By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000.

The Cell: A Molecular Approach. 2nd edition.
Show detailsThe internal composition of the cell is maintained because the plasma membrane is selectively permeable to small molecules. Most biological molecules are unable to diffuse through the phospholipid bilayer, so the plasma membrane forms a barrier that blocks the free exchange of molecules between the cytoplasm and the external environment of the cell. Specific transport proteins (carrier proteins and channel proteins) then mediate the selective passage of small molecules across the membrane, allowing the cell to control the composition of its cytoplasm.
Passive Diffusion
The simplest mechanism by which molecules can cross the plasma membrane is passive diffusion. During passive diffusion, a molecule simply dissolves in the phospholipid bilayer, diffuses across it, and then dissolves in the aqueous solution at the other side of the membrane. No membrane proteins are involved and the direction of transport is determined simply by the relative concentrations of the molecule inside and outside of the cell. The net flow of molecules is always down their concentration gradient—from a compartment with a high concentration to one with a lower concentration of the molecule.
Passive diffusion is thus a nonselective process by which any molecule able to dissolve in the phospholipid bilayer is able to cross the plasma membrane and equilibrate between the inside and outside of the cell. Importantly, only small, relatively hydrophobic molecules are able to diffuse across a phospholipid bilayer at significant rates (Figure 12.15). Thus, gases (such as O2 and CO2), hydrophobic molecules (such as benzene), and small polar but uncharged molecules (such as H2O and ethanol) are able to diffuse across the plasma membrane. Other biological molecules, however, are unable to dissolve in the hydrophobic interior of the phospholipid bilayer. Consequently, larger uncharged polar molecules such as glucose are unable to cross the plasma membrane by passive diffusion, as are charged molecules of any size (including small ions such as H+, Na+, K+, and Cl-). The passage of these molecules across the membrane instead requires the activity of specific transport and channel proteins, which therefore control the traffic of most biological molecules into and out of the cell.

Figure 12.15
Permeability of phospholipid bilayers. Gases, hydrophobic molecules, and small polar uncharged molecules can diffuse through phospholipid bilayers. Larger polar molecules and charged molecules cannot.
Facilitated Diffusion and Carrier Proteins
Facilitated diffusion, like passive diffusion, involves the movement of molecules in the direction determined by their relative concentrations inside and outside of the cell. No external source of energy is provided, so molecules travel across the membrane in the direction determined by their concentration gradients and, in the case of charged molecules, by the electric potential across the membrane. However, facilitated diffusion differs from passive diffusion in that the transported molecules do not dissolve in the phospholipid bilayer. Instead, their passage is mediated by proteins that enable the transported molecules to cross the membrane without directly interacting with its hydrophobic interior. Facilitated diffusion therefore allows polar and charged molecules, such as carbohydrates, amino acids, nucleosides, and ions, to cross the plasma membrane.
Two classes of proteins that mediate facilitated diffusion are generally distinguished: carrier proteins and channel proteins. Carrier proteins bind specific molecules to be transported on one side of the membrane. They then undergo conformational changes that allow the molecule to pass through the membrane and be released on the other side. In contrast, channel proteins (see the next section) form open pores through the membrane, allowing the free diffusion of any molecule of the appropriate size and charge.
Carrier proteins are responsible for the facilitated diffusion of sugars, amino acids, and nucleosides across the plasma membranes of most cells. The uptake of glucose, which serves as a primary source of metabolic energy, is one of the most important transport functions of the plasma membrane, and the glucose transporter provides a well-studied example of a carrier protein. The glucose transporter was initially identified as a 55-kd protein in human red blood cells, in which it represents approximately 5% of total membrane protein. Subsequent isolation and sequence analysis of a cDNA clone revealed that the glucose transporter has 12 α-helical transmembrane segments—a structure typical of many carrier proteins (Figure 12.16). These transmembrane α helices contain predominantly hydrophobic amino acids, but several also contain polar amino acid residues that are thought to form the glucose-binding site in the interior of the protein.

Figure 12.16
Structure of the glucose transporter. The glucose transporter has 12 transmembrane α helices. Polar amino acid residues located within the phospholipid bilayer are indicated as dark purple circles. (Adapted from G. I. Bell, C. F. Burant, J. Takeda (more...)
As with many membrane proteins, the three-dimensional structure of the glucose transporter is not known, so the molecular mechanism of glucose transport remains an open question. However, kinetic studies indicate that the glucose transporter functions by alternating between two conformational states (Figure 12.17). In the first conformation, a glucose-binding site faces the outside of the cell. The binding of glucose to this exterior site induces a conformational change in the transporter, such that the glucose-binding site now faces the interior of the cell. Glucose can then be released into the cytosol, followed by the return of the transporter to its original conformation.

Figure 12.17
Model for the facilitated diffusion of glucose. The glucose transporter alternates between two conformations in which a glucose-binding site is alternately exposed on the outside and the inside of the cell. In the first conformation shown (A), glucose (more...)
Most cells, including erythrocytes, are exposed to extracellular glucose concentrations that are higher than those inside the cell, so facilitated diffusion results in the net inward transport of glucose. Once glucose is taken up by these cells it is rapidly metabolized, so intracellular glucose concentrations remain low and glucose continues to be transported into the cell from the extracellular fluids. Because the conformational changes of the glucose transporter are reversible, however, glucose can be transported in the opposite direction simply by reversing the steps in Figure 12.17. Such reverse flow occurs, for example, in liver cells, in which glucose is synthesized and released into the circulation.
Ion Channels
In contrast to carrier proteins, channel proteins simply form open pores in the membrane, allowing small molecules of the appropriate size and charge to pass freely through the lipid bilayer. One group of channel proteins, discussed earlier, is the porins, which permit the free passage of ions and small polar molecules through the outer membranes of bacteria (see Figure 12.8). Channel proteins also permit the passage of molecules between cells connected at gap junctions, which are discussed later in the chapter. The plasma membranes of many cells also contain water channel proteins (aquaporins), through which water molecules are able to cross the membrane much more rapidly than they can diffuse through the phospholipid bilayer. The best-characterized channel proteins, however, are the ion channels, which mediate the passage of ions across plasma membranes. Although ion channels are present in the membranes of all cells, they have been especially well studied in nerve and muscle, where their regulated opening and closing is responsible for the transmission of electric signals.
Three properties of ion channels are central to their function (Figure 12.18). First, transport through channels is extremely rapid. More than a million ions per second flow through open channels—a flow rate approximately a thousand times greater than the rate of transport by carrier proteins. Second, ion channels are highly selective because narrow pores in the channel restrict passage to ions of the appropriate size and charge. Thus, specific channel proteins allow the passage of Na+, K+, Ca2+, and Cl- across the membrane. Third, most ion channels are not permanently open. Instead, the opening of ion channels is regulated by “gates” that transiently open in response to specific stimuli. Some channels (called ligand-gated channels) open in response to the binding of neurotransmitters or other signaling molecules; others (voltage-gated channels) open in response to changes in electric potential across the plasma membrane.
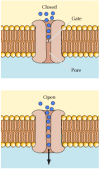
Figure 12.18
Model of an ion channel. In the closed conformation, the flow of ions is blocked by a gate. Opening of the gate allows ions to flow rapidly through the channel. The channel contains a narrow pore that restricts passage to ions of the appropriate size (more...)
The fundamental role of ion channels in the transmission of electric impulses was elucidated through a series of elegant experiments reported by Alan Hodgkin and Andrew Huxley in 1952. These investigators used the giant nerve cells of the squid as a model. The axons of these giant neurons have a diameter of about 1 mm, making it possible to insert electrodes and measure the changes in membrane potential that take place during the transmission of nerve impulses. Using this approach, Hodgkin and Huxley demonstrated that these changes in membrane potential result from the regulated opening and closing of Na+ and K+ channels in the plasma membrane. It subsequently became possible to study the activity of individual ion channels, using the patch clamp technique developed by Erwin Neher and Bert Sakmann in 1976 (Figure 12.19). In this method, a micropipette with a tip diameter of about 1 μm is used to isolate a small patch of membrane, allowing the flow of ions through a single channel to be analyzed and greatly increasing the precision with which the activities of ion channels can be studied.

Figure 12.19
The patch clamp technique. A small patch of membrane is isolated in the tip of a micropipette. Stimuli can then be applied from within the pipette, allowing the behavior of the trapped channel to be measured. (Adapted from E. Neher and B. Sakmann, 1992. (more...)
The flow of ions through membrane channels is dependent on the establishment of ion gradients across the plasma membrane. All cells, including nerve and muscle, contain ion pumps (discussed in the next section) that use energy derived from ATP hydrolysis to actively transport ions across the plasma membrane. As a result, the ionic composition of the cytoplasm is substantially different from that of extracellular fluids (Table 12.1). For example, Na+ is actively pumped out of cells while K+ is pumped in. In the squid axon, therefore, the concentration of Na+ is about 10 times higher in extracellular fluids than inside the cell, whereas the concentration of K+ is approximately 20 times higher in the cytosol than in the surrounding medium.
Table 12.1
Extracellular and Intracellular Ion Concentrations.
Because ions are electrically charged, their transport results in the establishment of an electric gradient across the plasma membrane. With resting squid axons there is an electric potential of about 60 mV across the plasma membrane, with the inside of the cell negative with respect to the outside (Figure 12.20). This electric potential arises both from ion pumps and from the flow of ions through channels that are open in the resting cell plasma membrane. The plasma membrane of resting squid axons contains open K+ channels, so it is more permeable to K+ than to Na+ or other ions. Consequently, the flow of K+ makes the largest contribution to the resting membrane potential.

Figure 12.20
Ion gradients and resting membrane potential of the giant squid axon. Only the concentrations of Na+ and K+ are shown, because these are the ions that function in the transmission of nerve impulses. Na+ is pumped out of the cell while K+ is pumped in, (more...)
As discussed in Chapter 10, the flow of ions across a membrane is driven by both the concentration and voltage components of an electrochemical gradient. For example, the 20-fold higher concentration of K+ inside the squid axon as compared to the extracellular fluid drives the flow of K+ out of the cell. However, because K+ is positively charged, this efflux of K+ from the cell generates an electric potential across the membrane, with the inside of the cell becoming negatively charged. This membrane potential opposes the continuing flow of K+ out of the cell, and the system approaches the equilibrium state, in which the membrane potential balances the K+ concentration gradient.
Quantitatively, the relationship between ion concentration and membrane potential is given by the Nernst equation:

where V is the equilibrium potential in volts, R is the gas constant, T is the absolute temperature, z is the charge of the ion, F is Faraday's constant, and Co and Ci are the concentrations of the ion outside and inside of the cell, respectively. An equilibrium potential exists separately for each ion, and the membrane potential is determined by the flow of all the ions that cross the plasma membrane. However, because resting squid axons are more permeable to K+ than to Na+ or other ions (including Cl-), the resting membrane potential (-60 mV) is close to the equilibrium potential determined by the intracellular and extracellular K+ concentrations (-75 mV).
As nerve impulses (action potentials) travel along axons, the membrane depolarizes (Figure 12.21). The membrane potential changes from -60 mV to approximately +30 mV in less than a millisecond, after which it becomes negative again and returns to its resting value. These changes result from the rapid sequential opening and closing of voltage-gated Na+ and K+ channels. Relatively small initial changes in membrane potential (from -60 to about -40 mV) lead to the rapid opening of Na+ channels. This allows Na+ to flow into the cell, driven by both its concentration gradient and the membrane potential. The sudden entry of Na+ leads to a large change in membrane potential, which increases to nearly +30 mV, approaching the Na+ equilibrium potential of approximately +50 mV. At this time, the Na+ channels are inactivated and voltage-gated K+ channels open, substantially increasing the permeability of the membrane to K+. K+ then flows rapidly out of the cell, driven by both the membrane potential and the K+ concentration gradient, leading to a rapid decrease in membrane potential to about -75 mV (the K+ equilibrium potential). The voltage-gated K+ channels are then inactivated and the membrane potential returns to its resting level of -60 mV, determined by the flow of K+ and other ions through the channels that remain open in unstimulated cells.

Figure 12.21
Membrane potential and ion channels during an action potential. (A) Changes in membrane potential at one point on a squid giant axon following a stimulus. ENa and EK are the equilibrium potentials for Na+ and K+, respectively. (B) The membrane potential (more...)
Depolarization of adjacent regions of the plasma membrane allows action potentials to travel down the length of nerve cell axons as electric signals, resulting in the rapid transmission of nerve impulses over long distances. For example, the axons of human motor neurons can be more than a meter long. The arrival of action potentials at the terminus of most neurons then signals the release of neurotransmitters, such as acetylcholine, which carry signals between cells at a synapse (Figure 12.22). Neurotransmitters released from presynaptic cells bind to receptors on the membranes of postsynaptic cells, where they act to open ligand-gated ion channels. One of the best-characterized of these channels is the acetylcholine receptor of muscle cells. Binding of acetylcholine opens a channel that is permeable to both Na+ and K+. This permits the rapid influx of Na+, which depolarizes the muscle cell membrane and triggers an action potential. The action potential then results in the opening of voltage-gated Ca2+ channels, leading to the increase in intracellular Ca2+ that signals contraction (see Figure 11.25).

Figure 12.22
Signaling by neurotransmitter release at a synapse. The arrival of a nerve impulse at the terminus of the neuron signals the fusion of synaptic vesicles with the plasma membrane, resulting in the release of neurotransmitter from the presynaptic cell into (more...)
The acetylcholine receptor, initially isolated from the electric organ of Torpedo rays in the 1970s, is the prototype of ligand-gated channels. The receptor consists of five subunits arranged as a cylinder in the membrane (Figure 12.23). In its closed state, the channel pore is thought to be blocked by the side chains of hydrophobic amino acids. The binding of acetylcholine induces a conformational change in the receptor such that these hydrophobic side chains shift out of the channel, opening a pore that allows the passage of positively charged ions, including Na+ and K+. However, the channel remains impermeable to negatively charged ions, such as Cl-, because it is lined by negatively charged amino acids.

Figure 12.23
Model of the acetylcholine receptor. The receptor consists of five subunits arranged around a central pore. The binding of acetylcholine to a site in the extracellular region of the receptor induces allosteric changes that open the channel gate. The channel (more...)
A greater degree of ion selectivity is displayed by the voltage-gated Na+ and K+ channels. Na+ channels are more than ten times more permeable to Na+ than to K+, whereas K+ channels are more than a thousand times more permeable to K+ than to Na+. The selectivity of the Na+ channel can be explained, at least in part, on the basis of a narrow pore that acts as a size filter. The ionic radius of Na+ (0.95 Å) is smaller than that of K+ (1.33 Å), and it is thought that the Na+ channel pore is narrow enough to interfere with the passage of K+ or larger ions (Figure 12.24).

Figure 12.24
Ion selectivity of Na+channels. A narrow pore permits the passage of Na+ bound to a single water molecule but interferes with the passage of K+ or larger ions.
K+ channels also have narrow pores, which prevent the passage of larger ions. However, since Na+ has a smaller ionic radius, this does not account for the selective permeability of these channels to K+. Selectivity of the K+ channel is based on a different mechanism, which was elucidated with the determination of the three-dimensional structure of a K+ channel by X-ray crystallography in 1998 (Figure 12.25). The channel pore contains a narrow selectivity filter that is lined with carbonyl oxygen (C=O) atoms from the polypeptide backbone. When a K+ ion enters the selectivity filter, interactions with these carbonyl oxygens displace the water molecules to which K+ is bound, allowing dehydrated K+ to pass through the pore. In contrast, a dehydrated Na+ is too small to interact with these carbonyl oxygens in the selectivity filter, which is held rigidly open. Consequently, Na+ remains bound to water molecules in a hydrated complex that is too large to pass through the channel.

Figure 12.25
Selectivity of K+channels. The K+ channel contains a narrow selectivity filter lined with carbonyl oxygen (C=O) atoms. The pore is just wide enough to allow the passage of dehydrated K+ from which all associated water molecules have been displaced as (more...)
Voltage-gated Na+, K+, and Ca2+ channels all belong to a large family of related proteins (Figure 12.26). For example, the genome sequence of C. elegans has revealed nearly 200 genes encoding ion channels, which presumably are needed to play diverse roles in cell signaling. K+ channels consist of four identical subunits, each containing either two or six transmembrane α helices. Na+ and Ca2+ channels consist of a single polypeptide chain, but each polypeptide contains four repeated domains that correspond to the K+ channel subunits. Voltage gating is mediated by one of the transmembrane α helices, which contains multiple positively charged amino acids. Membrane depolarization induces the movement of these positive charges toward the outside of the cell, shifting the position of this transmembrane segment and opening the channel. Rapid inactivation of Na+ and K+ channels during the propagation of action potentials is then mediated by cytoplasmic portions of the polypeptide chain, which bind to the cytoplasmic mouth of the channel pore and prevent further ion flow (Figure 12.27).

Figure 12.26
Structures of voltage-gated cation channels. The K+, Na+, and Ca2+ channels belong to a family of related proteins. The K+ channel is formed from the association of four identical subunits, one of which is shown. The Na+ channel consists of a single polypeptide (more...)

Figure 12.27
Inactivation of K+and Na+channels. Following voltage-gated opening, the K+ and Na+ channels are rapidly inactivated by the binding of cytoplasmic portions of the polypeptide chains to the pore. For the K+ channel, inactivation is mediated by a ball-and-chain (more...)
A wide variety of ion channels (including Ca2+ and Cl- channels) respond to different neurotransmitters or open and close with different kinetics following membrane depolarization. The concerted actions of these multiple channels are responsible for the complexities of signaling in the nervous system. Moreover, as discussed in the next chapter, the roles of ion channels are not restricted to the electrically excitable cells of nerve and muscle; they also play critical roles in signaling in other cell types. The regulated opening and closing of ion channels thus provides cells with a sensitive and versatile mechanism for responding to a variety of environmental stimuli.
Active Transport Driven by ATP Hydrolysis
The net flow of molecules by facilitated diffusion, through either carrier proteins or channel proteins, is always energetically downhill in the direction determined by electrochemical gradients across the membrane. In many cases, however, the cell must transport molecules against their concentration gradients. In active transport, energy provided by another coupled reaction (such as the hydrolysis of ATP) is used to drive the uphill transport of molecules in the energetically unfavorable direction.
The ion pumps responsible for maintaining gradients of ions across the plasma membrane provide important examples of active transport driven directly by ATP hydrolysis. As discussed earlier (see Table 12.1), the concentration of Na+ is approximately ten times higher outside than inside of cells, whereas the concentration of K+ is higher inside than out. These ion gradients are maintained by the Na+-K+ pump (also called the Na+-K+ ATPase), which uses energy derived from ATP hydrolysis to transport Na+ and K+ against their electrochemical gradients. This process is a result of ATP-driven conformational changes in the pump (Figure 12.28). First, Na+ ions bind to high-affinity sites inside the cell. This binding stimulates the hydrolysis of ATP and phosphorylation of the pump, inducing a conformational change that exposes the Na+-binding sites to the outside of the cell and reduces their affinity for Na+. Consequently, the bound Na+ is released into the extracellular fluids. At the same time, high-affinity K+-binding sites are exposed on the cell surface. The binding of extracellular K+ to these sites then stimulates hydrolysis of the phosphate group bound to the pump, which induces a second conformational change, exposing the K+-binding sites to the cytosol and lowering their binding affinity so that K+ is released inside the cell. The pump has three binding sites for Na+ and two for K+, so each cycle transports three Na+ and two K+ across the plasma membrane at the expense of one molecule of ATP.

Figure 12.28
Model for operation of the Na+-K+pump.
The importance of the Na+-K+ pump is indicated by the fact that it is estimated to consume nearly 25% of the ATP utilized by many animal cells. One critical role of the Na+ and K+ gradients established by the pump is the propagation of electric signals in nerve and muscle. As will be discussed shortly, the Na+ gradient established by the pump is also utilized to drive the active transport of a variety of other molecules. Yet another important role of the Na+-K+ pump in most animal cells is to maintain osmotic balance and cell volume. The cytoplasm contains a high concentration of organic molecules, including macromolecules, amino acids, sugars, and nucleotides. In the absence of a counterbalance, this would drive the inward flow of water by osmosis, which if unchecked would result in swelling and eventual bursting of the cell. The required counterbalance is provided by the ion gradients established by the Na+-K+ pump (Figure 12.29). In particular, the pump establishes a higher concentration of Na+ outside than inside the cell. As already discussed, the flow of K+ through open channels further establishes an electric potential across the plasma membrane. This membrane potential in turn drives Cl- out of the cell, so the concentration of Cl- (like that of Na+) is about ten times higher in extracellular fluids than in the cytoplasm. These differences in ion concentrations balance the high concentrations of organic molecules inside cells, equalizing the osmotic pressure and preventing the net influx of water.

Figure 12.29
Ion gradients across the plasma membrane of a typical mammalian cell. The concentrations of Na+ and Cl- are higher outside than inside the cell, whereas the concentration of K+ is higher inside than out. The low concentrations of Na+ and Cl- balance the (more...)
The active transport of Ca2+ across the plasma membrane is driven by a Ca2+ pump that is structurally related to the Na+-K+ pump and is similarly powered by ATP hydrolysis. The Ca2+ pump transports Ca2+ out of the cell, so intracellular Ca2+ concentrations are extremely low: approximately 0.1 μM, in comparison to extracellular concentrations of about 1 mM. This low intracellular concentration of Ca2+ makes the cell sensitive to small increases in intracellular Ca2+ levels. Such transient increases in intracellular Ca2+ play important roles in cell signaling, as noted already with respect to muscle contraction (see Figure 11.25) and discussed further in the next chapter.
Similar ion pumps in the plasma membranes of bacteria, yeasts, and plant cells are responsible for the active transport of H+ out of the cell. In addition, H+ is actively pumped out of cells lining the stomach, resulting in the acidity of gastric fluids. Structurally distinct pumps are responsible for the active transport of H+ into lysosomes and endosomes (see Figure 9.35). Yet a third type of H+ pump is exemplified by the ATP synthases of mitochondria and chloroplasts: In these cases the pumps can be viewed as operating in reverse, with the movement of ions down the electrochemical gradient being used to drive ATP synthesis.
The largest family of membrane transporters consists of the ABC transporters, so called because they are characterized by highly conserved ATP-binding domains or ATP-binding cassettes (Figure 12.30). More than a hundred family members have been identified in both prokaryotic and eukaryotic cells. In bacteria, ABC transporters utilize energy derived from ATP hydrolysis to transport a wide range of molecules, including ions, sugars, and amino acids. In eukaryotic cells, the first ABC transporter was discovered as the product of a gene (called the multidrug resistance, or mdr, gene) that makes cancer cells resistant to a variety of drugs used in chemotherapy. Two MDR transporters have now been identified. They are normally expressed in a variety of cells, where they function to remove potentially toxic foreign compounds. For example, expression of an MDR transporter in capillary endothelial cells of the brain appears to play an important role in protecting the brain from toxic chemicals. Unfortunately, the MDR transporters are frequently expressed at high levels in cancer cells where they recognize a variety of drugs and pump them out of cells, thereby making the cancer cells resistant to a broad spectrum of chemotherapeutic agents and posing a major obstacle to successful cancer treatment.

Figure 12.30
Structure of an ABC transporter. The basic structural unit of ABC transporters consists of six transmembrane domains followed by an ATP-binding cassette. In plasma membrane transporters (such as the MDR transporter illustrated), two of these units are (more...)
Another medically important member of the ABC transporter family is the gene responsible for cystic fibrosis. Although it is a member of the ABC family, the product of this gene (called the cystic fibrosis transmembrane conductance regulator, or CFTR) functions as a Cl- channel in epithelial cells, and defective Cl- transport is characteristic of the disease. The CFTR Cl- channel is also unusual in that it appears to require both ATP hydrolysis and cAMP-dependent phosphorylation in order to open. The structural basis for the function of CFTR as a regulated ion channel remains to be elucidated by future research.
Active Transport Driven by Ion Gradients
The ion pumps and ABC transporters discussed in the previous section utilize energy derived directly from ATP hydrolysis to transport molecules against their electrochemical gradients. Other molecules are transported against their concentration gradients using energy derived not from ATP hydrolysis but from the coupled transport of a second molecule in the energetically favorable direction. The Na+ gradient established by the Na+-K+ pump provides a source of energy that is frequently used to power the active transport of sugars, amino acids, and ions in mammalian cells. The H+ gradients established by the H+ pumps of bacteria, yeast, and plant cells play similar roles.
The epithelial cells lining the intestine provide a good example of active transport driven by the Na+ gradient. These cells use active-transport systems in the apical domains of their plasma membranes to take up dietary sugars and amino acids from the lumen of the intestine. The uptake of glucose, for example, is carried out by a transporter that coordinately transports two Na+ and one glucose into the cell (Figure 12.31). The flow of Na+ down its electrochemical gradient provides the energy required to take up dietary glucose and to accumulate high intracellular glucose concentrations. Glucose is then released into the underlying connective tissue (which contains blood capillaries) at the basolateral surface of the intestinal epithelium, where it is transported down its concentration gradient by facilitated diffusion (Figure 12.32). The uptake of glucose from the intestinal lumen and its release into the circulation thus provides a good example of the polarized function of epithelial cells, which results from the specific localization of active transport and facilitated diffusion carriers to the apical and basolateral domains of the plasma membrane, respectively.

Figure 12.31
Active transport of glucose. Active transport driven by the Na+ gradient is responsible for the uptake of glucose from the intestinal lumen. The transporter coordinately binds and transports one glucose and two Na+ into the cell. The transport of Na+ (more...)

Figure 12.32
Glucose transport by intestinal epithelial cells. A transporter in the apical domain of the plasma membrane is responsible for the active uptake of glucose (by cotransport with Na+) from the intestinal lumen. As a result, dietary glucose is absorbed and (more...)
The coordinate uptake of glucose and Na+ is an example of symport, the transport of two molecules in the same direction. In contrast, the facilitated diffusion of glucose is an example of uniport, the transport of only a single molecule. Active transport can also take place by antiport, in which two molecules are transported in opposite directions (Figure 12.33). For example, Ca2+ is exported from cells not only by the Ca2+ pump but also by an Na+-Ca2+ antiporter that transports Na+ into the cell and Ca2+ out. Another example is provided by the Na+-H+ exchange protein, which functions in the regulation of intracellular pH. The Na+-H+ antiporter couples the transport of Na+ into the cell with the export of H+, thereby removing excess H+ produced by metabolic reactions and preventing acidification of the cytoplasm.
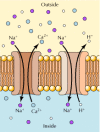
Figure 12.33
Examples of antiport. Ca2+ and H+ are exported from cells by antiporters, which couple their export to the energetically favorable import of Na+.
Box
Molecular Medicine: Cystic Fibrosis.
- Transport of Small Molecules - The CellTransport of Small Molecules - The Cell
- Trifolium alpestre USDA NPGS PI 325494 2000i SD maturase K (matK) gene, partial ...Trifolium alpestre USDA NPGS PI 325494 2000i SD maturase K (matK) gene, partial cds; chloroplastgi|2003932607|gb|MW073039.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...