NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton (FL): CRC Press/Taylor & Francis; 2011.
18.1. INTRODUCTION
A person stands transfixed before a Mark Rothko abstract painting oblivious to everything else. Embedded in this scene are questions that strike at the very heart of this book. What is the sensation being experienced? If the color or form of the painting were altered slightly, would the experience be the same? Why do some visitors to the museum glance at the same painting and shrug their shoulders before being absorbed by a Cezanne landscape or a Rembrandt portrait? Why do visitors bother to gaze at paintings at all? What is the nature of the reward that compels them to travel distances, pay entrance fees, and negotiate crowds to stare at pieces of canvas? What, if anything, distinguishes this rewarding experience from the pleasure of gazing at an attractive person or from the anticipation of a good meal?
In this chapter, I explore these issues in aesthetics through the lens of cognitive neuroscience. The term aesthetics is used broadly here, to encompass the perception, production, and response to art, but also to include the responses to objects and scenes that evoke a response that could be considered aesthetic. The nature of this response is something to which I shall return later in this chapter. I start by reviewing recent comments on the relationship of art and the brain made by visual neuroscientists. I then describe a framework that might guide research in neuroaesthetics. Following that, I review empirical work conducted thus far. Finally, I suggest how progress could be made in this nascent field.
At the outset, I should be clear about limits to defining art (Carroll 2000). Some philosophers have claimed that defining art with necessary and sufficient conditions is not possible (Weitz 1956). In response to such claims, recent theoreticians have defined art by its social and institutional (Dickie 1969) or its historical context (Danto 1964). Cognitive neuroscientists are unlikely to address sociological or historical conceptions of art. They are likely to sidestep definitional issues and focus on accepted examples of artwork or properties of these works as probes for experiments.
18.3. OBSERVATIONS ON THE RELATIONSHIP OF ART AND THE BRAIN
With rare exceptions, it is not clear that neuroscientists consider aesthetics worthy of inquiry. And some aestheticians probably consider neuroscientific inquiry into aesthetics an abomination. Only recently has neuroscience joined a tradition of empirical aesthetics that dates back to Fechner in the nineteenth century (Fechner 1876; and see Chapter 2 in this volume for more information about Fechner). The first wave of writings on aesthetics by neuroscientists points to parallels between art and organizational principles of the brain.
Zeki (1999) argued forcefully that no theory of aesthetics is complete without an understanding of its neural underpinnings. He suggested that the goals of the nervous system and of artists are similar. Both are driven to understand essential attributes of the world. The nervous system decomposes visual information into different components, such as color, luminance, and motion. Similarly, many artists, particularly within the last century, isolated different visual attributes. For example, Matisse emphasized color and Calder emphasized motion. Zeki suggests that artists, like visual neuroscientists, endeavor to uncover important distinctions in the visual world. In doing so, they discover modules of the visual brain. Recently, Cavanagh (2005) has similarly claimed that the artists’ goals resemble those of the nervous system. He observes that paintings often violate the physics of shadows, reflections, colors, and contours. Rather than adhering to physical properties of the world, these paintings reflect perceptual shortcuts used by the brain. Artists, in experimenting with forms of depiction, discovered what psychologists and neuroscientists are now identifying as principles of perception.
Livingstone (2002) and Conway (Conway and Livingstone 2007) focused on how artists make use of complex interactions between different components of vision in creating their paintings. The dorsal (where) and ventral (what) processing distinction is a central tenet in visual neuroscience (Ungerleider and Mishkin 1982) (though see Chapter 8 in this volume). The dorsal stream is sensitive to contrast differences, motion, and spatial location. The ventral stream is sensitive to simple form and color. Livingstone suggests that the shimmering quality of water or the sun’s glow on the horizon seen in some impressionist paintings (e.g., the sun and surrounding clouds in Monet’s Impression Sunrise) is produced by isoluminant objects distinguishable only by color. The dorsal stream is insensitive to these elements of the image. Since the dorsal stream identifies motion (or the lack thereof) and spatial location, Livingstone argues that isoluminant forms are not fixed with respect to motion or spatial location and are experienced as unstable or shimmering. Conversely, since shape can be derived from luminance differences, she argues that artists use contrast to produce shapes, leaving color for expressive rather than descriptive purposes (as in Derain’s portrait of Matisse). Livingstone highlights the combinatorial properties of visual attributes. Artists use these combinatorial properties to produce specific aesthetic effects.
Ramachandran and Hirstein (1999) proposed a set of perceptual principles underlying aesthetic experiences. For example, they emphasize the “peak shift” phenomena as offering insight into the aesthetics of abstract art by relying on Tinbergen’s (1954) work. Tinbergen demonstrated that seagull chicks beg for food from their mothers by pecking on a red spot near the tip of the mother’s beak. It turns out that a disembodied long thin stick with three red stripes near the end evokes an exaggerated response from these chicks. Ramachandran and Hirstein propose that neural structures that evolved to respond to specific visual stimuli respond more vigorously (a shift in their peak response) to underlying primitives of that form even when the viewer is not aware of the primitive. Their insight is that abstract art may be tapping into such visual primitives.
These examples of recent comments reflect an emerging recognition by neuroscientists that visual aesthetics are an important part of human experience, which ought to conform to principles of neural organization. Intriguing parallels exist between the concerns and techniques of artists and the organization of the visual brain. Some components of visual aesthetics should be amenable to empirical methods from cognitive neuroscience. The challenge ahead is to transform these comments into testable hypotheses and experimental paradigms.
18.3. A FRAMEWORK FOR NEUROAESTHETICS RESEARCH
A cognitive neuroscience research program in visual aesthetics rests on two principles (Chatterjee 2002). First, visual aesthetics, like vision in general, has multiple components. Second, an aesthetic experience emerges from a combination of responses to different components of a visual object. The process by which humans visually recognize objects offers a framework from which to consider these components. Investigations can be focused on these components and on their combinatorial properties.
The nervous system processes visual information both hierarchically and in parallel (Van Essen et al. 1990; Zeki 1993; Farah 2000). The levels of this processing can be classified as early, intermediate, and late vision (Marr 1982). Early vision extracts simple elements from the visual environment, such as color, luminance, shape, motion, and location (Livingstone and Hubel 1987; Livingstone 1988). These elements are processed in different parts of the brain. Intermediate vision segregates some elements and groups others together to form coherent regions in what would otherwise be a chaotic and overwhelming sensory array (Biederman and Cooper 1991; Grossberg, Mingolla, and Ros 1997; Vecera and Behrmann 1997; Ricci, Vaishnavi, and Chatterjee 1999). Late vision selects which of these coherent regions to scrutinize and evokes memories from which objects are recognized and meanings attached (Chatterjee 2003a; Farah 2000).
This sequence of visual processing is likely to be reflected in aesthetics (Chatterjee 2003b) (for a related model, see Helmut et al. 2004). Any work of art can be decomposed into its early, intermediate, and late vision components, and individual works of visual art (paintings) can be identified that exemplify each of these different componential stages (Figure 18.1). Aesthetic writings commonly distinguish between form and content (e.g., Russell and George 1990; Woods 1991). Similarly, scientists observe that early and intermediate vision process form and later vision processes content. Figure 18.2 shows a model of how the neuroscience of visual aesthetics might be mapped. The early features of an art object might be its color and its spatial location. These elements would be grouped together to form larger units in intermediate vision. Such grouping occurs automatically. The neural basis of grouping is not well understood, but it likely involves extrastriate cortex (Biederman and Cooper 1991; Grossberg, Mingolla, and Ros 1997). Grouping creates “unity in diversity,” a central notion of compositional balance.
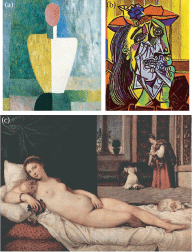
FIGURE 18.1
(See Color Insert)Visual aesthetics recapitulates visual processing: a hierarchical progression as documented through the brushstrokes of three renowned artists. Each painting depicts the female figure, through successive levels of representational complexity, (more...)

FIGURE 18.2
A general information-processing model to guide research in neuroaesthetics. See text for details.
If compositional form is apprehended automatically by intermediate vision, then sensitivity to such form should also be automatic. Subjects are sensitive to compositional form “at a glance,” with exposures as short as 50 msec (Locher and Nagy 1996). Intriguingly, preference for form predominates when images are shown over short exposure times, while preference for detail predominates when images are shown for slightly longer times (Ognjenovic 1991). Combinations of early and intermediate visual properties (e.g., color, shape, composition) engage attentional circuits mediated by frontal-parietal neural networks. Attentional modulation of early vision (Motter 1993, 1994; Shulman et al. 1997; Watanabe et al. 1998) is likely to contribute to a more vivid experience of the stimulus.
Beyond perception in visual aesthetics, two other aspects of aesthetics are important. The first is the emotional response to an aesthetic image; the second is how aesthetic judgments are made. The anterior medial temporal lobe, medial and orbitofrontal cortices, and subcortical structures mediate emotions in general, and reward systems in particular (Schultz, Dayans, and Mortague 1997; O’Doherty et al. 2001; Elliott, Friston, and Dolan 2000; Delgado et al. 2000; Breiter et al. 2001; Berridge and Kringelbach 2008). Aesthetic judgments about stimuli, as measured by preference ratings or appraisals, are likely to engage widely distributed circuits, most importantly the dorsolateral frontal and medial frontal cortices.
18.4. EMPIRICAL EVIDENCE: LESION STUDIES
Investigations of patients with brain damage have contributed greatly to our understanding of cognitive and affective systems. This approach also has substantial promise in advancing neuroaesthetics. Diseases of the brain can impair our ability to speak or comprehend language, to coordinate movements, to recognize objects, to apprehend emotions, and to make logical decisions. By contrast, while damage to the brain can certainly impair the ability to produce art, paradoxically, in some cases art abilities seem to improve. Brain damage can create a disposition to produce visual art, provide artists with a unique visual vocabulary, add to artists’ descriptive accuracy, and enhance their expressive powers. These paradoxical improvements offer unique insights into the creative underpinnings of artistic output. They are reviewed elsewhere (Chatterjee 2004, 2006, 2009) and will not be discussed further in this chapter, in keeping with the present focus on sensation and reward.
Studies of people with brain damage also can advance our understanding of the perception and experience of art. Some people with brain damage probably do not perceive art in the same way that non-brain-damaged individuals do and their emotional responses to artwork may very well differ from those of people without brain damage (cf. Chapter 16). However, neuropsychological investigations of aesthetic perception to date are non-existent. There is no adequate instrument to provide basic quantitative assessments of a person’s apprehension artwork. We are currently developing such a tool, The Assessment of Art Attributes (Chatterjee et al. 2010). This assessment assumes that the perception of art can be organized along different perceptual and conceptual attributes (see Table 18.1). Using such an assessment, one could begin to investigate groups of patients and ascertain the relationship of brain damage to selective deficits or enhancements in art perception. Much remains to be learned if we can develop adequate methods and measurements for this line of inquiry.
TABLE 18.1
A Pervasive Concern in Empirical Aesthetics has been Distinguishing between Form and Content of Artwork.
18.5. EMPIRICAL EVIDENCE: IMAGING STUDIES OF BEAUTY
Beauty is integral to most people’s concept of aesthetics (Jacobsen et al. 2004). Of course, not all art is beautiful and artists do not always intend to produce beautiful things. But beauty remains a central concept in discussions of art. Understanding the neural basis of the apprehension of and response to beauty might give us insight into the apprehension of and response to visual art. Facial beauty has received particular attention.
The response to facial beauty is likely to be deeply encoded in our biology. Cross-cultural judgments of facial beauty are quite consistent (Etcoff 1999; Perrett, May, and Yoshikawa 1994; Jones and Hill 1993). Adults and children within and across cultures agree in their judgments of facial attractiveness (Langlois et al. 2000), suggesting that universal principles of facial beauty exist. Similarly, infants look longer at attractive faces within a week of being born and the effects of facial attractiveness on infants’ gaze generalize across race, gender, and age by 6 months (Langlois et al. 1991; Slater et al. 1998). Thus, the disposition to engage attractive faces is present in brains that have not been modified greatly by experience. Some components of beauty are shaped further by cultural factors (Cunningham et al. 2002), but the universal components are likely to have distinct neural underpinnings.
Several studies report that attractive faces activate neural circuitry involved in reward systems, including the orbitofrontal cortex, the nucleus accumbens, the ventral striatum (Kampe et al. 2001; Aharon et al. 2001; O’Doherty et al. 2003; Ishai 2007; Kranz and Ishai 2006), and the amygdala (Winston et al. 2007). These regional activations are interpreted as reflecting emotional valences attached to attractive faces (Senior 2003). The particular emotional valences are those involved in the expectation of rewards and the satisfaction of appetites. The idea that attractive faces are rewarding stimuli, at least for men, is evident behaviorally. Heterosexual men discount higher future rewards for smaller immediate rewards with attractive female faces (Wilson and Daly 2004). Presumably these patterns of neural activation reflect ways in which attractive faces influence mate selection (Ishai 2007).
Perceptual features of faces, such as averageness, symmetry, the structure of cheek bones, the relative size of the lower half of the face, and the width of the jaw, influence people’s judgments of facial beauty (Grammer and Thornhill 1994; Enquist and Arak 1994; Penton-Voak et al. 2001). Winston et al. (2007) found left posterior occipito-temporal activity was enhanced by facial attractiveness. Similarly, Kranz and Ishai (2006) found greater activations in the lateral fusiform gyrus for attractive female faces than for unattractive female faces.
We conducted a study in which participants judged the attractiveness or matched the identity of pairs of faces. Attractiveness judgments evoked neural activity within a distributed network involving ventral visual association cortices and parts of dorsal posterior parietal and prefrontal cortices (Chatterjee et al. 2009). We interpreted the parietal, medial, and dorsolateral frontal activations as representing the neural correlates of the attention and decision-making components of this task. We also found positively correlated activity within the insula and negatively correlated activations within the anterior and posterior cingulate cortex. We inferred that these patterns represent the emotional responses to attractiveness. Importantly, when subjects matched the identity of faces, attractiveness continued to evoke neural responses in ventral visual areas, with a strength indistinguishable from that when participants considered beauty explicitly, suggesting that this ventral occipital region responds to beauty automatically.
Facial attractiveness is apprehended automatically (Palermo and Rhodes 2007; Olson and Marshuetz 2005) and has pervasive social effects beyond its specific role in mate selection. Attractive individuals are considered intelligent, honest, pleasant, and natural leaders (Kenealy, Frude, and Shaw 1988; Lerner et al. 1991; Ritts, Patterson, and Tubbs 1992), and are viewed as having socially desirable traits, such as strength and sensitivity (Dion, Berscheid, and Walster 1972). The cascade of neural events that bias social decisions is likely to be triggered by an early perceptual response to attractiveness. We proposed that neural activity within ventral visual cortices in response to facial attractiveness serves as the initial trigger for this cascade. The fact that this ventral occipital region of activation extended beyond cortical regions especially sensitive to faces per se raises the possibility that this area may be responsive to aesthetic objects more generally (Chatterjee et al. 2009).
18.6. EMPIRICAL EVIDENCE: IMAGING STUDIES OF ART
Very few studies have used art to examine the neural bases of aesthetics. While the goals in these studies were similar, their experimental approaches differed and the results at first glance appear varied. Kawabata and Zeki (2004) asked participants to rate abstract, still life, landscape, or portraitures paintings as beautiful, neutral, or ugly. They used a fixed-effects analysis in a 3 × 4 factor event-related design with event types segregated as beautiful, neutral, or ugly in one of the four painting categories. Not surprisingly, they found that the pattern of activity within the ventral visual cortex varied depending on whether subjects were looking at portraits, landscapes, or still-lives. In orbitofrontal cortex (BA 11) they found greater activity for beautiful than for ugly or neutral stimuli. In the anterior cingulate (BA 32) and left parietal cortex (BA 39), they found greater activity for beautiful than for neutral stimuli. Only activity within the orbitofrontal cortex increased with the beauty of all the painting types and the authors interpreted this activity as representing the neural underpinnings of the aesthetic emotional experience.
Vartanian and Goel (2004) used images of representational and abstract paintings in an fMRI study. They found that activity within the occipital gyri bilaterally and the left anterior cingulate increased with preference ratings. They also found that activity within the right caudate decreased as preference ratings decreased. Representational paintings evoked more activity within the occipital poles, the precuneus, and the posterior middle temporal gyrus than did abstract paintings.
Cela-Conde et al. (2004) used magnetoencephalography to record event potentials when participants viewed images of artworks and photographs. Participants judged whether or not the images were beautiful. Beautiful images evoked greater neural activity than not-beautiful images over the left dorsolateral prefrontal cortex with a latency of 400–1000 msec. The authors infer that this region is involved in making aesthetic judgments.
Jacobsen et al. (2005) used a different strategy to investigate the neural correlates of beauty in an fMRI study. Rather than use actual artworks as their stimuli, they used a set of geometric shapes designed in the laboratory. Participants judged whether the images were beautiful or whether the images were symmetric. Participants found symmetric patterns more beautiful than non-symmetric ones. Aesthetic judgments more than symmetry judgments activated the medial frontal cortex (BA 9/10), the precuneus, and the ventral prefrontal cortex (BA 44/47). The left intraparietal sulcus was conjointly active for symmetry and beauty judgments. Both beauty and complexity of the images evoked activity within orbitofrontal cortex. In a follow-up study using the same stimuli (Hofel and Jacobsen 2007), they found that beauty generated a lateral positive evoked potential in a temporal window between 360 and 1225 msec.
De Tommaso, Sardaro, and Livrea (2008) also used artworks that were rated as beautiful, neutral, and ugly. They found that gazing at beautiful paintings raised pain thresholds and at the same time inhibited P2 evoked potentials. The generators of the P2 potentials were localized to the anterior cingulate. These results suggest that aesthetic objects even in this laboratory setting are sufficiently engaging as to distract participants from unpleasant environmental stimuli.
One might be disheartened that these studies, all investigating aesthetics, report different patterns of activation. Nadal et al. (2008) suggest that the results of these studies are compatible within the general model (see Figure 18.2) that I proposed (Chatterjee 2003b). Engaging visual properties of paintings increase activity within ventral visual cortices (Vartanian and Goel 2004). Aesthetic judgments activate parts of dorsolateral prefrontal and medial prefrontal cortices (Cela-Conde et al. 2004; Jacobsen et al. 2005), and emotional responses to these stimuli activate the orbitofrontal (Kawabata and Zeki 2004; Jacobsen et al. 2005) as well as anterior cingulate (Kawabata and Zeki 2004; Vartanian and Goel 2004; de Tommaso, Sardaro, and Livrea 2008) cortices.
18.7. WHY ART?
Returning to the questions posed at the beginning of this chapter, why do people stand in hermetically sealed buildings that we call museums and stare at patches of paint on canvases? And why at some canvases, and not others? Any answer to these questions is necessarily speculative. I suggest that three factors are at play: the drive to beauty, the aesthetic attitude, and the institutional frameworks that promote and display art.
18.7.1. Drive for Beauty
Most people are drawn to beauty. As we have suggested in our own work, neural responses to attractive faces occur automatically, even when people are not explicitly judging beauty (Chatterjee et al. 2009). Three kinds of evolutionary arguments are made for the attraction to beauty. The first and most obvious is the way that beauty influences mate selection. When it comes to faces and bodies, the link between mate selection and beauty is clear (Rhodes et al. 2002). Attractive features represent phenotypic attributes that are desirable in selecting mates, such as genetic health and levels of immunocompetence (Etcoff 1999; Grammer et al. 2003; Penton-Voak et al. 2001; Perrett et al. 1998; Symons 1979; Thornhill and Gangestad 1999). (Also see Chapter 17 for more discussion of genetic health vis a vis olfactory phenotypes.) In this view, the nervous system has evolved to be attracted to specific configurations of facial features that signal “good genes,” configurations that we have come to regard as beautiful. A variation of this view is the “costly signal” proposal (Zahavi and Zahavi 1997). Male birds attract female birds with extravagant plumage or elaborate songs that appear to be maladaptive. They interfere with movement and also attract predators. The costly signal proposal is that such displays advertise the unusual vigor of the displayer. The displayer can afford to indulge in these seemingly maladaptive behaviors because they are so fit to begin with. Art making requires considerable time and effort and, as a costly display, would similarly advertise one’s fitness in the competition for mates.
Others have argued that conceptualizing art as derived from an attraction to beauty that is directly linked to mating behavior takes an unnecessarily narrow stance on its adaptive significance for humans. Dissanayake (2008) marshals considerable evidence for art’s role in promoting social cohesion. She rejects relatively recent and specifically Western notions embedded in aesthetic discussions, such as the importance of novelty or individual creativity, and takes an ethnographic view of the adaptive significance of art to humanity. For her, the behavior of “making special” is critical to art. Ordinary objects, movements, patterns, and sounds are transformed into something extraordinary by exaggeration, repetition, embellishment, and so on (Brown and Dissanayake 2009). Beauty, virtuosity, costliness, and emotional investment are all ingredients in the process of making something special. By focusing on the ritualistic nature of art making and appreciation she emphasizes the adaptive importance of art in enhancing cooperation, and encouraging cohesion and continuity within local societies.
A different kind of argument for why we have come to regard things as beautiful has to do with the nature of mental processing involved when apprehending objects (Rentschler et al. 1999). On this account preferences arise as a by-product of a general information-processing mechanism. As mentioned before, a leading candidate for such a mechanism is the extraction of a prototype, or the central exemplar of a category. People prefer prototypes of different kinds of stimuli, such as color (Martindale and Moore 1988) and music (Smith and Melara 1990). Faces would presumably be another category of stimuli subject to this biased preference for prototypes (Halberstadt and Rhodes 2000). A variation on the information-processing account for preference is the idea that people prefer to look at things that are processed “fluently.” Fluency, or the ease with which one processes objects, is rendered by specific physical features of objects as well their conceptual characteristics. Features of the object, such as symmetry and figure ground relationships, as well as the experiences of the viewer, influence fluent processing. The important point for our discussion is that processing fluency is associated with a positive affective response and aesthetic pleasure (Reber, Schwarz, and Winkielman 2004; Armstrong and Detweiler-Bedell 2008). People like what they process easily. Thus, familiarity as established by mere exposure and which contributes to processing fluency influences people’s preferences for simple displays in laboratories (Moreland and Zajonc 1976). These influences also extend beyond the laboratory. Familiarity also influences which impressionist paintings are regarded highly (Cutting 2007).
Given that specific configurations of physical objects contribute to the experience of beauty, regardless of whether this contribution is driven by mating desires and rituals, promotes social cohesion, or facilitates processing, how do these experiences relate to the aesthetic experience? Herein lies a paradox, as I describe below.
18.7.2. The Aesthetic Attitude
Evolutionary arguments for the importance of beauty emphasize its adaptive significance. Mate selection, social cohesion, and better information processing all have utility. The point of adaptation is to be useful in propagating the species. This utilitarian casting to aesthetics is at odds with an idea proposed in the eighteenth century (Kant 1790/1987) that the aesthetic attitude is one of “disinterested interest.” While Kant’s idea is by no means agreed on by all aestheticians, I base my speculations on its central role. Aesthetic pleasures are self-contained. They do not intrinsically encompass additional desires. That is not to say that an artwork cannot evoke utilitarian desires, such as the desire to own it or to display it to impress others. However, these rewards are not part of the aesthetic experience.
Can neuroscience contribute to an understanding of disinterested interest? Berridge and colleagues have drawn a distinction between “liking” and “wanting” (Wyvell and Berridge 2000; Berridge and Kringelbach 2008). Liking seems to be instantiated in the nucleus accumbens shell and the ventral pallidum mediated by opioid and GABAergic neurotransmitter systems. By contrast, the mesolimbic dopaminergic system, which includes the nucleus accumbens core, might mediate wanting, and cortical structures such as the cingulate and orbitofrontal cortex contribute to further conscious modulations of these liking and wanting experiences. This liking/wanting distinction is made in a rodent model with experiments using sweet and bitter tastes. Whether it generalizes to humans and in response to visual stimuli remains to be seen. However, the idea of a self-contained reward system could be the neural basis for aesthetic disinterested interest.
I suggest that neural circuitry for liking is an exaptation within our reward systems. That is, it is co-opted within our reward systems, cleaved off related reward systems with clear utilitarian designs of satisfying appetitive desires. Perhaps it developed to allow humans to maintain some distance from the objects of desire and has now come to serve the aesthetic attitude. However, at the extremes of aesthetic experiences lies a potential problem: it would be maladaptive. The most profound aesthetic experiences involve a refined liking, often described as awe or feeling the sublime, in which wanting has been tossed aside, but also in which individuals lose themselves in the experience. As mentioned earlier (de Tommaso, Sardaro, and Livrea 2008), they might not even feel pain! Imagine an early human in the savannah struck in rapture at a beautiful sunset, oblivious to the predator lying in wait in the tall grass. Or a modern human crossing the street suddenly immobilized by the soft evening light on a beautiful building, oblivious to cars whizzing by. The cost of the aesthetic experience is rendering the individual vulnerable. Entering an aesthetic attitude is dangerous.
18.7.3. The Institutional Context for Art
The display and contemplation of objects that evoke aesthetic experiences are better done in safe havens. The havens could be protective caves at Lascaux or sanctuaries of medieval Europe. Until recently much art was displayed in religious institutions. The religious rapture such imagery can evoke is close to the aesthetic attitude. One is lifted beyond utilitarian desires and everyday concerns and in fact lifted beyond oneself. Religious meditations while gazing at visual icons serve as a vehicle to spirituality (Nouwen 1987).
In secular societies, I suggest that private collections and museums play the role of providing a sanctuary within which one can safely contemplate images. They also bracket aesthetic experiences and publicly recognize the sense in which artworks are “special,” as described by Dissanyake (2008). Of course providing a sanctuary for and bracketing that which is special are not the only roles for museums. As discussed extensively by contemporary theoreticians, museums are embedded within a broader “art world” that encompasses a rich environment of comment, critique, and internal dialogues (Dickie 1969; Danto 1964), and is far removed from getting lost in a beautiful sunset. Thus, within internal dialogues of the art world, things regarded as ugly or incomprehensible by most might be highlighted and promoted. The drive to beauty that I suggest is the initiating trigger for artistic experiences might be regarded as naïve by some that establish institutional norms for art. But these movements are recent institutional embellishments on general human tendencies. Evolutionary biology in general and neuroscience in particular has little to contribute to this level of analysis of conceptions of art.
18.8. CONCLUDING COMMENTS
The neuroscience of visual aesthetics is in its infancy. With a field so wide open, progress in any direction would be an advance. Here, I suggest four areas worthy of focus. (1) A finer-grained exploration of the nature and contributions of sensations to aesthetic experiences. As already mentioned, visual art like any visual object can be decomposed into distinct attributes, such as color, line, texture, form, and so on. How do these attributes contribute to the aesthetic experience? How do we measure the contributions of these attributes? We have begun to develop a scale to do so for lesion studies (Chatterjee et al. 2010), but much work needs to be done.
How much of the aesthetic experience resides in a perceptual experience and how much resides in the emotional response to artwork? Paintings of landscapes are likely to activate the parahippocampus, still-lives the lateral occipital cortex, and portraits the fusiform gyrus. Does beauty modify these activations further? Perhaps these activations simply reflect category-specific activations evoked by perception itself, and the aesthetic work is done within reward systems. However, many feel that we perceive beautiful objects more vividly than non-beautiful objects. Some studies show neural responses to beauty within the ventral occipito-temporal cortex. Are these activations modulated by attention or is there an independent aesthetic factor that modulates neural activity? Moreover, within the ventral visual cortex, do general “visual beauty detectors” exist?
(2) Understanding the nature of aesthetic judgment. People vary in their aesthetic sensitivities. Aesthetic sensitivity has been referred to as a “T-factor” for taste (Eysenck 1941; Eysenck and Hawker 1994). People can also develop taste with training. Behavioral studies consistently show differences in the way that art-experienced individuals and art-naïve individuals look at artwork (Locher, Stappers, and Overbeeke 1999). Understanding the neural basis for taste and the ways aesthetic judgment might be modified with training would be of great interest.
The studies conducted thus far suggest that parts of the dorsolateral and medial prefrontal cortex are involved in making aesthetic judgments. These studies are unable to distinguish whether these brain activations are specific to aesthetic judgments or are part of neural systems that makes judgments regardless of the domain under consideration. Do aesthetic judgments engage neural circuits that are not engaged in other judgments?
(3) Characterizing the “reward” of aesthetic experiences. The imaging studies reviewed here implicate the orbitofrontal cortex, the anterior and posterior cingulate, the ventral striatum including the nucleus accumbens, the caudate, and the amygdala in one or another study as mediating the emotional response to beauty or to artwork. Presumably these structures differ in their functions (Berridge and Kringelbach 2008). Clearly a better sense of how these structures contribute to an overall emotional aesthetic experience is needed. Such an understanding would be necessary, for example, to address what is meant by “sublime,” an emotional experience mentioned frequently in aesthetics (Kant 1790/1987), but not in affective neuroscience.
(4) Constraining evolutionary theories. Evolutionary biology and psychology provide principles that help organize and interpret complex behavior. The universal nature of producing ornamentations and being pleased by these ornamentations certainly encourages evolutionary analyses of art. The challenge for evolutionary aestheticians is how to constrain this theorizing and test hypotheses. Evidence is gathered from disparate sources to argue for example whether art is adaptive (and in what way) or whether it is a by-product of adaptation. Comparative neuroscience has not contributed to the evolutionary discussions about art, but may provide some constraints on the theorizing. Even if such contributions from neuroscience were to be made, these hypotheses remain inherently post hoc (not unlike my speculations on “why art?”). The research enterprise lacks clear prospective tests of hypotheses and explicit conditions of falsifiability of experimental sciences.
In conclusion, aesthetics and a concern for beauty are central human experiences. They are integrally related to a specific set of sensations and rewards. While a small but dedicated group of investigators has been engaged in empirical aesthetics since the mid-nineteenth century, only recently has neuroscience wandered into this area. Some of the most basic questions about neuroaesthetics remain to be answered. The basic frameworks and methods from cognitive and affective neuroscience are in place for neuroaesthetics to mature as a science.
ACKNOWLEDGMENT
I would like to thank Lisa Santer for a critical reading of an earlier draft of this chapter and Jay Gottfried for pushing me to speculate beyond my natural inclinations.
REFERENCES
- Aharon I., Etcoff N., Ariely D., Chabris C. F., O’Connor E., Breiter H. C. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–51. [PubMed: 11709163]
- Armstrong T., Detweiler-Bedell B. Beauty as an emotion: The exhilarating prospect of mastering a challenging world. Review of General Psychology. 2008;12(4):305–29.
- Berridge K., Kringelbach M. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology. 2008;199(3):457–80. [PMC free article: PMC3004012] [PubMed: 18311558]
- Biederman I., Cooper E. E. Priming contour-deleted images: Evidence for intermediate representations in visual object recognition. Cognitive Psychology. 1991;23:393–419. [PubMed: 1884597]
- Breiter H. C., Aharon I., Kahneman D., Dale A., Shizgal P. Functional imaging of neural response to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. [PubMed: 11395019]
- Brown S., Dissanayake E. The arts are more than aesthetics: Neuroaesthetics as narrow aesthetics. Skov M., Vartanian O. Amityville, NY: Baywood Publishing Company; Neuroaesthetics. 2009:43–57.
- Carroll N. Madison, WI: The University of Wisconsin Press; Theories of Art Today. 2000
- Cavanagh P. The artist as neuroscientist. Nature. 2005;434(7031):301–7. [PubMed: 15772645]
- Cela-Conde C. J., Marty G., Maestu F., Ortiz T., Munar E., Fernandez A., Roca M., Rossello J., Quesney F. Activation of the prefrontal cortex in the human visual aesthetic perception. PNAS. 2004;101(16):6321–25. [PMC free article: PMC395967] [PubMed: 15079079]
- Chatterjee A. Universal and relative aesthetics: A framework from cognitive neuroscience. Paper read at International Association of Empirical Aesthetics, at Takarazuka. 2002 Japan.
- Chatterjee A. Neglect. A disorder of spatial attention. D’Esposito M. Cambridge, MA: The MIT Press; Neurological Foundations of Cognitive Neuroscience. 2003a:1–26.
- Chatterjee A. Prospects for a cognitive neuroscience of visual aesthetics. Bulletin of Psychology and the Arts. 2003b;4:55–59.
- Chatterjee A. The neuropsychology of visual artists. Neuropsychologia. 2004;42:1568–83. [PubMed: 15246293]
- Chatterjee A. The neuropsychology of visual art: Conferring capacity. International Review of Neurobiology. 2006;74:39–49. [PubMed: 16730504]
- Chatterjee A. Prospects for a neuropsychology of art. Skov M., Vartanian O. Amityville, NY: Baywood Publishing Company; Neuroaesthetics. 2009:131–43.
- Chatterjee A., Thomas A., Smith S. E., Aguirre G. K. The neural response to facial attractiveness. Neuropsychology. 2009;23:135–143. [PubMed: 19254086]
- Chatterjee A., Widick P., Sternschein R., Smith W. B. II. The assessment of art attributes. Empirical Studies of the Arts. 2010;28(2):207–22.
- Conway B. R., Livingstone M. S. Perspectives on science and art. Current Opinion in Neurobiology. 2007;17(4):476–82. [PMC free article: PMC2813684] [PubMed: 17851068]
- Cunningham M. R., Barbee A. P., Philhower C. L. Dimensions of facial physical attractiveness: The intersection of biology and culture. Rhodes G., Zebrowitz L. Westport, CT: Ablex; Facial Attractiveness. Evolutionary, Cognitive, and Social Perspectives. 2002
- Cutting J. E. Mere exposure, reproduction, and the impressionist canon. Brzyski A. Durham: Duke University Press; Partisan Canons. :79–93.
- Danto A. C. The artworld. Journal of Philosophy. 1964;61:571–84.
- de Tommaso M., Sardaro M., Livrea P. Aesthetic value of paintings affects pain thresholds. Consciousness and Cognition. 2008;17:1152–62. [PubMed: 18762434]
- Delgado M. R., Nystrom L. E., Fissell K., Noll D. C., Fiez J. A. Tracking the hemodynamic responses for reward and punishment. Journal of Neurophysiology. 2000;84:3072–77. [PubMed: 11110834]
- Dickie G. Defining art. American Philosophical Quarterly. 1969;6:253–56.
- Dion K., Berscheid E., Walster E. What is beautiful is good. Journal of Personality and Social Psychology. 1972;24:285–90. [PubMed: 4655540]
- Dissanayake E. The arts after Darwin: Does art have an origin and adaptive function? Zijlemans K., van Damme W. Amsterdam: Valiz; World Art Studies: Exploring Concepts and Approaches. 2008:241–63.
- Elliott R., Friston K. J., Dolan R. J. Dissociable neural responses in human reward systems. Journal of Neuroscience. 2000;20:6159–65. [PMC free article: PMC6772605] [PubMed: 10934265]
- Enquist M., Arak A. Symmetry, beauty and evolution. Nature. 1994;372(6502):169–72. [PubMed: 7969448]
- Etcoff N. New York: Anchor Books; Survival of the Prettiest. 1999
- Eysenck H. J. The empirical determination of an aesthetic formula. Psychological Review. 1941;48(1):83–92.
- Eysenck H. J., Hawker G. W. The taxonomy of visual aesthetic preferences: An empirical study. Empirical Studies of the Arts. 1994;12(1):95–101.
- Farah M. J. Malden, MA: Blackwell; The Cognitive Neuroscience of Vision. 2000
- Fechner G. Leipzig: Vorschule der Aesthetik. 1876 Breitkopf & Hartel.
- Grammer K., Fink B., Moller A. P., Thornhill R. Darwinian aesthetics: Sexual selection and the biology of beauty. Biological Review. 2003;78:385–407. [PubMed: 14558590]
- Grammer K., Thornhill R. Human (Homo sapiens) facial attractiveness and sexual selection: The role of symmetry and averageness. Journal of Comparative Psychology. 1994;108(3):233–42. [PubMed: 7924253]
- Grossberg S., Mingolla E., Ros W. D. Visual brain and visual perception: How does the cortex do perceptual grouping? Trends in Neuroscience. 1997;20:106–11. [PubMed: 9061863]
- Halberstadt J., Rhodes G. The attractiveness of non-face averages: Implications for an evolutionary explanation of the attractiveness of average faces. Psychological Science. 2000;11:285–89. [PubMed: 11273386]
- Helmut L., Benno B., Andries O., Dorothee A. A model of aesthetic appreciation and aesthetic judgments. British Journal of Psychology. 2004;95:489–508. [PubMed: 15527534]
- Hofel L., Jacobsen T. Electrophysiological indices of processing aesthetics: Spontaneous or intentional processes? International Journal of Psychophysiology. 2007;65(1):20–31. [PubMed: 17400317]
- Ishai A. Sex, beauty and the orbitofrontal cortex. International Journal of Psychophysiology. 2007;63(2):181–85. [PubMed: 16759727]
- Jacobsen T., Buchta K., Kohler M., Schroger E. The primacy of beauty in judging the aesthetics of objects. Psychological Reports. 2004;94:1253–60. [PubMed: 15362400]
- Jacobsen T., Schubotz R. I., Hofel L., v Cramon D. Y. Brain correlates of aesthetic judgments of beauty. Neuroimage. 2005;29:276–85. [PubMed: 16087351]
- Jones D., Hill K. Criteria of facial attractiveness in five populations. Human Nature. 1993;4(3):271–96. [PubMed: 24214367]
- Kampe K. K. W., Frith C. D., Dolan R. J., Frith U. Reward value of attractiveness and gaze. Nature. 2001;413 [PubMed: 11595937]
- Kant I. Critique of Judgment. 1790/1987 trans. W.S. Pluhar. Indianapolis: Hackett.
- Kawabata H., Zeki S. Neural correlates of beauty. Journal of Neurophysiology. 2004;91(4):1699–1705. [PubMed: 15010496]
- Kenealy P., Frude N., Shaw W. Influence of children’s physical attractiveness on teacher expectations. The Journal of Social Psychology. 1988;128:373–83.
- Kranz F., Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–68. [PubMed: 16401423]
- Langlois J. H., Kalakanis L. E., Rubenstein A. J., Larson A. D., Hallam M. J., Smoot M. T. Maxims or myths of beauty: A meta-analytic and theoretical review. Psychological Bulletin. 2000;126:390–423. [PubMed: 10825783]
- Langlois J. H., Ritter J. M., Roggman L. A., Vaughn L. S. Facial diversity and infant preferences for attractive faces. Developmental Psychology. 1991;27(1):79–84.
- Lerner R., Lerner J., Hess L., Schwab J. Physical attractiveness and psychsocial functioning among early adolescents. Journal of Early Adolescence. 1991;11:300–20.
- Livingstone M. New York: Abrams; Vision and Art: The Biology of Seeing. 2002
- Livingstone M., Hubel D. H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. The Journal of Neuroscience. 1987;7:3416–68. [PMC free article: PMC6569044] [PubMed: 3316524]
- Livingstone M., Hubel D. Segregation of form, colour, movement, and depth: Anatomy, physiology, and perception. Science. 1988;240:740–49. [PubMed: 3283936]
- Locher P. J., Stappers P. J., Overbeeke K. An empirical evaluation of the visual rightness theory of pictorial composition. Acta Psychologica. 1999;103(3):261–80. [PubMed: 10668300]
- Locher P., Nagy Y. Vision spontaneously establishes the percept of pictorial balance. Empirical Studies of the Arts. 1996;14(1):17–31.
- Marr D. New York: W.H. Freeman and Company; Vision. A Computational Investigation into the Human Representation and Processing of Visual Information. 1982
- Martindale C., Moore K. Priming, prototypicality, and preference. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:661–67.
- Moreland R. L., Zajonc R. B. A strong test of exposure effects. Journal of Experimental Social Psychology. 1976;12:170–79.
- Motter B. C. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. Journal of Neurophysiology. 1993;70:909–19. [PubMed: 8229178]
- Motter B. C. Neural correlates of attentive selection for color or luminance in extrastriate area V4. Journal of Neuroscience. 1994;14:2178–89. [PMC free article: PMC6577115] [PubMed: 8158264]
- Nadal M., Munar E., Capo M. A., Rosselo J., Cela-Conde C. J. Towards a framework for the study of the neural correlates of aesthetic preference. Spatial Vision. 2008;21(3):379–96. [PubMed: 18534110]
- Nouwen H. J. M. Notre Dame, IN: Ave Maria Press; Behold the Beauty of the Lord: Praying with Icons. 1987
- O’Doherty J., Kringelbach M. L., Rolls E. T., Hornack J., Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. [PubMed: 11135651]
- O’Doherty J., Winston J., Critchley H., Perret D., Burt D. M., Dolan R. J. Beauty in a smile: The role of orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–55. [PubMed: 12459213]
- Ognjenovic P. Processing of aesthetic information. Empirical Studies of the Arts. 1991;9(1):1–9.
- Olson I. R., Marshuetz C. Facial attractiveness is appraised in a glance. Emotion. 2005;5:498–502. [PubMed: 16366753]
- Palermo R., Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. [PubMed: 16797607]
- Penton-Voak I. S., Jones B. C., Little A. C., Baker S., Tiddeman B., Burt D. M., Perrett D. I. Symmetry, sexual dimorphism in facial proportions and male facial attractiveness. Proceedings of the Royal Society of London: Series B. 2001;268:1617–23. [PMC free article: PMC1088785] [PubMed: 11487409]
- Perrett D. I., Lee K. J., Penton-Voak I., Rowland D., Yoshikawa S., Burt D. M., Henzi S. P., Castles D. L., Akamatsu S. Effects of sexual dimorphism on facial attractiveness. Nature. 1998;394:884–87. [PubMed: 9732869]
- Perrett D. I., May K. A., Yoshikawa S. Facial shape and judgements of female attractiveness. Nature. 1994;368:239–42. [PubMed: 8145822]
- Ramachandran V. S., Hirstein W. The science of art: A neurological theory of aesthetic experience. Journal of Consciousness Studies. 1999;6(6–7):15–51.
- Reber R., Schwarz N., Winkielman P. Processing fluency and aesthetic pleasure: Is beauty in the perceiver’s processing experience? Personality & Social Psychology Review (Lawrence Erlbaum Associates). 2004;8(4):364–82. [PubMed: 15582859]
- Rentschler I., Jüttner M., Unzicker A., Landis T. Innate and learned components of human visual preference. Current Biology. 1999;9(13):665–71. [PubMed: 10395537]
- Rhodes G., Harwood K., Yoshikawa S., Miwi N., McLean I. The attractiveness of average faces: Cross-cultural evidence and possible biological basis. Rhodes G., Zebrowitz L. Westport, CT: Ablex; Facial Attractiveness. Evolutionary, Cognitive, and Social Perspectives. 2002
- Ricci R., Vaishnavi S., Chatterjee A. A deficit of preattentive vision: Experimental observations and theoretical implications. Neurocase. 1999;5(1):1–12.
- Ritts V., Patterson M., Tubbs M. Expectations, impressions, and judgments of physically attractive students: A review. Review of Educational Research. 1992;62:413–26.
- Russell P. A., George D. A. Relationships between aesthetic response scales applied to paintings. Empirical Studies of the Arts. 1990;8(1):15–30.
- Schultz W., Dayans P., Montague P. R. A neural substrate of prediction and reward. Science. 1997;275:1593–99. [PubMed: 9054347]
- Senior C. Beauty in the brain of the beholder. Neuron. 2003;38:525–28. [PubMed: 12765605]
- Shulman G. L., Corbetta M., Buckner R. L., Raichle M. E., Fiez J. A., Miezin F. M., Petersen S. E. Top-down modulation of early sensory cortex. Cerebral Cortex. 1997;7:193–206. [PubMed: 9143441]
- Slater A., Von Der Schulenburg C., Brown E., Badenoch M., Butterworth G., Parsons S., Samuels C. Newborn infants prefer attractive faces. Infant Behavior and Development. 1998;21(2):345–54.
- Smith D. J., Melara R. J. Aesthetic preference and syntactic prototypicality in music: ’Tis the gift to be simple. Cognition. 1990;34:279–98. [PubMed: 2328564]
- Symons D. Oxford: Oxford University Press; The Evolution of Human Sexuality. 1979
- Thornhill R., Gangestad S. W. Facial attractiveness. Trends in Cognitive Sciences. 1999;3(12):452–60. [PubMed: 10562724]
- Tinbergen N. New York: Basic Books; Curious Naturalist. 1954
- Ungerleider L. G., Mishkin M. Two cortical visual systems. Ingle D. J., Goodale M. A., Mansfield R. J. W. Cambridge: MIT Press; Analysis of Visual Behavior. 1982
- Van Essen D. C., Feleman D. J., DeYoe E. A., Ollavaria J., Knierman J. Modular and hierarchical organization of extrastriate visual cortex in the macaque monkey. Cold Springs Harbor Symp Quant Biol. 1990;55:679–96. [PubMed: 1966771]
- Vartanian O., Goel V. Neuroanatomical correlates of aesthetic preference for paintings. NeuroReport. 2004;15(5):893–97. [PubMed: 15073538]
- Vecera S. P., Behrmann M. Spatial attention does not require preattentive grouping. Neuropsychology. 1997;11:30–43. [PubMed: 9055267]
- Watanabe T., Sasaki Y., Miyauchi S., Putz B., Fujimaki N., Nielsen M., Takino R., Miyakawa S. Attention-regulated activity in human primary visual cortex. Journal of Neurophysiology. 1998;79:2218–21. [PubMed: 9535981]
- Weitz M. The role of theory in aesthetics. Journal of Aesthetics and Art Criticism. 1956;15:27–35.
- Wilson M., Daly M. Do pretty women inspire men to discount the future? Proceedings of the Royal Society of London. 2004;271:177–79. [PMC free article: PMC1810021] [PubMed: 15252976]
- Winston J. S., O’Doherty J., Kilner J. M., Perrett D. I., Dolan R. J. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. [PubMed: 16828125]
- Woods W. A. Parameters of aesthetic objects: Applied aesthetics. Empirical Studies of the Arts. 1991;9(2):105–14.
- Wyvell C. L., Berridge K. C. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: Enhancement of reward “wanting” without enhanced “liking” or response reinforcement. Journal of Neuroscience. 2000;20:8122–30. [PMC free article: PMC6772712] [PubMed: 11050134]
- Zeki S. Oxford: Blackwell Scientific Publications; A Vision of the Brain. 1993
- Zeki S. Art and the brain. Journal of Consciousness Studies. 1999;6:76–96.
- Zeki S. New York: Oxford University Press; Inner Vision: An Exploration of Art and the Brain. 1999
- Neuroaesthetics: The Cognitive Neuroscience of Aesthetic Experience.[Perspect Psychol Sci. 2016]Neuroaesthetics: The Cognitive Neuroscience of Aesthetic Experience.Pearce MT, Zaidel DW, Vartanian O, Skov M, Leder H, Chatterjee A, Nadal M. Perspect Psychol Sci. 2016 Mar; 11(2):265-79.
- A Farewell to Art: Aesthetics as a Topic in Psychology and Neuroscience.[Perspect Psychol Sci. 2020]A Farewell to Art: Aesthetics as a Topic in Psychology and Neuroscience.Skov M, Nadal M. Perspect Psychol Sci. 2020 May; 15(3):630-642. Epub 2020 Feb 6.
- Review Neuroscience of aesthetics.[Ann N Y Acad Sci. 2016]Review Neuroscience of aesthetics.Chatterjee A, Vartanian O. Ann N Y Acad Sci. 2016 Apr; 1369(1):172-94. Epub 2016 Apr 1.
- Review Move me, astonish me… delight my eyes and brain: The Vienna Integrated Model of top-down and bottom-up processes in Art Perception (VIMAP) and corresponding affective, evaluative, and neurophysiological correlates.[Phys Life Rev. 2017]Review Move me, astonish me… delight my eyes and brain: The Vienna Integrated Model of top-down and bottom-up processes in Art Perception (VIMAP) and corresponding affective, evaluative, and neurophysiological correlates.Pelowski M, Markey PS, Forster M, Gerger G, Leder H. Phys Life Rev. 2017 Jul; 21:80-125. Epub 2017 Feb 27.
- Complementarity As Generative Principle: A Thought Pattern for Aesthetic Appreciations and Cognitive Appraisals in General.[Front Psychol. 2017]Complementarity As Generative Principle: A Thought Pattern for Aesthetic Appreciations and Cognitive Appraisals in General.Bao Y, von Stosch A, Park M, Pöppel E. Front Psychol. 2017; 8:727. Epub 2017 May 9.
- Visual Art - Neurobiology of Sensation and RewardVisual Art - Neurobiology of Sensation and Reward
- Syt7 synaptotagmin 7 [Rattus norvegicus]Syt7 synaptotagmin 7 [Rattus norvegicus]Gene ID:59267Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
