All Jasper's Basic Mechanisms of the Epilepsies content, except where otherwise noted, is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported license, which permits copying, distribution and transmission of the work, provided the original work is properly cited, not used for commercial purposes, nor is altered or transformed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Noebels JL, Avoli M, Rogawski MA, et al., editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012.
This title is an author manuscript version first made accessible on the NCBI Bookshelf website July 2, 2012.
Post-mortem studies of patients with temporal lobe epilepsy show that astrocytes become reactive with altered protein expression. When taken together with the observation that astrocytes release chemical transmitters the idea has developed that these glia contribute to the generation of seizures. In the epileptic brain there is a reduction in expression of an astrocyte-specific enzyme glutamine synthetase (GS) which converts synaptically released glutamate into glutamine, a precursor for glutamate and GABA synthesis. It is shown that selective reactive astrocytosis, and the accompanying loss of GS, leads to reduced synaptic inhibition and increased spread of excitation. In addition, reactive astrocytes exhibit increased expression of adenosine kinase, the enzyme responsible for converting adenosine, an endogenous anticonvulsant, to AMP. Consequently, a reduction in adenosine accompanies reactive astrocytosis. Astrocytes also regulate surface expression of neuronal NMDA receptors and contribute to their excitation through the release of glutamate and D-serine. Astrocytic Ca2+ signals, which are dampened by some anticonvulsants, stimulate the release of glial glutamate which can provide excitation to neurons. When this excitatory pathway is taken together with the reduction in GABA- and adenosine-dependent inhibition seen during reactive astrocytosis it is not hard to envision how these support cells might contribute to seizure generation.
INTRODUCTION
Glia, Greek for ‘glue’, was discovered by Rudolph Virchow, a German anatomist in the mid-nineteenth century. The name reflects the original view that glia played merely a structural or metabolic support role for neurons. Glial cells, especially astrocytes, are much more than ‘glue’ or merely quiescent, and display their own set of activities. Studies over the last 20 years show that astrocytes perform a series of complex functions that go well beyond the uptake and recycling of neurotransmitters and the buffering of extracellular potassium.1,2
Morphologically, astrocytes are characterized by a highly ramified structure of thin processes with which they contact neurons, blood vessels and other astrocytes. Astrocyte-astrocyte contacts mediate gap-junction coupling between adjacent glial cells to form a cellular network called astrocytic syncytium.3 Although astrocytes form a highly interconnected network, they are structurally organized in non-overlapping spatial domains with limited interdigitation of processes between adjacent cells.4,5 Within its own domain of occupancy, each astrocyte can contact tens of thousands of synapses and hundreds of dendrites4,5(Figure 1A) . The contact between the astrocyte and the neuron is a highly dynamic structure6,7 and the extent of astrocytic coverage of the neuronal terminals is activity-dependent.8
While astrocytes have been considered as non-excitable cells, because unlike neurons they do not fire action potentials,9 they display a form of excitability that is based on variations of the intracellular Ca2+ concentration.10 Astrocytes express a plethora of receptors for neurotransmitters whose activation leads to increases in intracellular Ca2+ concentration that can propagate to neighboring astrocytes as an intercellular Ca2+ wave.10–12 These Ca2+ increases promote the release of neuroactive substances, the so-called gliotransmitters (Figure 1B). These gliotransmitters can control diverse brain processes, such as vasculature tone13 and neuronal activity1,2 and can also modulate mammalian behavior such as sleep.1,2
In keeping with the currently accepted concept that astrocytes form an integral and active part of excitatory and inhibitory synaptic transmission and communicate back to synapses,14 emerging evidence has suggested a critical role for these glial cells in the pathogenesis of neurological disorders such as epilepsy.15–17 Astrocytes become reactive in the epileptic brain and show changes in the expression of metabolic enzymes such as glutamine synthetase and adenosine kinase leading to modification of neuronal excitability. Astrocytes also release glutamate through a Ca2+-dependent mechanism that can synchronize neuronal firing and modulate neuronal excitability and synaptic transmission. In this chapter, we will focus on current lines of evidence suggesting the involvement of reactive astrocytes and gliotransmission in experimental studies of epilepsy, and possible underlying mechanisms.
Ca2+ SIGNALS IN ASTROCYTES
In Vitro and In Situ Studies
The development of video imaging techniques and fluorescent indicators of Ca2+ has allowed us to observe dynamic spatiotemporal changes in Ca2+ concentration18 in neurons and glial cells simultaneously. Unlike neurons, astrocytes do not produce action potentials, and thus they were thought to be quiescent. However, in the early 1990s, initial cell culture studies reported that the excitatory neurotransmitter glutamate elicited Ca2+ elevations in individual astrocytes that can propagate to neighboring astrocytes as an intercellular Ca2+ wave involving dozen of cells, suggesting long-distance communication between these cells.10–12 Further evidence for neuronal activity-dependent Ca2+ elevations in astrocytes comes from studies showing that the stimulation of Schaffer collateral in hippocampal slices preparation also increases the intracellular Ca2+ concentration in these glials cells.19
The synaptic control of astrocytic Ca2+ signals is due to the fact that astrocytes express a wide range of functional receptors for different neurotransmitters.20 Many of these receptors are of the metabotropic type, such as metabotropic glutamate receptor 5 (mGluR5) and metabotropic receptor activated by purines such as P2Y1. These receptors are associated with G proteins that, upon activation, stimulate phospholipase C and formation of diacylglycerol and inositol (1,4,5)-triphosphate (IP3). In turn, IP3 increases the intracellular concentration of Ca2+ through the release of Ca2+ from intracellular IP3-sensitive Ca2+ stores.21 Subsequently, several lines of evidence coming from brain slice preparations demonstrated that excitatory neuronal activity can trigger Ca2+ elevations in astrocytes.21
In Vivo Studies
Recent studies using two-photon microscopy22,23 and specific fluorescent dyes that selectively label astrocytes24 convincingly demonstrate that neuronal activity can trigger Ca2+ signals in astrocytes in vivo. Using these two revolutionary techniques, Hirase et al.25 were the first to analyze changes in Ca2+ signals in cortical astrocytes from living, anesthetized rat. More than 60% of the imaged astrocytes showed a complex pattern of changes in intracellular Ca2+ concentration. However, these changes occured with a relatively low frequency under basal conditions and showed a limited degree of correlation with nearby astrocytes.
The first evidence that astrocytes respond to neuronal activity by increasing their intracellular Ca2+ concentration in vivo was the observation that the application of gamma-aminobutyric acid A (GABAA) receptor antagonists such as bicuculline25 or picrotoxin,26, which increase neuronal activity by triggering epileptic-like discharges, resulted in an increase in Ca2+ signaling in cortical astrocytes. Additionally, these Ca2+ signals were correlated between pairs of nearby astrocytes,25 suggesting that in vivo neuronal activity leads to synchronous Ca2+ signals in multiple astrocytes. Subsequently, sensory stimulation was shown to increase Ca2+ signals in astrocytes in anesthetized animals. In the mouse, whisker,27 limb,28 and odor stimulation29 causes Ca2+ elevations in astrocytes in the whisker barrel, the primary somatosensory cortex and the olfactory bulb respectively. Generally, these Ca2+ increases in astrocytes were delayed by a few seconds compared with the neuronal responses27,30 and were significantly correlated with the strength of the sensory stimulation.27 Moreover, a study in ferrets30 demonstrates that visual stimulation can also induce Ca2+ signals in astrocytes in the visual cortex. Interestingly, this study found that astrocytes were even more sharply tuned for stimulus orientation and frequency than neurons.
More recently, neuronal activity in awake, behaving mice was shown to correlate with Ca2+ elevations in astrocytes. Two-photon microscopy through a cranial window of an awake, head-restrained mouse allowed to run on a Styrofoam ball was used to visualize Ca2+ signals in cortical neurons and astrocytes.31 Repetitive Ca2+ signals were associated with the running behavior and were temporally correlated in multiple astrocytes over a distance of almost 100 μm.31 Similar studies were performed in the cerebellum, where radial Bergmann glia signals were found to correlate with locomotor behavior and were sensitive to blockade of neuronal activity.32 Interestingly, in these studies, Nimmerjahn et al.32 described three different forms of Ca2+ signals; one of them was initiated during locomotor behavior and correlated with changes in blood perfusion, suggesting that these glia networks modulate macroscopic changes in brain dynamics and blood flow.32
In vivo pharmacological studies have been performed to elucidate the mechanisms underlying activity-mediated Ca2+ signals in astrocytes. In vivo application of the mGluR1 or the mGluR5 antagonists LY367385 or 6-methyl-2-(phenylethynyl)-pyridine (MPEP), respectively, reduced the whisker activity-induced Ca2+ elevations in astrocytes, while the application of the ionotropic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione(CNQX) had no effect on the astrocytic Ca2+ response,27 indicating a role for both mGluR1and mGluR5 but not for ionotropic receptors in astrocyte activation. Additionally, another in vivo study has shown that application of the astrocytic glutamate transporter inhibitor DL-threo-β-benzyloxyaspartate (TBOA) reduced Ca2+ elevations in astrocytes induced by visual stimulation,30 suggesting a role for glutamate transporters in the modulation of Ca2+ signals in astrocytes.
Together, these in vivo results demonstrate that, in response to sensory stimulations, astrocytes exhibit complex and extremely finely tuned intracellular Ca2+ signals generated by the synaptic release of glutamate that activates mGluRs and glutamate transporters at the astrocytic surface. More importantly, recent studies suggest that these astrocytic Ca2+ signals are important for the regulation of the arteriole diameter,33–40 the modulation of the hemodynamic response that generates the intrinsic optical signal,30 and the control of the blood flow associated with motor behavior.32 While astrocytic Ca2+ signals appear to play important physiological roles as evidenced by the aforementioned studies, they also are activated by pathological neuronal activities, such as during epileptiform discharges,25,26 and might participate in seizure generation and maintenance, as described later in this chapter.
ASTROCYTES RELEASE CHEMICAL TRANSMITTERS TO MODULATE NEURONAL AND SYNAPTIC FUNCTIONS
The functional consequence of the astrocytic Ca2+ signals is the release of gliotransmitters that have been shown to modulate neuronal and synaptic functions 1,2,41(Figure 1B). Several of these gliotransmitters, such as glutamate, adenosine triphosphate (ATP), adenosine, and D-serine, are released in a Ca2+-dependent manner21,42 through vesicle43–46 and lysosome exocytosis.47–49 Astrocytes in culture express the soluble N-ethylmaleimide-sensitive fusion protein receptor (SNARE) complex43,50–52 which is colocalized with small vesicles positive for vesicular glutamate transporters,43,50–52 ATP-storing vesicles,49,53 and D-serine-containing vesicles.54 Furthermore, ultrastructural studies in situ have shown that astrocytic processes contain small synaptic-like vesicles with a diameter of 30 nm, which are located in close proximity to synapses.43,44 While Ca2+-dependent exocytosis represents the better-characterized pathways of astrocytic release of glutamate, ATP, adenosine, and D-serine, alternative release mechanisms have also been proposed. These mechanisms include reversal of glutamate transporters, connexin/pannexin hemichannels, pore-forming P2X7 receptors and swelling-induced activation of volume-regulated anion channels.55
In accordance with the concept of the tripartite synapse (Figures 1A and B) in which astrocytes are considered to be functionally associated with the pre- and postsynaptic nerve terminals as a third, signaling element at the synapse,56 it is now well accepted that astrocytes not only respond to neuronal activity but also regulate neuronal excitability, synaptic transmission, and behavior by releasing gliotransmitters.1,2,14 The mechanisms and consequences of this bidirectional communication between neurons and astrocytes are grouped in a process called gliotransmission.2 The better-characterized gliotransmitters involved in gliotransmission are discussed below.
Release of Glutamate
Glutamate, one of the first gliotransmitters released from astrocytes to be identified, has been reported to exert many effects on synaptic transmission and neuronal excitability. Several studies have shown that glutamate release from astrocytes through a Ca2+-dependent mechanism activated receptors located at the presynaptic terminals. Through activation of mGluR157,58 or N-methyl-D-aspartate (NMDA) receptors,44 astrocytes enhance the frequency of spontaneous and evoked excitatory synaptic currents. Additionally, astrocytic glutamate induces the depression or potentiation59 of inhibitory synaptic transmission by activation of presynaptic mGluR2/360 or kainate receptors,61 respectively. Moreover, it has been shown that Ca2+-dependent glutamate release from astrocytes at single hippocampal synapses participated in the generation of long-term potentiation (LTP) through the activation of the presynaptic mGluR1.58 Thus, all of these studies indicate that Ca2+-dependent release of glutamate from astrocytes modulates synaptic strength and plasticity.
Furthermore, various studies have shown that glutamate release from astrocytes activates postsynaptic NMDA receptors to generate slow inward currents (SICs).62 The observation that spontaneous Ca2+ spikes in astrocytes correlates in thalamic neurons with the detection of SICs, suggests that these spontaneous Ca2+ signals mediate the release of glutamate from astrocytes to modulate neuronal excitability. Following this observation, multiple experiments performed to determine the nature of these SICs have shown that different protocols used to induce Ca2+ signals in astrocytes also increased the occurrence of the SICs.2,41 For example, ligands that induce astrocytic Ca2+ signals,63–68 photolysis of caged Ca2+ that has been selectively loaded into astrocytes,64,69 single astrocyte depolarization,70 and electrical stimulation of excitatory presynaptic terminals64,69 all resulted in Ca2+ elevations in astrocytes and occurrence of SICs in nearby neurons. Additionally, SICs have been described in different brain regions71 such as the thalamus, the cortex, the hippocampus, the nucleus accumbens, the olfactory bulb and, more recently, the brainstem.72 Typically, SICs are mediated by the activation of a specific group of extrasynaptic NMDA receptors containing the NR2B subunit since they are inhibited by ifenprodil, a selective antagonist of NR2B-NMDA receptors.64 Furthermore, SICs are insensitive to tetrodotoxin (TTX) and tetanus neurotoxin (TeNT), two substances that block the generation of action potential and the synaptic release of neurotransmitters, respectively,64 confirming their non-neuronal origin. A recent study also demonstrated that the generation of SICs depends on the duration and kinetics of the Ca2+ signals in astrocytes,68 although Fiacco et al.73 suggested an alternative interpretation. More importantly, when SICs reach sufficient amplitude, they can trigger burst of action potentials that occur with a high degree of synchronicity in hippocampal pyramidal neurons over short distances of 100 μm.63,64 Thus, glutamatergic gliotransmission increases neuronal excitability and operates as a non-synaptic mechanism for neuronal synchronization. This form of communication between neurons and astrocytes could represent a significant source of excitation during epileptic discharges as discussed later in this chapter.
Release of D-Serine
The presence of serine racemase, an enzyme required for the conversion of L- to D-serine, in astrocytes has led to the idea that the amino acid might be a significant player in the regulation of the NMDA receptors by acting as the natural substrate for glycine binding sites on the receptor.74,75 D-Serine-containing vesicles are released from astrocytes in a Ca2+-dependent manner and act as a coagonist of the NMDA receptor.54 In the supraoptic nucleus of the hypothalamus, the dynamic astrocytic coverage of synapses dependent on physiological signals influences extracellular levels of astrocytic D-serine and consequently leads to a certain form of metaplasticity.76 For example, the high degree of synaptic coverage by astrocytes seen in virgin rodents induces LTP, whereas the reduced astrocytic coverage of synapses that occurs during lactation induces long-term depression.76 More recent work has addressed the role of D-serine release in NMDA receptor-dependent LTP in the Schaffer collateral-CA1 synapses.77 In this work, the selective inhibition of serine racemase in one astrocyte suppressed local LTP induction, demonstrating that one astrocyte is the direct source of D-serine in the hippocampus and can modulate synaptic input plasticity on nearby neurons.
Release of ATP
The release of ATP from astrocytes has been known for a long time and was initially proposed as a mechanism for the propagation of intercellular Ca2+ waves through the astrocytic syncytium.3,11,78 First, ATP is released from astrocytes during Ca2+ wave propagation.11,79 Second, the propagation can be abolished by antagonists of purinergic P2Y receptors11,79–81 or the ATP-degrading enzyme apyrase.11,81 Third, visualization of the release of ATP demonstrates that its velocity correlates with that of the Ca2+ wave in astrocytes.81 These results, suggest that ATP could be an extracellular messenger and a primary signal for the Ca2+ wave propagation. While mechanisms of glutamate and D-serine release from astrocytes are experimentally well described, the mechanisms underlying the release of ATP from astrocytes are less well understood. The release of ATP is reduced by inhibitors of several anion channels,82,83 gap junctions hemichannels,78,84,85 and pore-forming P2X7 receptors,86 suggesting the involvement of different pathways in the release. Additionally, ATP release from astrocytes is partly Ca2+- and SNARE proteins-dependent.84,87 Further, astrocytes also display vesicles that contain ATP, and vesicular adenosine triphospatase (ATPase) inhibitors block the release of ATP.53,87 More importantly, Pascual et al.88 generated inducible transgenic mice that express a dominant-negative SNARE (dnSNARE) domain of vesicle-associated membrane protein 2 (VAMP2) selectively in astrocytes to suppress the exocytotic release of chemical transmitters from astrocytes. Interestingly, using these transgenic astrocyte-specific dnSNARE mice, it was shown that astrocytes regulate the activation of neuronal A1 receptors that are responsible for presynaptic inhibition of excitatory synaptic transmission in the hippocampus and cortex.88–90 Bioluminescence imaging demonstrated that this molecular genetic manipulation led to reduced extracellular ATP, and pharmacological evidence was consistent with the notion that astrocyte-derived extracellular ATP is hydrolyzed to adenosine to cause a tonic suppression of synaptic transmission.88 The mechanism of release of these purines has not been investigated further. The recent discovery of Sawada et al.91 demonstrated that a novel member of an anion transporter family functioning as a vesicular nucleotide transporter was highly expressed in astrocytes. Together these findings raise the possibility that astrocytes release ATP by exocytosis. However, before such a conclusion is drawn, considerable additional studies will be required.
Once ATP is released from astrocytes, it can exert physiological effects modulating neuronal excitability. For example, in hypothalamic slices, astrocytes express α-1 adrenergic receptors, and in response to adrenergic input they release ATP, which acts on P2X7 receptors localized on nearby magnocellular neurosecretory cells (MNCs). As a result, there is an enhancement of AMPA receptor surface expression and an increase in the amplitude of miniature excitatory postsynaptic current in these cells.92 More recent studies have confirmed postsynaptic effects of ATP on MNCs. Using combined two-photon Ca2+ imaging, photolysis of caged compounds and electrophysiology, it has been shown that there is a mGluR1-dependent Ca2+ increase in astrocytes along with ATP release resulting in an increase in the amplitude of miniature excitatory postsynaptic currents of MNCs through the activation of postsynaptic P2X7 receptors.93 Additionally, another study from Zhang et al.94 in hippocampal cultures has shown that the release of ATP from stimulated astrocytes was able to depress glutamatergic neuronal transmission through the direct activation of P2Y receptors. Interestingly, using hippocampal slices, Zhang et al.94 also stated that the glutamatergic synaptic depression was due to the ATP metabolite adenosine, which acted on the A1 adenosine receptors. Altogether, these results suggest that ATP release from astrocytes can modulate neuronal excitability and synaptic transmission through direct and indirect actions. The indirect action of astrocytic ATP requires its conversion to adenosine, a metabolite known to have multiple effects on neuronal and synaptic functions, as described below.
Adenosine Derived from Astrocyte-Released ATP
Once ATP is released into the extracellular space, a variety of ectonucleotidases hydrolyze ATP to AMP and then a 5′-nucleotidase converts AMP to adenosine,95 which is known to be a powerful modulator of synaptic activity via its actions on the G-protein-coupled adenosine receptor subtypes (A1, A2, and A3).96 In the retina, adenosine derived from astrocyte-released ATP can activate A1 receptors coupled to K+ channels, which hyperpolarize neurons and decrease their excitability.97 In the hippocampus, the adenosine thus produced results in presynaptic inhibition of excitatory synaptic transmission mediated by the activation of A1 receptors.88,94,98 Moreover, chelating Ca2+ in astrocytic syncytium and the use of glial-specific toxins interfere with the A1-dependent synaptic depression, confirming the glial origin of this process.98 In addition to the mechanisms described above, astrocytes can directly release adenosine, especially in response to hypoxic stimulation99,100 even though release of adenosine is more typical of neurons.101 In that case, the release of adenosine from astrocytes depends on export of adenosine through the equilibrative nucleoside transporters.
Recent work using the dnSNARE animals has shown that astrocytic adenosine is important for sleep homeostasis by participating in the accumulation of sleep pressure and contributing to cognitive deficits associated with sleep loss.90 Moreover, another study has shown that the dnSNARE animals displayed a 50% reduction in surface expression of NMDA receptors, resulting in a decrease in cortical slow oscillations.89 Thus, these studies identify astrocytes as a major regulator of the activation of neuronal A1 receptors, and thus presumably as a source of adenosine in the brain, and suggest that purinergic gliotransmission plays an important role in synaptic transmission, plasticity, and behavior.
REACTIVE ASTROCYTOSIS AND EPILEPSY
Reactive changes in astrocytes are frequently encountered in the hippocampus in association with temporal lobe epilepsy (TLE) in humans102 and with animal models of epilepsy.103,104 These reactive changes, termed reactive astrocytosis, generally involve increases in astrocyte size and number103,104 and often occur together with neuronal loss and synaptic rearrangements.103,105 Reactive astrocytes exhibit an increased expression of glial cytoskeletal proteins, glial fibrillary acidic protein (GFAP), and vimentin, which are therefore used to assess the development of reactive astrocytosis.106,107 Interestingly, recent studies have shown that reactive astrocytosis was also accompanied by a loss of the astrocytic domain organization108 and the generation of new astrocytes from stem cells.109 More importantly, in addition to morphological changes, many proteins are up- or downregulated in reactive astrocytes, leading to changes in cellular functions.17 Whether these functional changes modify seizure susceptibility is an intriguing notion receiving increased attention. In the following sections, we discuss recent evidence suggesting that changes in expression of two astrocyte-specific enzymes, glutamine synthetase (GS) and adenosine kinase (ADK), could promote seizures in the epileptic brain.
Reactive Astrocytosis and GS Downregulation in Epilepsy
In the brain, GS is expressed almost exclusively by astrocytes110 and is responsible for the conversion of synaptically released glutamate into glutamine after the neurotransmitter is taken up by transporters into the synaptically associated glia111 (Figure 2). This glutamate-glutamine cycle normally serves as a major mechanism for ammonia detoxification in the brain and also as a buffered reservoir of a precursor (glutamine) for glutamate and GABA synthesis112 (Figure 2).
Early studies performed during epilepsy surgery demonstrated that the accumulation and impaired clearance of hippocampal glutamate in TLE were due to a slowing in the conversion of glutamate to glutamine,113 suggesting a role for GS deficiency in epilepsy. Later, this observation was supported by two discoveries showing the loss of GS in clinical and experimental epilepsies. First, hippocampal tissue removed from patients with TLE during epilepsy surgery was characterized by downregulation of GS.114 Second, GS was downregulated with elevated GFAP immunoreactivity during the chronic phase of epilepsy in an animal model of TLE.115 More recently, an animal model of chronic GS deficiency in the hippocampus has been developed to replicate the situation in human TLE.116,117 In these studies, freely moving rats monitored with video and electroencephalography developed clusters of spontaneous recurrent seizures and neuropathological changes like those seen in human TLE after a continuous intrahippocampal infusion of the GS inhibitor methionine sulfoximine (MSO).
More importantly, recent studies suggest that downregulation of GS and the consequent reduction in the pool of the GABA precursor glutamine could partially deplete inhibitory synaptic terminals of GABA and impair GABAergic inhibition. In support of this notion, Liang et al.118 showed that selective blockade of GS by MSO reduced the amplitude of inhibitory postsynaptic currents (IPSCs) in CA1 pyramidal neurons during repetitive stimulus trains. Interestingly, the MSO effect was prevented by the replenishment of glutamine in the bath perfusion.118 Additionally, recent experiments performed with the same preparation showed no significant effects of MSO on glutamatergic transmission.119 Together, these results suggest that the glial glutamate-glutamine cycle is the major contributor to synaptic GABA release and regulates inhibitory synaptic strength.
More recently, we tested the hypothesis that GS deficits may be contributing to TLE by reducing synaptic inhibition in the vicinity of reactive astrocytes.120 First, in this study, a higher-titer viral transduction of astrocytes with enhanced green fluorescent protein (eGFP) via bilateral injections of adeno-associated virus into the mice hippocampus led to reactive astrocytosis. Reactive eGFP-astrocytes showed high levels of expression of GFAP and vimentin, while nearby neurons and microglia were not altered. Second, consistent with the studies suggesting that reduced GS expression levels are associated with the development of astrocytosis,111,114,115 a pronounced downregulation of GS associated with enhanced GFAP and vimentin expression was observed. Third, we hypothesized that the GS downregulation and, therefore, the reduced glutamine could generate a deficit in the inhibitory synaptic transmission in neurons located in the eGFP-positive areas. To test this hypothesis, we used brain slices to record evoked IPSCs (eIPSCs) and spontaneous miniature IPSCs (mIPSCs) in CA1 pyramidal neurons located in the eGFP-positive areas from mice treated with the adeno-associated virus. As expected, the amplitude of eIPSCs and the amplitude/frequency of mIPSCs were reduced while evoked excitatory postsynaptic currents (eEPSCs) were not altered, suggesting that only inhibitory synaptic transmission was impaired in CA1 pyramidal neurons proximal to reactive astrocytes. Furthermore, MSO had no effect on the amplitude of eIPSCs in CA1 neurons located in the vicinity of eGFP-positive reactive astrocytes, confirming that the evoked failure was due to GS deficiency. Interestingly, bath application of glutamine increased the amplitude of eIPSCs, providing further proof that eIPSCs failure was mediated by neuronal glutamine starvation. These results suggest that reactive astrocytosis-induced GS downregulation leads to an interrupted neuronal glutamine supply, impaired neuronal production of GABA, and compromised inhibitory synaptic transmission (Figure 2). Finally, to determine whether reactive astrocytosis was associated with network hyperexcitability, we used voltage-sensitive dye imaging techniques and stimulation of the temporoammonic pathway between the entorhinal cortex and CA1, a particular technique of stimulation known to constrain excitatory postsynaptic potentials (EPSPs) by triggering a feedforward inhibition. In eGFP-positive hippocampal slices, the EPSPs propagated much further than in control slices from untreated animals. Furthermore, bath perfusion of eGFP-positive hippocampal slices with exogenous glutamine reduced the areas activated by the temporoammonic pathway stimulation. Thus, inhibitory deficits associated with reactive astrocytosis lead to hyperexcitability of the hippocampal network and this can be prevented by exogenously supplied glutamine. Altogether, these results suggest that the GS loss seen during reactive astrocytosis could contribute to elevated seizure susceptibility in TLE by reducing neuronal inhibition (Figure 2). Consequently, protecting GS function might represent a promising therapeutical strategy to prevent seizures.
Reactive Astrocytosis and ADK Upregulation in Epilepsy
Under physiological conditions extra- and intracellular levels of adenosine are rapidly equilibrated via distinct families of nucleoside transporters.121 Intracellularly, adenosine is rapidly metabolized by phosphorylation to AMP via ADK,121 the key enzyme of adenosine metabolism,122 which, in adult brain, is predominantly expressed in astrocytes.123 Thus, ADK is ideally located to control the astrocyte-based adenosine cycle by driving the influx of adenosine into the cell via bidirectional nucleoside transporters122 (Figure 3). Adenosine, in particular, plays a prominent role in seizure regulation and has been found to be elevated in patients following seizures, leading to the conclusion that adenosine released during a seizure mediates seizure arrest and postictal refractoriness.124 The adenosine A1 receptor-mediated functions are largely responsible for the anti-convulsant and neuroprotective activity of adenosine.96 Thus, binding of adenosine to A1 receptors that are highly expressed in the hippocampus, leads to decreased neuronal excitability and synaptic transmission through postsynaptic membrane hyperpolarization and inhibition of presynaptic release, respectively96 (Figure 3). Consequently, changes in the homeostasis of the astrocyte-based adenosine cycle are to be expected in the epileptic brain.
Recent studies from Boison’s group have identified ADK as molecular link between reactive astrocytosis and neuronal dysfunction in epilepsy leading to the ADK hypothesis of epileptogenesis.122 Thus, transgenic mice overexpressing ADK in the brain displayed a reduced tone of the endogenous anti-convulsant adenosine, leading to the emergence of spontaneous chronic seizures.125 Furthermore, a direct association between the development of reactive astrocytosis and the upregulation of ADK has been shown.125,126 Conversely, transgenic mice with a forebrain-selective reduction of ADK were resistant to seizure development.126 Together, these studies indicate that reactive astrocytosis causes overexpression of ADK, which was shown to be sufficient to trigger seizures (Figure 3). Thus, reconstitution or augmentation of adenosine and/or inhibition of ADK constitutes a pharmacological rationale for seizure suppression. In support of this notion, it has been demonstrated that intrahippocampal implants of ADK-deficient stem cell-derived neuronal precursors suppress kindling epileptogenesis, suggesting that any therapy leading to focal augmentation of the adenosine system has the potential to prevent epileptogenesis.121,127,128
ASTROCYTIC Ca2+ SIGNALS, GLUTAMATE RELEASE AND EPILEPSY
Several lines of evidence indicate that Ca2+ elevations in astrocytes leading to glutamate release and synchronization of neuronal firing (see above) could be involved in epilepsy. Thus, below we discuss recent studies supporting the potential role for astrocytic Ca2+ signals and glutamate release in the generation of epileptiform activity.
Astrocytic Ca2+ Signals and Epilepsy
Under physiological conditions, Ca2+ signals in astrocytes arise to activation of mGluRs (see above). Interestingly, it has been shown that the protein expression levels of mGluRs were increased in reactive astrocytes in animal models of epilepsy129,130 and in hippocampal specimen from patients with TLE.131 Furthermore, hippocampal cultured astrocytes derived from patients with TLE show increases in Ca2+ signals.111 More recently, studies have shown that cortical epileptiform activity induced in vivo in anesthetized mice with bicuculline,25 picrotoxin,26 or the A-type K+ channel blocker 4-aminopyridine132 (4-AP) were associated with increases in Ca2+ signals in astrocytes, which could be suppressed by intraperitoneal injections of several anti-epileptic drugs including valproate, gabapentin and phenytoin.132 This positive correlation between increased astrocytic Ca2+ signaling and epileptiform activity onset suggests that these Ca2+ signals might contribute to seizure generation.
Astrocytic Glutamate Release and Epilepsy
Studies performed by Kang et al.66 first demonstrated that Ca2+-dependent release of glutamate from astrocytes might be involved in the generation of epileptiform discharges. In brain slices, the infusion of IP3 through a patch-clamp pipette to increase Ca2+ signals into astrocytes was followed by the occurrence of slow, decayed transient inward currents (STCs) in nearby CA1 pyramidal neurons.66 In current-clamp, STCs were able to depolarize the neuronal membrane and trigger firing of action potentials. This neuronal depolarization was reminiscent of the paroxysmal depolarization shift (PDS) underlying an interictal epileptiform event133 that is known to be synchronized over many millimeters of epileptic brain.134
Later work from the same group supports the role of astrocytes in generating epilepsy.132 Using 4-AP to induce interictal epileptiform activity in hippocampal slices, Tian et al.132 showed that PDSs persisted in the presence of both TTX and different Ca2+ channel blockers that suppressed presynaptic release but were blocked by the ionotropic glutamate receptor antagonists CNQX and D-AP5. Thus, these results suggest that PDSs were triggered by release of glutamate from extrasynaptic sources. To determine whether Ca2+ signals in astrocytes are associated with PDSs, the authors used photolysis of caged Ca2+. However, photolysis of caged Ca2+ in one astrocyte induced local PDS in the presence of TTX, suggesting that Ca2+ elevations in astrocytes lead to glutamate release, which targets nearby neurons to generate PDSs, the hallmark of epileptic activity.133
In contrast to studies from Nedergaard’s group described above, our work135 suggested that glutamate release from astrocytes was not necessary for the generation of epileptiform activity but rather that it could be modulatory. In this work, we induced both ictal and interictal epileptiform activity in hippocampal slices by removing Mg2+ (0 Mg2+) in the presence of picrotoxin. The epileptiform activity thus generated triggered Ca2+ signals in astrocytes and increased the frequency of NMDA-receptor-mediated SICs that share certain properties with STCs. However, when slices were pre-incubated with D-AP5 to block NMDA-receptor mediated SICs, treatment of the slices with 0 Mg2+ and picrotoxin was still able to trigger epileptiform activity, suggesting that glutamate release from astrocytes and SICs per se were not required to initiate epileptiform activity. Interestingly, D-AP5 reversibly reduced the duration of both ictal and interictal epileptiform events, suggesting that astrocytic glutamate, even though it is not necessary for the generation of epileptiform activity, might be important in determining the strength of epileptiform discharges.135
Faced with these two conflicting studies,132,135 recent work by Gomez-Gonzalo et al.65 attempted to determine the role of astrocytes in the generation of focal ictal discharges. First, the authors showed in enthorinal cortex (EC) slices from rats that while 0 Mg2+/picrotoxin induced both ictal and interictal discharges, only ictal discharges were associated with Ca2+ elevations in astrocytes. Second, the frequency and duration of ictal discharges, as well as their associated astrocytic Ca2+ signals, were attenuated by mGluR5 and P2Y receptor antagonists, whereas interictal discharges were unaffected, indicating that astrocytic Ca2+ elevations mediated by mGluR5 and P2Y receptors do not have a role in the generation of interictal discharges. Furthermore, the selective stimulation of astrocytes with the peptide Thr-Phe-Leu-Leu-Arg-NH2 (TFLLR-NH2), known to induce astrocytic glutamate release and SICs via activation of the PAR-1 thrombin receptor,68,136 was able to generate ictal discharges in the presence of 0 Mg2+/picrotoxin, suggesting that astrocytic glutamate release was sufficient to initiate ictal discharges in EC slices prone to generate epileptiform activity. Through a series of elegant experimental manipulations, Gomez-Gonzalo et al. were able to show that while neuronal activity is critical for the generation of ictal discharges, astrocytes can modulate the threshold for the generation of this epileptiform activity.65
In addition to its potential role in the generation of epileptiform activity, our recent study suggested that Ca2+-dependent release of glutamate from astrocytes could also contribute to the typical delayed neuronal death observed after status epilepticus (SE).137 Using two-photon in vivo microscopy and the pilocarpine model of epilepsy to induce SE in mice, we have shown that SE enhanced Ca2+ signals in astrocytes for 3 days and that this enhancement was associated with the period of delayed neuronal death.137 More importantly, we found that post-SE administration of MPEP to block mGluR5 mediated Ca2+ signals in astrocytes (see above) and ifenprodil to selectively block NMDA-NR2B receptors mediated SICs (see above) provided significant neuronal protection.137 Furthermore, we have shown that selective loading of Ca2+ chelators into astrocytes after SE also led to neuronal protection, suggesting neurotoxic roles for glutamatergic gliotransmission in epilepsy.137 This notion is also supported by previous studies demonstrating that the activation of extrasynaptic NMDAR-NR2B receptors stimulates cyclic adenosine monophosphate response element binding protein (CREB) dephosphorylation and neuronal death.138
While controversial observations exist concerning the involvement of astrocytes in the generation of seizures it is clear that astrocytic Ca2+ signals and astrocytic glutamate play an important role in the mechanisms of epilepsy. However, we now await the introduction of astrocyte selective inhibitors to define the role of astrocytes in the process. Thus, gliotransmission should be considered as a potential therapeutic target in epilepsy.
CONCLUDING REMARKS
The extraordinary evolution of in vivo Ca2+ imaging techniques allowed us to appreciate the highly dynamic nature of Ca2+ signaling in astrocytes under physiological conditions. This Ca2+ excitability represents an original pathway for astrocytes to integrate and process the neuronal information in the brain. In response to neuronal activity, astrocytes release gliotransmitters to modulate both excitatory and inhibitory synaptic transmission, and consequently affect brain plasticity and mammalian behavior. It is striking to see how astrocytes react to epilepsy by changing their shape and functions. This glial reactivity leads to increases in neuronal excitability and consequently accelerates the evolution of this neuronal disorder. In addition to astrocytic reactivity, extensive experimental research suggests that astrocytic Ca2+ signaling and gliotransmitter release participate in the generation of seizures. However, data coming from this research are currently controversial mainly due to the different experimental approaches and lack of selective pharmacological tools to block astrocytic Ca2+ signaling and gliotransmission. In the future, the development of transgenic animals bearing specific deficiencies interfering with astrocytic Ca2+ signals and gliotransmission and the use of chronic models of epilepsy that more closely mimic the complex feature of seizures in epileptic patients will represent new approaches to identify the role of astrocytes in the generation of seizures and rigorously evaluate the idea that these glial cells represent a target for developing new therapeutic strategies for epilepsy.
ACKNOWLEDGMENTS
This work was supported by grants from the Epilepsy Foundation to Jerome Clasadonte and the National Institute of Neurological Disorders and Stroke to Philip G. Haydon.
DISCLOSURE STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. [PMC free article: PMC3117429] [PubMed: 20148679]
- 2.
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86(3):1009–1031. [PubMed: 16816144]
- 3.
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11(2):87–99. [PubMed: 20087359]
- 4.
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22(1):183–192. [PMC free article: PMC6757596] [PubMed: 11756501]
- 5.
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27(24):6473–6477. [PMC free article: PMC6672436] [PubMed: 17567808]
- 6.
- Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26(35):8881–8891. [PMC free article: PMC6675342] [PubMed: 16943543]
- 7.
- Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20(8):2235–2239. [PubMed: 15450103]
- 8.
- Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4(11):e343. [PMC free article: PMC1609127] [PubMed: 17048987]
- 9.
- Sontheimer H, Black JA, Waxman SG. Voltage-gated Na+ channels in glia: properties and possible functions. Trends Neurosci. 1996;19(8):325–331. [PubMed: 8843601]
- 10.
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470–473. [PubMed: 1967852]
- 11.
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19(2):520–528. [PMC free article: PMC6782195] [PubMed: 9880572]
- 12.
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54(7):716–725. [PMC free article: PMC2605018] [PubMed: 17006900]
- 13.
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10(11):1369–1376. [PubMed: 17965657]
- 14.
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2(3):185–193. [PubMed: 11256079]
- 15.
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13(2):54–63. [PubMed: 17207662]
- 16.
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. [PubMed: 16495941]
- 17.
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58(2):168–178. [PMC free article: PMC4124883] [PubMed: 18439402]
- 18.
- Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264(14):8171–8178. [PubMed: 2498308]
- 19.
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16(16):5073–5081. [PMC free article: PMC6579292] [PubMed: 8756437]
- 20.
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78(1):99–141. [PubMed: 9457170]
- 21.
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421–431. [PubMed: 19615761]
- 22.
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–76. [PubMed: 2321027]
- 23.
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2(12):932–940. [PubMed: 16299478]
- 24.
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1(1):31–37. [PubMed: 15782150]
- 25.
- Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2(4):E96. [PMC free article: PMC387267] [PubMed: 15094801]
- 26.
- Gobel W, Helmchen F. In vivo calcium imaging of neural network function. Physiology (Bethesda ). 2007;22:358–365. [PubMed: 18073408]
- 27.
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9(6):816–823. [PubMed: 16699507]
- 28.
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27(23):6268–6272. [PMC free article: PMC6672142] [PubMed: 17554000]
- 29.
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58(6):897–910. [PMC free article: PMC2922004] [PubMed: 18579080]
- 30.
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320(5883):1638–1643. [PubMed: 18566287]
- 31.
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56(1):43–57. [PMC free article: PMC2268027] [PubMed: 17920014]
- 32.
- Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62(3):400–412. [PMC free article: PMC2820366] [PubMed: 19447095]
- 33.
- Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci. 2007;27(15):4036–4044. [PMC free article: PMC6672520] [PubMed: 17428981]
- 34.
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95(10):e73–e81. [PubMed: 15499024]
- 35.
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. [PubMed: 17013381]
- 36.
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456(7223):745–749. [PMC free article: PMC4097022] [PubMed: 18971930]
- 37.
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26(11):2862–2870. [PMC free article: PMC2270788] [PubMed: 16540563]
- 38.
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–199. [PubMed: 15356633]
- 39.
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. [PubMed: 16388306]
- 40.
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6(1):43–50. [PubMed: 12469126]
- 41.
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters. Nat Rev Neurosci. 2010;11(4):227–238. [PubMed: 20300101]
- 42.
- Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium. 2005;38(3–4):375–382. [PubMed: 16105683]
- 43.
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7(6):613–620. [PubMed: 15156145]
- 44.
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10(3):331–339. [PubMed: 17310248]
- 45.
- Martineau M, Galli T, Baux G, Mothet JP. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia. 2008;56(12):1271–1284. [PubMed: 18615566]
- 46.
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54(7):700–715. [PubMed: 17006898]
- 47.
- Jaiswal JK, Fix M, Takano T, Nedergaard M, Simon SM. Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proc Natl Acad Sci U S A. 2007;104(35):14151–14156. [PMC free article: PMC1955787] [PubMed: 17715060]
- 48.
- Li D, Ropert N, Koulakoff A, Giaume C, Oheim M. Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J Neurosci. 2008;28(30):7648–7658. [PMC free article: PMC6670856] [PubMed: 18650341]
- 49.
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9(8):945–953. [PubMed: 17618272]
- 50.
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24(11):2633–2642. [PMC free article: PMC6729507] [PubMed: 15028755]
- 51.
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci U S A. 2004;101(25):9441–9446. [PMC free article: PMC438995] [PubMed: 15197251]
- 52.
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004;279(13):12724–12733. [PubMed: 14722063]
- 53.
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278(2):1354–1362. [PubMed: 12414798]
- 54.
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102(15):5606–5611. [PMC free article: PMC556243] [PubMed: 15800046]
- 55.
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52(1–2):142–154. [PMC free article: PMC2267911] [PubMed: 17669556]
- 56.
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. [PubMed: 10322493]
- 57.
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24(3):722–732. [PMC free article: PMC6729258] [PubMed: 14736858]
- 58.
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317(5841):1083–1086. [PubMed: 17717185]
- 59.
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17(20):7817–7830. [PMC free article: PMC6793927] [PubMed: 9315902]
- 60.
- Liu QS, Xu Q, Kang J, Nedergaard M. Astrocyte activation of presynaptic metabotropic glutamate receptors modulates hippocampal inhibitory synaptic transmission. Neuron Glia Biol. 2004 Nov;1(4):307–316. [PMC free article: PMC1474019] [PubMed: 16755304]
- 61.
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004;101(9):3172–3177. [PMC free article: PMC365762] [PubMed: 14766987]
- 62.
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4(8):803–812. [PubMed: 11477426]
- 63.
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24(31):6920–6927. [PMC free article: PMC6729611] [PubMed: 15295027]
- 64.
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43(5):729–743. [PubMed: 15339653]
- 65.
- Gómez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M, Vetri F, Uva L, Pozzan T, de Curtis M, Ratto GM, Carmignoto G. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010;8(4):e1000352. [PMC free article: PMC2854117] [PubMed: 20405049]
- 66.
- Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94(6):4121–4130. [PubMed: 16162834]
- 67.
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57(6):883–893. [PubMed: 18367089]
- 68.
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28(26):6659–6663. [PMC free article: PMC2866443] [PubMed: 18579739]
- 69.
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104(6):1995–2000. [PMC free article: PMC1794302] [PubMed: 17259307]
- 70.
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25(9):2192–2203. [PMC free article: PMC6726085] [PubMed: 15745945]
- 71.
- Fellin T. Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity. J Neurochem. 2009;108(3):533–544. [PubMed: 19187090]
- 72.
- Reyes-Haro D, Müller J, Boresch M, Pivneva T, Benedetti B, Scheller A, Nolte C, Kettenmann H. Neuron-astrocyte interactions in the medial nucleus of the trapezoid body. J Gen Physiol. 2010;135(6):583–594. [PMC free article: PMC2888059] [PubMed: 20479112]
- 73.
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54(4):611–626. [PubMed: 17521573]
- 74.
- Mothet JP, Parent AT, Wolosker H, Brady RO Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97(9):4926–4931. [PMC free article: PMC18334] [PubMed: 10781100]
- 75.
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92(9):3948–3952. [PMC free article: PMC42079] [PubMed: 7732010]
- 76.
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–784. [PubMed: 16713567]
- 77.
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463(7278):232–236. [PMC free article: PMC2807667] [PubMed: 20075918]
- 78.
- Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: novel roles of “hemi-channels” Pflugers Arch. 2009;457(6):1207–1226. [PMC free article: PMC2656403] [PubMed: 18853183]
- 79.
- Cotrina ML, Lin JH, López-García JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20(8):2835–2844. [PMC free article: PMC6772203] [PubMed: 10751435]
- 80.
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95(26):15735–15740. [PMC free article: PMC28113] [PubMed: 9861039]
- 81.
- Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A. 2003;100(19):11023–11028. [PMC free article: PMC196920] [PubMed: 12958212]
- 82.
- Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88(1):246–256. [PubMed: 14675168]
- 83.
- Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA. ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol. 2003;89(4):1870–1877. [PubMed: 12686569]
- 84.
- Bal-Price A, Moneer Z, Brown GC. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia. 2002;40(3):312–323. [PubMed: 12420311]
- 85.
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33(2):93–102. [PubMed: 20022389]
- 86.
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26(5):1378–1385. [PMC free article: PMC2586295] [PubMed: 16452661]
- 87.
- Maienschein V, Marxen M, Volknandt W, Zimmermann H. A plethora of presynaptic proteins associated with ATP-storing organelles in cultured astrocytes. Glia. 1999;26(3):233–244. [PubMed: 10340764]
- 88.
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–116. [PubMed: 16210541]
- 89.
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci U S A. 2009;106(35):15037–15042. [PMC free article: PMC2736412] [PubMed: 19706442]
- 90.
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–219. [PMC free article: PMC2673052] [PubMed: 19186164]
- 91.
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105(15):5683–5686. [PMC free article: PMC2311367] [PubMed: 18375752]
- 92.
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8(8):1078–1086. [PubMed: 15995701]
- 93.
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64(3):391–403. [PMC free article: PMC4107870] [PubMed: 19914187]
- 94.
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40(5):971–982. [PubMed: 14659095]
- 95.
- Zimmermann H, Braun N. Extracellular metabolism of nucleotides in the nervous system. J Auton Pharmacol. 1996;16(6):397–400. [PubMed: 9131425]
- 96.
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. [PubMed: 15797469]
- 97.
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23(5):1659–1666. [PMC free article: PMC2322877] [PubMed: 12629170]
- 98.
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26(20):5370–5382. [PMC free article: PMC6675310] [PubMed: 16707789]
- 99.
- Bjorklund O, Shang M, Tonazzini I, Dare E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol. 2008;596(1–3):6–13. [PubMed: 18727925]
- 100.
- Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55(1):36–45. [PubMed: 17004232]
- 101.
- Parkinson FE, Sinclair CJ, Othman T, Haughey NJ, Geiger JD. Differences between rat primary cortical neurons and astrocytes in purine release evoked by ischemic conditions. Neuropharmacology. 2002;43(5):836–846. [PubMed: 12384169]
- 102.
- Cohen-Gadol AA, Pan JW, Kim JH, Spencer DD, Hetherington HH. Mesial temporal lobe epilepsy: a proton magnetic resonance spectroscopy study and a histopathological analysis. J Neurosurg. 2004;101(4):613–620. [PubMed: 15481715]
- 103.
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182(1):21–34. [PubMed: 12821374]
- 104.
- Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;49(Suppl 2):33–41. [PubMed: 18226170]
- 105.
- Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci. 2010;30(6):2051–2059. [PMC free article: PMC6634015] [PubMed: 20147533]
- 106.
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–434. [PubMed: 15846805]
- 107.
- Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24(21):5016–5021. [PMC free article: PMC6729371] [PubMed: 15163694]
- 108.
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28(13):3264–3276. [PMC free article: PMC6670598] [PubMed: 18367594]
- 109.
- Borges K, McDermott D, Irier H, Smith Y, Dingledine R. Degeneration and proliferation of astrocytes in the mouse dentate gyrus after pilocarpine-induced status epilepticus. Exp Neurol. 2006;201(2):416–427. [PMC free article: PMC4090707] [PubMed: 16793040]
- 110.
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195(4284):1356–1358. [PubMed: 14400]
- 111.
- Eid T, Williamson A, Lee TS, Petroff OA, de Lanerolle NC. Glutamate and astrocytes--key players in human mesial temporal lobe epilepsy? Epilepsia. 2008;49(Suppl 2):42–52. [PubMed: 18226171]
- 112.
- Hassel B, Dingledine R. Glutamate. In: Siegel GJ, Albers RW, Brady ST, Price DL, editors. Basic Neurochemistry. 7th ed. Burlington, MA: Elsevier; 2006. pp. 267–290.
- 113.
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43(7):703–710. [PubMed: 12102672]
- 114.
- Eid T, Thomas MJ, Spencer DD, Rundén-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363(9402):28–37. [PubMed: 14723991]
- 115.
- Hammer J, Alvestad S, Osen KK, Skare O, Sonnewald U, Ottersen OP. Expression of glutamine synthetase and glutamate dehydrogenase in the latent phase and chronic phase in the kainate model of temporal lobe epilepsy. Glia. 2008;56(8):856–868. [PubMed: 18381650]
- 116.
- Eid T, Ghosh A, Wang Y, Beckström H, Zaveri HP, Lee TS, Lai JC, Malthankar-Phatak GH, de Lanerolle NC. Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain. 2008;131(Pt 8):2061–2070. [PMC free article: PMC2724901] [PubMed: 18669513]
- 117.
- Wang Y, Zaveri HP, Lee TS, Eid T. The development of recurrent seizures after continuous intrahippocampal infusion of methionine sulfoximine in rats: a video-intracranial electroencephalographic study. Exp Neurol. 2009;220(2):293–302. [PMC free article: PMC2989153] [PubMed: 19747915]
- 118.
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26(33):8537–8548. [PMC free article: PMC2471868] [PubMed: 16914680]
- 119.
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27(34):9192–9200. [PMC free article: PMC6672195] [PubMed: 17715355]
- 120.
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13(5):584–591. [PMC free article: PMC3225960] [PubMed: 20418874]
- 121.
- Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009;85(2–3):131–141. [PMC free article: PMC2713801] [PubMed: 19428218]
- 122.
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol. 2008;84(3):249–262. [PMC free article: PMC2278041] [PubMed: 18249058]
- 123.
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142(1):125–137. [PubMed: 16859834]
- 124.
- During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32(5):618–624. [PubMed: 1449242]
- 125.
- Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rülicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128(Pt 10):2383–2395. [PubMed: 15930047]
- 126.
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118(2):571–582. [PMC free article: PMC2157568] [PubMed: 18172552]
- 127.
- Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics. 2009;6(2):278–283. [PMC free article: PMC2682344] [PubMed: 19332320]
- 128.
- Boison D, Stewart KA. Therapeutic epilepsy research: from pharmacological rationale to focal adenosine augmentation. Biochem Pharmacol. 2009;78(12):1428–1437. [PMC free article: PMC2766433] [PubMed: 19682439]
- 129.
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12(7):2333–2344. [PubMed: 10947812]
- 130.
- Steinhauser C, Seifert G. Glial membrane channels and receptors in epilepsy: impact for generation and spread of seizure activity. Eur J Pharmacol. 2002;447(2–3):227–237. [PubMed: 12151014]
- 131.
- Tang FR, Lee WL. Expression of the group II and III metabotropic glutamate receptors in the hippocampus of patients with mesial temporal lobe epilepsy. J Neurocytol. 2001;30(2):137–143. [PubMed: 11577252]
- 132.
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11(9):973–981. [PMC free article: PMC1850946] [PubMed: 16116433]
- 133.
- Prince DA, Connors BW. Mechanisms of interictal epileptogenesis. Adv Neurol. 1986;44:275–299. [PubMed: 3518347]
- 134.
- Korn SJ, Giacchino JL, Chamberlin NL, Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987;57(1):325–340. [PubMed: 3559679]
- 135.
- Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci. 2006;26(36):9312–9322. [PMC free article: PMC6674496] [PubMed: 16957087]
- 136.
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J Physiol. 2007;581(Pt 3):1057–1081. [PMC free article: PMC2170820] [PubMed: 17412766]
- 137.
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci. 2007;27(40):10674–10684. [PMC free article: PMC2917229] [PubMed: 17913901]
- 138.
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600(1–2):148–153. [PubMed: 12445470]
Figures
Figure 1The tripartite synapse
(A) A single astrocyte sends out processes that enwrap a number of synapses. One synapse results from the association between the pre- and postsynaptic terminals. (B) At the synapse, neurotransmitters released from the presynaptic terminal activate an astrocyte which responds with Ca2+ elevations. In turn, Ca2+ elevations lead to the release of neuroactive substances called gliotransmitters which act back to the synapse to regulate the presynaptic function and modulate the postsynaptic response.
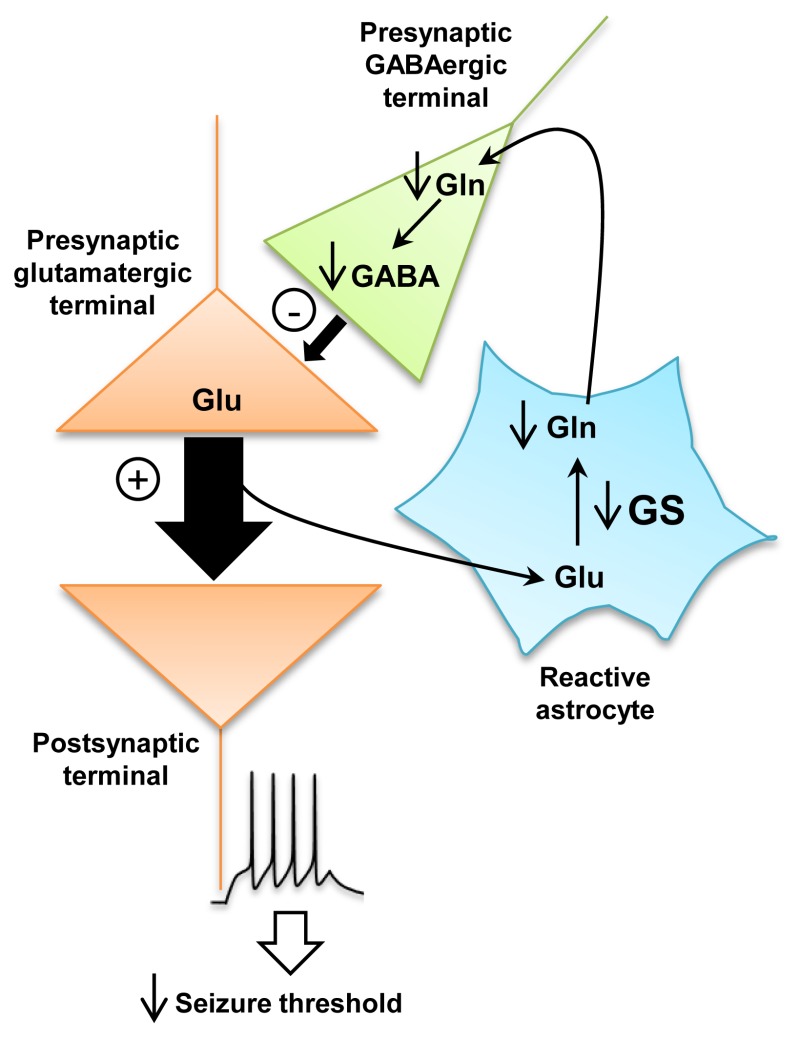
Figure 2The activity change of glutamine synthetase (GS) in reactive astrocytes contributes to seizure development
Glutamate (Glu) released at the excitatory synapse is converted into glutamine (Gln) through activity of GS in astrocytes. Glutamine is used as a precursor for synthesis of GABA in GABAergic neurons. The loss of GS in reactive astrocytes leads to a decrease in Gln and GABA levels in GABAergic terminals. Consequently, presynaptic GABAergic inhibition is reduced, increasing presynaptic release of Glu. In turn, Glu enhances the excitability of postsynaptic neurons, leading to a reduction in the seizure threshold.
Figure 3The activity change of adenosine kinase (ADK) in reactive astrocytes contributes to seizure development
Extracellular ATP is rapidly degraded into adenosine (ADO) by ectonucleotidases (ENT). Adenosine exerts both pre- and postsynaptic inhibitory effects via adenosine A1 receptors (A1R). The extra- and intracellular levels of ADO are equilibrated via nucleoside transporters (NT). The intracellular level of ADO depends on the activity of ADK in astrocytes that converts ADO into 5′-adenosine-monophosphate (5′-AMP). Overexpression of ADK in reactive astrocytes increases the influx of ADO into the cell, decreasing extracellular levels of ADO. Consequently, pre- and postsynaptic inhibitory effects of ADO are reduced leading to a decrease in seizure threshold.