NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
General Concepts
Clinical Manifestations
The genera Escherichia, Klebsiella, Enterobacter, Serratia, and Citrobacter (collectively called the coliform bacilli) and Proteus include overt and opportunistic pathogens responsible for a wide range of infections. Many species are members of the normal intestinal flora. Escherichia coli (E coli) is the most commonly isolated organism in the clinical laboratory.
Enteric Infections: E coli is a major enteric pathogen, particularly in developing countries. The principal groups of this organism responsible for enteric disease include the classical enteropathogenic serotypes (EPEC), enterotoxigenic (ETEC), enteroinvasive (EIEC), enterohemorrhagic (EHEC), and enteroggregative (EAEC) strains which are described in detail in Chapter 25.
Nosocomial Infections: Coliform and Proteus bacilli currently cause 29 percent of nosocomial (hospital-acquired) infections in the United States. In order of decreasing frequency, the major sites of nosocomial infection are the urinary tract, surgical sites, bloodstream, and pneumonias. This group of nosocomial pathogens are responsible for 46% of urinary tract and 24% of surgical site infections, 17% of the bacteremias, and 30% of the pneumonias. E coli is the premier nosocomial pathogen.
Community-Acquired Infections: E coli is the major cause of urinary tract infections, including prostatitis and pyelonephritis; Proteus, Klebsiella,and Enterobacter species are also common urinary tract pathogens. Proteus mirabilis is the most frequent cause of infection-related kidney stones. Klebsiella pneumoniae causes a severe pneumonia; K rhinoscleromatis causes rhinoscleroma; and K ozaenae is associated with ozena, an atrophic disease of the nasal mucosa.
Structure, Classification, and Antigenic Types
The coliforms and Proteus are Gram negative bacilli. All genera except Klebsiella are flagellated. Some strains produce capsules. Virulence often depends on the presence of attachment pili (which can be characterized by specific hemagglutinating reactions). Sex pili also may be present. The major classes of antigens used in defining strains are H (flagellar), O (somatic), and K (capsular).
Pathogenesis
E coli enteropathogens have diverse mechanisms for disease production which include different toxins and colonization factors (see Ch. 25 ). Specific serotypes of coliforms and Proteus with particular virulence factors often preferentially infect specific extraintestinal sites. E coli bacilli in extraintestinal infections have soluble and cell-bound hemolysins, siderophores, capsules, and adherence pili.
Host Defenses
Coliforms and Proteus species rarely cause extraintestinal disease unless host defenses are compromised. Disruption of the normal intestinal flora by antibiotic therapy may allow resistant nosocomial strains to colonize or overgrow. The skin and mucosae may be breached by disease, trauma, operation, venous catheterization, tracheal intubation, etc. Immunosuppressive therapy also increases the risk of infection.
Epidemiology
The epidemiology of coliform and Proteus infections involves many reservoirs and modes of transmission. The infecting organism may be endogenous or exogenous. Transmission may be direct or indirect; vehicles include hospital food and equipment, intravenous solutions, and the hands of hospital personnel. Nosocomial strains progressively colonize the intestine and pharynx with increasing length of hospital stay, resulting in an increased risk of infection.
Diagnosis
The clinical picture depends on the site of infection; diagnosis relies on culturing the organism and on biochemical and/or serologic identification. A variety of phenotypic (i.e., biotyping, serotyping, antibiograms, bacteriocin and phage typing) and genotypic (i.e., plasmid analysis, RFLP, ribotyping, and PCR) methods are used for epidemiological investigations.
Control
The most effective way to reduce transmission of nosocomial organisms is for all hospital personnel to wash hands meticulously after attending to each patient. Vaccines and hyperimmune sera are not currently available. Various antibiotics are the backbone of treatment; drug resistance (often multiple) due to conjugative plasmids is a major problem.
Introduction
The Gram-negative bacilli of the genera Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter,and Proteus(Table 26- 1) are members of the normal intestinal flora of humans and animals and may be isolated from a variety of environmental sources. With the exception of Proteus, they are sometimes collectively referred to as the coliform bacilli because of shared properties, particularly the ability of most species to ferment the sugar lactose.
Table 26-1
Taxonomy of Selected Coliform Bacilli and Proteus in Human Clinical Specimens.
Many of these microorganisms used to be dismissed as harmless commensals. Today, they are known to be responsible for major health problems worldwide. A limited number of species, including E coli, K pneumoniae, Enterobacter aerogenes, Enterobacter cloacae, S marcescens,and P mirabilis, are responsible for most infections produced by this group of organisms. The increasing incidence of the coliforms, Proteus, and other Gram-negative organisms in diseases reflects in part a better understanding of their pathogenic potential but more importantly the changing ecology of bacterial disease. The widespread and often indiscriminate use of antibiotics has created drug-resistant Gram-negative bacilli that readily acquire multiple resistance through transmission of drug resistance plasmids (R factors). Also, development of new surgical procedures, health support technology, and therapeutic regimens has provided new portals of entry and compromised many host defenses.
Clinical Manifestations
As opportunistic pathogens, the coliforms and Proteus take advantage of weakened host defenses to colonize and elicit a variety of disease states (Fig. 26-1). Together, the many disease syndromes produced by these organisms are among the most common infections in humans requiring medical intervention.

Figure 26-1
Sites of colonization and extraintestinal disease production by the coliforms and Proteus.
Enteric Infections
The role of E coli as a major enteric pathogen, particularly in developing countries, is discussed in detail in Ch. 25.However, the different types of E coli associated with enteric infections and which are classified into five groups according to their virulence properties are briefly described here: Enteropathogenic (EPEC) serotypes in the past were associated with serious outbreaks of diarrhea in newborn nurseries in the US. They remain an important cause of acute infantile diarrhea in developing countries. Disease is rare in adults. Enteroinvasive (EIEC) types produce disease resembling shigellosis in adults and children. Enterotoxigenic (ETEC) types are a major cause of traveler's diarrhea, and of infantile diarrhea in developing countries. Enterohemorrhagic E. coli(EHEC) occur largely as a single serotype (O157:H7) causing sporadic cases and outbreaks of hemorrhagic colitis characterized by bloody diarrhea. EHEC also may cause hemolytic uremic syndrome (HUS), an association of hemolytic anemia, thrombocytopenia, and acute renal failure. Enteroaggregative (EAEC) types exhibit a characteristic aggregative pattern of adherence and produce persistent gastroenteritis and diarrhea in infants and children in developing countries.
Nosocomial Infections
The etiology of nosocomial infections has markedly changed during past decades. Streptococci were the major nosocomial pathogens in the preantibiotic era. However, following the introduction and use of sulfonamides and penicillin, Staphylococcus aureus became the predominant pathogen in the 1950's. Aerobic gram negative rods gained prominence as nosocomial pathogens with widespread use of aminoglycosides and first generation cephalosporins through the early 1970's. Subsequent widespread use of broad spectrum cephalosporins was associated with changes in the frequency and etiology of nosocomial infections into the 1980's with the trend towards certain gram-positive pathogens. For example, in nosocomial bloodstream infections from 1980 to 1989 marked increases in the incidence of coagulase-negative staphylococci, S. aureus, enterococci, and Candida albicans infections occurred.
The coliforms and Proteus were responsible for 29 percent of nosocomial (hospital-acquired) infections in the United States from 1990 through 1992 based on data from hospitals participating in the National Nosocomial Infections Survey (NNIS) (Table 26-2). Estimates of nosocomial infections in US hospitals suggest that about 5 percent of the estimated 40 million annual admissions, or 2 million patients, had at least one nosocomial infection. Thus, the coliforms and Proteus probably are responsible for hospital-acquired infections in approximately 600,000 patients each year. Aside from the enormous cost measured in human life, nosocomial infections prolong the duration of hospitalization by an average of 4 days and increase the cost of medical care by $4.5 billion a year in 1992 dollars.
Table 26-2
Frequency of Selected Pathogens Causing Nosocomial Infections a.
The highest numbers of nosocomial infections in the NNIS occur in surgical and medicine services. Among surgical patients, highest rates of nosocomial infections occur with surgery on the stomach (21%) and bowel (19%), craniotomies (18%), coronary artery bypass graft procedures (11%) and other cardiac surgery (10%). High rates also are observed with burn (15%) and high-risk nursery patients (14%). In order of decreasing frequency, the major sites of nosocomial infection are the urinary tract, surgical sites, bloodstream, and lower respiratory tracts. The coliforms and Proteus were responsible for 46% of urinary tract and 24% of surgical site infections, 17% of the bacteremias, and 30% of the pneumonias from 1990 through 1992. Escherichia coli, the predominant nosocomial pathogen, is the major cause of infection in the urinary tract and is common in other body sites. Staphylococcus aureus and Pseudomonas aeruginosa are currently the most common pathogens in nosocomial pneumonias, followed by Enterobacter and Klebsiella. Coagulase-negative staphylococci have replaced E coli as the predominant pathogen in primary bloodstream infections. The major causes of surgical site infections are S aureus, coagulase- negative staphylococci, and enterococci.
Other coliform bacilli and Proteus have been incriminated in various hospital-acquired infections. Klebsiella, Enterobacter,and Serratia species are frequent causes of bacteremia at some medical centers and also are frequently involved in infections associated with respiratory tract manipulations, such as tracheostomy and procedures using contaminated inhalation therapy equipment. Klebsiella and Serratia species commonly cause infections following intravenous and urinary catheterization and infections complicating burns. Proteus species frequently cause nosocomial infections of the urinary tract, surgical wounds, and lower respiratory tract. Less frequently, Proteus species cause bacteremia, most often in elderly patients. A series of nationwide outbreaks of bacteremia (1970 to 1971 and 1973), caused by contaminated commercial fluids for intravenous injections, involved Enterobacter cloacae, Enterobacter agglomerans,and C freundii.
The role of Citrobacter species in human disease is not as great as that of the other coliforms and Proteus. Citrobacter freundii and C diversus (C koseri) have been isolated predominantly as superinfecting agents from urinary and respiratory tract infections. Citrobacter septicemia may occur in patients with multiple predisposing factors; Citrobacter species also cause meningitis, septicemia, and pulmonary infections in neonates and young children. Neonatal meningitis produced by C diversus, while uncommon, is associated with a very high frequency of brain abscesses, death, and mental retardation in survivors. Although E coli and group B streptococci cause most cases of neonatal meningitis, the most common cause of brain abscesses in neonatal meningitis is P mirabilis.
Immunocompromised patients often develop non-hospital-acquired infections with coliforms. For example, group B streptococci and E coli are responsible for most cases of neonatal meningitis, with the latter accounting for about 40 percent of cases. Infections seen in cancer patients with solid tumors or malignant blood diseases frequently are caused by E coli, Klebsiella, Serratia,and Enterobacter species. Such infections often have lethal course. Individuals who are immunosuppressed by therapy (e.g., cancer patients or transplant recipients) or by congenital defects of the immune system may develop Klebsiella, Enterobacter, and Serratia infections. Many additional factors such as diabetes, trauma, and chronic lung disease may predispose to infection by coliforms and other microbes.
Community-Acquired Infections
The coliform organisms and Proteus species are major causes of diseases acquired outside the hospital; many of these diseases eventually require hospitalization. Escherichia coli causes approximately 85 percent of cases of urethrocystitis (infection of the urethra and bladder), about 80 percent of cases of chronic bacterial prostatitis, and up to 90 percent of cases of acute pyelonephritis (inflammation of the renal pelvis and parenchyma). Approximately one half of females have had a urinary tract infection by their late twenties due to E coli from their fecal flora. Proteus, Klebsiella, and Enterobacter species are among the other organisms most frequently involved in urinary tract infections. Proteus, particularly P mirabilis, is believed to be the most common cause of infection-related kidney stones, one of the most serious complications of unresolved or recurrent bacteriuria.
Klebsiella was first recognized clinically as an agent of pneumonia. Klebsiella pneumoniae accounts for a small percentage of pneumonia cases; however, extensive damage produced by the organism results in high case fatality rates (up to 90 percent in untreated patients). Klebsiella rhinoscleromatis is the agent of rhinoscleroma, a chronic destructive granulomatous disease of the respiratory tract that is endemic in Eastern Europe and Central America. Klebsiella ozaenae, a rare cause of serious infection, is classically associated only with ozena, an atrophy of nasal mucosal membranes with a mucopurulent discharge that tends to dry into crusts; however, recent studies indicate that the organism may cause various other diseases including infections of the urinary tract, soft tissue, middle ear, and blood.
Distinctive Properties
Structure and Antigens
The generalized structure and antigenic composition of coliform bacilli, as well as of Proteus and other members of the family Enterobacteriaceae, are depicted schematically in Figure 26-2. A more detailed figure of the structure is presented in Chapter 2. The major antigens of coliforms are referred to as H, K, and O antigens. The coliforms and Proteus are divided into serotypes on the basis of combinations of these antigens; different serotypes may have different virulence properties or may preferentially colonize and produce disease in particular body habitats. The H antigen determinants are flagellar proteins. Escherichia coli, Enterobacter, Serratia, Citrobacter,and Proteus organisms are peritrichous (i.e., they have flagella that grow from many places on the cell surface). Klebsiella species are nonmotile and nonflagellated and thus have no H antigens.

Figure 26-2
Structure and antigenic composition of coliforms and Proteus species.
Some strains of coliform and Proteus species have pili (fimbriae). Pili are associated with adhesive properties and, in some cases, are correlated with virulence. Different pilial colonization factors generally are detectable as hemagglutinins that can be distinguished by the type of erythrocyte agglutinated and by the susceptibility of the hemagglutination to inhibition by the sugar mannose. Sex pili, which have receptors for ‘male’ specific bacterial viruses and are genetically determined by extrachromosomal plasmids, are important in coliform ecology and in the epidemiology of diseases produced by coliforms and Proteus species in that sex pili are involved in genetic transfer by conjugation (e.g., chromosome-mediated and plasmid-mediated drug resistances or virulence factors).
Major Surface Antigens
K antigens (capsule antigens) are components of the polysaccharide capsules. Certain K antigens (e.g., K88 and K99 of E coli) are pilus-like proteins. The K antigens often block agglutination by specific O antisera. In the past, K antigens routinely were differentiated into A, L, and B groups on the basis of differences in their lability to heat; however, these criteria are subject to difficulties that make the distinction tenuous. Some Citrobacter serotypes produce Vi (virulence) antigen, a K antigen also found in Salmonella typhi. Species of Proteus, Enterobacter,and Serratia apparently have no regular K antigens. However, the K antigens are important in the pathogenesis of some coliforms. A diffuse slime layer of variable thickness (the M antigen) also may be produced but, unlike the K antigens, it is nonspecific and is serologically cross-reactive among different organisms.
The outer membrane of the bacterial cell wall of these species contains receptors for bacterial viruses and bacteriocins (plasmid-encoded, antibiotic like bactericidal proteins called colicins in E coli that are active against the same or closely related species). The outer membrane also contains lipopolysaccharide (LPS), of which the lipid A portion is endotoxic and the O (somatic) antigen is serotype specific. The serologic specificity of the O antigens is based on differences in sugar components, their linkages, and the presence or absence of substituted acetyl groups. Loss of the O antigen by mutation results in a smooth-to-rough transformation, which often involves changes in colony type and saline agglutination, as well as loss of virulence . Certain strains of P vulgaris (OX-19, OX-2, and OX-K) produce O antigens that are shared by some rickettsiae. These Proteus strains are used in an agglutination test (the Weil-Felix test) for serum antibodies produced against rickettsiae of the typhus and spotted fever groups (see Ch. 38).
Toxins
Enterotoxigenic strains of Klebsiella, Enterobacter, Serratia,Citrobacter, and Proteus also have been isolated from infants and children with acute gastroenteritis. The enterotoxins of at least some of these organisms are of the heat-labile and heat-stable types and have other properties in common with the E coli toxins (see Ch. 25). However, the importance of the coliforms and Proteus, other than E coli, in enteric infections is questionable
Pathogenesis
The process of disease production by coliforms is, in many cases, poorly understood. Production of disease by coliforms or Proteus species in extraintestinal sites often involves specific serotypes of the organisms and special virulence factors. For example, respiratory tract infections by K pneumoniae predominantly involve capsular types 1 and 2, whereas urinary tract infections often involve types 8, 9, 10, and 24. Similarly, only a few polysaccharide K antigens (types 1, 2, 3, 5, 12, and 13) of E coli are found with high frequency in urinary tract and other extraintestinal infections. These observations suggest that different serotypes may have specific pathogenicities. An alternative explanation is that such strains may simply be the most prevalent types in the normal gut flora.
There is good evidence for specific pathogenicity in E coli strains that cause extraintestinal infections (Table 26-3). Approximately 80 percent of E coli isolates involved in neonatal meningitis carry the K1 antigen, a fact attributable, at least in part, to the higher resistance to phagocytosis of K1-positive strains. Certain O antigens (O7 and O18) are found in combination with K1, usually in strains that are isolated from cases of neonatal bacteremia and meningitis and that show increased resistance to the bactericidal effects of serum complement. Interestingly, the E coli K1 antigen, composed of neuraminic acid, shows immune cross-reactivity with the group B meningococcal polysaccharide capsule.
Table 26-3
Virulence Factors of E coli Isolates from Extraintestinal Infections.
Escherichia coli strains isolated from extraintestinal infections often possess a number of properties not usually found in random fecal isolates. These include production of soluble and cell-bound hemolysins, the colicin V plasmid, production of the siderophores aerobactin and enterochelin, and special pilial antigens for adherence to target cells. The hemolysin kills host cells and makes iron more available by releasing hemoglobin-bound iron from lysed red cells. To strip iron from the host iron-binding proteins ( transferrin and lactoferrin), E coli produces siderophores of both the hydroxamate (aerobactin) and phenolate (enterochelin) types. Common or type 1 pili may mediate adherence to bladder cells; P-pili are virulence factors for strains causing pyelonephritis; S-pili, which recognize O-linked sialo-oligosaccharides of glycophorin A, are associated with meningitis and urinary tract infections. Certain afimbrial adhesions and outer membrane proteins also have been associated with urinary tract infections.
The enzyme urease, produced by Proteus, and to a lesser extent by Klebsiella species, is thought to play a major role in the production of infection-induced urinary stones. Urease hydrolyzes urea to ammonia and carbon dioxide. Alkalinization of the urine by ammonia can cause magnesium phosphate and calcium phosphate to become supersaturated and crystallize out of solution to form, respectively, struvite and apatite stones. Bacteria within the stones may be refractory to antimicrobial therapy. Large stones may interfere with renal function. The ammonia produced by urease activity may also damage the pithelium of the urinary tract.
Except in cases of bacteremia and other systemic infection, there is little evidence that endotoxin plays a role in most coliform and Proteus diseases. Humans with coliform bacteremia show many of the typical effects of endotoxin, including fever, depletion of complement, release of inflammatory mediators, lactic acidosis, hypotension, vital organ hypoperfusion, irreversible shock, and death.
Host Defenses
It cannot be overemphasized that coliforms (except for E coli in enteric diseases) and Proteus species are unlikely to cause disease unless the local or generalized host defenses fail in some way. The normal gastrointestinal flora, which includes E coli and, frequently, other coliforms and Proteus species in small numbers, is important in preventing disease through bacterial competition. Prolonged antibiotic therapy compromises this defense mechanism by reducing susceptible components of the normal flora, permitting nosocomial coliform strains or other bacteria to colonize or overgrow.
The organisms may breach anatomic barriers through third-degree burns, ulcers associated with solid tumors of the skin and mucous membranes, intravenous catheters, and surgical or instrumental procedures on the biliary, gastrointestinal, and genitourinary tracts. The lungs may be violated by instrumentation, as in tracheal intubation, or even by aerosols from contaminated nebulizers or humidifiers, which carry organisms to the terminal alveoli.
Corticosteroid administration, radiotherapy, and the increased steroid levels associated with pregnancy tend to decrease host control over infections (e.g., by depressing the immune response). Cytotoxic drugs also are immunosuppressive. Cancer-or drug-induced neutropenia is an important predisposing factor in bacteremia. Devitalized tissue or foreign bodies may be a source of organisms and may also shelter the organisms from phagocytes and antimicrobial factors.
The interaction of multiple predisposing factors often determines the clinical course and outcome of coliform or Proteus infection. For example, the mortality of bacteremia increases progressively when the underlying disease (e.g., cancer or diabetes) is rated as nonfatal, ultimately fatal (death within 5 years), or rapidly fatal (death within 1 year). Similarly, coliform and Proteus infections commonly are more severe in the very old and very young.
Epidemiology
The epidemiology of coliform and Proteus infections is complex and involves multiple reservoirs and modes of transmission. Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus species live in water, soil, and occasionally food and, in many cases, form part of the intestinal flora of humans and animals. Escherichia coli is believed not to be free living, and its presence in environmental samples is taken as indicating recent fecal contamination. In fact, water quality is determined by the presence of the rapid lactose fermenting E coli, Klebsiella, and Enterobacter (coliform counts ) and E coli(fecal coliform counts) using special selective media.
Coliform and Proteus organisms causing infection may be exogenous or endogenous. While most nosocomial infections appear to arise from endogenous flora, studies of hospitalized adults and infants have shown that the intestinal tract is progressively colonized by nosocomial coliforms with increasing length of hospitalization. Patients being treated with antibiotics, severely ill patients, and (probably) infants are more likely to be colonized, and other sites of colonization such as the nose and throat may be important in such patients. Colonized patients have a higher risk of nosocomial infection than patients who are not colonized.
The bacteria may be acquired indirectly via various vehicles or by direct contact . A variety of vehicles have been implicated in the spread of nosocomial pathogens. For example, Klebsiella, Enterobacter, and Serratia species have all been recovered in large numbers from hospital food, particularly salads, with the hospital kitchen being a primary source . An outbreak of urinary tract infections due to multiply drug-resistant S marcescens was associated with contaminated urine- measuring containers and urinometers. Serious outbreaks or individual cases of bacteremia due to coliforms have been associated with extrinsic contamination of intravenous fluids or caps during manufacture and with extrinsic contamination of intravenous fluids and administration sets in the hospital environment. Other medical devices and medications have served as vehicles for the spread of nosocomial pathogens. Occasionally, transmission may be via members of the hospital staff who are colonized with nosocomial pathogens in the rectum or vagina or on the hands; however, passive carriage on the hands of medical personnel constitutes the major mode of transmission.
Certain properties of the coliforms may be important in the epidemiology of hospital-acquired infections. Coliform bacteria other than E coli frequently are found in tap water or even distilled or deionized water. They may persist or actively multiply in water associated with respiratory therapy or hemodialysis equipment. Klebsiella, Enterobacter, and Serratia species, like Pseudomonas species, may exhibit increased resistance to antiseptics and disinfectants. The same group of coliforms has a selective ability over other common nosocomial pathogens (including E coli, Proteus species, Pseudomonas aeruginosa,and staphylococci) to proliferate rapidly at room temperature in commercial parenteral fluids containing glucose.
Diagnosis
Because the coliforms and Proteus can cause many types of infection, the clinical symptoms rarely permit a diagnosis. Culturing and laboratory identification are usually required. Selected characteristics that are useful in the differentiation of coliform bacilla and Proteus species found in human clinical specimens are shown in Table 26-4. The organisms have simple nutritional requirements and grow well on mildly selective media commonly used for members of the Enterobacteriaceae, but not on some moderately and highly selective enteric plating media (Salmonella-Shigella, bismuth sulfite, and brilliant green agar). Extraintestinal specimens such as urine, purulent material from wounds or abscesses, sputum, and sediment from cerebrospinal fluid should be plated for isolation on blood agar and a differential medium such as MacConkey or eosin-methylene blue agar. The finding of more than 105 organisms/ml in clean voided midstream urine is often taken as ‘significant bacteriuria.’ However, in acutely symptomatic females and with other types of specimens (i.e., those obtained by catheterization or suprapubic aspiration) from either sex, a more appropriate threshold, particularly in the presence of pus cells and the absence of epithelial cells, might be more than 102 colonies of a known uropathogen/ml. Because urine is a good growth medium for many microbes, specimens should be refrigerated (4°C) if transport to the laboratory is delayed longer than 30 minutes, unless a urine transport container with preservative is used.
Table 26-4
Differentiation of Coliform Bacilli and Proteus Found in Human Clinical Specimens.
Isolation of certain coliforms or Proteus species from fecal specimens may be facilitated by adding a moderately selective medium such as xylose-lysine-desoxycholate (XLD) or Hektoen enteric agar. Use of tetrathionate or selenite broth for enrichment of enterotoxigenic strains from feces is not recommended because both media inhibit various genera of coliforms. The strong (E coli, K pneumoniae, Enterobacter aerogenes) and occasionally the slow or weak (Serratia, Citrobacter) lactose-fermenting coliforms produce characteristic pigmented colonies on the enteric plating media. A striking characteristic of Proteus species is their propensity to swarm over the surface of most plating media, making the isolation of other organisms in mixed cultures difficult. The swarming growth appears as a rapidly spreading thin film, sometimes with changing patterns of whirls and bands. Sorbitol MacConkey agar is useful for screening EHEC (commonly E. coli O157:H7) on which sorbitol-negative colonies are nonpigmented and considered suspicious for the organism. Unless the physician specifically requests that the laboratory look for the possibility of E coli as an enteropathogen, tests for pathogenic strains, including toxin assays, serotyping, and serogrouping, will not be done.
In cases of suspected bacteremia, replicate bottles (one cultured aerobically, the other anaerobically) containing 25 to 100 ml of appropriate medium with anticoagulant (e.g., sodium polyanetholesulfonate) are inoculated with 10-ml portions of blood. It is usually necessary to take multiple specimens, both before and after antibiotic therapy is started. It is important to take specimens after antibiotic treatment is started so that therapeutic failure can be recognized while the bacteremia may still be amendable to more aggressive medical or surgical treatment.
All of the coliforms and Proteus species are Gram negative, facultative anaerobic, non-spore- forming rods that are typically motile, except for Klebsiella, which is nonmotile. The oxidase test is negative, and nitrates are reduced to nitrites. Proteus species and all coliforms ferment glucose, but fermentation of other carbohydrates varies. Lactose usually is fermented rapidly by Escherichia, Klebsiella and some Enterobacter species and more slowly by Citrobacter and some Serratia species. Proteus, unlike the coliforms, deaminates phenylalanine to phenylpyruvic acid, and it does not ferment lactose. Typically, Proteus is rapidly urease positive. Some species of Klebsiella, Enterobacter, and Serratia produces a positive urease reaction, but they do so more slowly. A battery of tests for biochemical properties is required to identify the coliforms and Proteus to the species level. Commercial identification systems are now widely used by most US clinical laboratories and consist of ‘kits’ or miniaturized biochemical tests which are read manually (e.g., API-20E and BBL Crystal) or automatically (e.g., Vitek or MicroSCAN).
The coliforms are characterized by great antigenic diversity caused by various combinations of specific H, K, and O antigens. For example, approximately 50 H, 90 K, and 160 O antigens have been identified among various strains of E coli. In contrast, Klebsiella, with no H antigens, has 10 O antigens and approximately 80 K antigens. Serologic identification of the coliforms and Proteus species, commonly by reference laboratories, is an extremely important epidemiologic tool. Similarly, other phenotying methods including biotyping (biochemical profiles), antibiograms (patterns of resistance to antimicrobal agents), and bacteriocin and phage typing have been widely used in epidemiologic studies, particularly of multiresistant isolates of coliforms and Proteus. Recently, genotyping methods such as plasmid profiles (determined by agarose gel electrophoresis), RFLP (restriction fragment link polymorphism) of total DNA, pulsed-field gel electrophoresis, targeted analysis of DNA polymorphism, ribotype, and arbitrarily primed PCR (polymerase chain reaction) have been used in epidemiological studies. In hospital-acquired infections, for example, the same or a small number of serologic or plasmid types suggests single sources of infection. The finding of multiple serotypes or plasmid profiles suggests multiple sources of infection or endogenous infections.
Control
Prevention of coliform and Proteus infections, particularly those that are hospital acquired, is difficult and perhaps impossible. Sewage treatment, water purification, proper hygiene, and other control methods for enteric pathogens will reduce the incidence of E coli enteropathogens. However, these control measures are rarely available in less developed regions of the world. Breast-feeding is an effective means of limiting outbreaks of enteropahogens in infants. Aggressive infection control committees in hospitals can do much to reduce nosocomial infections through identification and control of predisposing factors, education and training of hospital personnel, and limited microbial surveillance. Except for investigations of potential outbreaks, routine culturing of personnel, patients, and the environment is not warranted. Selective decontamination of the digestive tract with a suitable nonabsorbable antimicrobial regimen may be useful during outbreaks caused by nosocomial coliforms and Proteus. Meticulous hand washing after each patient contact a highly effective means of reducing the transmission of nosocomial pathogens (Fig. 26-3)is infrequently or poorly performed by some hospital personnel. In a study conducted in an intensive care unit following an educational campaign on the importance of hand washing, the compliance was 17 percent for physicians, 100 percent for nurses, 82 percent for respiratory technicians, and 88 percent for diagnostic services personnel.
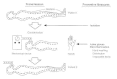
Figure 26-3
Major routes of transmission and prevention of spread of nosocomial pathogens.
Active or passive immunization against coliforms and Proteus species is not practiced. However, vaccines or hyperimmune sera for the six common Gram negative pathogens (E coli, Klebsiella, Enterobacter, Serratia, Pseudomonas aeruginosa,and Proteus) probably would have a major impact on morbidity and mortality from nosocomial infections. In a trial, the mortality was reduced markedly in a group of patients with Gram-negative bacteremia who had been given antiserum against a mutant E coli with an exposed lipopolysaccharide core.
Ampicillin, sulfonamides, cephalosporins, tetracycline, trimethoprim-sulfamethoxazole, nalidixic acid, ciprotloxacin, and nitrofurantoin have been useful in treating urinary tract infections by coliforms and Proteus species. Gentamicin, amikacin, tobramycin, ticarcillin/clavulate, imipenem, aztreonam, and a variety of third-generation cephalosporins may be effective for systemic infections; however, laboratory tests for drug susceptibility are essential. For example, resistence of E coli to ampicillin, and first generation cephalosporins is increasing rapidly to the extent that they can no longer be considered primary drugs of choice in empirical treatment of urinary tract infections. Likewise, emergence of coliforms with chromosomal or plasmid-encoded extended spectrum B-lactamase activity is causing global problems with resistance to third generation cephalosporins. Some coliforms have multiple resistance due to the presence of R plasmids transmissible by conjugation. Conjugative resistance plasmids allow the transfer of resistance genes among species and genera that normally do not exchange chromosomal DNA (Ch. 5). In some cases, resolution of the infection may require drainage of abscesses or other surgical intervention.
Measures commonly used to control epidemics of antibiotic resistant Gram-negative bacilli have included: (1) closing the unit to new admissions until control of the outbreak is underway; (2) reinforcing hand-washing practices; (3) gown and glove isolation, often combined with isolation of patients in separate quarters; and (4) restricting the use of the antibiotic to which the offending clone is resistant.
References
- Beck-Sague C, Villarino E, Giuliano D. et al. Infectious diseases and death among nursing home residents: results of surveillance in 13 nursing homes. Infect Control Hosp Epidemiol. 1994;15:494. [PubMed: 7963443]
- Bergeron MG. Treatment of pyelonephritis in adults. Med Clin North Amer. 1995;79:619. [PubMed: 7752732]
- Bingen, E: Applications of molecular methods to epidemiologic investigations of nosocomial infections in a pediatric hospital. Infect Control Hosp Epidemiol 15, 1994 . [PubMed: 7963442]
- Bodey, GP, Elting LS, Rodriguez S, Hernandez M. Klebsiella bacteremia: a 10-year review in a cancer institution. Cancer. 1989;64:2368. [PubMed: 2804929]
- Brun-Buisson C, Legrand, P Can topical and nonabsorbable antimicrobials prevent cross transmission of resistant strains in ICUs? Infect Control Hosp Epdemiol. 1994;15:447. [PubMed: 7963436]
- Conley JM, Hill S, Ross J. et al. Handwashing practices in an intensive care unit: the effects of an educational program and its relationship to infection rates. Am J Infect Control. 1989;17:330. [PubMed: 2596730]
- Emori GT, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428. [PMC free article: PMC358296] [PubMed: 8269394]
- Foxman, B, Zhang L, Palin K. et al. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. Infect Dis. 1995;171:1514. [PubMed: 7769286]
- Gordon MC, Hankins GDV. Urinary tract infections and pregnancy. Comp Ther. 1989;15:52. [PubMed: 2676334]
- Johnson JR, Stamm WE. Urinary tract infections in women: diagnosis and treatment. Ann Intern Med. 1989;111:906. [PubMed: 2683922]
- Horan TC, Culver DH. et al. Nosocomial infections in surgical patients in the United States, January 1986-June 1992. Infect Control Hosp Epidemiol. 1993;14:73. [PubMed: 8440883]
- Lipsky BA. Urinary tract infections in men: epidemiology, pathophysiology, diagnosis, and treatment. Ann Intern Med. 1989;110:138. [PubMed: 2462391]
- Marshall JC, Christou NV, Horn R, Meakins JL. The microbiology of multiple organ failure: the proximal gastrointestinal tract as an occult reservoir of pathogens. Arch Surg. 1988;123:309. [PubMed: 3341911]
- Mobley HLT, Chippendale GR. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis. J Infect Dis. 1990;161:525. [PubMed: 2179424]
- Morris JG, Lin FYC, Morrison CB. et al. Molecular epidemiology of neonatal meningitis due to Citrobacter diversus: a study of isolates from hospitals in Maryland. J Infect Dis. 1986;154:409. [PubMed: 3734491]
- Nyström B. Impact of handwashing on mortality in intensive care: examination of the evidence. Infect Control Hosp Epidemiol. 1994;15:435. [PubMed: 7963433]
- Saito H, Elting L, Bodey GP, Berky P. Serratia bacteremia: review of 118 cases. Rev Infect Dis. 1989;11:912. [PubMed: 2602776]
- Schaberg, DR, Culver, DH, Gaynes, RP Major trends in the microbial etiology of nosocomial infection. Amer Med. 1991;91(suppl 3B):72S. [PubMed: 1928195]
- Stamm, WE Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Amer Med. 1991;91(suppl 3B):65S. [PubMed: 1928194]
- Swartz MN. Hospital-acquired infections: diseases with increasingly limited therapies. Proc. Natl. Acad. Sci. USA. 1994;91:2420. [PMC free article: PMC43382] [PubMed: 8146133]
- Toltzis P, Blumer JL. Antibiotic-resistant gram-negative bacteria in the critical care setting. Pediatric Clin North Amer. 1995;42:687. [PubMed: 7761147]
- van Saene HKF, Nunn AJ. et al. Viewpoint: survival benefit by selective decontamination of the digestive tract (SDD). Infect Control Hosp Epidemiol. 1994;15:443. [PubMed: 7963435]
- [Post-marketing surveillance of antibacterial activities of cefozopran against various clinical isolates--II. Gram-negative bacteria].[Jpn J Antibiot. 2002][Post-marketing surveillance of antibacterial activities of cefozopran against various clinical isolates--II. Gram-negative bacteria].Igari J, Oguri T, Hiramatsu N, Akiyama K, Koyama T. Jpn J Antibiot. 2002 Feb; 55(1):22-60.
- [Resistance to beta-lactam antibiotics and aminoglycosides in gram negative bacteria. 1. Molecular and genetic characterization of R-factors (author's transl)].[Zentralbl Bakteriol Orig A. 1976][Resistance to beta-lactam antibiotics and aminoglycosides in gram negative bacteria. 1. Molecular and genetic characterization of R-factors (author's transl)].Schmid B, Kayser FH. Zentralbl Bakteriol Orig A. 1976 Apr; 234(3):371-83.
- [Antibacterial activities of monobactams against fresh clinical isolates].[Jpn J Antibiot. 1988][Antibacterial activities of monobactams against fresh clinical isolates].Deguchi K, Yokota N, Koguchi M, Nakane Y, Fukayama S, Nishimura Y, Oda S, Tanaka S, Kato M, Sato K. Jpn J Antibiot. 1988 Nov; 41(11):1600-22.
- Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004.[J Clin Microbiol. 2007]Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004.Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV. J Clin Microbiol. 2007 Oct; 45(10):3352-9. Epub 2007 Aug 22.
- Review Gram-negative bacillary infections. Pathogenic and pathophysiologic correlates.[Am J Med. 1985]Review Gram-negative bacillary infections. Pathogenic and pathophysiologic correlates.Duma RJ. Am J Med. 1985 Jun 7; 78(6A):154-64.
- Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus - Medi...Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus - Medical Microbiology
- Profile neighbors for GEO Profiles (Select 120950910) (199)GEO Profiles
- Profile neighbors for GEO Profiles (Select 120949939) (199)GEO Profiles
- Chromosome neighbors for GEO Profiles (Select 120940208) (20)GEO Profiles
- Chromosome neighbors for GEO Profiles (Select 120953642) (20)GEO Profiles
Your browsing activity is empty.
Activity recording is turned off.
See more...
