NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton (FL): CRC Press/Taylor & Francis; 2011.
1.1. SCOPE AND GOALS
This monograph constitutes a singular and bold initiative toward a synthesis of sensation and reward. Although these topics have developed in parallel, this may be largely a historical “accident.” On the other hand, the complexity of each domain may be so great that an extensive separate research on sensation and reward was necessary before an attempted synthesis could be initiated (see chapter by Marks in this volume). In any event, the current state of affairs does not yet permit an overarching specification of lines of integration between these topics. Thus, while introductory chapters are often concerned with such matters, the Preface by Jay Gottfried adequately sets the rationale for and context of this volume. Accordingly, this introductory chapter has another goal, which is to deal with both very old and very new aspects of one of the issues specified in the Preface: “the ability to predict and anticipate reward.” The very old issue concerns the long-established, but rarely discussed, exclusion of learning, memory, and related cognitive processes from primary sensory cortical fields in audition, somesthesis, and vision. The very new topic concerns recent surprising findings about the effects of reward level on associative plasticity in the primary auditory cortex. So, in one sense, this chapter serves as a set of proximal “bookends” for the contents of this monograph.
1.2. CONCERNING THE TRADITIONAL EXCLUSION OF LEARNING, MEMORY, AND COGNITION FROM PRIMARY SENSORY CORTICES
A foundation for traditional theories of cortical organization has been that primary sensory cortices provide only an analysis of the external world, i.e., sensations. In one scenario, the neural substrates of novel “neutral” sensations are conveyed from primary sensory cortical regions to cortical “association areas,” where they are given meaning by uniting with sensations from rewards or punishments. This conception was initiated by Pavlov. Another, more dominant scenario is that neutral sensations are analyzed in primary sensory cortical zones, then passed on to “higher” sensory zones where processes of interpretation and comprehension take place. Finally, this information is combined elsewhere with sensations from reinforcers, at which point associations are formed. In short, the end-point of these processes is that sensations acquire the ability to predict and anticipate rewards with other reinforcers. However, the discovery, and its aftermath during the past 20 years or so, that primary sensory fields participate in all these processes, i.e., they are not simply analyzers of sensations, should render both conceptualizations moot. While awareness of the cognitive aspects of primary sensory cortices is increasing, this is a continuing, and in some circles contentious, enterprise.
The dominant traditional view, so deeply woven into the fabric of neuroscience that it is rarely actually discussed, is that primary sensory cortices analyze sensory input, while “higher order” sensory and association cortices interpret and comprehend the sensory analyses that they receive from primary cortical fields. This view is a heritage of the nineteenth century. Some of the first structural–functional relationships discovered concerned the spinal cord; Bell and Magendie are credited with finding that the dorsal roots are sensory and the ventral roots are motor (Fearing 1930). Much of the research program for the rest of the century concerned the extent to which the entire neuraxis was organized on sensory-motor principles (Young 1970). Subsequently, Fritsch and Hitzig discovered the motor cortex, and lesion studies provided the approximate locations of sensory cortices. By the end of the nineteenth century, the cerebral cortex was conceived of only in sensory-motor terms:
For the cerebral hemispheres consist only of centres related respectively to the sensory and motor tracts… Ideas are revived associations of sensations and movements… thought is internal speech… intellectual attention is ideal vision. (Ferrier 1886)
As studies of the brain burgeoned, later workers concluded that sensory and motor areas did not comprise the entire neocortex, which also had “association” areas. Fleshsig (1901) reported differential myelination in the cortex: sensory and motor cortices exhibited myelination at birth, other areas thereafter. He concluded, erroneously as it turned out, that only the sensory and motor cortices received subcortical projections; the association areas were thought to receive inputs only from other cortical regions (Fleshsig 1901). In short, Fleshsig’s schema was that the cortex consisted of (a) sensory-motor zones that were connected to the thalamus and brain stem and were functional at birth; and (b) the association cortex that was connected only to other cortical regions and was not functional until well after birth. The late Irving Diamond pointed out that as association cortical areas myelinate later, this sequence of myelination “is just what would be expected if an infant sees sensory qualities such as color and brightness before these impressions are associated with another to form the perception of objects” (Diamond and Weinberger 1986). Thus, Fleshsig had provided an anatomical basis for the distinction between “lower” (i.e., sensory-motor) and higher psychological functions.
The final step was to parse sensory cortices into finer-grain functions. This was supplied by the impressive cytoarchitectonic studies of A.W. Campbelf’s (1905) Histological Studies on the Localization of Cerebral Function. His influence has been profound. Campbell labeled the region now identified as primary visual cortex (V1) “visual sensory,” and called regions nearby (e.g., areas 18 and 19) “visual psychic.” Similarly, the region now known as A1 was termed “auditory sensory,” while adjacent areas (in modern parlance, auditory “belt” areas) (Kaas and Hackett 2000) were “auditory psychic” (cf. chapter by Camalier and Kaas in this volume). In this, Campbell intended to make a clear distinction between cortical regions he considered to subserve only sensations from those he believed to concern the comprehension of sensations. Accordingly, this distinction, made purely on cytoarchitectonic grounds, removed learning, memory, and other cognitive functions from primary sensory fields (Diamond 1979). Campbell’s “ghost” still walks the halls of neuroscience.
1.3. ASSOCIATIVE REPRESENTATIONAL PLASTICITY
There has been a sea change in the concept of the sensory cortex. It had long been known that primary sensory cortices develop increased responses to stimuli that gain behavioral importance via learning (e.g., classical or instrumental conditioning) (reviewed in Weinberger and Diamond 1987). However, such plasticity could not be understood within the context of sensation or sensory processing because a single stimulus (or two stimuli in the case of discrimination learning) constitutes too limited a stimulus set to reveal whether associative processes had modified receptive fields or other fundamental descriptors of sensory representation. New experimental paradigms, which combined the study of learning with standard analyses in sensory physiology, could do so. Initiated in the period 1985–1990, this “unified” approach revealed that associative learning was accompanied by systematic changes in the representation of sound in the primary auditory cortex (A1). More specifically, Pavlovian conditioned stimuli (CS) gained increased representation, as indexed both by tuning shifts of frequency receptive fields to the CS and gain in area within the tonotopic map (Diamond and Weinberger 1986; Gonzalez-Lima and Scheich 1986; Gonzalez-Lima 1989; Bakin and Weinberger 1990). For convenience, we refer to learning-dependent, systematic changes in cortical representation of a stimulus parameter as high-order (cortical) associative representational plasticity (HARP).
The involvement of A1 in learning and memory is extensive. HARP possesses all the major attributes of associative memory. In addition to being associative, it is highly specific to the signal frequency (within fractions of an octave), rapidly acquired (in as few as five trials), discriminative (increased responses to a reinforced [CS +] tone and decreased responses to an unreinforced [CS −] tone), consolidates (becomes stronger over hours and days in the absence of further training), and exhibits long-term retention (tracked for up to 8 weeks post-training) (reviewed in Weinberger 2007; Weinberger 2010 in press). Therefore, HARP is an excellent candidate for serving as a substrate of specific auditory memory, despite the fact that it develops in a primary sensory zone.
Subsequently, associative processes in both Pavlovian and instrumental conditioning were found to be responsible for HARP in A1 in a wide variety of tasks, for both reward and punishment, and for all other acoustic parameters investigated, e.g., stimulus level (Polley et al. 2004), rate of tone pulses (Bao et al. 2004), envelope frequency of frequency-modulated tones (Beitel et al. 2003), tone sequence (Kilgard and Merzenich 2002), and auditory localization (Kacelnik et al. 2006). Furthermore, HARP develops during associative learning in all investigated taxa, e.g., big brown bat (Gao and Suga 1998), cat (Diamond and Weinberger 1986), guinea pig (Bakin and Weinberger 1990), owl monkey (Recanzone, Schreiner, and Merzenich 1993), rat (Kisley and Gerstein 2001; Hui et al. 2009), and human (Molchan et al. 1994; Morris, Friston, and Dolan 1998; Schreurs et al. 1997). Similar findings have been reported in other sensory systems, as reported elsewhere in the sensory chapters in this volume. The contrast between the traditional “sensation” and contemporary “cognitive” conceptions of primary sensory cortical fields (Scheich et al. 2007) cannot be overemphasized.
1.4. AUDITORY CORTICAL PLASTICITY AND LEARNING STRATEGY
Neurophysiological correlates of learning have been found in virtually all brain structures studied over the past 60 years (e.g., reviewed in John 1961; Thompson, Patterson, and Teyler 1972; Farley and Alkon 1985; Nakahara et al. 1998; Suzuki and Eichenbaum 2000; Schouenborg 2004). An implicit assumption has been that the magnitude of learning-related plasticity is linked to the magnitude of learning, as indexed by appropriate behavioral performance measures. Recent findings in A1 have challenged this assumption. For example, whereas two-tone discrimination learning for reward in the owl monkey was found to be correlated with the amount of expanded representation of the training frequency band (Recanzone et al. 1993), similar discrimination learning for reward in the cat apparently yielded no plasticity in A1 (Brown, Irvine, and Park 2004). This “failure to replicate” has been interpreted as casting grave doubts about the importance of HARP in the auditory cortex, and has cast suspicion on associative plasticity in other sensory modalities. One explanation is that the failure to replicate is due to a “species difference” between the monkey and cat (Brown, Irvine, and Park 2004). If so, then while the findings in the monkey could be accepted, at best such cortical plasticity would not be a general process; at worst, the findings might be idiosyncratic to the species used.
However, unexpected findings from studies of “simple” auditory learning in the rat suggest another type of explanation, and indeed add another dimension to the search for understanding the role of sensory systems in reward learning. Animals were trained to bar press in the presence of a 10 sec tone to receive a water reward. (A maximum of two rewards could be obtained on each trial.) They were “punished” for bar pressing in silence (e.g., after tone offset) by a time-out period signaled by a flashing light (Figure 1.1a). While apparently a simple task, rats could solve this problem in two ways. One strategy would be to start bar pressing at tone onset and stop bar pressing at tone offset. For convenience, this is called the tone-duration strategy (T-Dur) (Figure 1.1b-1). Alternatively, they could use the strategy of beginning to bar press at tone onset, but continue to respond until a bar press after the rewarded period elicited the flashing light error signal, i.e., effectively ignoring tone offset; hereafter called the tone-onset-to-error strategy (TOTE) (Figure 1.1b-2). The standard protocol cannot easily distinguish between these strategies because the error signal can practically co-occur with tone offset (Figure 1.1a-1).
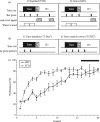
FIGURE 1.1
Learning to respond to tones for rewards can be solved using different behavioral strategies. (a) Two protocols were used to train animals to respond to tone cues for rewards. The grace protocol differed from the standard protocol only in the inclusion (more...)
The two learning strategies can be disambiguated by inserting a “grace period” immediately following tone offset (Figure 1.1a-2). Bar presses during this period would not be rewarded and would not be “punished,” although all subsequent responses during silence on that trial would produce a flashing light and time-out. This pattern of behavior would indicate the use of the TOTE strategy (Figure 1.1b-2).
In this initial study, different groups were trained with different protocols, either the standard protocol, or with the grace period protocol. Both groups learned to achieve about the same level of high performance (Figure 1.1c). Representational areas in A1 were determined by obtaining complete maps of the tonotopic organization of frequency in a terminal mapping experiment. Despite a high level of performance, the STD group exhibited no detectable plasticity (Figure 1.2).
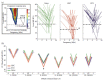
FIGURE 1.2
(See Color Insert) Tone-onset-to-error learning strategy results in reduced threshold and bandwidth in A1. Frequency response areas (FRA) were constructed for each recording site in A1 to determine threshold and bandwidth across A1 tonotopy (inset). (more...)
This “negative finding” apparently supports the views of Brown, Irvine, and Park (2004) that HARP in A1 is not important. However, the group trained in the grace protocol exhibited HARP in the form of signal-specific decreases in absolute threshold and bandwidth, i.e., learning was accompanied by highly specific increases in neural sensitivity and selectivity, respectively (Figure 1.2).
The “grace group (GRC)” could have solved the problem of obtaining water by responding only during tones by using either the T-Dur or TOTE strategies. In fact, they tended to use the latter strategy, i.e., they continued to bar press after tone offset until they received the error signal. Insofar as both groups solved the problem to the same approximate level, the differences in cortical plasticity cannot be due to differences in performance, but rather appear to reflect the use of different learning strategies (Berlau and Weinberger 2008). (We will consider why the different strategies could lead to differential plasticity in A1 later.)
If the use of the TOTE strategy is responsible for plasticity, then a greater use of this strategy should produce greater plasticity. In the first study, the GRC group used the TOTE strategy about 20% of the time, and they developed specific decreases in cortical threshold and bandwidth, but not in enlargement of representational area. We therefore hypothesized that HARP has several forms. The most modest would be local decreases in threshold and bandwidth. A stronger form would be local tuning shifts to the signal frequency. Finally, the strongest form would be an actual increase in the area of representation, probably reflecting global shifts in tuning to the signal frequency. To test this hypothesis, we used a protocol that greatly increased the likelihood that subjects would use the TOTE strategy. Indeed, the use of TOTE increased from about 20% to about 80% of trials. This was accompanied by a significant increase in the area of representation of the signal frequency in A1, supporting the hypothesis of different forms of HARP (Bieszczad and Weinberger 2010c).
Overall, the findings reveal that a hitherto ignored factor can be critical for the formation of plasticity in associative reward learning. It is not sufficient to demonstrate learning, or even to obtain a high level of learning performance in order to obtain associative plasticity in the primary auditory cortex. Rather, it appears necessary to also determine how subjects learn to solve problems.
In addition to the general implications for understanding the neural bases of sensation and reward effects, learning strategy may explain the failure to replicate in the cat the development of HARP in the owl monkey (see above). It may indeed be the case that “species differences” are the cause, but only because cats and owl monkeys may use different learning strategies. Owl monkeys probably rely on onset transients in their con-specific vocalizations, and therefore would be expected to use the tone-onset-dependent TOTE strategy, which confers HARP in A1.
1.5. LEARNING STRATEGY TRUMPS LEVEL OF REWARD IN DETERMINING CORTICAL PLASTICITY
In the initial study, animals had been trained at a moderate level of motivation, i.e., with moderate water restriction. We expected that animals trained with a greater magnitude of reward, i.e., those with stricter water deprivation, would develop HARP of even greater magnitude. Therefore, we trained two groups of rats to bar press to tones for water rewards in an identical grace period protocol (Figure 1.1a-2) at different levels of water deprivation. One group was moderately motivated (ModMot). This group was expected to replicate our prior findings for learning-induced plasticity. Indeed, it did replicate the finding of signal-specific threshold and bandwidth decreases in A1, i.e., increases in neural sensitivity and selectivity, without area gain. The second group was highly motivated (HiMot), i.e., with higher water restriction. Because these animals were thirstier, water would be expected to have a higher subjective reward value. Indeed, the HiMot animals responded to tones faster and learned to a higher level than the ModMot group (Figure 1.3a). Remarkably, however, the HiMot animals did not develop detectable plasticity—either in the form of specific decreases in cortical threshold and bandwidth or in enlargements of representational area (Figure 1.3b). What might be the basis for this counter-intuitive result?
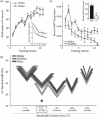
FIGURE 1.3
Learning strategy trumps reward level in determining HARP. (a) As expected, highly motivated animals (HiMot) attained a higher performance level at asymptote and responded faster throughout learning to tone signals that predict reward (inset) than animals (more...)
The underlying reason for the lack of HARP in these HiMot animals can be explained by returning to a consideration of learning strategy. An analysis of the patterns of behavior during learning revealed that the ModMot group used the TOTE strategy (Figure 1.1b-2), while the HiMot subjects used the T-Dur strategy (Figure 1.3c). In effect, the TOTE type of learning strategy “trumped” the level of subjective reward for the development of specific plasticity in A1. Thus, how animals learn to associate sensation and rewards has a dominant role for the formation of cortical plasticity, even over the potent behavioral influence of a reward’s perceived magnitude (Bieszczad and Weinberger 2010b).
1.6. PLASTICITY IN SENSATION MAY DEPEND ON “MATCHING” OF CRITICAL CUES AND NEURONAL RESPONSE PROCLIVITIES
How is it possible that learning can take place apparently without plasticity in a structure thought to be critical for a signal’s sensation? For example, animals that did not use the TOTE strategy still learned the association between tones and rewards but exhibited no HARP in A1. We suggest that the key to understanding sensory plasticity in the prediction of reward (and other reinforcers) is a “concordance” between the cue features that are the basis of a learning strategy and the particular response “proclivities” of sensory neurons to respond to those features. Thus, the TOTE strategy is based on responding to the onset of a tone, while ignoring its offset. As it happens, neurons in A1 appear to be particularly sensitive to onset transients (Masterton 1993; Heil and Irvine 1998; Phillips and Heining 2002). In this way, cells in A1 might develop specific plasticity during TOTE learning because of their proclivity to respond to acoustic onset transients. By contrast, subjects that employed other strategies apparently used additional behavioral guidance by tone offset, or exclusive guidance by offset, without the use of tone onset. We hypothesize that in both groups, learning was supported by the development of HARP in neurons whose response proclivities matched the cues that guided the learning strategies, i.e., in “on-off” cells for animals using an on-off strategy (i.e., T-Dur) and “off” cells when only tone offsets are used. Such off-sensitive cells could not be detected during mapping of A1 with animals necessarily under barbiturate anesthesia, which precludes detection of “off” responses. Also, other auditory cortical regions might contain such cells. Thus, one cannot assume that representations of relevant sensations will develop plasticity by virtue of serving as signals for reward. Rather, it may be necessary to determine how a problem is solved, and then match the critical cue with cells that are particularly “tuned” to that cue. The intriguing implication is that the expression of reward plasticity in a given sensory neuron (or set of neurons) is directly tied to its intrinsic physiological response profile evoked by a particular sensory input. This idea underscores the intimate, perhaps inextricable, interplay between processing of sensation and processing of reward, a recurrent theme appearing throughout this book.
1.7. CONCLUSIONS
We hope that this chapter has provided some insight into the reasons why sensory mechanisms for “the ability to predict and anticipate reward” have traditionally been excluded from primary sensory cortices. We further trust that we have adequately conveyed the importance of learning strategy for understanding how initially neutral sensations can produce specific reorganizations of their representations. We are well aware that the findings summarized here complicate the search for understanding the relationships between neural mechanisms of sensation and reward. Nonetheless, the finding that the effects of reward magnitude depend on how problems are solved, provides a promising entry point into this domain. Achievement of an adequate synthesis between sensation and reward may well require a generation. In this quest, we will need to become fully cognizant of the influences of many factors, some of which have received scant attention in the past. Learning strategy appears to be such a factor.
1.8. CODA
The leitmotif of this volume is the unification of sensation and reward, with the goal beyond its pages of a conceptual and neural synthesis of these two historically separate fields of inquiry. For navigation in such a quest, we propose sensory-specific HARP as an instrument for unison.
HARP posits that sensation and reward transcend cooperation, e.g., that the neural representation of a sensation’s “sensory” characteristics and “behavioral” meaning are one and the same. Cortical topology along various modalities and dimensions of the sensory world identifies a sensation’s specific features, but at the same time (and perhaps even more importantly), can attribute feature-specific subjective value (meaning) by the relative amount of cortical representation along a pertinent stimulus dimension.
Is a function of HARP to track the value of specific sensations? One might predict that the subjective value of sensation will be greatest for sensory events that predict the largest rewards, and that HARP will be greatest for signals of high reward magnitude. Furthermore, these salient sensations are likely to be most strongly remembered due to their behavioral importance. If so, the magnitude of a sensation’s representational gain in the cortex should also index its memory strength. Remarkably, learning-induced gains in primary sensory cortical area have been found at the junction of sensation, reward value, and memory.
Recent work from our laboratory (Rutkowski and Weinberger 2005) showed that the more water deprived the subjects were during learning to respond to tones for water rewards, the larger the cortical expansion of tone-specific representational area in A1. Therefore, as the salience of the sensory tone signal increased (with the level of deprivation), the more representational area it gained in the cortex. Subsequently, Bieszczad and Weinberger (2010a) determined a tone signal’s strength in memory by measuring its resistance to extinction. They found that animals with the greatest gains in tone-specific A1 representational area were the most resistant to response extinction. Therefore, gains in sensory cortical area conferred the strength of tone-signal memory. Overall, the findings indicate that HARP indexes a synthesis among sensation, reward, and strength of memory.
Thus, the representation of sensation-specific value might be intrinsic to sensory cortices by experience-dependent allocation of representational areas “casting bets” on the sensations with the highest probability of positive return. Sensory cortical over-representation of specific sensations and their enhanced presence in memory could provide the necessary neural impetus to elicit processes for behavioral reactions as complex as decision making, subjective preference, or aesthetic pleasure. Undoubtedly, there is an orchestration of competing sensory inputs in the reward-circuitry and executive-control areas of the brain. However, these control circuits also influence the inherent activity of their component sensory inputs. For example, acetylcholine is a potent modulator of auditory cortical plasticity (Ashe and Weinberger 1991; Miasnikov, Chen, and Weinberger 2008), as is dopamine (Bao, Chan, and Merzenich 2001; Schicknick et al. 2008; Hui et al. 2009), and the affective amygdala drives the nucleus basalis to release acetylcholine in the cerebral cortex (discussed in Chavez, Mcgaugh, and Weinberger 2009).
The linkage between sensation and reward via learning-induced representational plasticity and memory eases navigation toward their unification in concept and in the brain. That learning produces various forms of HARP in sensory systems and depends on factors such as how learning connects rewards to sensations, and under what motivational conditions, alludes to the possibility that one function of representational plasticity is, in fact, to unite sensation identity with its reward value.
ACKNOWLEDGMENTS
We thank Gabriel K. Hui and Jacquie Weinberger for assistance. This research was supported by research grants from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIDCD), DC-02938, DC-05592, and DC-010013.
REFERENCES
- Ashe J.H., Weinberger N.M. Acetylcholine modulation of cellular excitability via muscarinic receptors: Functional plasticity in auditory cortex. In: Richardson R.T., editor. Activation to Acquisition: Functional Aspects of the Basal Forebrain Cholinergic System. Boston; Birkhauser: 1991. pp. 189–246.
- Bakin J.S., Weinberger N.M. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–86. [PubMed: 2085753]
- Bao S., Chan V.T., Merzenich M.M. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. [PubMed: 11452310]
- Bao S., Chang E.F., Woods J., Merzenich M.M. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–81. [PubMed: 15286790]
- Beitel R.E., Schreiner C.E., Cheung S.W., Wang X., Merzenich M.M. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci USA. 2003;100:11070–75. [PMC free article: PMC196928] [PubMed: 12941865]
- Berlau K.M., Weinberger N.M. Learning strategy determines auditory cortical plasticity. Neurobiol Learn Mem. 2008;89:153–66. [PMC free article: PMC3601836] [PubMed: 17707663]
- Bieszczad K.M., Weinberger N.M. Representational gain in cortical area underlies increase of memory strength. Proc Natl Acad Sci USA. 2010a;107:3793–98. [PMC free article: PMC2840533] [PubMed: 20133679]
- Bieszczad K.M., Weinberger N.M. Learning strategy trumps motivational level in determining learning-induced auditory cortical plasticity. Neurobiol Learn Mem. 2010b;93:229–39. [PMC free article: PMC3192530] [PubMed: 19853056]
- Bieszczad K.M., Weinberger N.M. Remodeling the cortex in memory: Increased use of a learning strategy increases the representational area of relevant acoustic cues. Neurobiol Learn Mem. 2010c;94(2):124–44. [PMC free article: PMC2922489] [PubMed: 20434577]
- Brown M., Irvine D.R.F., Park V.N. Perceptual learning on an auditory frequency discrimination task by cats: Association with changes in primary auditory cortex. Cereb Cortex. 2004;14:952–65. [PubMed: 15115736]
- Campbell A.W. Histological Studies on the Localization of Cerebral Function. Cambridge: Cambridge University Press; 1905.
- Chavez C.M., Mcgaugh J.L., Weinberger N.M. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol Learn Mem. 2009;91:382–92. [PMC free article: PMC3635825] [PubMed: 19028592]
- Diamond D.M., Weinberger N.M. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–60. [PubMed: 3708366]
- Diamond I.T. The subdivisions of the neocortex: A proposal to revise the traditional view of sensory, motor, and associational areas. Progr Psychobiol Physiol Psychol. 1979;8:1–43.
- Farley J., Alkon D.L. Cellular mechanisms of learning, memory, and information storage. AnnuRev Psychol. 1985;36:419–94. [PubMed: 2983604]
- Fearing F. Reflex Action: A Study in the History of Physiological Psychology. Baltimore: William & Wilkins Co; 1930.
- Fleshsig P. Developmental (myelogenetic) localization of the cerebral cortex in the human subject. Lancet. 1901;2:1027–29.
- Gao E., Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA. 1998;95:12663–70. [PMC free article: PMC22888] [PubMed: 9770543]
- Gonzalez-Lima F. Functional brain circuitry related to arousal and learning in rats. In: Ewert J.-P., Arbib M.A., editors. Visuomotor Coordination. New York: Plenum; 1989. pp. 729–765.
- Gonzalez-Lima F., Scheich H. Behav Brain Res. Vol. 20. 1986. Neural substrates for tone-conditioned bradycardia demonstrated with 2-deoxyglucose. II. Auditory cortex plasticity; pp. 281–93. [PubMed: 3741589]
- Heil P., Irvine D.R. The posterior field P of cat auditory cortex: Coding of envelope transients. Cereb Cortex. 1998;8:125–41. [PubMed: 9542892]
- Hui G.K., Wong K.L., Chavez C.M., Leon M.I., Robin K.M., Weinberger N.M. Conditioned tone control of brain reward behavior produces highly specific representational gain in the primary auditory cortex. Neurobiol Learn Mem. 2009;92:27–34. [PMC free article: PMC2891027] [PubMed: 19249380]
- John E.R. High nervous functions: Brain functions and learning. Annu Rev Physiol. 1961;23:451–84. [PubMed: 13790206]
- Kaas J.H., Hackett T.A. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–99. [PMC free article: PMC34351] [PubMed: 11050211]
- Kacelnik O., Nodal F.R., Parsons C.H., King A.J. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol. 2006;4:e71. [PMC free article: PMC1393755] [PubMed: 16509769]
- Kilgard M.P., Merzenich M.M. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:3205–9. [PMC free article: PMC122497] [PubMed: 11880653]
- Kisley M.A., Gerstein G.L. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. Eur J Neurosci. 2001;13:1993–2003. [PubMed: 11403693]
- Masterton R.B. Central auditory system. ORL J Otorhinolaryngol Relat Spec. 1993;55:159–63. [PubMed: 8321548]
- Miasnikov A.A., Chen J.C., Weinberger N.M. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiol Learn Mem. 2008;90:443–54. [PMC free article: PMC2556567] [PubMed: 18573347]
- Molchan S.E., Sunderland T., Mcintosh A.R., Herscovitch P., Schreurs B.G. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA. 1994;91:8122–26. [PMC free article: PMC44557] [PubMed: 8058767]
- Morris J.S., Friston K.J., Dolan R.J. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc Biol Sci. 1998;265:649–57. [PMC free article: PMC1689028] [PubMed: 9608726]
- Nakahara K., Ohbayashi M., Tomita H., Miyashita Y. The neuronal basis of visual memory and imagery in the primate: A neurophysiological approach. Adv Biophys. 1998;35:103–19. [PubMed: 9949767]
- Phillips M.L., Heining M. Neural correlates of emotion perception: From faces to taste. In: Rouby C., Schaal B., editors. Olfaction, Taste, and Cognition. New York: Cambridge University Press; 2002. pp. 196–208.
- Polley D.B., Heiser M.A., Blake D.T., Schreiner C.E., Merzenich M.M. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci USA. 2004;101:16351–56. [PMC free article: PMC528983] [PubMed: 15534214]
- Recanzone G.H., Schreiner C.E., Merzenich M.M. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. [PMC free article: PMC6576321] [PubMed: 8423485]
- Scheich H., Brechmann A., Brosch M., Budinger E., Ohl F.W. The cognitive auditory cortex: Task-specificity of stimulus representations. Hear Res. 2007;229:213–24. [PubMed: 17368987]
- Schicknick H., Schott B.H., Budinger E., Smalla K.-H., Riedel A., Seidenbecher C.I., Scheich H., Gundelfinger E.D., Tischmeyer W. Dopaminergic modulation of auditory cortex-dependent memory consolidation through mTOR. Cereb Cortex. 2008;18:2646–58. [PMC free article: PMC2567422] [PubMed: 18321872]
- Schouenborg J. Learning in sensorimotor circuits. Curr Opin Neurobiol. 2004;14:693–97. [PubMed: 15582370]
- Schreurs B.G., Mcintosh A.R., Bahro M., Herscovitch P., Sunderland T., Molchan S.E. 1997. Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response J Neurophysiol 77 2153 63. [PubMed: 9114262]
- Suzuki W.A., Eichenbaum H. The neurophysiology of memory. Ann NY Acad Sci. 2000;911:175–91. [PubMed: 10911874]
- Thompson R.F., Patterson M.M., Teyler T.J. The neurophysiology of learning. Annu Rev Psychol. 1972;23:73–104. [PubMed: 4335280]
- Weinberger N.M. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learn Mem. 2007;14:1–16. [PMC free article: PMC3601844] [PubMed: 17202426]
- Weinberger N.M. Reconceptualizing the primary auditory cortex: Learning, memory and specific plasticity. In: Winer J. A., Schreiner C.E., editors. The Auditory Cortex. New York: Springer-Verlag; 2010 in press.
- Weinberger N.M., Diamond D.M. Physiological plasticity in auditory cortex: Rapid induction by learning. Prog Neurobiol. 1987;29:1–55. [PubMed: 3295997]
- Young R.M. Mind, Brain and Adaptation in the Nineteenth Century. Oxford: Clarendon Press; 1970.
- SCOPE AND GOALS
- CONCERNING THE TRADITIONAL EXCLUSION OF LEARNING, MEMORY, AND COGNITION FROM PRIMARY SENSORY CORTICES
- ASSOCIATIVE REPRESENTATIONAL PLASTICITY
- AUDITORY CORTICAL PLASTICITY AND LEARNING STRATEGY
- LEARNING STRATEGY TRUMPS LEVEL OF REWARD IN DETERMINING CORTICAL PLASTICITY
- PLASTICITY IN SENSATION MAY DEPEND ON “MATCHING” OF CRITICAL CUES AND NEURONAL RESPONSE PROCLIVITIES
- CONCLUSIONS
- CODA
- ACKNOWLEDGMENTS
- REFERENCES
- Review Visualizing Adult Cortical Plasticity Using Intrinsic Signal Optical Imaging.[In Vivo Optical Imaging of Bra...]Review Visualizing Adult Cortical Plasticity Using Intrinsic Signal Optical Imaging.Frostig RD, Chen-Bee CH. In Vivo Optical Imaging of Brain Function. 2009
- Review Human brain plasticity: evidence from sensory deprivation and altered language experience.[Prog Brain Res. 2002]Review Human brain plasticity: evidence from sensory deprivation and altered language experience.Neville H, Bavelier D. Prog Brain Res. 2002; 138:177-88.
- Learning strategy refinement reverses early sensory cortical map expansion but not behavior: Support for a theory of directed cortical substrates of learning and memory.[Neurobiol Learn Mem. 2015]Learning strategy refinement reverses early sensory cortical map expansion but not behavior: Support for a theory of directed cortical substrates of learning and memory.Elias GA, Bieszczad KM, Weinberger NM. Neurobiol Learn Mem. 2015 Dec; 126:39-55. Epub 2015 Oct 24.
- Review Performance of a Computational Model of the Mammalian Olfactory System.[Neuromorphic Olfaction. 2013]Review Performance of a Computational Model of the Mammalian Olfactory System.Benjaminsson S, Herman P, Lansner A. Neuromorphic Olfaction. 2013
- Review Associative representational plasticity in the auditory cortex: a synthesis of two disciplines.[Learn Mem. 2007]Review Associative representational plasticity in the auditory cortex: a synthesis of two disciplines.Weinberger NM. Learn Mem. 2007 Jan-Feb; 14(1-2):1-16. Epub 2007 Jan 3.
- Introduction: From Traditional Fixed Cortical Sensationism to Contemporary Plast...Introduction: From Traditional Fixed Cortical Sensationism to Contemporary Plasticity of Primary Sensory Cortical Representations - Neurobiology of Sensation and Reward
Your browsing activity is empty.
Activity recording is turned off.
See more...
