NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Carson S, Lee N, Thakurta S. Drug Class Review: Newer Antihistamines: Final Report Update 2 [Internet]. Portland (OR): Oregon Health & Science University; 2010 May.
This publication is provided for historical reference only and the information may be out of date.
Overview
Literature searches for Update 2 identified 1754 new citations. We received dossiers from the manufacturers of azelastine, desloratadine, and levocetirizine. By applying the eligibility and exclusion criteria to titles and abstracts of all identified citations, we obtained full-text copies of 140 citations. After re-applying the criteria for inclusion, we ultimately included 61 publications, representing 58 unique studies. See Appendix D for a list of excluded studies and reasons for exclusion at this stage. Figure 1 shows the flow of study selection.
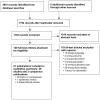
Figure 1
Results of literature search for Update 2a. a A modified PRISMA flow diagram was used.
Key Question 1. For outpatients with seasonal or perennial allergic rhinitis or urticaria, do newer antihistamines differ in effectiveness?
Summary of findings
Adults
Seasonal allergic rhinitis
- Eleven short-term trials (1 good quality, 1 fair) showed no significant difference in comparisons of cetirizine to fexofenadine and loratadine, fexofenadine to loratadine and desloratadine, levocetirizine to loratadine, and azelastine nasal spray to desloratadine and olopatadine nasal spray.
- Two fair-quality trials found azelastine nasal spray superior to oral cetirizine for reduction in symptoms and quality of life.
- Quality of life was better with fexofenadine than loratadine in 1 fair-quality study.
Perennial allergic rhinitis
- Two head-to-head trials (1 poor quality, 1 fair) showed no significant difference in reduction in symptoms with levocetirizine compared with loratadine and desloratadine.
- Two fair-quality 6-month trials of levocetirizine 5 mg showed improved quality of life at 6 months relative to placebo.
- Ten placebo-controlled trials demonstrated efficacy for azelastine nasal spray, cetirizine, desloratadine, levocetirizine, and loratadine, but did not provide information about comparative effectiveness.
Urticaria
- Loratadine was superior to cetirizine for reduction in symptoms in 2 fair-quality trials. Response (defined as asymptomatic) rates were higher with loratadine, but the differences were not statistically significant.
- Levocetirizine was superior to desloratadine for symptom reduction in 1 fair-quality trial, but there was no difference in quality of life.
- Cetirizine was more efficacious than fexofenadine in 1 fair-quality trial limited by a high dropout rate and no intention-to-treat analysis.
Children
Seasonal allergic rhinitis
- No head-to-head studies were identified.
- Placebo-controlled trials showed efficacy for cetirizine and fexofenadine.
- Cetirizine and loratadine were similarly efficacious compared with first-generation antihistamines.
Perennial allergic rhinitis
- One fair-quality study suggested that cetirizine may be more efficacious than loratadine.
- Cetirizine was superior to levocetirizine for symptoms in 1 fair-quality study, but there was no difference in quality of life.
- There was insufficient evidence for other drug comparisons.
Urticaria
- No head-to-head studies were identified.
- Cetirizine was similar to oxatomide for efficacy in children ages 2 to 6 years in 1 fair-quality trial.
- Cetirizine prevented urticaria in atopic children over 18 months in 1 fair-quality trial.
Detailed Assessment
Adults
Seasonal allergic rhinitis
Direct evidence
Eleven head-to-head trials with a duration of 2 weeks or longer assessed efficacy in adults with seasonal allergic rhinitis (Tables 2 and 3; Evidence Tables 1 and 2).26–36 The trials varied in country, season, number of patients, and baseline Total Symptom Score. One trial was of 4 weeks duration and the rest were of 2 weeks duration. One trial was rated good quality30 and the rest were fair.
Table 2
Head-to-head trials in adults with seasonal allergic rhinitis.
Table 3
Total Symptom Score change from baseline in head-to-head trials in adults with seasonal allergic rhinitis.
Most studies found no significant difference between newer antihistamines in the change from baseline in Total Symptom Score, with few exceptions (Table 3). A comparison of azelastine nasal spray to oral cetirizine 10 mg found a greater reduction in Total Symptom Score with azelastine (29.3% compared with 23.0%; P=0.015).30 One other trial compared azelastine nasal spray to cetirizine and also found a greater reduction in Total Symptom Score with azelastine, although the difference was not statistically significant (23.9% compared with 19.6%; P=0.08).26 A trial of loratadine 10 mg compared to fexofenadine 10 mg found a significantly greater reduction in Total Symptom Score with loratadine as rated by the patient (39% compared with 33%; P=0.019). The difference between treatment groups in investigator-rated change in Total Symptom Score was not statistically significant in this same trial (35% compared with 29%; P=0.063).33 Six studies also had a placebo arm, and all found the active treatment superior to placebo.
Three head-to-head trials measured quality of life outcomes, all using the Rhinoconjunctivitis Quality of Life Questionnaire.26, 30, 36 Quality-of-life scores at 2 weeks were better for patients taking azelastine nasal spray compared with cetirizine in 2 studies26, 30 and better with fexofenadine than loratadine in 1 study.36
Indirect evidence
Fifteen placebo-controlled trials demonstrated short-term efficacy of newer antihistamines in adults with seasonal allergic rhinitis, including 4 studies of desloratadine,37–40 2 of levocetirizine,41, 42 6 of azelastine nasal spray,43–48 and 3 of olopatadine nasal spray.49–51 Details of these studies are presented in Evidence Tables 1 and 2.
Comparisons of newer antihistamines to active controls revealed mixed results. Cetirizine was generally comparable to rupatadine (an antihistamine).52 Loratadine demonstrated few significant differences from ebastine (an antihistamine),53 mixed results compared to montelukast (a selective leukotriene receptor antagonist),54 and was generally less efficacious than rupatadine.55 In 1 trial,56 loratadine was as effective as clemastine.
Perennial allergic rhinitis
Direct evidence
We identified 2 head-to-head trials in adults with perennial allergic rhinitis (Evidence Tables 3 and 4).57, 58 One of these was not published,57 but results are available at ClinicalTrials.gov. In this 2-week trial, there was no significant difference between levocetirizine 5 mg and loratadine 10 mg in the change from baseline in Total Symptom Score.57 A 4-week placebo-controlled trial compared levocetirizine to desloratadine, both at 5 mg daily.58 Although both treatments improved total nasal symptom scores more than placebo, there was no significant difference between the treatment groups.
Indirect evidence
Ten placebo-controlled trials demonstrated efficacy in adults with perennial allergic rhinitis. Details of these studies are shown in Evidence Tables 3 and 4. We identified 2 trials of azelastine,59, 60 2 of cetirizine,61–63 3 of desloratadine,64–66 3 of levocetirizine (in 4 publications),67–71 and 1 of loratadine.72 Most of the efficacy trials were short term, however 2 trials of levocetirizine 5 mg reported improved quality of life compared with placebo at 6 months.67, 68
Urticaria
Direct evidence
Five head-to-head trials in adults with urticaria are shown in Table 4 and in Evidence Tables 5 and 6.73–77 Two fair-quality, head-to-head trials compared cetirizine to loratadine.74, 77 In 1 trial, loratadine reduced mean Total Symptom Score more than cetirizine. Response rates were higher with loratadine in both trials, but the difference was not statistically significant in one (63% compared with 45%)74 and the P value was not reported in the other (81% compared with 60%).77 The latter study reported that the number, size, and duration of lesions was significantly improved in patients taking loratadine (P<0.05) and the mean score of pruritus was significantly greater with loratadine (P<0.05), but data were not given.
Table 4
Head-to-head trials in adults with urticaria.
One trial compared cetirizine to fexofenadine. Cetirizine 10 mg daily was more efficacious than fexofenadine 180 mg daily at 28-day follow-up.75 This study was limited by an attrition rate of 16%, and data were presented only for those completing the study.
Two head-to-head trials compared levocetirizine to another newer antihistamine.73, 76 A trial of 886 adults with urticaria compared mean pruritus score of levocetirizine 5 mg compared with desloratadine 5 mg after 4 weeks of treatment.76 Levocetirizine decreased pruritus severity significantly more than desloratadine after 1 week, the primary outcome. Mean symptom scores were improved more with levocetirizine. Levocetirizine was also significantly better than desloratadine on patients’ global satisfaction at 1 week and 4 weeks, and on investigators’ global satisfaction at 1 week, but not on endpoint. Quality-of-life was assessed, and was improved in both treatment groups, but no analysis was done. In a crossover trial, 45 patients who had achieved complete symptomatic control with cetirizine 10 mg after 6 weeks were then switched to levocetirizine 5 mg for an additional 6 weeks.73 Wheal and flare response was similar with both drugs, but the itch response was better with cetirizine in 70% of patients. This study was open-label and had a high dropout rate.
Indirect evidence
Nine placebo-controlled trials examined efficacy in adults with chronic idiopathic urticaria.78–86 Two of these were rated poor quality and are listed in Appendix F.85, 86 Of the 7 fair- or better-quality trials, 4 included desloratadine 5 mg,78, 81, 83, 84 2 included levocetirizine 5 mg,80, 82 and 1 included fexofenadine 180 mg.79 All found the active treatment group superior to placebo for reducing symptoms. Indirect comparisons that can be made from these studies were limited due to differences in outcome measures and reporting. Improved quality-of-life was reported with desloratadine and levocetirizine compared with placebo in 2 studies, both using the validated Dermatology Life Quality Index.78, 80
In a 4-week trial of 188 patients,87 cetirizine had a faster onset than the first-generation antihistamine hydroxyzine but was effective in a similar proportion of patients.
A search for literature on the efficacy or effectiveness of newer antihistamines in other types of urticaria in adults identified only poor-quality studies.88–92
Children
Seasonal allergic rhinitis
Ten studies examined the efficacy of newer antihistamines among children (Evidence Tables 7 and 8), and 2 of these studies were of poor quality (See Appendix F for poor-quality studies).93, 94 One head-to head study of azelastine nasal spray compared with olopatadine nasal spray enrolled both adolescents and adults, but the results for children were not reported separately.34 The results of placebo-controlled trials of cetirizine95–99 and fexofenadine100 demonstrated significant improvements in symptoms with the study drug compared with placebo.
Active-control studies compared cetirizine101 and loratadine102 to first-generation antihistamines, with no significant differences between groups. Jordana and colleagues103 found that fluticasone nasal spray was more efficacious than loratadine for nasal symptoms, but there were no significant differences for eye symptoms.
Perennial allergic rhinitis
Eleven studies (see Table 5 and Evidence Tables 9 and 10) were identified which examined the efficacy of newer antihistamines among children with perennial allergic rhinitis,99–101, 104–111,1 of which was of poor quality. 103 Two studies examined children 2 to 6 years old,110, 112 most examined children 6 to 12 or 14 years old. Two studies primarily focused on adults, but included participants 12 years of age and older.55, 113 These studies are presented with the adult studies, as data were not stratified by age group to allow for examination of adolescents only.
Table 5
Outcomes from trials in children with perennial allergic rhinitis.
Inclusion criteria generally required a positive response to a skin test for house-dust mite allergy or other non-seasonal respiratory allergens along with a clinical history consistent with perennial allergic rhinitis. Children with major systemic illnesses were excluded.
Two head-to-head trials were identified. One trial compared cetirizine to loratadine among children 2 to 6 years of age.110 The primary outcome was the histamine skin prick test and cetirizine produced greater inhibition of the wheal response than loratadine (P<0.001). Both drugs produced improvements in parent- and investigator-assessed symptoms, with loratadine significantly more efficacious than cetirizine (P<0.001) for parent assessment of rhinorrhea, sneezing, nasal obstruction, and nasal pruritus. No significant differences were noted between groups in investigator-assessed global evaluation score or in nasal eosinophil count.
The second head-to-head trial compared cetirizine to levocetirizine over 12 weeks in 80 children ages 6 to 12 years with perennial allergic rhinitis.114 Both drugs improved Total Symptom Score and quality of life as measured by the Pediatric Rhinoconjunctivitis Quality of Life Questionnaire compared with placebo. There was significantly more improvement in Total Symptom Score at 8 and 12 weeks with cetirizine, but no difference between groups in improvement in quality of life.
In 3 studies with active controls, cetirizine improved symptoms compared with placebo arms and compared with ketotifen and oxatomide.109 Cetirizine was comparable to montelukast in 1 study,107 but similar in efficacy in another.112 Three fair-quality, placebo-controlled studies104, 105, 108 found cetirizine efficacious for nasal symptoms, particularly at a dosage of 10 mg daily (either at bed time or divided doses twice daily) for children 6 to 12 years.
A single study examining loratadine noted it to be efficacious at a dosage of 5 to 10 mg daily when compared to placebo.111 One study found azelastine nasal spray efficacious compared to placebo at 6 weeks.115 There were no data on any of the other newer antihistamines in children.
Urticaria
Two studies examined the efficacy of newer antihistamines for the treatment of urticaria in children (Evidence Tables 11 and 12). In 1 study examining the efficacy of cetirizine compared with oxatomide in children 2 to 6 years of age with chronic idiopathic urticaria,116 no significant differences were noted between groups. The other study examined the efficacy of cetirizine in preventing acute urticaria among young children with atopic dermatitis who were at high risk of acute urticaria.117 Efficacy was demonstrated during the 18-month treatment period in this placebo-controlled, randomized study, but positive effects did not persist after treatment was stopped.
Key Question 2. For outpatients with seasonal or perennial allergic rhinitis or urticaria, do newer antihistamines differ in harms?
Summary of findings
Adults
- Total withdrawals and withdrawals due to adverse events were low with cetirizine, levocetirizine, loratadine, desloratadine, fexofenadine, azelastine, and olopatadine and were comparable to rates observed with placebo.
- Sedation and headaches were commonly reported adverse events.
- First generation antihistamines (diphenhydramine, chlorpheniramine) were more sedating than newer generation antihistamines.
- Of the newer agents, cetirizine and levocetirizine were more sedating than loratadine and desloratadine, and possibly more sedating than fexofenadine.
- Rates of headaches were similar in cetirizine, loratadine, and fexofenadine-treated patients.
- Bitter taste or taste alteration and nasal discomfort were common with both azelastine and olopatadine nasal sprays relative to placebo.
- Direct evidence found higher rate of bitter taste and nasal discomfort with azelastine while indirect assessment of the evidence suggested minimal difference between the groups.
- One cohort study suggested a greater risk of cardiovascular events with cetirizine when compared to non-users. A nonsignificant increase in risk was observed with loratadine in this study. Eleven short-term trials detected no clinically relevant electrocardiogram changes.
Children
- There was insufficient evidence on comparative harms.
- Overall, newer antihistamines were well-tolerated in this population with low withdrawal rates due to adverse events.
- Minor neurologic and respiratory symptoms were frequently reported in both treatment and placebo groups. These included headaches, insomnia, nervousness, somnolence, and upper respiratory tract infections.
- Based on short-term fair evidence, newer antihistamines including cetirizine did not significantly prolong QTc interval.
Detailed assessment
Adults
In this update we identified 14 trials,38, 40, 50, 58, 60, 68, 69, 76, 78, 80, 82–84, 118 2 observational studies,119, 120 and 1 systematic review121 of adults only, while 16 trials26, 28, 34, 43, 45–49, 51, 65, 69, 77, 122–124 included mixed populations of children and adults. Because most patients in the 16 trials were adults, we included results from these trials here.
Total withdrawals and withdrawals due to adverse events
Withdrawal rates due to adverse events were generally low, in the range of 2% to 5% for azelastine, olopatadine, levocetirizine, desloratadine, and fexofenadine. Serious adverse events were rare. One patient taking fexofenadine had an asthma attack requiring hospitalization 79 and 1 patient taking desloratadine and 3 patients using placebo had heart rhythm disorder that did not require study discontinuation.65
Commonly reported adverse events
Four observational studies125–128 and 1 systematic review121 from this update provided additional data on adverse effects of long-term use of newer antihistamines.129–132 Three of these studies 121, 127, 128 assessed effects of newer antihistamines in pregnancy and are discussed in Key Question 3. Sedation was the main focus of 6 studies,125, 126, 129–132 and the overall incidence of sedation was both variable and low. Results from the above studies and 8 new head-to-head trials26, 28, 34, 57, 76, 77, 133, 134 are discussed below in addition to publications from previous updates.
First-generation antihistamines compared with newer antihistamines
A fair-quality meta-analysis,135 a 1-week active-control trial,123 and a 2-week placebo-controlled trial48 suggested that both first-generation and newer antihistamines resulted in sedation compared to placebo, and that first-generation agents diphenhydramine or chlorpheniramine caused more sedation than cetirizine, fexofenadine, loratadine, desloratadine, and azelastine nasal spray. A fair-quality cohort study resulted in more claims for serious injury with diphenhydramine compared with loratadine.129
Cetirizine compared with loratadine
A fair-quality cohort study showed that cetirizine had significantly higher odds of sedation than loratadine and fexofenadine; loratadine was not significantly different from fexofenadine.130 Similar results were seen in a fair- to poor-quality trial where cetirizine produced greater sedative effects and adverse effects on motivation than loratadine.136 Only 1 head-to-head trial did not find significant differences in adverse events in patients using cetirizine, loratadine, and fexofenadine.137
No trial evidence was found on tolerance to the sedation with antihistamines. The labeling for cetirizine included a statement of caution when driving a car or operating potentially dangerous machinery and to avoid concomitant use of alcohol or other central nervous system depressants, as an additional reduction in alertness or performance may occur.
Levocetirizine compared with desloratadine
A fair-quality observational study125 compared the risk of drowsiness and sedation between levocetirizine and desloratadine using prescription-event monitoring in the UK (N=24 195). The first occurrence of sedation was low for both groups but was significantly lower with desloratadine (0.08%; P<0.0001) than levocetirizine (0.37%). Crude odds ratio for levocetirizine and sedation was almost 5 times greater (odds ratio, 4.9; 95% CI, 2.40 to 10.02). Adjusting for gender, indication, and previous antihistamine use, the risk remained similar. Patients with or without asthma were assessed and patients without asthma were 6 times more likely to report sedation than patients with asthma (odds ratio, 6.75; 95% CI, 2.37 to 19.22).
Results from 2 smaller head-to-head studies comparing levocetirizine with desloratadine concurred with the findings above.76, 133 In these 2 head-to-head trials, common adverse events included headaches, somnolence, and dry mouth. Four additional smaller head-to-head trials comparing cetirizine, levocetirizine, and loratadine also confirmed that levocetirizine and cetirizine were more sedating (or having nervous system disorders) than loratadine.57, 77, 134, 138
Cetirizine compared with fexofenadine
One fair- to poor-quality head-to-head trial75 compared cetirizine and fexofenadine (83% of randomized analyzed). Cetirizine-treated patients noted more drowsiness complaints than fexofenadine but no statistical difference was found (7.7% compared with 4.5%, P=0.29). No significant differences were found for constipation, abdominal pain, epigastric pain, or cough.
Desloratadine compared with fexofenadine
Rates for somnolence and upper respiratory tract infection were comparable and low in both groups (≤1%).28 Compared with desloratadine, a few more fexofenadine-treated patients withdrew from a 2-week study primarily because of headaches (3.8% compared with 1.0%).
Astelin nasal spray compared with olopatadine nasal spray
In a 16-day head-to-head trial, total withdrawal from treatment groups was low (overall 2.5%). This trial reported adverse events for all screened patients which included patients during run-in phase. Of the commonly reported events, azelastine-treated patients reported more frequent bitter taste (19.7% compared with 12.2%; placebo, 1.7%) and nasal discomfort (3.7% compared with 1.7%; placebo, 1.7%) than in olopatadine-treated patients. One case of nasal ulceration with azelastine occurred at day 30; this resolved in 7 days.34
We identified 3 placebo-controlled trials of azelastine spray and 3 placebo-controlled trials of olopatadine spray.43, 46, 49–51, 60 Indirectly, azelastine 0.137 mg and olopatadine 0.4% exhibited similar rates of bitter taste (range: azelastine, 5.8% to 9.5%; olopatadine, 5.8% to 8.7%; placebo, 0% to 2.2%). Patients using higher-strength olopatadine 0.6% reported the highest incidence of bitter taste (range: 9.2% to 16.1%).
One of the azelastine trials also evaluated a newer formulation product, Astepro®.43 This trial found less bitter taste with the new formulation than with the standard product at both the low and high doses (Astepro® 1 spray twice daily, 5.8% compared with azelastine 1 spray twice daily, 9.5% compared with placebo, 1.5%; Astepro 2 sprays twice daily, 6.8% compared with azelastine 2 sprays twice daily, 8.0% compared with placebo, 2.2%).
Azelastine nasal spray compared with cetirizine
Total withdrawal rate and withdrawals due to adverse events were low for azelastine nasal spray users and for cetirizine-treated patients over a 2-week study period (Evidence Table 1).26 More reports of bitter taste were however associated with azelastine spray than cetirizine (7.7% compared with 0%). Rates of somnolence, headaches, epistaxis, and sore throat were not significantly different between treatment groups and occurred in <2% of patients.
Electrocardiogram changes
Prolongation of the QT interval is a concern with this class of agents since the withdrawal of terfenadine and astemizole.
A fair-quality nested-case control cohort study132 using the UK-based General Practice Research Database reported that for 5 newer-generation antihistamines combined, a 4.2 times higher risk of ventricular arrhythmias compared to non use. Astemizole posed the highest risk (relative risk, 19.0); the relative risk for cetirizine was 7.9 (95% CI, 1.6 to 39.3) and loratadine 3.2 (95% CI, 0.4 to 26.9). The safety and tolerability of fexofenadine (an active metabolite of terfenadine) was shown in over 16 638 patients in a UK Prescription-event monitoring cohort131 as well as in a placebo-controlled trial where no significant electrocardiogram changes were noted.79 A total of 11 studies37, 53, 65, 66, 79, 81–83, 113, 139–141 noted no clinically significant electrocardiogram changes compared with the placebo group. One patient using desloratadine (0.2%) and 3 placebo-treated patients (0.5%) reported heart and rhythm disorders; patients did not require study withdrawal.65
Children
Total withdrawals and withdrawals due to adverse events
Overall, newer antihistamines were well tolerated in children. Across 16 placebo-controlled trials95, 98, 100, 104, 108, 112, 114–117, 144–149 that reported total withdrawals, up to 18.9% of patients treated with newer antihistamines (cetirizine, levocetirizine, desloratadine, azelastine, and fexofenadine) and up to 23% of children treated with placebo withdrew from the trials. Up to 3.1% of those treated with newer antihistamines withdrew as a result of an adverse event compared with up to 4.7% of placebo-treated patients.
Commonly reported adverse events
In this update, we identified 7 additional trials76, 112, 114, 115, 145, 150, 151 and 1 observational study.152 Adverse events in studies of children are summarized Evidence Tables 9,76, 112, 114, 11513,152 20,145, 150 and 22.151 Adverse events reported from the last update are listed in Appendix E. There were no major events reported. In the only head-to-head trial in children,110 2 adverse events were reported in the cetirizine group, with none reported in the loratadine group (N=80). One participant developed somnolence and irritability, the other a generalized rash. These 2 adverse events necessitated participant withdrawal from study. Three observational studies143, 152, 153 presented data on adverse events but were of poor quality (Appendix F).
Minor neurologic and respiratory symptoms were the most common adverse events, particularly headache, insomnia, nervousness, somnolence, and upper respiratory tract infections with oral antihistamines. Rates varied widely, however, and adverse events were also very common among placebo groups. Three placebo-controlled trials112, 114, 151 reported somnolence in <2% to 10% of patients treated with cetirizine compared with <2% patients treated with placebo. One trial150 of desloratadine reported somnolence in 5.3% of patients compared with 7.3% of those receiving placebo.
Upper respiratory tract infections were observed in 3 trials145, 146, 151 with no significant difference between active treatment and placebo (Evidence Tables 20, 9, and 22 respectively).
One trial evaluating azelastine nasal spray found increased incidence of pharyngitis (7.8% compared with 4.9%) and cough (4.7% compared with 1.6%) relative to placebo.115
Electrocardiogram changes
Nine studies examined the effects of newer antihistamines on electrocardiogram changes, particularly on the QT and QTc interval.99, 136, 145, 147–150, 154, 155 No study demonstrated significant prolongation of the QT interval with cetirizine,99, 144, 147–149 fexofenadine,144, 145 or desloratadine.150, 156 One poor-quality study examined concurrent use of cetirizine or loratadine and erythromycin estolate154 and noted no abnormality of the QT or QTc interval.
One head-to-head study examined the effects of terfenadine, astemizole, loratadine, and cetirizine on electrocardiogram among children with perennial allergic rhinitis.154 Erythromycin estolate was administered to all study participants, and a significant increase in QT interval was noted in the terfenadine group, but not in the other groups. The QTc interval, however, was not prolonged or different in any group.
Other
The Early Treatment of the Atopic Child (ETAC)117, 147, 157, 158 was a prospective, double-blind, parallel-group study examining the efficacy of cetirizine in preventing onset of asthma among children 12 to 24 months old with atopic dermatitis (Evidence Tables 22 and 23). Study participants were treated for 18 months and adverse events were assessed at the end of treatment. Although this study did not meet inclusion criteria for this report with respect to population characteristics (the study did not involve allergic rhinitis or urticaria), it was included in this paper because it provided long-term data on the safety of cetirizine in a large population of young children.
In the ETAC study, serious adverse events were less common with cetirizine (9.3%) than placebo (13.6%; P=0.053). Serious adverse events attributed to the study medication occurred in 1 child receiving cetirizine and 5 children receiving placebo. Hospitalization rates did not differ between the treatment groups (P=0.189). There were 10 accidental overdoses of study medications by study participants; 2 of these participants were receiving cetirizine. Symptoms and events (Evidence Table 22) were reported with similar frequency in cetirizine- and placebo-treated groups. Age-appropriate increases in height and weight were observed during the study period. No clinically relevant differences between groups for changes in electrocardiograms were observed, and cetirizine therapy was not associated with prolongation of the QTc interval in any participant.
Key Question 3. Are there subgroups of patients based on demographics (age, racial groups, gender), concomitant medications (drug-drug interactions), co-morbidities (drug-disease interactions or pregnancy), for which one newer antihistamine is more effective or associated with fewer harms?
Summary of findings
- Insufficient evidence was available to determine whether any of the newer antihistamines had an advantage in efficacy or harms based on age, gender, or race/ethnicity.
- Patients with allergic rhinitis with mild intermittent asthma or atopic dermatitis tolerated newer antihistamines similar to patients without these comorbidities.
- There was minimal increased risk of birth defects observed with H-1 receptor antagonists including cetirizine, fexofenadine, and loratadine.
Detailed assessment
Age, gender, race/ethnicity
No direct evidence was available to determine whether any antihistamine has an advantage in efficacy or harms for any gender or racial group.
Asthma
Three fair-quality placebo-controlled trials were identified in patients with allergic rhinitis and asthma (Evidence table 24).139, 159, 160 Patient assessment of asthma significantly improved on cetirizine compared with placebo in 2 studies,159, 160 however no improvement (or worsening) of pulmonary function tests occurred. Berger and colleagues139 examined desloratadine in patients with seasonal allergic rhinitis and asthma and found a significant decrease in total asthma symptom scores in the treatment group.
There were no significantly different adverse events reported in primarily adult patients with allergic rhinitis and asthma compared with patients without asthma. There were no reports of worsening asthma with active treatment; only 2 placebo-treated patients146 reported asthma aggravated. Most patients included in these studies had mild intermittent asthma and were likely not using inhaled corticosteroids. Two trials evaluated cetirizine,159, 160 levocetirizine (Evidence Table 3),68, 146 and azelastine nasal spray (Evidence Table 1).45, 48 One trial evaluated desloratadine.139 Commonly reported adverse events for oral antihistamines were headache, fatigue, nausea, dry mouth, and sedation. Nasal burning, bitter taste or altered taste, and epistaxis were observed more often in azelastine-treated patients than with placebo.
Atopic dermatitis
An 18-month, placebo-controlled trial studied levocetirizine in 510 children 12 to 24 months in age who had atopic dermatitis, allergy to grass pollen or house dust mites, and family history of allergies.151 The dose of levocetirizine was in the higher range (0.125 mg/kg twice daily; total daily dose ranged from 2.8 mg to 3.8 mg) but the overall withdrawal rates due to adverse events were low between levocetirizine and placebo (2.0% compared with 1.2%) and there were no significant differences in achieving developmental milestones in treatment groups.
About 96% of enrolled children reported at least 1 adverse event during the trial. The most commonly reported event was upper respiratory tract infections (levocetirizine, ~51% compared with placebo, ~50%). Worsening atopic dermatitis was low and occurred similarly between groups (levocetirizine, ~5% compared with placebo, ~6%). Febrile convulsions, however, were reported more often in levocetirizine-treated children than placebo (2.0% compared with 0.4%). Although the investigators suspected the convulsions were not study medication-related, they could not rule out the possibility and recommend that this be explored further. There was one 30-month-old child that developed lymphadenopathy and was diagnosed with acute lymphoblastic leukemia. The investigators judged that a relationship of study drug and this occurrence was unlikely.
Pregnancy
Rhinitis is one of the most common conditions during pregnancy, affecting more than 20% of pregnant women.161 However, women who are pregnant, lactating, or not using adequate birth control are excluded from clinical trials. Thus safety data must come solely from observational studies.
We identified 1 additional cohort study (N=1882) that evaluated cetirizine exposure in the first trimester of pregnant women.128 The findings from this observational study concurred with 5 other observational studies131, 162–165 and a meta-analysis,166 which found no significant increase risk in birth defects in women exposed to H-1 receptor blockers, including fexofenadine and loratadine (Evidence Tables 13 and 24).
Results from a systematic review with meta-analysis,121 a nested case-control study,127 and data from the National Birth Defects Prevention Study,167 indicated that loratadine exposure during pregnancy does not significantly increase risk of hypospadias in male infants. Additional analyses of these 2 studies121, 167 showed that non-sedating and sedating antihistamines did not significantly increase the risk of hypospadias.
- Overview
- Key Question 1. For outpatients with seasonal or perennial allergic rhinitis or urticaria, do newer antihistamines differ in effectiveness?
- Key Question 2. For outpatients with seasonal or perennial allergic rhinitis or urticaria, do newer antihistamines differ in harms?
- Key Question 3. Are there subgroups of patients based on demographics (age, racial groups, gender), concomitant medications (drug-drug interactions), co-morbidities (drug-disease interactions or pregnancy), for which one newer antihistamine is more effective or associated with fewer harms?
- Results - Drug Class Review: Newer AntihistaminesResults - Drug Class Review: Newer Antihistamines
Your browsing activity is empty.
Activity recording is turned off.
See more...
