By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsThe intermediate mesoderm generates the urogenital system—the kidneys, the gonads, and their respective duct systems. Saving the gonads for our discussion of sex determination in Chapter 17, we will concentrate here on the development of the mammalian kidney.
Progression of kidney types
The importance of the kidney cannot be overestimated. As Homer Smith noted (1953), “our kidneys constitute the major foundation of our philosophical freedom. Only because they work the way they do has it become possible for us to have bone, muscles, glands, and brains.”
While this statement may smack of hyperbole, the kidney is an incredibly intricate organ. Its functional unit, the nephron, contains over 10,000 cells and at least 12 different cell types, with each cell type located in a particular place in relation to the others along the length of the nephron.
The development of the mammalian kidney progresses through three major stages. The first two stages are transient; only the third and last persists as a functional kidney. Early in development (day 22 in humans; day 8 in mice), the pronephric duct arises in the intermediate mesoderm just ventral to the anterior somites. The cells of this duct migrate caudally, and the anterior region of the duct induces the adjacent mesenchyme to form the tubules of the initial kidney, the pronephros (Figure 14.18A). While the pronephric tubules form functioning kidneys in fish and in amphibian larvae, they are not thought to be active in mammalian amniotes. In mammals, the pronephric tubules and the anterior portion of the pronephric duct degenerate, but the more caudal portions of the pronephric duct persist and serve as the central component of the excretory system throughout its development (Toivonen 1945; Saxén 1987). This remaining duct is often referred to as the nephric or Wolffian duct.

Figure 14.18
General scheme of development in the vertebrate kidney. (A) The original tubules, constituting the pronephros, are induced from the nephrogenic mesenchyme by the pronephric duct as it migrates caudally. (B) As the pronephros degenerates, the mesonephric (more...)
As the pronephric tubules degenerate, the middle portion of the nephric duct induces a new set of kidney tubules in the adjacent mesenchyme. This set of tubules constitutes the mesonephros, or mesonephric kidney (Figure 14.18B; Sainio and Raatikainen-Ahokas 1999). In some mammalian species, the mesonephros functions briefly in urine filtration, but in humans and rodents, it does not function as a working kidney. In humans, about 30 mesonephric tubules form, beginning around day 25. As more tubules are induced caudally, the anterior mesonephric tubules begin to regress through apoptosis (although in mice, the anterior tubules remain while the posterior ones regress: Figure 14.18C,D). During its brief existence, however, the mesonephros provides important developmental functions. First, as will be discussed in Chapter 15, it is the source of hematopoietic stem cells necessary for blood cell development (Medvinsky and Dzierzak 1996; Wintour et al. 1996). Second, in male mammals, some of the mesonephric tubules persist to become the sperm-carrying tubes (the vas deferens and efferent ducts) of the testes (see Chapter 17).
The permanent kidney of amniotes, the metanephros, is generated by some of the same components as the earlier, transient kidney types (Figure 14.18C). It is thought to originate through a complex set of interactions between epithelial and mesenchymal components of the intermediate mesoderm. In the first steps, the metanephrogenic mesenchyme forms in posteriorly located regions of the intermediate mesoderm, and it induces the formation of a branch from each of the paired nephric ducts. These epithelial branches are called the ureteric buds. These buds eventually separate from the nephric duct to become the ureters that take the urine to the bladder. When the ureteric buds emerge from the nephric duct, they enter the metanephrogenic mesenchyme. The ureteric buds induce this mesenchymal tissue to condense around them and differentiate into the nephrons of the mammalian kidney. As this mesenchyme differentiates, it tells the ureter bud to branch and grow.
Reciprocal interaction of kidney tissues
Thus, the two intermediate mesodermal tissues—the ureteric bud and the metanephrogenic mesenchyme—interact and reciprocally induce each other to form the kidney (Figure 14.19). The metanephrogenic mesenchyme causes the ureteric bud to elongate and branch. The tips of these branches induce the loose mesenchyme cells to form epithelial aggregates. Each aggregated nodule of about 20 cells will proliferate and differentiate into the intricate structure of the renal nephron. Each nodule first elongates into a “comma” shape and then forms a characteristic S-shaped tube. Soon afterward, the cells of this epithelial structure begin to differentiate into regionally specific cell types, including the capsule cells, the podocytes, and the distal and proximal tubule cells. While this is happening, the epithelializing nodules break down the basal lamina of the ureter bud ducts and fuse with them. This creates a connection between the ureteric bud and the newly formed tube, thereby enabling material to pass from one into the other (Bard et al. 2000). The tubes derived from the mesenchyme form the secretory nephrons of the functioning kidney, and the branched ureteric bud gives rise to the renal collecting ducts and to the ureter, which drains the urine from the kidney.
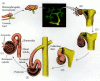
Figure 14.19
Reciprocal induction in the development of the mammalian kidney. (A) As the ureteric bud enters the metanephrogenic mesenchyme, the mesenchyme induces the bud to branch. (B-F) At the tips of the branches, the epithelium induces the mesenchyme to aggregate (more...)
Clifford Grobstein (1955, 1956) documented this reciprocal induction in vitro. He separated the ureteric bud from the metanephrogenic mesenchyme and cultured them either individually or together. In the absence of mesenchyme, the ureteric bud does not branch. In the absence of the ureteric bud, the mesenchyme soon dies. When they are placed together, however, the ureteric bud grows and branches, and nephrons form throughout the mesenchyme (Figure 14.20).

Figure 14.20
Kidney induction observed in vitro. (A) An 11-day mouse metanephric rudiment includes both ureteric bud and metanephrogenic mesenchyme. (B) After the first day of culture, nephrons can be seen at the tips of the branching ureters. (C) The branching collecting (more...)
The mechanisms of reciprocal induction
The induction of the metanephros can be viewed as a dialogue between the ureteric bud and the metanephrogenic mesenchyme. As the dialogue continues, both tissues are altered. There appear to be at least eight sets of signals operating in the reciprocal induction of the metanephros.
Step 1: Formation of the metanephrogenic mesenchyme
Only the metanephrogenic mesenchyme has the competence to respond to the ureteric bud to form kidney tubules, and if induced by other tissues (such as embryonic salivary gland or neural tube tissue), the metanephrogenic mesenchyme will respond by forming kidney tubules and no other structures (Saxén 1970; Sariola et al. 1982). Thus, the metanephrogenic mesenchyme cannot become any tissue other than nephrons. Its competence to respond to ureteric bud inducers is thought to be regulated by a transcription factor called WT1, and if the metanephrogenic mesenchyme lacks this factor, the uninduced cells die (Kriedberg et al. 1993). In situ hybridization shows that WT1 is normally first expressed in the intermediate mesoderm prior to kidney formation and is then expressed in the developing kidney, gonad, and mesothelium (Pritchard-Jones et al. 1990; van Heyningen et al. 1990; Armstrong et al. 1992). Although the metanephrogenic mesenchyme appears homogeneous, it may contain both mesodermally derived tissue and some cells of neural crest origin (Le Douarin and Tiellet 1974; Sariola et al. 1989; Sainio et al. 1994).
Step 2: The metanephrogenic mesenchyme secretes GDNF and HGF to induce and direct the ureteric bud
The second signal in kidney development is a set of diffusible molecules that cause the two ureteric buds to grow out from the nephric ducts. Recent research has shown that glial-derived neurotrophic factor (GDNF) is a critical component of this signal. GDNF is synthesized in the metanephrogenic mesenchyme, and mice whose gdnf genes were knocked out died soon after birth from their lack of kidneys (Moore et al. 1996; Pichel et al. 1996; Sánchez et al. 1996). The GDNF receptor (the c-Ret protein) is synthesized in the nephric ducts and later becomes concentrated in the growing ureteric buds (Figure 14.21; Schuchardt et al. 1996). Mice lacking the GDNF receptor also die of renal agenesis. Another protein synthesized by the metanephrogenic mesenchyme is hepatocyte growth factor (HGF; also known as scatter factor), and the receptor for HGF (the c-met protein) is made by the ureteric buds. Antibodies to HGF will block ureteric bud outgrowth in cultured kidney rudiments (Santos et al. 1994; Woolf et al. 1995). The synthesis of GDNF and HGF by the mesenchyme is thought to be regulated by the WT1 protein.
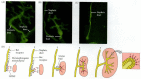
Figure 14.21
Ureteric bud growth is dependent on GDNF and its receptor. (A) The ureteric bud from a 11.5-day wild-type mouse embryonic kidney cultured for 72 hours has a characteristic branching pattern. (B) In embryonic mice heterozygous for the genes encoding GDNF, (more...)
In another mouse mutation, the Danforth short-tail mutant, the ureteric bud is initiated but does not enter the metanephrogenic mesenchyme (Gluecksohn-Schoenheimer 1943). Here, too, the kidney does not form. This failure of the ureteric bud to grow has been correlated with the absence of Wnt11 expression in the tips of the ureteric bud. Wnt11 expression is maintained by proteoglycans made by the mesenchyme. It appears that once the ureteric bud has entered the mesenchyme, these mesenchymal proteoglycans stimulate its continued growth by maintaining the expression and secretion of Wnt11 (Davies et al. 1995; Kispert et al. 1996).
Step 3: The ureteric bud secretes FGF2 and BMP7 to prevent mesenchymal apoptosis
The third signal in kidney development is sent from the ureteric bud to the metanephrogenic mesenchyme, and it alters the fate of the mesenchyme cells. If left uninduced by the ureteric bud, the mesenchyme cells undergo apoptosis (Grobstein 1955; Koseki et al. 1992). However, if induced by the ureteric bud, the mesenchyme cells are rescued from the precipice of death and are converted into proliferating stem cells (Bard and Ross 1991; Bard et al. 1996). The factors secreted from the ureteric bud include fibroblast growth factor 2 (FGF2) and bone morphogenetic protein 7 (BMP7). FGF2 has three modes of action in that it inhibits apoptosis, promotes the condensation of mesenchyme cells, and maintains the synthesis of WT1 (Perantoni et al. 1995). BMP7 has similar effects, and in the absence of BMP7, the mesenchyme of the kidney undergoes apoptosis (see Figure 4.21; Dudley et al. 1995; Luo et al. 1995).
Step 4: LIF from the ureteric bud induces the mesenchyme cells to aggregate and to secrete wnt4
The ureteric bud also causes dramatic changes in the behavior of the metanephrogenic mesenchyme cells, converting them into an epithelium. The newly induced mesenchyme synthesizes two adhesive proteins, E-cadherin and syndecan (Figure 14.22; Vainio et al. 1989, 1992), which clump the mesenchyme together. These aggregated nodes of mesenchyme now synthesize an epithelial basal lamina containing type IV collagen and laminin. At the same time, the mesenchyme cells synthesize receptors for laminin, and this allows the aggregated cells to become an epithelium (Ekblom et al. 1994; Müller et al. 1997). The cytoskeleton also changes from one characteristic of mesenchyme cells to one typical of epithelia* (Ekblom et al. 1983; Lehtonen et al. 1985).

Figure 14.22
Syndecan expression in induced and uninduced kidney mesenchymes. (A) In situ hybridization localizing syndecan mRNA in the mesenchymal aggregates of a 15-day embryonic mouse kidney. Visualization of the autoradiograph is by dark-field illumination (so (more...)
The transition from mesenchymal to epithelial organization may be mediated by several molecules. FGF2 is needed to induce the aggregation of the mesenchyme cells, but it is not capable of turning these aggregates into epithelial cells (Karavanova et al. 1996). Leukemia inhibitory factor (LIF) is able to convert these mesenchymal aggregates into kidney tubule epithelium (but only if they have been exposed to FGF2) (Figure 14.23; Barasch et al. 1999). The ureteric bud secretes FGFs and LIF, and the mesenchyme has receptors for these proteins.

Figure 14.23
LIF induces kidney tubule formation. (A) Metanephrogenic mesenchyme treated with FGF2 or TGFα will form clumps but will not form epithelia. (B) Tubular epithelium is induced when FGF-treated mesenchyme is exposed to ureter bud secretions or LIF. (more...)
Once induced, and after it has started to condense, the mesenchyme begins to secrete Wnt4, which acts in an autocrine fashion to complete the transition from mesenchymal mass to epithelium (Stark et al. 1994; Kispert et al. 1998). Wnt4 expression is found in the condensing mesenchyme cells, in the S-shaped tubes, and in the region where the newly epithelialized cells fuse with the ureteric bud tips. In mice lacking the Wnt4 gene, the mesenchyme becomes condensed but does not form epithelia. Therefore, the ureteric bud induces the changes in the metanephrogenic mesenchyme by secreting FGFs and LIF, but these changes are mediated by the effects of the mesenchyme's secretion of Wnt4 on itself.
Step 5: Conversion of the aggregated cells into a nephron
Not much is known about the transition from pretubular aggregate to nephron. One molecule that may be involved in this transition is the Lim-1 homeodomain transcription factor (Karavanov et al. 1998). This protein is found in the mesenchyme cells after they have condensed around the ureteric bud, and its expression persists in the developing nephron (Figure 14.24). Two other proteins that may be critical for the conversion of the aggregated cells into a nephron are polycystins 1 and 2. These proteins are the products of the genes whose loss-of-function alleles give rise to human polycystic kidney disease. Mice deficient in these genes have abnormal, swollen nephrons (Ward et al. 1996; van Adelsberg et al. 1997).

Figure 14.24
Lim-1 expression (dark stain) in the 19-day embryonic mouse kidney. In situ hybridization shows high levels of expression in the newly epithelialized comma-shaped and S-shaped bodies. (From Karavanov et al. 1998; photograph courtesy of A. A. Karavanov.) (more...)
Step 6: Signals from the mesenchyme induce the branching of the ureteric bud
We still do not know for certain the identities of those molecules secreted by the metanephrogenic mesenchyme that are responsible for inducing the epithelial branching patterns of the ureteric bud. Recent evidence has implicated several paracrine factors in these events, and these factors probably work as pushes and pulls. Some factors may preserve the extracellular matrix surrounding the epithelium, thereby preventing branching from taking place. Conversely, other factors may cause the digestion of this extracellular matrix, permitting branching to occur. The first candidate for regulating kidney branching is GDNF (Sainio et al. 1997). Not only can GDNF from the mesenchyme induce the initial ureteric bud from the nephric duct; it can also induce secondary buds from the ureteric bud once the bud has entered the mesenchyme (Figure 14.25). The second candidate molecule is transforming growth factor b1 (TGF-b1). When exogenous TGF-β1 is added to cultured kidneys, it prevents the epithelium from branching (Figure 14.26B; Ritvos et al. 1995). TGF-β1 is known to promote the synthesis of extracellular matrix proteins and to inhibit the metalloproteinases that can digest these matrices (Penttinen et al. 1988; Nakamura et al. 1990). It is possible that TGF-β1 stabilizes branches once they form. A third molecule that may be important in epithelial branching is activin. When activin is added exogenously to embryonic mouse kidney rudiments, it severely distorts the normal branching pattern (Figure 14.26C; Ritvos et al. 1995). The epithelial cells do not die and are still capable of inducing the mesenchyme cells to form nephrons, but the branches are grossly disordered. Activin may trigger the digestion of the extracellular matrix at the site of a new branch, and adding it exogenously may cause the breakdown of the extracellular matrix throughout the epithelium.
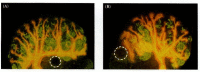
Figure 14.25
The effect of GDNF on the branching of the ureteric epithelium. The ureteric bud and its branches are stained orange (with antibodies to cytokeratin 18), while the nephrons are stained green (with antibodies to nephron brush border antigens). (A) 13-day (more...)

Figure 14.26
The effect of TGF-β1 and activin on the morphogenesis of kidney epithelium. (A) An 11-day mouse kidney cultured for 4 days in control medium has a normal branching pattern. (B) An 11-day mouse kidney cultured in TGF-β1 shows no branching (more...)
Steps 7 and 8: Induction of mesenchymal stem cells by the ureteric bud, and the differentiation of the nephron and growth of the ureteric bud
After the initial interactions create the first pretubular aggregates, the metanephrogenic mesenchyme cells near the kidney border begin to proliferate to form stem cells. These stem cells can interact with the ureteric bud branches to form new nephrons, or they can produce stromal cells. The stromal cells migrate to the central portion of the kidney and produce factors (as yet unknown) that (1) enable the continued growth of the ureteric bud and (2) stimulate the differentiation of the nephron into the convoluted renal tubules, Henle's loop, the glomerulus, and the juxtaglomerular apparatus.
The transcription factor BF-2 is synthesized in these stromal cells. When it is knocked out in mouse embryos, the resulting kidney lacks a branched ureteric tree (it branches only three or four times instead of the normal seven or eight, resulting in an 8- to 16-fold reduction in the number of branches), and the aggregates do not differentiate into nephrons (Hatini et al. 1996). So it appears that the factors necessary for ureteric epithelial growth and nephron differentiation are synthesized by the stromal cells and are regulated by transcription factor BF-2.
The stromal cells have been found to secrete FGF7, a growth factor whose receptor is found on the ureteric bud. FGF7 is seen to be critical for maintaining ureteric epithelial growth, and consequently, ensuring an appropriate number of nephrons in the kidney (Qiao et al. 1999).
In the developing kidney, we see an epitome of the reciprocal interactions needed to form an organ. We also see that we have only begun to understand how organs form.
Footnotes
- *
The mesenchymal to epithelial transition appears to be mediated by the expression of Pax2 in the newly induced mesenchyme cells. When antisense RNA to Pax2 prevents the translation of the Pax2 mRNA that is transcribed as a response to induction, the mesenchyme cells of cultured kidney rudiments fail to condense (Rothenpieler and Dressler 1993).
- Intermediate Mesoderm - Developmental BiologyIntermediate Mesoderm - Developmental Biology
- Homo sapiens ectonucleoside triphosphate diphosphohydrolase 3 (ENTPD3), mRNAHomo sapiens ectonucleoside triphosphate diphosphohydrolase 3 (ENTPD3), mRNAgi|4557424|ref|NM_001248.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...