NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Data Points Publication Series [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011-.
From 2001 to June 2011, the number of centers providing proton beam therapy grew from 3 to 10. From 2006 to 2009, the number of Medicare beneficiaries receiving proton beam therapy nearly doubled.
The near doubling of Medicare beneficiaries receiving proton beam therapy from 2006 to 2009 was due to a 68 percent increase in use for “conditions of possible benefit,” mostly prostate cancer, with no increase in use for commonly accepted indications.
Prostate cancer is the most common condition for which a Medicare beneficiary recieves proton beam therapy.
CMS has yet to issue a national coverage rule for proton beam therapy or its specific indications.
Proton beam radiotherapy is a form of external beam radiation that offers better precision for localized dosage than other types of external beam radiotherapy. Because proton beams deposit most of their energy during the final portion of their trajectory, they diminish the risk of damage to tissue surrounding the tumor and thus allow for higher treatment doses with fewer side effects. Proton beam radiotherapy has been used in research applications since the 1950s and entered clinical practice in the United States in 1990.1
No randomized controlled trials and only a few well-conducted cohort studies have compared proton beam radiation to other treatments.2,3 In the absence of evidence of clinical superiority, proton beam radiotherapy has gained acceptance based on a theoretical advantage for the treatment of specific cancers. Agreement is strongest for the use of proton radiotherapy for (1) tumors surrounded by critical structures such as the eye, brain, and spinal cord that preclude or complicate resection or other radiation techniques, or (2) tumors for which other treatments are not very effective. For example, proton beam radiotherapy is preferred for solid tumors in children because it minimizes detrimental effects of radiation on developing structures surrounding the tumor and reduces the risk of long-term side effects.3
In January 2001, three proton beam treatment centers were operating in the United States (Loma Linda, California; Massachusetts General Hospital in Boston; and the University of California, San Francisco). By 2006, three additional centers had opened at Indiana University in Bloomington, M.D. Anderson Cancer Center in Houston, and the University of Florida in Gainesville, followed in 2009 by another in Oklahoma City. By June 2011, the United States was home to 10 proton beam treatment centers, with many more proposed or under construction (Figure 1).

Figure 1
Locations of current and proposed proton beam treatment facilities in the United States.
The number of proton beam centers also increased worldwide, from 17 centers operating outside of the United States in 2001 to 29 in 2011.4
The Centers for Medicare & Medicaid Services (CMS) has yet to release a national coverage or noncoverage determination for proton beam radiotherapy, so local Medicare administrative contractors (previously known as fiscal intermediaries or carriers) have the authority to develop local coverage decisions (LCDs). Local advisory committees (with membership primarily comprising physicians5) provide input for developing LCDs, which specify conditions for payment of claims, including acceptable procedure and diagnosis codes. The first LCDs for proton beam radiotherapy went into effect in 2009,6,7 prior to which LCDs included proton beam radiotherapy along with external beam radiotherapy in general but without identifying specific indications.8
Currently, LCDs vary by contractor regarding their indications for coverage of proton beam radiotherapy, but most LCDs include one or more of the following:
- A list of conditions for which proton beam radiotherapy is medically reasonable (e.g., eye, brain, and spinal cord) and a second list of conditions for which proton beam radiotherapy may be medically reasonable if specified requirements are met and documentation is adequate (e.g., lung, prostate).
- A requirement that the medical record include evidence of benefit for proton beam radiotherapy over other treatment modalities.
- A requirement (for some indications) that the patient be treated as part of a clinical trial.
- Special documentation requirements for prostate cancer.
- A statement that proton beam radiotherapy will be evaluated on a case-by-case basis. Providers must contact the contractor to discuss indications and payment.
Despite the rarity of commonly accepted indications such as tumors of the eye, skull base, and spinal cord, use of proton beam radiotherapy has accelerated in the last decade. Proponents argue that the theoretical advantages of the proton beam's precision apply to more common conditions such as prostate cancer and non-small cell lung cancer; however, no evidence exists for the comparative effectiveness or harms of this therapy.2 Financial factors may in part be driving this trend of including more common conditions among the indications for proton beam therapy, since expanding its use allows for faster recovery of the substantial investment needed to construct a proton beam center.9 A major concern among detractors of proton therapy is cost; one report cited costs of providing proton therapy that were more than double those of other radiation therapies.9 The difference in Medicare payment rates for proton beam radiotherapy versus other radiation therapies is not trivial (Table 1).
Table 1
Medicare payment rates for three-dimensional conformal radiation therapy (3D CRT), intensity-modulated radiation therapy (IMRT), and proton beam radiotherapy in 2007.
Payment rates (which include both Medicare trust fund reimbursement and patient cost sharing) for proton beam radiotherapy vary by the type of facility providing the services and its location. Hospital-based treatment centers receive payments based on the Hospital Outpatient Prospective Payment System (HOPPS) ambulatory payment classifications (APCs), which are wage adjusted according to provider location.10 Rates for payments to freestanding centers are set by local Medicare administrative contractors based on Healthcare Common Procedure Coding System (HCPCS) codes. APC codes 664 and 667 and HCPCS codes 77520, 77522, 77523, and 77525 are used to bill for proton beam radiotherapy. Changes in payment for proton beam therapy between 2006 and 2009 varied across providers. Hospital outpatient-based facilities experienced a rate decrease from 2007 to 2009 followed by a return to 2007 levels in 2010 and 2011. Freestanding centers experienced variable changes. Some contractors reduced payment rates approximately 5 percent from 2008 to 2009, while others granted small increases (1 percent) in rates during the same period.11
This report details the increased use of proton beam radiotherapy among Medicare beneficiaries from 2006 to 2009 in terms of both recipients and indications.
METHODS
This analysis included all Medicare beneficiaries with claims indicating a diagnosis in the malignant or benign neoplasms range of the ICD-9 codes (140-239) and receipt of proton beam therapy (HCPCS 77520-77525) between January 1, 2006, and December 31, 2009.
Measures
For each year, we calculated the number of unique beneficiaries, diagnoses, and reimbursements. We counted each beneficiary only once, in the year treatment started, regardless of whether treatment duration spanned two calendar years. We grouped diagnoses by the degree of consistency in LCD policy regarding proton beam therapy (Table 2). Group 1 diagnoses include cancers for which proton beam is considered medically reasonable in all of the LCDs; we termed these “commonly accepted indications.” Group 2 contains diagnoses for which proton beam may be reasonable; we termed these “conditions of possible benefit.” This category also includes some diagnoses (such as multiple myeloma) that are not included in any LCD but do appear in the claims. In cases where multiple diagnosis codes are associated with proton beam radiotherapy for a single patient, we choose the most common diagnosis code within the neoplasm range of ICD-9 codes (140-239). Fewer than 11 cases offered no diagnosis code for proton beam claims other than a radiation therapy diagnosis code (V58.0), and these were classified as “other.”
Table 2
“Medically reasonable” conditions .
When possible, we report use by specific diagnosis, and we uniformly report prostate and non-small cell lung cancers separately due to their high frequency among proton beam recipients. We assigned proton beam recipients to their provider by matching ZIP Codes for service providers to those of the proton beam centers operational at the time of the service. Fewer than 11 apparent users could not be matched to an existing center, and Medicare reimbursed none of the claims they submitted. Patients who received State assistance with Medicare premiums or cost sharing when proton beam radiotherapy treatment was initiated are identified by Dual Status codes 01 to 09.
Throughout this report, “reimbursement” will refer to the amount paid by the Medicare program, and “payments” will refer to total payments (Medicare reimbursement and patient cost sharing). For hospital-based facilities, we obtained it from the line payment amount. These estimates include only payments related to the use of the facility, equipment, and technician fees (the technical components of the service). Our estimates do not include payments to physicians for treatment planning and management, because these cannot be differentiated from payments for standard external beam radiation therapy.
We calculated mean payment per beneficiary and treatment course (several weeks for some tumors) as the sum of all paid claims. In cases where treatment took place in two years (e.g., December to January), we considered the total amount to have taken place in the year treatment began. Patients beginning treatment in December 2009 were not included in the calculation of mean, median, and total payments because it is not possible to determine whether the treatment was completed or continued into 2010. Denied claims were excluded from the analysis. We separately analyzed the number and proportion of denied claims by year, provider, and indication to assess whether usage differed importantly from an analysis of paid claims.
RESULTS
Providers
The number of centers operating in the United States remained constant at six from 2006 to 2008. One center was added in 2009. However, from 2006 to 2009, the number of beneficiaries treated increased from about 740 to almost 1,200. Centers varied considerably in treatment volume over the study period. Three centers maintained stable or decreased caseloads (SF, MA, LL) and two others doubled theirs. Florida's caseload rose dramatically, from 13 in 2006 to 286 in 2009 (Figure 2).
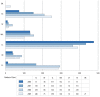
Figure 2
Growth in number of beneficiaries treated with proton beam radiotherapy by facility, 2006-2009. OK=Oklahoma City, OK; TX=Houston, TX; SF=San Francisco, CA; MA=Boston, MA; LL=Loma Linda, CA; IN=Indianapolis, IN; FL=Gainesville, FL.
Patients
Patients receiving proton beam therapy were more likely to be younger than age 75 and white (Table 3). Approximately 5 percent of recipients were under age 65 and received Medicare benefits due to disability. The high percentage of males (85 percent) is consistent with the large number who received proton therapy for prostate cancer. A small percentage of patients (5.8 percent) received assistance with their Medicare premiums and/or full Medicaid benefits. Prostate cancer is the most common diagnosis on Medicare claims for proton beam radiotherapy (69.9%). Other diagnoses that appeared frequently included lung (7.1%), eye (6.6%), malignant and benign brain tumors (4.1%), bone tumors (2.2%), and metastatic tumors in the brain and bones (1.0%; Table 3).
Table 3
Characteristics of proton beam radiotherapy users in Medicare, by demography and indication, 2006-2009.
Between 2006 and 2009, we observed a small decrease in the proportion of patients receiving treatment for Group 1/commonly accepted indications (18.7 percent in 2006 to 10.6 percent in 2009; Figure 3 and Table 4).

Figure 3
Growth in proton beam radiotherapy by diagnosis, 2006-2009.
Table 4
Distribution of diagnoses associated with proton radiotherapy claims over time, 2006-2009.
Over this same period, the number of beneficiaries treated for conditions of possible benefit (Group 2) nearly doubled, from 595 to 1,025.
A similar increase was observed for prostate and lung cancers. Across the four study years, the bulk of the proton beam beneficiaries covered by Medicare were individuals with prostate cancer (66% to 72%).
Reimbursement Associated With Proton Beam Radiotherapy
Medicare reimbursements for proton beam therapy peaked at $28 million in 2007 and dropped to $27 million in 2009 (Table 5), with the drop owing partly to payment decreases and the shift toward treating the majority of patients in freestanding centers instead of hospital outpatient settings (see Table 6). Payments per treatment day averaged $897 for Medicare and $197 for the patient (or the patient's payer). This amount includes no physician management or planning fees. Reimbursement per patient varies by treatment indication. An analysis of claims suggests that ocular tumors typically receive a relatively short course of therapy (one week), whereas other cancers, such as prostate, receive daily doses for six to eight weeks. Average total treatment reimbursements range from about $5,000 for ocular tumors to $25,000 for prostate cancers (Table 7). In 2009, prostate cancer comprised 73 percent of the cases and 79 percent of the amount paid by Medicare.
Table 5
Annual total reimbursement by Medicare to proton beam treatment centers by claim year, 2006-2009.
Table 6
Annual range of payments for proton radiotherapy claims, by type of center, 2006-2009.
Table 7
Annual mean, median, and total reimbursement for proton beam radiotherapy, by diagnosis and year, 2006-2009.
Impact of Denied Claims on Assessment of Proton Beam Therapy Use
As always, analysis of denied claims proved difficult. Most commonly, claims were denied for being submitted multiple times. Denied claims accounted for 5 percent of the claims submitted in each year. Eight to 19 percent of patients had at least one claim denied between 2006 and 2009 (Table 8). In very few cases were all claims for proton beam radiotherapy denied, and a small portion of these came from ZIP Codes with no proton beam radiotherapy providers. Thus, it is likely that these fully denied claims represent billing errors rather than actual receipt of proton beam radiotherapy. In general, providers whose programs were established prior to 2002 were less likely to have any claim rejected than newer providers, with the exception of San Francisco (established in 1994), with 51 percent of beneficiaries having some or all claims denied. The frequency of any claim denial did not vary between indicated (Group 1) and potentially indicated (Group 2) conditions. Of note, most denied claims for indicated conditions were for ocular tumors where the exact location in the eye was initially unspecified.
Table 8
Distribution of diagnoses associated with proton radiotherapy claims over time, by year, location, and diagnosis.
DISCUSSION
As a highly specific form of advanced radiotherapy, proton beam therapy may play an important role in treating tumors surrounded by critical structures such as the eye, brain, and spinal cord. All of these are commonly accepted indications for this precise therapy that allows dose escalation to the tumor site while minimizing the unintended consequences in adjacent tissue. However, many are being treated for tumors for which there is far less consensus about this therapy's use and for which no clinical trial data yet suggest superiority of proton beam therapy over other modalities such as intensity modulated radiotherapy and brachytherapy. The specific tumors for which precise targeting of dose offers overwhelming potential benefits (commonly accepted indications) are rare, and few facilities would likely treat enough such patients to support a proton beam center.
The major growth in proton beam therapy from 2006 to 2009 was due to a 68 percent growth in utilization in “conditions of possible benefit,” mostly prostate cancer. Several factors likely explain the trend toward using proton beam therapy for prostate cancer. First, concerns about impotence, incontinence, and procitis caused by radiation damage to adjacent tissue may be driving patients and physicians to seek more specific forms of radiotherapy. Second, evidence of improved disease-free survival with higher doses of conventional prostate radiation has led some physicians to seek safer ways to escalate dose.13 Third, prostate cancer is more common than the other conditions of possible benefit.
With 217,730 new cases diagnosed in 2010,14 even a small percentage of prostate cancer patients seeking to use proton beam therapy will lead to a large increase in use of the technology. While less than one-half a percent of incident prostate cancer patients received proton beam radiation therapy in 2009, those cases accounted for almost 75 percent of all proton therapy use. The potential for treating a greater percentage of prostate cancer cases with proton beam therapy likely explains the planned opening of several new treatment facilities in the United States in the coming years. The cost of such an expansion will be further multiplied by the fact that with 6 to 8 weeks of therapy (compared to one week for ocular neoplasms), prostate tumors are by far the most expensive to treat with proton beams. Even a minimal trend toward proton therapy in prostate cancer treatment is likely to be widely felt by proton beam centers and have economic implications for the Medicare program.
REFERENCES
- 1.
- Levin WP, Kooy H, et al. Proton beam therapy. Br J Cancer. 2005;93(8):849–54. [PMC free article: PMC2361650] [PubMed: 16189526]
- 2.
- Terasawa T, Dvorak T, Ip S, et al. Systematic review: charged-particle radiation therapy for cancer. Ann Intern Med. 2009;151(8):556–65. [PubMed: 19755348]
- 3.
- Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25(8):953–64. [PubMed: 17350944]
- 4.
- Particle Therapy Co-Operative Group. Particle therapy facilities in operation. [June 24, 2011]. Available at: http://ptcog
.web.psi.ch/ptcentres.html. - 5.
- Foote SB. Focus on locus: evolution of Medicare's local coverage policy. Health Aff. 2003;22(4):137–46. [PubMed: 12889761]
- 6.
- Highmark Medicare Services. Local coverage determination for proton beam therapy, LCD Database ID Number L30314. 2009. Available at: https://www
.cms.gov/medicare-coverage-database/ - 7.
- First Coast Service Options, Inc. Local coverage determination for proton beam radiotherapy, LCD Database ID Number L29263. 2009. Available at: https://www
.cms.gov/medicare-coverage-database/ - 8.
- Noridian Administrative Services, LLC. Local coverage determination for radiation oncology: external beam/teletherapy, LCD Database ID Number L23754. 2007. [July 11, 2011]. Available at: https://www
.cms.gov/medicare-coverage-database/ - 9.
- Zietman A, Goitein M, Tepper JE. Technology evolution: is it survival of the fittest? J Clinl Oncol. 2010;28(27):4275–79. [PubMed: 20697074]
- 10.
- Prospective payment system for hospital outpatient services. 2000. 65 FR 18434. [PubMed: 9025727]
- 11.
- Caron J. Ensuring appropriate incentives for proton beam therapy: a review of the Medicare reimbursement landscape. 2009. [June 8, 2011]. Available at: http://www
.ebglaw.com /files/34618_Caron_RAPNov09.pdf. - 12.
- Wisconsin Physicians Service Insurance Corporation. Local coverage determination for proton beam therapy, LCD Database ID Number DL31617. 2011.
- 13.
- Peeters STH, Heemsbergen WD, Koper PCM, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch Multicenter Randomized Phase III Trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–96. [PubMed: 16648499]
- 14.
- Cancer facts and figures 2010. Atlanta: American Cancer Society; 2010. [July 5, 2011]. Available at: http://www
.cancer.org /acs/groups/content /@nho/documents/document/acspc-024113 .pdf.
Acknowledgments
The authors wish to thank Jessica Zeglin and Mary A. Leonard for their graphic design expertise.
This project was funded under Contract No. HH-SA29020100013I from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Suggested Citation: Jarosek S, Elliott S, Virnig BA. Proton beam radiotherapy in the U.S. Medicare population: growth in use between 2006 and 2009. Proton Beam Radiotherapy. Data Points # 10 (prepared by the University of Minnesota DEcIDE Center, under Contract No. HHSA29020100013I ). Rockville, MD: Agency for Healthcare Research and Quality; January 2012. AHRQ Publication No. 12-EHC005. [PubMed: 22649801]
- Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer.[Cancer. 2016]Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer.Halpern JA, Sedrakyan A, Hsu WC, Mao J, Daskivich TJ, Nguyen PL, Golden EB, Kang J, Hu JC. Cancer. 2016 Aug 15; 122(16):2496-504. Epub 2016 May 25.
- Review Medicare Part D: selected issues for pharmacists and beneficiaries in 2007.[J Manag Care Pharm. 2007]Review Medicare Part D: selected issues for pharmacists and beneficiaries in 2007.Kilian J, Stubbings J. J Manag Care Pharm. 2007 Jan-Feb; 13(1):59-65.
- Medicare program; Medicare prescription drug discount card. Interim final rule with comment period.[Fed Regist. 2003]Medicare program; Medicare prescription drug discount card. Interim final rule with comment period.Centers for Medicare & Medicaid Services (CMS), HHS. Fed Regist. 2003 Dec 15; 68(240):69839-927.
- Improving Comprehensive Medication Review Acceptance by Using a Standardized Recruitment Script: A Randomized Control Trial.[J Manag Care Spec Pharm. 2017]Improving Comprehensive Medication Review Acceptance by Using a Standardized Recruitment Script: A Randomized Control Trial.Miguel A, Hall A, Liu W, Garrett J, Ballew A, Yang TH, Segal R. J Manag Care Spec Pharm. 2017 Jan; 23(1):13-21.
- Review Medicare Part D-a roundtable discussion of current issues and trends.[J Manag Care Pharm. 2009]Review Medicare Part D-a roundtable discussion of current issues and trends.Balfour DC 3rd, Evans S, Januska J, Lee HY, Lewis SJ, Nolan SR, Noga M, Stemple C, Thapar K. J Manag Care Pharm. 2009 Jan-Feb; 15(1 Suppl A):3-9.
- Proton beam radiotherapy in the U.S. Medicare population: growth in use between ...Proton beam radiotherapy in the U.S. Medicare population: growth in use between 2006 and 2009 - Data Points Publication Series
Your browsing activity is empty.
Activity recording is turned off.
See more...
