 3 months (n= 99) or 6 months (n= 103); 3 mg/kg/day of propranolol divided twice daily for 3 months (n= 101) or 6 months (n= 102).92 Two independent, trained, validated readers centrally assessed digital photographs taken at each patient’s 15 study visits for complete or nearly complete resolution, hemangioma evolution, and change in hemangioma size and color. Investigators at each site performed these same assessments, and assessed complications, adverse events, and use of other treatment for IH. Parents or guardians also assessed changes in IH since the previous visit.
Overall, 61 of 101 patients (60%) assigned to propranolol 3mg/kg/day for 6 months and 2 of 55 patients (4%) assigned to placebo had complete or near complete resolution of hemangioma at week 24 (p<0.001). Fifty of 102 children (49%) receiving 1mg/kg/day for 6 months had complete or nearly complete resolution (p<0.001 versus placebo). This propranolol regimen remained superior to placebo when adjusting for age group, hemangioma location, and randomization ratio. However, only 40 percent of the cases judged centrally as “complete resolution” and “complete or nearly complete resolution” were assessed similarly by the on-site investigators. The on-site investigators noted sustained improvement from week 5 through week 24 in 71 percent of cases, which was similar to the rate determined by the centralized assessments.
The most frequent reason for discontinuation was treatment inefficacy. Of the 133 patients (29%) who discontinued treatment, 36 were receiving the 6-month placebo regimen, 35 were receiving the 3-month 1 mg/kg/day propranolol regimen, and 35 were receiving the 3-month 3 mg/kg/day regimen. Those with the lowest rates of discontinuation were patients receiving propranolol for 6 months at the 1 mg/kg/day dosing (n=14) and 3 mg/kg/day dosing (n= 13) regimens. Six (10%) patients assigned to the selected propranolol regimen required reintroduction of treatment from week 24 to week 96.
A small pilot RCT99 conducted by the same investigators of the larger, multi-center RCT92 described above included 14 infants (<16 weeks of age) with non-problematic IH. 99 Participants received 3 to 4 mg/kg/day of propranolol. IH thickness decreased by a mean of 44.9 percent (95% CI: 36.0 to 76.2%) in the propranolol group compared with an increase of 11.3 percent in the placebo arm.
Another good quality RCT conducted in Australia randomized 40 children with IH that did not require urgent treatment to receive propranolol at 2 mg/kg/day divided three times daily or placebo for 6 months.17 Nineteen patients were treated with propranolol, and IH growth stopped before week 4 of propranolol treatment in all patients. The largest difference in mean percent change in volume between the propranolol and placebo groups (based on serial hemispheric measurements of tumor volume) occurred at week 12 (−66.4%, p = 0.03). IH redness and elevation improved significantly more at weeks 12 and 24 in the propranolol compared to placebo group (p values ≤ 0.07). Of the 19 patients treated with propranolol, two responded only minimally (start of treatment at ages 5.5 and 11 months).
In one fair quality cohort study conducted in India, thirty-three children up to 10 years of age with IH requiring treatment due to airway obstruction, ocular occlusion or compression, aesthetic disfigurement or ulceration, who may have failed other treatment modalities, and those patients greater than 12 months of age with continuous proliferation of their IH without signs of resolution were treated with propranolol at a dose of 2 mg/kg/day divided twice daily.94 The study compared these participants with historical controls who had not previously received therapy. Significant involution defined as a score of 5 to 9 on a 10-point scale (10=no change in original IH, 0=normal skin) was seen in 28/31 (90.3%). All children 6 months of age and younger responded (20/20, 100%). No child greater than 36 months of age (0/2, 0%) responded to propranolol. Sixty-five to 80 percent of involution occurred in the first 8 weeks of propranolol therapy. The overall mean involution score for the propranolol group compared with the control group was 4.37 versus 8.38 (p< 0.0001). Table 10 outlines resolution outcomes in these studies.
Table 10
Key resolution outcomes in RCTs comparing propranolol and placebo or observation.
Propranolol Versus Other Active Modalities
Oral Propranolol Versus Oral or Intralesional Steroids
Six studies compared oral propranolol with steroids: one compared oral propranolol and oral prednisolone;98 one compared oral propranolol with prednisolone and with propranolol plus prednisolone;100 two compared oral propranolol and oral prednisone,96,132,133 one compared oral propranolol and unspecified oral steroids;97 and one compared oral propranolol and intralesional triamcinolone and betamethasone;131 (Table 11). A good quality RCT compared prednisolone (2 mg/kg/day) with propranolol (2 mg/kg/day) in 19 infants. 98 The mean change in total surface area did not differ significantly between prednisolone and prednisone (0.41 vs 0.64 mm2, p=0.12). The rate of total surface area decline was faster in the prednisolone group, and this discrepancy persisted when baseline lesion characteristics were taken into account. Three patients (2 in propranolol group, 1 in prednisolone group) had IH regrowth after medication weaning. This trial was halted early due to withdrawal of 75% (6/8) of the participants in the prednisolone group.
Table 11
Resolution outcomes in studies comparing beta-blockers and steroids.
A fair quality RCT compared three treatment regimens: propranolol (2–3mg/kg/day), prednisolone (1–4 mg/kg/day), and both agents in 30 children between 1 week and 8 months old.100 Thirty percent of children with IH in the head and neck area had parotid IH, and 53 percent of lesions overall were superficial (27% mixed, 20% deep). IH reduction from baseline was greater in the propranolol alone and propranolol plus prednisolone arms compared with the prednisolone arm (p values <0.01). Size reduction in the prednisolone arm was significantly different from baseline only at the 6-month followup (p=0.008). Size reduction did not differ by lesion type in any group although time to respond was less for mixed lesions compared with superficial and deep lesions (p<0.02).
A fair quality retrospective cohort study compared 12 patients treated with propranolol (mean dose 2.7 mg/kg/day, range 2.5–3.5) matched with 12 historical patients treated with prednisone (mean dose 2.8 mg/kg/day, range 2.0–4.0).96 At all time points, propranolol was rated as more effective than prednisone (p=0.007 at 1 month, p=0.002 at 2 months, and p<0.001 at 6 months). Mean improvement using the VAS was 78.7 percent with propranolol versus 44.8 percent with prednisone (p<0.001). In another poor quality cohort study comparing oral propranolol (2mg/kg/day) and prednisone (2mg/kg/day for 2 week tapered downwards) in 60 infants, propranolol produced significantly greater size reduction than did prednisone (median 2.0 cm2 vs. 3.5 cm2, p=0.006).132,133 Improvements in redness, IH height, and turgor were also greater in the propranolol arm vs. prednisone (p<0.001).
A fair quality retrospective cohort study compared propranolol (target dose 2 mg/kg/day) with an unspecified oral corticosteroid (dose ranged from 2–4 mg/kg/day, most took 4 mg/kg/day).97 There were 75 infants in the propranolol group and 42 in the corticosteroid group. Overall, more patients in the propranolol group (56/68, 82%) than the corticosteroid group (12/42, 29%) achieved clearance of 75 percent or more (p<0.01). Some of the patients in the propranolol group had received corticosteroids prior to propranolol treatment. There was no significant difference in the proportion of propranolol-participants with at least 75 percent clearance when subanalyzed according to previous corticosteroid use.
Finally, a fair quality retrospective cohort study compared effects on amblyopia in children with periorbital or cheek IH receiving oral propranolol (up to 3 mg/kg/day) or intralesional steroids (up to 1 mL).131 Children receiving steroids received injections 8 weeks apart and had a significantly longer median duration of therapy compared with those receiving propranolol (median 15.9 months, interquartile range [IQR] 10.28 vs. 6.5 months, IQR 4.87, p<0.001). Improvement in amblyopia did not differ significantly between groups (no amblyopia in 61% of the steroid group and 86% of propranolol group at followup). Two children in the steroid arm and one in the propranolol group, all of whom began therapy in the proliferative phase, had no improvement in amblyopia. The study did not assess resolution outcomes.
Intralesional Propranolol Versus Intralesional Triamcinolone
A fair quality prospective cohort study compared a single intralesional propranolol injection with a single intralesional triamcinolone injection in 22 infants with periocular capillary hemangioma (Table 12).130 Among the 12 participants who received propranolol, the response was excellent for five (42%), good for three (25%), fair for two (17%), and poor for two (17%). Among the 10 participants who received triamcinolone, the response was excellent for four (40%), good for two (20%), fair for two (20%), and poor for two (20%). Seven participants (four in the propranolol group and three in the triamcinolone group) experienced rebound growth after responding to treatment. All of these participants received and responded to a second injection. There were statistically significant reductions in astigmatic error and degree of ptosis in both groups, and the differences between the two treatment groups were not statistically significant.
Table 12
Resolution outcomes in studies comparing intralesional propranolol and triamcinolone.
Propranolol Plus Pulsed Dye Laser Versus Propranolol Alone
A fair quality retrospective cohort study compared three treatments for facial segmental IH: concurrent propranolol and pulsed dye laser (n=12), propranolol followed by pulsed dye laser (n=5), and propranolol alone (n=8) (Table 13).150 Mean hemangioma size was larger in the concurrent treatment group (41.65 cm2) than the sequential (20.1 cm2) and propranolol-only groups (18.0 cm2). Among the 12 participants who received concurrent propranolol and pulsed dye laser, six (50%) had complete clearance and six (50%) had near-complete clearance. All five of the participants in the propranolol followed by pulsed dye laser group also had complete (n=2, 40%) or near-complete (n=3, 60%) clearance. Among the eight participants who receive propranolol alone, one (13%) had complete clearance, two (25%) near-complete clearance, and five (63%) partial clearance. The difference in effectiveness between combined therapy, either concurrently or sequentially, and propranolol alone was statistically significant. The number of days of propranolol treatment until near-complete clearance was significantly lower (p<0.001) for those receiving concurrent therapy (mean 92.3 ± 50.9 days) or sequential therapy (mean 181.2 ± 101.1 days) than those receiving propranolol alone (mean 288.0 ± 83.5 days).
Table 13
Resolution outcomes in studies comparing propranolol with laser and propranolol alone.
Oral Propranolol Versus Intralesional Bleomycin
A poor quality prospective cohort study compared oral propranolol with intralesional bleomycin in 20 children with cutaneous hemangioma (Table 14).95 Participants either received daily oral propranolol for six weeks or three bleomycin injections given at 6-week intervals. In the bleomycin group (n=7 at final follow up), one participant had a grade I response, five a grade II response, and two a grade III response. In the propranolol group (n=10), two participants had a grade I response, four a grade II response, three a grade III response, and one a grade IV response. Children who received propranolol began responding to treatment more quickly than those who received bleomycin.
Table 14
Resolution outcomes in studies comparing propranolol and bleomycin.
Propranolol Versus No Propranolol
A fair quality retrospective cohort study examined the effect of propranolol on the incidence of invasive procedures in 58 children with nasal IH.149 Participants fell into three groups: treated in the pre-propranolol era (n=20), treated in the post-propranolol era and received propranolol (n=25), and treated in the post-propranolol era and did not receive propranolol (n=13). Many participants received other therapies including corticosteroids, laser treatments, and/or surgery. Participants who received propranolol had a lower likelihood of laser treatment than those treated in the pre-propranolol era (hazard ratio 0.44, 95% CI: 0.27 to 0.78). The risks of surgical excision did not differ significantly (hazard ratio 0.45, 95% CI: 0.15 to1.38).
Another fair quality cohort study conducted in the Netherlands compared 20 children with ulcerated IH treated with propranolol with 20 historical controls (matched on age at IH onset, extent of ulceration, and type, location and size of the IH).145 Children in the control group had received steroids (25%), PDL (1%), antibiotics (60%), and local wound care (100%). Mean age of the patients at the start of ulceration was 2.3 months, and complete healing occurred after an average total ulceration time of 8.7 weeks in the propranolol treated group versus 22.4 weeks (p= 0.012) in the historical control group. Four of 19 (20%) patients who completed propranolol treatment had regrowth. One (0.5%) patient restarted propranolol due to significant regrowth of the IH, affecting surrounding structures. Table 15 outlines key outcomes.
Table 15
Key outcomes in studies comparing propranolol and no propranolol.
Oral Propranolol Versus Other Beta-Blockers or Dosage Forms
Atenolol Versus Propranolol
In a fair quality RCT conducted in Chile, investigators randomized 23 infants from 1 to 15 months of age with IH displaying functional impairment, aesthetic disfigurement, ulceration, or location on skin folds to receive oral atenolol (1 mg/kg/day in a daily dose) or oral propranolol (2 mg/kg/day divided into 3 daily doses) for 6 months.102 Of 13 patients randomized to atenolol, seven had complete response (53.8%) compared with six of 10 children randomized to propranolol (60%) (p = 0.68). Upon cessation of treatment, four (40%) children in the propranolol group and two (15.4%) in the atenolol group had rebound growth.
In a fair quality cohort study conducted in the Netherlands, 30 consecutive infants ages 1.5 to 30 months (median age 6.4 months) with problematic IH were treated with atenolol (final dose of 1–3 mg/kg/day) compared with a historical control cohort of 28 infants with IH treated with propranolol (mean dose 2 mg/kg/day).146,147 Of the 27 patients treated with atenolol and 24 patients treated with propranolol, those treated with atenolol were significantly younger than those treated with propranolol (p = 0.01). In addition, while not significant, the atenolol group contained more patients with ulceration (30% versus 4%). There were no statistically significant differences noted in quantitative improvement of IH by VAS scores or change in HAS scores between the groups. Twenty-seven of 30 infants treated with atenolol (90%) and all patients treated with propranolol showed clinical involution at the end of the treatment period (p= 0.09). Table 16 outlines key outcomes.
Table 16
Resolution outcomes in studies comparing beta-blockers.
Nadolol Versus Propranolol
In a poor quality cohort study conducted in Canada, oral nadolol was used in the six month treatment of 10 infants 1-month to 1-year of age and compared to a historical group of nine similar infants matched for age and hemangioma location who were treated with oral propranolol for at least six months (Table 17).101 Infants were treated with oral nadolol starting at 0.5 mg/kg/day divided twice daily and increased weekly by 0.5 mg/kg to a maximum dose of 4 mg/kg/day (mean dose 2.19 ± 1.1 mg/kg). Propranolol was administered to a maximum of 2–3 mg/kg/day divided three times daily (mean dose 1.89 ± 0.29 mg/kg). The nadolol treated group had a mean percentage IH shrinkage of 97 ± 3.05 percent at the 24-week visit compared with 86 ± 14.82 percent shrinkage observed in the propranolol group (p< 0.001).
Table 17
Key resolution outcomes in studies comparing nadolol and propranolol.
Oral Propranolol Compared With Other Dosage Forms
In a fair quality single blinded RCT conducted in Egypt, 45 consecutive patients with problematic, superficial IH (rapidly progressive, compromising vital or normal physiological function, or causing disfigurement) were assigned to one of three treatments: oral propranolol (2 mg/kg/day divided into two daily doses, n=15), topical propranolol 1 percent ointment applied twice daily, or intralesional propranolol (1 mg propranolol hydrochloride as a 1 mL injection, n=15) repeated weekly (0.2 mL injected per 1 cm lesion diameter to a maximum of 1 mL, doses divided among multiple lesions, n=15) (Table 18).93 Twelve (80%) patients treated with oral propranolol had improvement in their IH: nine (60%) patients showed a complete response; 2 (13.3%) demonstrated a sustained plateau with ≥ 50 percent reduction in size; 1 (6.7%) showed a sustained plateau with <50 percent reduction in size. Two children (13.3%) had no response to treatment and one (6.7%) discontinued treatment.
Table 18
Resolution outcomes in studies comparing forms of propranolol.
Ten (66.7%) patients treated with topical propranolol had improvement in their IH. Three (20%) demonstrated complete response; 5 (33.3%) demonstrated a sustained plateau with ≥ 50 percent reduction in size, 2 (13.3%) showed a sustained plateau with < 50 percent reduction in size, and five (33.3%) had no response to treatment. Eight (53.3%) patients treated with intralesional propranolol showed improvement in their IH. Two (13.3%) participants had a complete response; three (20%) demonstrated a sustained plateau with ≥ 50 percent reduction in size; three (20%) had a sustained plateau with less than 50 percent reduction in size, seven children (46.7%) had no response. Rebound growth was documented in one (6.7%), one (6.7%) and two (13.3%) children treated with oral, topical, and intralesional propranolol, respectively. Time to achieve initial response and duration of treatment needed to achieve the final response were significantly greater in both the topical (3–8 weeks to initial response; 5–10 months treatment duration) and intralesional propranolol (4–8 weeks to initial response; 5–12 months treatment duration) groups as compared with the oral propranolol group (2–4 weeks to initial response; 3–9 months treatment duration, p values ≤ 0.01).
Timolol Versus Placebo/Observation or Other Modalities
Timolol Compared With Placebo or Observation
In a good quality double-blind, placebo-controlled RCT conducted in Australia, investigators randomly assigned 41 infants ages 5 to 24 weeks with small, focal, superficial IH not requiring systemic therapy to treatment with placebo (n=22) or timolol maleate 0.5 percent gel (n=19).104
Investigators reported a significant increase in the number of IH lesions decreasing in size by ≥5 percent in the timolol group compared with the placebo group at weeks 8 (37% vs. 5%, p= 0.04), 20 (47% vs.6%, p= 0.02), and 24 (60% vs.11%, p= 0.01). At 24 weeks, 47 percent of the timolol treated group had significantly increased difference in blinded photo score of 0 (no redness) compared with 6 percent in the placebo group, while the proportion of lesions completely red in the treatment group (6%) was significantly less than the placebo group (55%, p values <0.01).
In a fair quality trial conducted in the United States, children with non-vision-threatening IH (defined as absence of visually significant ptosis or induced astigmatism on initial examination) were either observed (those presenting between August 1, 2007 and March 30, 2009) or offered treatment (presenting April 1, 2009 and January 15, 2011) with topical 0.25 percent timolol maleate gel.144 At 2 months follow-up, a good response was observed in eight (61.5%) infants, moderate response in four (30.8%) and one (7.7%) infant had a poor response in the treatment group compared with good response observed in no (0%) infants, a moderate response seen in one (10%) infant, and nine (10%) infants with poor response in the control arm. In addition, five (100%) superficial lesions and three (42.9%) mixed lesions treated with timolol demonstrated a good response, while four (57.1%) deep lesions treated with timolol demonstrated a moderate response. Overall, timolol-treated patients had significantly improved responses compared with the observation group (p=0.001). One patient in whom timolol was prematurely stopped at 5 months of age had rebound growth, which again regressed with resumption of topical timolol.
In a poor quality prospective cohort study conducted in China, 124 infants ≤ 12 months of age with superficial IH (≤ 3 mm in height) and without prior treatment or tumor regression were treated with either topical 0.5% timolol maleate drops three times daily (n=101) or observed (n= 23).103 Timolol promoted regression in 57 patients (56.4%), controlled growth in 36 patients (35.6%), and was ineffective in 8 patients (7.9%) compared with the observation group where regression was seen in one patient (4.3%), controlled growth observed in seven (30.4%), and continued growth observed in 15 patients (65.2%). Regression and efficacy rates in the timolol group compared to the observation group were significantly improved (p<0.05). At 3 to 5 months followup, no regrowth was noted in 12 patients followed who had complete regression of their IH. Table 19 outlines key outcomes.
Table 19
Key resolution outcomes in studies comparing timolol and observation or placebo.
Timolol Ophthalmic Solution Versus Imiquimod Cream
One fair quality retrospective cohort study evaluated imiquimod cream versus timolol ophthalmic solution for treatment of superficial proliferating IH (Table 20).105 There were 40 treated IH among the participants. The mean duration of therapy was 4.6 months in the imiquimod group and 4.3 months in the timolol group. Duration of followup was not reported. The VAS score and change in the hemangioma activity score did not differ significantly between the two groups.
Table 20
Resolution outcomes in studies comparing timolol and imiquimod.
Topical Timolol Versus Laser
One fair quality RCT conducted in Egypt compared topically applied timolol (0.5% ophthalmic solution) and sequential PDL and Nd:YAG laser in 60 children (age range not clear) with superficial or mixed IH.14 Children received treatment for roughly 4 to 5.5 months. Forty percent of children in the timolol group and 20 percent in the laser group had an excellent response (defined as improvement of 76–100%), and IH hemoglobin level declined significantly from baseline in both groups. Improvement in IH in either group did not differ between children who were greater or less than 6 months of age, but response was greater in superficial lesions compared with mixed lesions in both groups. More mixed lesions responded to laser than to timolol, with deep components of superficial lesions not responding to timolol. Superficial lesions responded more quickly and more extensively to timolol than to laser (p=NR). The study provided few statistical comparisons of timolol versus laser.
In a poor quality retrospective cohort study comparing topical timolol alone with timolol plus PDL in 102 children with superficial IH, children received treatment for between 2 and 24 months.106 Overall, 97 percent of children had improvement in IH (3 children in the timolol arm had no change, 28 had >75% improvement), with greater improvement in the combination arm compared with the timolol alone arm (mean global assessment score change of 2.66 vs. 1.88, p=0.02, score range=−1 to 4 with higher number indicating more improvement). Table 21 outlines key outcomes.
Table 21
Resolution outcomes in studies comparing timolol and laser.
Harms of Beta-Blockers
Harms Reported in Studies Included in This Review
Thirteen comparative studies specifically defined harms of beta-blockers used to treat IH.17,92–94,98,101,102,104,105,132,133,144,145,147 Several studies specifically noted that no harms were observed: one study evaluating topical timolol maleate 0.5 percent gel compared to placebo;104 a cohort study evaluating topical 0.25 percent timolol maleate gel;144 one RCT of ophthalmic timolol,105 and a cohort study of timolol that informed parents of potential adverse effects to monitor for, reported evaluating for safety (non-specified), and stated that no adverse effects were reported.103 An RCT comparing atenolol versus propranolol102 and two other cohort studies of intralesional propranolol130 and up to 2mg/kg/day of oral propranolol95 reported that no harms were observed. Another RCT of propranolol (3–4 mg/kg/day) including 14 participants reported asymptomatic hypotension and bradycardia in an unstated number of infants and discontinuation of treatment in one child due to drowsiness.99
One RCT comparing propranolol and prednisolone reported side effects associated with 2 mg/kg/day dosing of propranolol in the categories of allergy/immunology (0.02% of lesions), dermatologic (0.05% of lesions), gastrointestinal (0.11% of lesions), infection (0.11% of lesions), pulmonary/respiratory (0.32% of lesions), vascular (0.07% of lesions).98 Fewer severe adverse events occurred in the propranolol arm compared with prednisolone (1 vs. 11, p=0.01); the one severe event in the propranolol arm was a case of dehydration necessitating hospitalization. Children in the propranolol group had more pulmonary events (typically upper respiratory tract infections) than those in the prednisolone arm (14 vs. 5, p<0.001). In another RCT comparing propranolol, prednisolone, and propranolol plus prednisolone, more children in the steroid and combination arms had adverse effects than those in the propranolol alone arm.100 The 10 children receiving propranolol had two side effects: one case of asymptomatic hypoglycemia, and one case of somnolence. In one cohort study (poor quality for harms reporting) ulceration and atrophic scarring occurred in one child receiving propranolol and laser treatment, and no adverse effects were observed in the propranolol only arm.150 One study of topical timolol reported shortness of breath and insomnia in one of 30 children,14 while another reported no harms associated with topical timolol in another cohort study comparing timolol and laser.106
Harms most frequently reported with use of oral beta-blockers (propranolol, atenolol, nadolol) included sleep disturbances, cold extremities, gastrointestinal symptoms, and bronchial irritation (classified as hyperreactivity, bronchospasm, bronchiolitis, cold induced wheezing). One study 93 reported hypotension and bradycardia in three of 15 children, and two syncopal episodes in another child. Few children receiving beta-blockers in comparative studies discontinued treatment due to adverse effects (n=24/1062, 2.3%). Studies typically included a limited number of participants and may not have been adequately powered to detect harms (Table 22).
Table 22
Harms/adverse effects in comparative studies of beta-blockers to treat IH.
The safety population in a large RCT92 included 456 patients in total (Table 23). Thirty-three serious events occurred in 26 patients, and no significant difference overall or in individual events between the placebo group and group receiving propranolol at 3 mg/kg/day for 6 months were noted. One serious adverse event of second-degree atrioventricular heart block (with a preexisting cardiac condition later documented) occurred after dose administration on day 0, and treatment was discontinued. While hypotension and hypoglycemia were both documented in this trial, neither was clinically significant enough to lead to treatment discontinuation.
Table 23
Harms/adverse events reported by dose in Leaute-Labreze et al. 2015.
Table 24 summarizes the incidence and type of adverse effects reported in case series. Consistent with the pharmacological action of propranolol, decreases in blood pressure and heart rate were the most frequently reported adverse events and were as high as 100 percent in some series.168,171 However, reductions in these parameters were not always clinically significant. In most prospective case series, clinically important hypotension and bradycardia were not reported; asymptomatic changes were specifically noted in several series.16,151–158,169,182,189,194, 199,205 The lack of cardiac events may be due to required cardiovascular evaluation prior to initiation of propranolol or discontinuation after short-term monitoring. The number of patients that did not qualify for propranolol therapy was not provided in any of these series. No adverse effects were reported in several case series,154,156,157,196 and most studies of topical beta-blockers reported that no adverse events were observed, though studies typically did not describe methods for harms monitoring.159,160,186 Two studies of topical applications reported recurrent itching associated with topical propranolol187 in 3 percent of children and sleep disturbances in 1 percent of children receiving topical timolol.161 The remaining case series reported few adverse events, and those reported rarely caused discontinuation of the medication. In total, 51/3810 (1.3%) children in case series discontinued treatment due to adverse events including sleep disturbances (n=13), bronchial hyperreactivity, wheezing, or asthma (n=9), and cold extremities (n=7).
Table 24
Adverse effects in case series of propranolol to treat IH.
Harms Reported in Package Insert Data
Hemangeol® is the only medication included in this review that has an FDA approved indication for infantile hemangioma. The safety of Hemageol® in pediatric patients has been reported in the medication package insert.206 FDA medical review packages were not available for this medication. The most common adverse events, occurring in greater than 10% of infants, were sleep disorders, aggravated respiratory tract infections such as bronchitis and bronchiolitis associated with cough and fever, diarrhea, and vomiting.206 In a study of pooled safety data (n=424), infants (63% aged 91–150 days) were treated with Hemangeol® 1.2 mg/kg/day or 3.4 mg/kg/day for 3 or 6 months. Treatment emergent adverse events occurring in 3% or greater in infants receiving the Hemangeol® 1.2 mg/kg/day (n=200) or Hemangeol® 3.4 mg/kg/day (n=224) compared to placebo were provided. Adverse events and frequencies for patients receiving Hemangeol® 1.2 mg/kg/day included: sleep disorders (17.5%), bronchitis (8%), peripheral coldness (8%), agitation (8.5%), diarrhea (4.5%), somnolence (5.0%), nightmare (2.0%), irritability (5.5%), decreased appetite (2.5%), and abdominal pain (3.5%). Adverse events and frequencies for patients receiving Hemangeol® 3.4 mg/kg/day (n=224) included: sleep disorders (16.1%), bronchitis (13.4%), peripheral coldness (6.7%), agitation (4.5%), diarrhea (6.3%), somnolence (0.9%), nightmare (6.3%), irritability (1.3%), decreased appetite (3.6%), and abdominal pain (0.4%). Additional adverse events reported in less than 1% of patients participating in clinical trials included: second degree atrioventricular heart block (occurring in a patient with underlying conduction disorder), urticaria, alopecia, decreased blood glucose, and decreased heart rate.
The safety and efficacy of the oral tablet, oral capsule, and injectable formulations of propranolol have not been investigated in pediatric patients.207–209 The package inserts for these formulations state that reports of bronchospasm and congestive heart failure have been reported in pediatric patients receiving propranolol. Additional adverse events revealed during post-marketing surveillance include agranulocytosis, hallucination, and purpura.206
Harms of Other Active Comparator Agents
Harms of corticosteroids and PDL are presented in those sections; this section only includes medications for which harms are not presented elsewhere in this review. In a study rated poor quality for harms reporting, reported complications of bleomycin included febrile episode, superficial ulceration, and raised alkaline phosphatase.95 The proportion of participants who experienced these complications is unclear. In another study, which was rated good quality for harms reporting, adverse effects in 20 participants using imiquimod included crusting of lesions (65%), superficial scars (15%), and skin pigmentation (29%).
Key Question 3. Effectiveness and Harms of Drugs Administered After the Failure of Corticosteroids or Beta-Blockers
We did not identify any comparative studies addressing this Key Question.
Key Question 4. Effectiveness and Harms of Surgical Interventions
Key Points
- Studies primarily addressed different laser modalities compared with observation or other laser modalities. PDL was the most commonly studied laser type, but multiple variations in treatment protocols did not allow for demonstration of superiority of a single method (low SOE for difference in effects on size reduction between longer pulse PDL and other lasers).
- Two small studies addressed different surgical techniques (cryotherapy, intense pulsed light photothermolysis, sclerosis) and reported some positive effects in reducing IH size or improving appearance, but their smaller size and low quality preclude conclusions (insufficient SOE).
- Many studies used historical controls, based on now superseded treatment regimens.
- In two RCTs reporting level of clearance, at least 40 percent of children in laser or observation arms had complete or near complete clearance of IH (low SOE for lack of difference between PDL and observation).
- Cohort studies assessed outcomes after CO2 and Nd:YAG (neodymium yttrium aluminum garnet) lasers and typically reported some resolution of lesion size, but heterogeneity among studies limits our abilities to draw conclusions (insufficient SOE).
- Harms associated with laser treatment included skin atrophy, bleeding, scarring, ulceration, purpura, and pigmentation changes. Bleeding and ulceration were observed in the immediate postoperative period, distinguishing these complications from the possible natural complications of IH themselves (moderate SOE for association of PDL with pigmentation changes; low for association with bleeding; and insufficient for scarring. Low SOE for association of Nd:YAG laser with scarring and insufficient for association with bleeding and pigmentation changes).
Overview of the Literature
Eleven comparative studies (three RCTs,210–212 seven retrospective cohort studies,213–219 and one prospective comparative study that used treated and untreated lesions and intervention and control groups220) and 30 case series addressed surgical approaches. The RCTs were conducted in the Netherlands,210 Japan,211 and the UK.212 Cohort studies were performed in the United States,216,217 Greece,218 Singapore,213 Russia,219 and Germany.214,215 Two RCTs210,212 compared PDL to observation; one used traditional PDL in infants aged 1 to 14 weeks,212 and the second used PDL with epidermal cooling in infants aged 0 to 6 months.210 The third RCT211 compared the use of non-cooled traditional PDL to longer pulse PDL with epidermal cooling in infants between 1 and 3 months old. We considered RCTs to be of good210 and fair quality.211,212
Cohort studies examined various comparisons between different laser types including PDL versus Nd:YAG,215 Argon versus Nd:YAG,217 short pulse PDL versus longer pulse PDL.213 One compared Nd:YAG and CO2 lasers and also included a non-surgical comparison group for airway IH.214 Two studies compared different skin cooling protocols with the same laser types, including Nd:YAG218 and PDL.216 One cohort study compared cryosurgery, photothermolysis with intense pulsed light, and photothermolysis plus sclerosis with alcohol and lidocaine.219 We considered two cohort studies as fair quality,213,218 and the rest as poor.214–217,219 We considered the self-controlled comparative study (rated using the Newcastle Ottawa tool) as poor quality.220
Overall, longer pulse PDL with epidermal cooling was the most commonly used laser for cutaneous lesions and Nd:YAG was the most commonly used intralesionally. Most studies reported a higher success rate with longer pulse PDL compared to observation in managing the size of IH, although the magnitude of effect differed substantially. CO2 laser was used for subglottic IH in a single study, and was noted to have a higher success rate and lower complication rate than both Nd:YAG and observation. Studies addressing other surgical approaches (cryosurgery, intense pulsed light thermolysis) reported some improvements in IH but included few participants in each arm (total n = 263).
SOE for outcomes after laser and surgical treatments ranged from insufficient to low for effectiveness outcomes. The evidence was limited by low sample size, and variations in the laser settings used including wavelength and cooling protocols. For Nd:YAG and CO2 lasers, all studies were limited by sample size, and SOE was insufficient for all outcome parameters.
Thirty case series reported on harms of surgical approaches for IH (3831 children). Seventeen case series reported on harms from laser treatments, including 10 studies of PDL,221–229,230 four studies of Nd:YAG lasers,231–234 one of combined PDL and Nd:YAG,235 one of long-pulse Alexandrite laser,236 and one report of carbon dioxide laser.237 Most studies included children with IH in multiple locations; one included children with only airway IH.237 Ages of children in these series, where clearly reported, ranged from less than 1 month to 11 years. We considered one study to be of good quality for harms reporting,223 two of fair quality,227,230 and 14 of poor quality.221,222,224–226,228,229,231–237 We rated one cohort study that compared propranolol with concurrent PDL or followed by PDL and two comparing laser and topical timolol as poor quality for harms reporting and discuss harms of PDL here and harms of propranolol in the beta-blocker section of KQ2 above.14,106,150
Thirteen case series (840 children) reported harms from surgical procedures, typically excision or resection, to treat IH.238–249 Ages ranged from 1 month to 19 years. The majority of studies focused on treatment of facial IH, including three studies of lip IH,240,244,248 two series of periocular/periorbital IH,242,245 two reports of various facial locations,241,247 and one study of nasal tip IH.243 All of the studies were rated as poor quality for assessment of harms as data collection was not predefined.
Detailed Analysis
Effectiveness of Laser Treatment
PDL Compared With Observation
Two RCTs compared PDL to observation. One good quality RCT210 randomized 22 children with IH between 0 and 6 months of age into equal groups of observation or PDL with epidermal cooling. Twelve-month size change scores were used for analysis. Further, parents were asked to answer quality of life questionnaires at enrollment and at age 12 months. There was no statistical difference seen in echo depth or total surface area between the two groups; however color was significantly improved in the PDL group compared with control (p=0.03). Photographs reviewed for overall improvement also showed a “significant improvement” for the PDL group (46%) over the observation group (18%), but this “significant improvement” was not quantitatively defined. Parent-reported quality of life scales showed no difference in the severity of skin problems between groups. Sixty-three percent of parents in the PDL group reported improvement in the IH at 12 months compared with 33 percent in the observation group (p=NR). Thirteen percent of parents perceived the treatments to be very painful.
The second, fair-quality RCT randomized 121 children to PDL (n=60) and observation (n=61) groups.212,250 The investigators attempted to reduce bias by including a blinded panel of parents of non-study children to describe whether they perceived the hemangioma to be a problem at 1 year of age. The investigators reported no differences in the number of children experiencing near complete resolution (42%–44% in each group) but more children in the PDL group (30%) than in the control arm (5%) experienced complete resolution (p=0.001). Outcomes between groups were similar at the 5-year followup of 117 children (32 of 57 in the PDL arm had complete clearance vs. 27 of 60 in the observation arm, p=0.31 and 41 of 57 and 48 of 60 had minimal residual signs, p=0.39). Table 25 outlines key outcomes.
Table 25
Key resolution outcomes in studies comparing PDL and observation.
Comparative Effectiveness of Various PDL Modalities
One fair quality Japanese RCT211 randomized 52 patients to a “traditional PDL” group and a “long-pulse” dye laser group (pulse durations of 0.45 milliseconds vs. 10–20 milliseconds). The percentage of patients achieving an excellent (76–100%) clearance of the lesion did not differ between groups, with rates of 54 to 65 percent in each group. Time to maximal proliferation was significantly shorter (106 days) in the longer pulse PDL group compared with the traditional PDL group (177 days, p=0.01). Another fair quality cohort study comparing short and longer pulse PDL similarly reported no significant differences in the number of children with complete or near-complete resolution by age 3 to 3.5 years.213
In a poor quality cohort study evaluating cryogen spray cooling as an adjunct to PDL versus no cooling in 164 children (mean age overall= 2 years, 11 months), c216hildren in the cryogen cooling arm required fewer treatments and had greater improvements in volume and texture than children in the non-cooled PDL arm (p values <0.01). 216 Changes in color did not differ between groups. Table 26 outlines key outcomes.
Table 26
Key resolution outcomes in studies comparing PDL modalities.
Nd:YAG Laser Compared With Other Lasers or Observation
Three poor quality cohort studies compared Nd:YAG laser to either argon laser,217 traditional PDL,215 or CO2 laser or observation.214 One study included 55 children with sequelae from hemangioma and reported similar rates of excellent clearance (defined as 90–100% clearance) between Nd:YAG and Argon groups and a higher rate of children attaining 50 percent or greater clearance in the Nd:YAG group (72% vs. 52%).217 Lesions were also scored for size length and width, which showed little difference between groups. Heights of lesions were sub-analyzed, which showed a greater ability of Nd:YAG to treat thicker lesions, with no excellent results in the argon group for lesions 0.5 cm in height and greater.
In a study comparing Nd:YAG and PDL and including 50 children, 41 percent of children receiving PDL and 30 percent receiving Nd:YAG had complete clearance of IH (p=NR).215 Similar numbers in each group had 70 to 99 percent or <70 percent clearance, and 7 percent in the PDL arm and 18 percent in the Nd:YAG arm had growth of IH. The average pain score was 5.6 for the PDL group and 3.9 for the Nd:YAG arm.
A final retrospective cohort reviewed outcomes after practice changes regarding the management of subglottic hemangioma.214 Fifteen children in the “pre-laser” era (1973–1986) were treated with observation and systemic steroids, 14 patients from 1986–1994 were treated with Nd:YAG, and 17 patients from 1995 and after were treated with CO2 laser. All patients with severe airway obstruction from the hemangioma were treated with tracheostomy. In children who did not present with previously placed tracheostomy, there was no statistical difference in the need for tracheostomy between the steroid treatment group and the Nd:YAG group; however, CO2 yielded a lower tracheostomy rate. There was also a reduction in the time to tracheostomy decannulation from 26.6 to 10.6 months with CO2 compared with Nd:YAG. There was no difference in time to decannulation with Nd:YAG compared to steroid treatment. Two of 10 Nd:YAG patients developed laser-related stenosis; no patients in the CO2 group developed stenosis. Speech and developmental issues were reported by more parents of children who had had tracheostomy compared with those who had no tracheostomy, and parental worry about the fate of the child lessened earlier if the child did not have a tracheostomy. Table 27 outlines key outcomes.
Table 27
Key resolution outcomes in comparative studies of Nd:YAG laser.
Nd:YAG Laser With Cooling Compared With No Cooling
In one fair quality cohort study, 235 patients (mean age= 9 months) received the same Nd:YAG laser treatment but different methods of epidermal cooling (ice chips during procedure, n=115; ice before, during, and after treatment, n=120).218 Children were treated until they received an excellent (90–100% resolution) or good (50–89% resolution) result. Patients with more extensive cooling required a mean 1.45 sessions of laser treatment compared to 2.11 in the less extensive cooling group (Table 28).
Table 28
Key resolution outcomes in comparative studies of Nd:YAG laser with cooling.
Effectiveness of Surgical Treatments
Photothermolysis With Intense Pulsed Light Compared With Cryosurgery Compared With Photothermolysis With Intense Pulsed Light Plus Sclerosis
One retrospective cohort study compared three treatment modalities in 250 infants <12 months old) with maxillofacial IH: cryosurgery, photothermolysis with intense pulsed light, and photothermolysis plus sclerosis with alcohol and lidocaine (Table 29).219 More children in the combined group had a size reduction of at least 50 percent after one treatment session than in the cryosurgery or laser arms (60 vs. 18 vs. 31, respectively, p=NR). Four children in the combined arm, 17 in the laser arm, and 24 in the cryosurgery arm had less than 10 percent decrease in IH size. Time to IH regression was reduced by 4.2 months for the combined arm compared with the laser arm and was 10.7 months shorter compared with the cryosurgery arm (p=NR). Children in combined arm required an average of 2.6 treatment sessions compared with 4.5 in the laser arm and 3.7 in the cryosurgery arm (p=NR).
Table 29
Key resolution outcomes in comparative studies of photothermolysis with intense pulsed light and cryosurgery.
Cryosurgery Versus No Treatment
One study assessed cryosurgical treatment of IH in preterm infants with multiple IH by treating one IH lesion and not treating another.220 Some children had more than one pair of treated/untreated lesions, and the study followed infants up to age 1 or 2 years. Thirteen of 17 treated IH and two of 17 untreated IH met the primary endpoint of intact, IH-free skin with mild or no pigmentation or scarring at 1 or 2 years of age (p<0.001). Fifteen of 17 IH in the control arm had residual signs at the final followup and one had scarring, compared with four IH with scarring in the cryosurgery arm (p<0.001). Table 30 outlines outcomes.
Table 30
Key resolution outcomes in comparative studies of cryosurgical therapy.
Harms of Laser and Surgical Interventions
Harms associated with laser treatment included skin atrophy, bleeding, scarring, ulceration purpura, and pigmentation changes. Bleeding and ulceration were observed in the immediate postoperative period, distinguishing these complications from the possible natural complications of IH themselves. In one RCT with 5-year followup of children treated early with PDL, the number of children in the PDL arm with skin atrophy did not differ significantly from that in the observation arm (13 of 57 vs. 7 of 60, p=0.14), but more children in the PDL arm had hypopigmentation (25 vs. 14, p=0.03).212,250 Numbers requiring surgical correction did not differ significantly between groups (4 in PDL arm vs. 2 in observation). In one RCT comparing traditional PDL and longer pulse PDL, hyperpigmentation, hypopigmentation, and negative textural changes were all significantly greater in the traditional PDL group (p values <0.01).211 In a cohort study comparing short and longer pulse PDL, minor skin complications were greater in the short pulse group, and typical sequelae or laser treatment, erythema, edema and purpura, lasted longer in the short pulse group, but the study did not provide statistical analysis of these outcomes.213 One study comparing topical timolol and timolol plus PDL reported that no harms occurred in either group, while another comparing timolol and PDL plus Nd:YAG laser reported minor crusting and hyperpigmentation in four of 30 children.14,106 Studies typically included a limited number of participants and may not have been adequately powered to detect harms. Table 31 outlines harms reported in comparative studies.
Table 31
Harms/adverse effects in comparative studies of lasers to treat IH.
Ten case series reported on 1785 children who were treated with PDL (Table 32). One Korean study (good quality for harms reporting) treated 47 superficial or mixed IH in 40 patients monitored for hyper- and hypo- pigmentation, skin atrophy, hypertrophic scarring, and ulceration during treatment.223 The only adverse event noted in this study was hyperpigmentation in two patients with superficial IH. The final assessment in this study was at the end of treatment so no long term follow information was available. A fair quality case series reported on PDL treatment for 65 children with ulcerated IH.227 There were no cases of the predefined complications of hypo- or hyperpigmentation or epidermal textural changes. Some scarring occurred in an unknown number of patients that was comparable to scarring associated with healing of conservative treatment. Another fair quality case series of hand hemangiomas noted atrophy, pigment change, ulceration and scarring.230 The most frequently reported harms were hyperpigmentation (1% to 14% in four studies221,223–225) and hypopigmentation (0–25% in five studies221,223–225,228). Ulceration was also noted in three studies. Two studies reported no adverse events,222,226 and another reported no permanent side effects but cases of hyper and hypopigmentation.225
Table 32
Adverse effects in case series of laser treatments for IH.
One thousand and seven children received treatment with Nd:YAG lasers reported in four case series. The most frequently reported adverse events from one large case series with 684 children included skin burn (11%), infection (6.6%), and scarring (4.4%).232 Another larger study with 160 participants reported complications including delayed healing, postoperative infection and scarring in 10 percent of their patients.231 A single case series of 31 patients with subglottic IH noted one case of respiratory distress related to the ventilation system.237
Table 33 outlines harms reported in surgical case series and in one comparative study of cryosurgery.220 Dehiscence rates ranged from 1.4 percent to 5.5 percent in five studies,238,240,246,248,251 and single cases of postoperative trauma-related wound dehiscence were reported in an additional three studies.238,241,243 Postoperative infections were noted in two studies.240,249 Scarring, skin necrosis, and alopecia were also noted in two reports. Other complications including facial paresis, permanent palsy, hematoma, intraoperative bleeding, cellulitis, hypopigmentation were reported in a single study each. One study reported no adverse events.247 One larger series of 127 patients with lip IH treated with liquid nitrogen cryotherapy reported five cases of hypopigmentation and three cases of hemorrhage and ulceration. Labial mucoceles were noted in three children 3 years after treatment.244 Harms reported in one study of cryosurgery included scarring in treated and untreated lesions.220
Table 33
Adverse effects in case series of surgical treatments for IH.
- Natural History of Untreated IH and Adverse Outcomes of Untreated IH
- Evidence for Association of Cutaneous IH and Occult IH
- Results of Literature Searches for Key Questions
- Effectiveness and Harms of Imaging Modalities
- Effectiveness and Harms of Corticosteroids or Beta-Blockers
- Effectiveness and Harms of Drugs Administered After the Failure of Corticosteroids or Beta-Blockers
- Effectiveness and Harms of Surgical Interventions
- Results - Diagnosis and Management of Infantile HemangiomaResults - Diagnosis and Management of Infantile Hemangioma
- Applicability Tables - Diagnosis and Management of Infantile HemangiomaApplicability Tables - Diagnosis and Management of Infantile Hemangioma
Your browsing activity is empty.
Activity recording is turned off.
See more...