NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2011.
3.1. INTRODUCTION
Aloe vera has a long history of popular and traditional use. It is used in traditional Indian medicine for constipation, colic, skin diseases, worm infestation, and infections (Heber 2007). It is also used in Trinidad and Tobago for hypertension (Lans 2006) and among Mexican Americans for the treatment of type 2 diabetes mellitus (DM; Coronado et al. 2004). In Chinese medicine, it is often recommended in the treatment of fungal diseases (Heber 2007). In Western society, Aloe vera is one of the few herbal medicines in common usage, and it has found widespread use in the cosmetic, pharmaceutical, and food industries. In the case of health, the therapeutic claims for the topical and oral application of Aloe vera cover a wide range of conditions, but few claims have been the subject of robust clinical investigation. The conditions for which clinical trials of Aloe vera have been conducted include skin conditions, management of burn and wound healing, constipation, DM, and gastrointestinal disorders.
3.2. TAXONOMY AND TRADITIONAL ORIGINS
Aloe vera is one of approximately 420 species of the genus Aloe (Dagne et al. 2000), which is variously classified as belonging to the Asphodelaceae, Liliaceae, or Aloaceae families. Commonly known as Aloe barbadensis Miller, its legitimate name according to the international rules of botanical nomenclature is A. vera (L.) Burm.f. (Grindlay and Reynolds 1986). The geographic origin of Aloe vera is believed to be in Sudan, with the plant subsequently being introduced in the Mediterranean region and most other warm areas of the world (Grindlay and Reynolds 1986).
Aloe has been used extensively by the Egyptians, Assyrians, Mediterranean civilizations and in Biblical times (Grindlay and Reynolds 1986). The first authentic record of Aloe as a plant with healing properties is accredited to a Mesopotamian clay tablet dated at ca 2100 bce. However, the first detailed depiction of the plant’s medicinal value is found in the Papyrus Ebers, an Egyptian document dated at ca 1550 bce, which sets out multiple Aloe-containing preparations for the treatment of external and internal ailments. The Aloe vera plant is described in detail in the Greek Herbal of Dioscorides (ca 70 ad), and its use promoted for the treatment of wounds, hair loss, genital ulcers, and hemorrhoids (Davis 1997). Aloe vera was officially listed as a purgative and skin protectant by the U.S. pharmacopoeia in 1820 (Park and Lee 2006) and was clinically used in the 1930s for the treatment of radiotherapy burns to the skin and mucous membranes (Collins and Collins 1935; Manderville 1939). Until today, Aloe is an important traditional medicine in many countries, including China, India, the West Indies, South Africa, and Japan (Grindlay and Reynolds 1986).
3.3. CURRENT USAGE
Aloe vera is one of the few herbal medicines widely used in Western society, with the manufacturing of Aloe vera extracts being one of the largest botanical industries worldwide (Grindlay and Reynolds 1986; Eshun and He 2004). In 2004, the value of the Aloe industry was estimated to be US$125 million for the cost of the raw Aloe material and US$110 billion for finished Aloe-containing products (International Aloe Science Council 2004). Aloe vera is used in the cosmetic, food, and pharmaceutical industries. In the cosmetic and toilet industry, it is used as a base material for skin moisturizers, soaps, shampoos, sun lotions, makeup creams, perfumes, shaving creams, bath aids, and many other products (Eshun and He 2004; Boudreau and Beland 2006). The food industry uses Aloe in the manufacture of functional foods, especially health drinks, and as a bitter agent (Saccu, Bogoni, and Procida 2001). Pharmaceutical products are available for topical applications (gels and ointments) and oral use (tablets and capsules; Hamman 2008).
3.4. STRUCTURE AND CHEMICAL CONSTITUENTS
Aloe vera is a perennial succulent xerophyte; it has elongated and pointed leaves that are joined at the stem in a rosette pattern and that grow to about 30–50 cm in length and 10 cm in breadth at the base in the adult plant (World Health Organization 1999). The leaf is protected by a thick, green epidermis layer (skin or rind), which surrounds the mesophyll. Immediately beneath the rind are located the vascular bundles, which are composed of three types of tubular structures: the xylem (transports water and minerals from roots to leaves), the phloem (transports starch and other synthesized products to the roots), and the large pericyclic tubules (contains the yellow leaf exudate commonly referred to as “aloes,” “sap,” or “latex”; Boudreau and Beland 2006). The pericyclic portion of the vascular bundle is adherent to the rind, whereas the remainder of the vascular bundle protrudes into the mesophyll layer (Danhof 1987). The mesophyll can be differentiated into chlorenchyma cells and thinner-walled parenchyma cells. The parenchyma (filet or pulp), which is the major part of the leaf by volume, contains a clear mucilaginous gel (known as Aloe vera gel; Femenia et al. 1999; Femenia et al. 2003).
Aloe vera is considered to be the most biologically active of the Aloe species (World Health Organization 1999). More than 75 potentially active constituents have been identified in the plant (Table 3.1) including vitamins, minerals, saccharides, amino acids, anthraquinones, enzymes, lignin, saponins, and salicylic acids. The leaf exudate contains anthraquinones, particularly barbaloin (Figure 3.1), which appear to be responsible for its bitter taste and cathartic effect (Dagne et al. 2000; Boudreau and Beland 2006). Barbaloin and other products of the phenylpropanoid pathway are commonly referred to as polyphenolic compounds. These are derived from the precursor phenolic acids, and they may act as antioxidants to inhibit free radical–mediated cytotoxicity and lipid peroxidation (Cook and Samman 1996). Aloe vera also contains products of the isoprenoid pathway, including carotenoids, steroids, terpenes, and phytosterols (Samman 1998). Isoprenoids can be regarded as sensory molecules because they contribute to the color and fragrance of the products in which they exist.
TABLE 3.1
Classes and Selected Examples of Phytochemicals in Aloe vera.
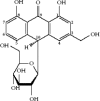
FIGURE 3.1
Structure of barbaloin (aloin), a glucoside of loe-emodin.
Aloe vera gel is rich in polysaccharides, including acemannan (partially acetylated glucomannans; Figure 3.2), which has been reported as the primary active substance in the parenchyma (t'Hart et al. 1989). However, given the number of other potentially active compounds in the plant, it is possible that the biological activities of Aloe vera result from the synergistic action of a variety of compounds, rather than from a single defined component (Dagne et al. 2000; Hamman 2008). Equally, the potential for constituents to exhibit antagonistic and competitive activities also influences the overall biological activity of particular Aloe vera preparations (Hamman 2008).
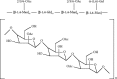
FIGURE 3.2
Structure of acemannan, a mucopolysaccharide that is extracted from Aloe vera leaves.
3.5. EFFECT OF CULTIVATION AND PROCESSING
The composition of Aloe vera extracts differs according to the plant variety, climatic and seasonal variations, and the age of the plant (Eshun and He 2004). However, the processing method has the largest effect on the number and amount of active ingredients in a product (Wang and Strong 1995). The commercial production process of Aloe vera products typically involves crushing, grinding, or pressing of the whole Aloe vera leaf to produce juice, followed by various steps of filtration and stabilization to achieve the desired extract (Eshun and He 2004). This method provides ease of processing and higher efficiency in the recovery of the solids (Agarwala 1997), but it can result in a product that contains little or no active ingredients (Eshun and He 2004). In an analysis of 18 commercial Aloe vera products, only 9 exhibited quantifiable amounts of mucilaginous polysaccharide (Ross, Elsohly, and Wilkins 1997). Only three of the nine commercial Aloe vera gel powders sourced from leading international suppliers demonstrated satisfactory amounts of the polysaccharide Acemannan (Bozzi et al. 2007). Variable polysaccharide content in Aloe vera has been attributed particularly to heating the plant extract to >60°C, which results in significant changes in molecular weight (Turner et al. 2004). A further issue with the commercial production process is that during the commercial extraction of Aloe vera gel, it is virtually impossible to prevent the contamination by leaf exudates (Eshun and He 2004). Finally, the adulteration of Aloe vera products using fillers such as maltodextrin, glucose, glycerin, and malic acid represents a major concern for the Aloe vera market (Bozzi et al. 2007). As a counter to such misrepresentations in the industry, the International Aloe Science Council developed a certification program that validates the quality and quantity of Aloe vera in approved commercial products.
3.6. HEALTH EFFECTS: THE SCIENTIFIC EVIDENCE
The therapeutic claims for Aloe vera cover a broad range of conditions. It is commonly used topically in the treatment of dermatological and wound healing conditions. The oral application of the Aloe vera latex is promoted as a laxative, whereas gel and whole-leaf oral preparations have been variously recommended for use as an adjunct to chemotherapy treatment and to ameliorate diverse disorders such as DM, infectious diseases, metastatic cancer, and ulcerative colitis. The clinical use of Aloe vera is supported primarily by anecdotal evidence and case reports. The number of clinical trials exploring its effectiveness has begun to increase (Table 3.2); however, a standardization of methodological trial quality has yet to be achieved.
TABLE 3.2
Controlled Trials Investigating the Effectiveness of Aloe vera in the Treatment of Various Health Conditions in Humans.
3.6.1. Topical Applications
The first case report of the beneficial effects of Aloe vera in the treatment of skin and wound healing was published in 1935, with fresh whole-leaf extract reported to provide rapid relief from the itching and burning associated with severe roentgen (radiation) dermatitis and complete skin regeneration (Collins and Collins 1935). Numerous subsequent reports have explored the role of topical Aloe vera administration in skin conditions and wound healing management, including psoriasis, dermatitis, oral mucositis, burn injuries, and surgical wounds.
3.6.1.1. Dermatological Conditions
Results of a number of clinical trials suggest that Aloe vera is positively indicated in the treatment of skin disorders. A trial of wound healing management after the full-faced dermabrasion of patients with acne vulgaris demonstrated that the saturation of a standard polyethylene wound gel dressing with Aloe vera significantly reduced time to reepithelization compared to use of the standard dressing alone (Fulton 1990). In a randomized, double-blind, controlled trial of Aloe vera or placebo cream in 60 patients with chronic psoriasis, the cure rate in the Aloe vera group was 83% (with no relapses at 12 months of follow-up) compared to only 7% in the placebo group (Syed et al. 1996). Converse results were reported in a later trial examining the efficacy of a commercial Aloe vera gel preparation in the treatment of slight to moderate psoriasis vulgaris. The Aloe vera or placebo gel was applied twice daily for 4 weeks to symmetrical test lesions using an intraindividual right/left comparison study design. The sum score of erythema, infiltration, and desquamation significantly favored the placebo treatment (Paulsen, Korsholm, and Brandrup 2005).
Further, despite case reports (Loveman 1937) and animal studies (Rowe 1940) to the contrary, Aloe vera extracts have either no effect or less effect than other topical treatments in acute radiation dermatitis. In the first of two randomized controlled trials in 194 women receiving radiation therapy for breast cancer, the topical self-administration of Aloe vera gel to radiation-exposed skin produced no difference in the severity of the dermatitis compared to a placebo gel. In the second study, the placebo group was replaced with a “no-treatment” group to account for any unintended beneficial effects of the inert carrier gel used as the placebo in the first trial. The results failed to show any benefit of the Aloe vera gel in preventing radiation-induced dermatitis (Williams, Burk, and Loprinzi 1996). Similarly, in 70 radiation therapy patients who were randomized to receive either commercially available Aloe vera gel or no treatment (other than mild soap), Aloe vera did not significantly protect against radiation-induced skin changes (Olsen et al. 2001). In a study involving 225 patients undergoing radiation therapy, the topical application of Aloe vera gel thrice a day throughout the treatment and for an additional 2 weeks after the completion of radiation therapy was significantly less efficacious in reducing the treatment-related side effects than aqueous cream (Heggie et al. 2002). In the pediatric setting, 45 patients undergoing radiation therapy for various diagnoses were treated with either an Aloe vera–based gel or a anionic polar phospholipid (APP)-based cream applied symmetrically within the irradiated field after each session. The authors reported statistically significant results favoring the APP-based cream on a number of skin assessment variables, including dryness, comfort, erythema, and peeling. The study was limited by a lack of description of randomization and blinding and the inclusion of patients with varied diagnoses, radiotherapy sites, and cancer treatment regimes (Merchant et al. 2007).
A 20 mL “swish and swallow” of Aloe vera solution (94.5% aloe juice) four times daily in addition to conventional treatment (baking soda mouth rinse, Benadryl and nystatin combination mouth-washes, and viscous lidocaine, as needed) did not improve radiation-related mucositis in patients with head-and-neck neoplasms. Study limitations included a small sample size, patient heterogeneity, a large distribution of primary cancer sites, and an inability to monitor compliance (Su et al. 2004).
3.6.1.2. Burn Injuries
Aloe vera has long been associated with the treatment of burns. With the advent of nuclear power, the U.S. government conducted research on the ability of Aloe vera to treat thermal and radiation burns with the aim of introducing its use into the military (Ashley et al. 1957). In 1959, the U.S. Food and Drug Administration approved the use of Aloe vera ointment as an over-the-counter medication for healing burns on the skin (Park and Lee 2006).
Heck et al. (1981) randomly assigned 18 patients with second-degree burns to be treated, after debridement, with gauze containing either Aloe vera cream or Silvadene ointment. The Aloe vera group had a mean healing time of 13 days compared to 16 days in the Silvadene group; however, the difference did not reach statistical significance. In a recent meta-analysis, a statistically significant benefit of Aloe vera for the treatment of burns was demonstrated. Using the duration of wound healing as an outcome measure, the meta-analysis of the efficacy of Aloe vera in burn wound healing concluded that Aloe vera treatments reduced healing time by approximately 9 days compared to conventional treatment groups (p = .006; Maenthaisong et al. 2007). Four controlled clinical trials (with a total of 371 subjects) met the inclusion criteria for the review. The four studies differed in their study design, intervention, and reported outcomes. The Aloe vera preparations included fresh Aloe vera mucilage (Thamlikitkul et al. 1991), gauze saturated with 85% Aloe vera gel (Visuthikosol et al. 1995), Aloe vera cream (Akhtar and Hatwar 1996), and 1% Aloe vera powder wrapped with Vaseline gauze (Sun et al. 1994). None of the studies standardized the amount of active Aloe vera ingredients administered. The outcomes measured were wound healing time, described as time to complete epithelization (Visuthikosol et al. 1995) or not defined (Akhtar and Hatwar 1996); the success rate of wound healing (Thamlikitkul et al. 1991); and epithelization rate (Sun et al. 1994). Maenthaisong et al. (2007) note that, due to differences in Aloe vera products and outcome measures used, it is difficult to draw a specific conclusion regarding the effect of Aloe vera on burn healing. Nonetheless, the results of the review combined with other evidence suggest that Aloe vera preparations at a range of different doses are beneficial in the treatment of burn wounds.
3.6.1.3. Surgical Wound Healing
Aloe vera has been reported to accelerate postoperative wound healing in periodontal flap surgery (Payne 1970). Conversely, a randomized controlled trial involving women with complications of wound healing after gynecological surgery found that the mean healing time in the conventional care group (53 days) was significantly shorter (p < .003) than in the Aloe vera gel group (83 days; Schmidt and Greenspoon 1991). The results of the trial must be interpreted with caution, as only 21 of 40 women completed the study and more patients were lost to follow-up from the gauze group (n = 12) than the Aloe vera group (n = 5). An intention-to-treat analysis was not performed (meaning that patients lost to follow-up were excluded from the analysis), which potentially introduces significant bias into the results.
3.6.2. Oral Applications
Therapeutic claims promote the use of oral Aloe vera in the treatment of a wide range of conditions, such as alopecia, Alzheimer’s disease, congenital heart failure, depression, glaucoma, hemorrhoids, hepatitis, multiple sclerosis, and varicose veins; however, scientific investigations of such claims are limited. Claims that have been the subject of clinical trials include the oral application of Aloe vera preparations in the treatment of constipation, DM, metastatic cancer, and ulcers and inflammation of the gastrointestinal tract.
3.6.2.1. Laxative
Aloe vera latex is commonly used in the treatment of constipation (de Witte 1993); the laxative effect of the anthraquinone glycosides found in Aloe vera latex is well established (Ulbricht et al. 2008). In a double-blind, randomized, controlled trial of 28 healthy adults, aloin was reported to have a laxative effect compared to a placebo that was stronger than the stimulant laxative phenolphthalein (Chapman and Pittelli 1974). In subjects with chronic constipation, a novel preparation containing Aloe vera, celandine, and psyllium was found to improve a range of constipation indicators (bowel movement frequency, consistency of stools, and laxative dependence) in a 28-day double-blind trial; however, the effect of Aloe vera alone was not investigated in this study (Odes and Madar 1991). Aloe vera laxative preparations have been approved by the German Commission E governmental regulatory agency for use in the treatment of constipation as a second-line agent; however, Aloe latex is no longer recognized as an over-the-counter drug by the U.S. Food and Drug Administration due to a lack of sufficient data to establish its safety for use as a laxative.
3.6.2.2. Diabetes Mellitus
Aloe vera is a traditional remedy for diabetes mellitus (DM) in many parts of the world, including Latin America (Coronado et al. 2004) and the Arabian Peninsula (Yeh et al. 2003). Some evidence in humans and animals suggests that Aloe vera is able to alleviate the chronic hyperglycemia and perturbed lipid profile that are characteristic of DM, which are major risk factors for cardiovascular complications in the disease.
Agarwal (1985) reported hypoglycemic and hypolipidemic effects from the long-term dietary administration of 100 g of an Aloe vera gel preparation combined with 20 g of psyllium seed husks. The study involved 5000 patients aged 35–65 years with atheromatous heart disease, a population that included 3167 noninsulin-dependent diabetic patients. Marked reductions were noted in serum cholesterol, triglycerides, and total lipid levels, along with an increase in high-density lipoprotein (HDL) cholesterol. All but 177 of the diabetic patients demonstrated a normalization of fasting and postprandial blood glucose levels that necessitated the withdrawal of all oral hypoglycemic agents by the end of 2 months of therapy. A beneficial effect of Aloe vera gel alone on blood glucose and lipid parameters in diabetic subjects also has been demonstrated. In the first of two related clinical trials, 72 diabetic women without drug therapy were administered one tablespoon of Aloe vera gel or placebo for 6 weeks. Blood glucose and serum triglyceride levels were significantly decreased with Aloe vera treatment, although cholesterol concentrations were unaffected (Yongchaiyudha et al. 1996). In the second trial, the effects of Aloe vera gel or placebo in combination with glibenclamide (a commonly prescribed antidiabetic medication) were investigated, similarly resulting in significant reductions in blood glucose and serum triglyceride concentrations in the Aloe vera group (Bunyapraphatsara et al. 1996).
In addition to gel preparations, Aloe vera latex has been shown to lower fasting blood glucose levels in case studies of five patients with noninsulin-dependent DM (Ghannam et al. 1986). Further, the whole-leaf Aloe vera extract administered to 60 patients with hyperlipidemia in a 12-week controlled clinical trial resulted in significantly decreased levels of total serum cholesterol, triglycerides, and low-density lipoproteins (LDLs; Nasiff, Fajardo, and Velez 1993). However, although studies in humans provide promising preliminary data that denote a beneficial effect of Aloe vera in diabetes and associated cardiovascular complications, effects have yet to be confirmed by controlled clinical trials that are both randomized and blinded to subjects and investigators.
Animal studies exploring the effects of Aloe vera on blood glucose and lipids have demonstrated less consistent results likely due to different combinations of animal models and Aloe vera preparations used. In rodent models, both the chronic administration of Aloe vera latex to alloxan-induced diabetic mice (Ajabnoor 1990) and Aloe vera gel to streptozotocin (STZ)-induced diabetic rats (Rajasekaran et al. 2004) resulted in significant reductions in fasting blood glucose. Conversely, Aloe vera gel was reported to increase plasma glucose levels in alloxan-induced diabetic rats (Koo 1994). More recently, the antidiabetic effects of processed Aloe vera gel were investigated in mice exhibiting diet-induced obesity (DIO), an animal model that has been shown to demonstrate metabolic abnormalities that closely resemble those found in human noninsulin-dependent DM, including hyperglycemia, obesity, and insulin resistance (Kim et al. 2009). Oral administration of the gel reduced circulating blood glucose concentrations to a normal level, significantly decreased plasma insulin, and lowered triglyceride levels in the liver and plasma of the DIO mice. Similarly, Aloe vera gel extract has been shown to normalize the fasting blood glucose and plasma insulin levels and reduce the concentrations of cholesterol, triglycerides, and free fatty acids in the plasma, liver, and kidney of STZ-induced diabetic rats (Rajasekaran et al. 2006).
3.6.2.3. Metastatic Cancer
The concomitant oral administration of 1 mL twice a day of Aloe vera tincture (10% Aloe vera and 90% alcohol) and 20 mg/day of melatonin compared to melatonin alone was studied in 50 patients with locally advanced or metastatic solid tumors for whom no other effective standard therapy was available. In the group treated with Aloe vera and melatonin combined, 12 of 24 patients had their disease stabilized compared to only 7 of 26 patients in the melatonin-only group. In addition, the percentage of individuals surviving 1 year was significantly higher with Aloe vera plus melatonin compared with melatonin treatment alone (Lissoni et al. 1998).
3.6.2.4. Ulcers and Inflammation of the Gastrointestinal Tract
Aloe vera preparations are widely promoted for the treatment of gastrointestinal disorders, including ulcers and inflammatory bowel disease, but evidence of their effectiveness is inconsistent. In 1963, clinical evidence of the successful use of Aloe vera gel (administered in a heavy liquid petrolatum emulsion) was reported for the treatment of 12 patients with peptic ulcers (Blitz, Smith, and Gerard 1963). In a 3-month randomized controlled trial of 58 patients with irritable bowel syndrome, no evidence was found to suggest that Aloe vera has any beneficial effect (Davis et al. 2006).
A recent attempt to formally evaluate the efficacy and safety of Aloe vera gel in the treatment of ulcerative colitis produced encouraging, although not conclusive, results. In a randomized controlled trial of 44 subjects with mild to moderately active ulcerative colitis, the oral administration twice daily of 100 mL Aloe vera gel to 30 subjects for 4 weeks generated clinical remission and improvement more often than in the placebo group (14 subjects); however, despite positive trends the results failed to reach statistical significance. The simple clinical colitis activity index and histological scores showed small statistically significant improvements in the Aloe vera group. Six patients (20%) who were given Aloe vera gel and three patients (21%) who were given placebo withdrew from the study because of deterioration or a failure to improve sufficiently but were included in the statistical analyses. The Aloe vera preparation used in this study was reported to contain a high proportion (>95%) of Aloe vera pulp, and the dose administered was the maximum recommended by the manufacturers. No adverse effects were observed during the trial, and the authors note that a higher dose may have been more efficacious and suggest the need for further, larger controlled trials of Aloe vera gel in active ulcerative colitis and in the maintenance of remission (Langmead et al. 2004).
3.7. ACTIVE INGREDIENTS AND MECHANISMS OF ACTION
A large number of biological activities have been ascribed to Aloe vera to explain its purported health benefits, including antimicrobial, anti-inflammatory, lipid and glucose lowering, antiproliferative, immunostimulatory, and antioxidant functions. A number of potentially active ingredients in the latex and gel of Aloe vera have been identified; however, much has yet to be determined about their mechanisms of action. Further studies are also required to determine the active properties of numerous other Aloe vera constituents and to explore the competitive or synergistic actions of particular combinations of ingredients.
3.7.1. Active Latex Constituents
The major C-glycosides, barbaloin (Figure 3.1) and isobarbaloin, have been shown to be the principal agents responsible for cathartic and other effects of Aloe vera latex in humans and animals. Both barbaloin and isobarbaloin undergo decomposition in the large intestine to form the active metabolites aloe-emodin-9-anthrone and aloe-emodin (Figure 3.3), which induce laxation via multiple mechanisms. In vitro and in vivo studies in rats demonstrated that aloe-emodin-9-anthrones reduce the absorption of water from the intestinal lumen by inhibiting the activity of Na+, K+-adenosine triphosphatase (ATPase) and stimulate water secretion by increasing the paracellular permeability across the colonic mucosa (Ishii, Tanizawa, and Takino 1990). Secretion of water into the lumen by a prostaglandin-dependent mechanism has also been reported (Capasso et al. 1983). The net result is a reduction in water absorption and the formation of softer stools (Boudreau and Beland 2006). Aloe-emodin has been suggested to have antiangiogenic properties; it has been demonstrated to be a potent inhibitor of urokinase secretion and tubule formation of endothelial cells, both key events in angiogenesis (Cárdenas, Quesada, and Medina 2006).
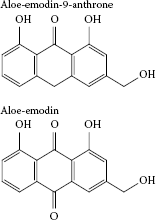
FIGURE 3.3
Aloe-emodins (anthraquinones) isolated from Aloe vera.
3.7.2. Active Gel Constituents
Polysaccharides, particularly mannose-containing polysaccharides, cellulose, and pectic polysaccharides, comprise the major part of Aloe vera gel. Acetylated glucomannan is primarily responsible for the gel’s mucilaginous properties (Hamman 2008) and has been found in vitro and in animal studies to modulate immune function (through macrophage activation and cytokine production) and accelerate wound healing (Ulbricht et al. 2008). Veracylglucan B and veracylglucan C (Figure 3.4), two maloyl glucans isolated from Aloe vera gel, have been demonstrated in vitro to have potent anti-inflammatory effects, although their effects on cell proliferation appear antagonistic (Esua and Rauwald 2006).
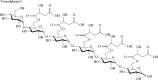
FIGURE 3.4
Veracylglucan C: a maloyl glucan isolated from Aloe vera.
Among the nonpolysaccharide gel constituents, salicylic acid and other antiprostaglandin compounds may contribute to the local anti-inflammatory activity of Aloe vera via the inhibition of cyclooxygenase (Ulbricht et al. 2008). Potent antioxidant effects, including the ability to scavenge superoxide anions, have been attributed to the caffeoyl group of isorabaichromone, a derivative of aloesin (C-glycosylated 5-methylchromone). Five phytosterols have been isolated from Aloe vera gel based on their ability to decrease the HbA1c level in a mouse model (db/db) of type 2 DM. Each of the phytosterols, namely lophenol, 24-methyl-lophenol, 24-ethyl-lophenol, cycloartanol, and 24-methylene-cycloartanol, was shown to significantly decrease fasting blood glucose levels in the db/db mice compared to controls at a dose of 1 μg/day (Tanaka et al. 2006). Phytosterols are not extensively absorbed from the intestine but can bind cholesterol and prevent it from being absorbed (Ralph and Provan 2000). Phytosterols have been shown to lower plasma cholesterol concentrations, including the atherogenic LDL fraction (Moghadasian and Frohlich 1999). The mechanisms of action by which Aloe vera modulates blood glucose are unknown, but it has been suggested that it may interact with insulin. It has been hypothesized that Aloe stimulates insulin synthesis or its release from pancreatic β cells (Ajabnoor 1990). Processed Aloe vera gel was found to suppress the expression of the adipogenic genes SREBP-1a, FAS, and GPAT, suggesting that the gel improves insulin resistance by the reducing toxic effects of lipids in the liver (Kim et al. 2009).
3.8. SAFETY AND EFFICACY
Determining the safety and efficacy of Aloe vera is difficult due to the lack of standardization of commercially available Aloe vera preparations. Similarly, the need for a more detailed understanding of the plant’s active components makes it difficult to evaluate the optimal doses of particular Aloe vera preparations for the treatment of specific disorders.
Despite these challenges, a recent systematic review of Aloe vera by the Natural Standard Research Collaboration concluded that topical application of Aloe vera gel or extract is safe for the treatment of mild to moderate skin conditions, burns, wounds, and inflammation (Ulbricht et al. 2008). In terms of efficacy, reasonable evidence in humans supports the topical use of Aloe vera for the treatment of burn wounds. Evidence for its use in psoriasis, dermatitis, and surgical wound healing is conflicting.
The Natural Standard Research Collaboration further concluded that the oral use of Aloe vera gel for its potential hypoglycemic effects and the short-term use of oral Aloe latex as a laxative are possibly safe; however, prolonged use of the latex is likely to be unsafe due to a theoretical risk of dehydration and electrolyte imbalance (Ulbricht et al. 2008). The cathartic properties of anthraquinone glycosides found in Aloe vera latex are well established. However, given the potential safety concerns with its use, there is a need for further clinical trials to investigate the benefits of latex administration over conventional laxative treatments. Although inconclusive, there is some preliminary evidence of a favorable effect of Aloe vera gel taken orally in type 2 DM, ulcerative colitis and the stabilization of metastatic cancer.
3.8.1. Toxicology
Until now there are no published controlled in vivo toxicology studies of Aloe vera in humans (Steenkamp and Stewart 2007). In animal studies, Aloe vera-derived ingredients were not found to be toxic in acute oral studies using mice and rats. In mice, the LD50 was >200 mg/kg and >80 mg/kg in parenteral and intravenous studies, respectively, whereas in rates the corresponding LD50 values were >50 mg/kg and >15 mg/kg, respectively. No significant toxicity was seen with acemannan given intravenously or intraperitoneally at 4-day intervals over 30 days at maximum dose levels of 200 mg/kg in mice and 50 mg/kg in rats (Cosmetic Ingredient Review Expert Panel 2007). The no observed adverse effect level (NOAEL) for whole-leaf Aloe vera powder was 87.7 and 109.7 mg/kg/day in male and female rats, respectively (Matsuda et al. 2007). Life-long Aloe vera gel ingestion (contributing 1% of total diet) in rats was demonstrated to produce no harmful effects or deleterious changes (Ikeno et al. 2002). In contrast, chronic ingestion of 100 mg/kg Aloe vera (extracted in ethanol) given orally in rats produced reproductive toxicity, significant sperm damage, inflammation, and mortality compared to control animals (Shah et al. 1989). In a recent safety assessment of Aloe, the Cosmetic Ingredient Review Expert Panel (2007) concluded that Aloe latex, but not the polysaccharide material derived from the inner gel, is cytotoxic.
3.8.2. Carcinogenicity
Tumor-promoting and antimutagenic activities have been ascribed to the latex of Aloe vera (Boudreau and Beland 2006). Multiple in vitro studies have demonstrated the potential genotoxicity of anthraquinones; however, anthraquinones in Aloe vera do not appear to be well absorbed, and four in vivo studies resulted in no genotoxicity from aloe-emodin and emodin (Brusick and Mengs 1997). Anthranoid-containing laxatives such as aloe-emodin have been suggested to cause colorectal cancer (Siegers et al. 1993); however, recent research has not shown any correlation. A 2-year carcinogenicity study in rats reported that whole-leaf Aloe powder was not carcinogenic at nontoxic dose levels in the colon (Yokohira et al. 2009). In many large epidemiological studies in humans, long-term laxative abuse has not been associated with colorectal cancer (Nusko et al. 2000; Park et al. 2009).
3.8.3. Photoxicity
Phototoxicity of aloe-emodin has been demonstrated in animal studies; however, phototoxicity was not observed in several clinical studies in humans using amounts of aloe-emodin that are commonly found in commercially available Aloe vera preparations (Cosmetic Ingredient Review Expert Panel 2007).
3.8.4. Adverse Effecrs
In the reviewed clinical trials, no serious adverse reactions were reported following Aloe vera administration. Three patients experienced allergic reactions after the topical application of an Aloe vera preparation (Williams, Burk, and Loprinzi 1996). In case reports, hypersensitivity and allergic responses to Aloe vera are the most commonly described adverse effects of Aloe vera use. The topical application of Aloe vera gel has resulted in contact dermatitis, and oral use may cause diarrhea or vomiting (Morrow, Rapaport, and Strick 1980; Ernst 2000; Wang et al. 2003; Chinnusamy et al. 2009). Many of these reactions appear to be associated with anthraquinone contaminants of the gel product.
In rare cases, severe adverse effects have been associated with the oral application of Aloe vera. Induced acute toxic hepatitis has been observed in four instances of Aloe vera ingestion (Luyckx et al. 2002; Rabe et al. 2005; Kanat, Ozet, and Ataergin 2006). In one case, a 47-year-old man presented with acute oliguric renal failure and liver dysfunction after consuming high oral doses of Aloe vera (Luyckx et al. 2002). Aloe vera was also believed to be the cause of hypothyroidism in one female patient (Pigatto and Guzzi 2005) and Henoch–Schonlein purpura in another after an Aloe vera remedy juice was taken for back pain (Evangelos, Spyros, and Spyros 2005).
3.8.5. Contraindications and Drug Interactions
Allergy: Use of Aloe vera preparations should be avoided in individuals with a known allergy to plants of the Liliaceae family (garlic, onions, and tulips; Ulbricht et al. 2008).
Pregnancy: Use of Aloe vera as a laxative during pregnancy may pose potential teratogenic and toxicological effects on the embryo and fetus (World Health Organization 1999; Ulbricht et al. 2008).
Renal or cardiac disease: Prolonged use of Aloe vera latex has been associated with watery diarrhea resulting in electrolyte imbalance (Cooke 1981; Boudreau and Beland 2006), and anecdotal reports suggest that the increasing loss of potassium may lead to hypokalemia. Therefore, the Aloe vera latex is contraindicated in patients with a history of renal or cardiac disorders.
Drug interactions: Potential interactions have been suggested for Aloe vera and drugs that may alter electrolyte balance, such as thiazide diuretics and corticosteroids. Possible hypokalemia-related arrhythmia suggests a potential herb–drug interaction with cardiac glycosides. Caution is warranted in patients taking hypoglycemic agents as interactions with Aloe vera gel have been reported (Boudreau and Beland 2006). There exists a case report of a 35-year-old woman who lost 5 L of blood during surgery as a result of a possible herb-drug interaction between Aloe vera and sevoflurane, an inhibitor of thromboxane A2 (Lee et al. 2004).
3.9. DRUG/VITAMIN BIOAVAILABILITY
Aloe vera gel has been shown to enhance vitamin C and E’s bioavailability in a double-blind, randomized, controlled trial (Vinson, Al Kharrat, and Andreoli 2005). The authors suggest that Aloe vera gel protects against the degradation of vitamins in the intestinal tract and the gel polysaccharides may bind to vitamins and thereby slow down their absorption rate.
Aloe vera gel has been shown to significantly increase the transport of insulin in a cell model, and limited information suggests that if coadministered, it may also enhance the intestinal absorption of other poorly absorbed drugs (Hamman 2008).
3.10. RESEARCH NEEDS
Analysis of the potential efficacy of Aloe vera in the treatment of particular disorders is complicated by differences in Aloe vera preparations, their means of administration, and the animal model or study design employed in individual studies. Research on standardized methodological quality is, therefore, needed to identify which Aloe vera components, individually or in combination, exhibit therapeutic properties and the exact mechanisms by which they act. Controlled in vivo toxicology and safety studies of Aloe vera preparations in humans are also required.
3.11. CONCLUSIONS
Despite its long history of use, there remains a lack of consistent scientific evidence to support many of the therapeutic claims for Aloe vera. Evidence of efficacy is strongest for the laxative effects of Aloe vera latex, however, whether the latex is more efficacious than conventional laxative treatments has not yet been determined, and the anthraquinones in the latex are associated with considerable risks. The topical application of Aloe vera gel is likely safe and demonstrates overall efficacy in healing burn wounds, whereas some promising preliminary evidence suggests that the oral use of the gel may have beneficial effects in lowering blood glucose levels in type 2 DM, stabilizing metastatic cancer, and treating mild to moderate ulcerative colitis. Further research in humans is required to confirm these effects.
REFERENCES
- Agarwal O.P. Prevention of atheromatous heart disease. Angiology. 1985;36:485–92. [PubMed: 2864002]
- Agarwala O.M. Whole leaf Aloe gel vs. standard Aloe gel. Drug Cosmet Ind. 1997;160:22–8.
- Ajabnoor M.A. Effect of aloes on blood glucose levels in normal and alloxan diabetic mice. J Ethnopharmacol. 1990;28:215–20. [PubMed: 2109811]
- Akhtar M.A, Hatwar S.K. Efficacy of Aloe vera extract cream in management of burn wound. J Clin Epidemiol. 1996;49 1:24.
- Ashley F.L, O'Loughlin B.J, Peterson R, Fernandez L, Stein H, Schwartz A.N. The use of Aloe vera in the treatment of thermal and irradiation burns in laboratory animals and humans. Plast Reconstr Surg. 1957;20:383–96. [PubMed: 13505124]
- Blitz J, Smith J.W, Gerard J.R. Aloe vera gel in peptic ulcer therapy: Preliminary report. J Am Ost Assoc. 1963;62:731–35. [PubMed: 13971654]
- Boudreau M.D, Beland F.A. An evaluation of the biological and toxicological properties of Aloe barbadensis (Miller), Aloe vera. J Environ Sci Health C. 2006;24:103–54. [PubMed: 16690538]
- Bozzi A, Perrin C, Austin S, Arce Vera F. Quality and authenticity of commercial Aloe vera gel powders. Food Chem. 2007;103:22–30.
- Brusick D, Mengs U. Assessment of the genotoxic risk from laxative senna products. Environ Mol Mutagen. 1997;29:1–9. [PubMed: 9020301]
- Bunyapraphatsara N, Yongchaiyudha S, Rungpitarangsi V, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L juice. II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine. 1996;3:245–48. [PubMed: 23195078]
- Capasso F, Mascolo N, Autore G, Duraccio M.R. Effect of indomethacin on aloin and 1.8 dioxianthraquinone-induced production of prostaglandins in rat isolated colon. Prostaglandins. 1983;26:557–62. [PubMed: 6658004]
- Cárdenas C, Quesada A.R, Medina M.A. Evaluation of the anti-angiogenic effect of aloe-emodin. Cell Mol Life Sci. 2006;63:3083–89. [PubMed: 17131052]
- Chapman D.D, Pittelli J.J. Double-blind comparison of alophen with its components for cathartic effects. Curr Ther Res Clin Exp. 1974;16:817–20. [PubMed: 4217249]
- Chinnusamy K, Nandagopal T, Nagaraj K, Sridharanet S. Aloe vera induced oral mucositis: A case report. Internet J Pediatr Neonatol. 2009;9(2)
- Choi S, Chung M.H. A review on the relationship between Aloe vera components and their biologic effects. Semin Integr Med. 2003;1:53–62.
- Collins E, Collins C. Roentgen dermatitis treated with fresh whole leaf of Aloe vera. Am J Roentgenol. 1935;33:396–7.
- Cook N.C, Samman S. Flavonoids: Chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem. 1996;7:66–76.
- Cooke W. Laxative abuse. Acta Gastroenterol Belg. 1981;44:448–58. [PubMed: 7342623]
- Coronado G.D, Thompson B, Tejeda S, Godina R. Attitudes and beliefs among Mexican Americans about type 2 diabetes. J Health Care Poor Underserved. 2004;15:576–88. [PubMed: 15531816]
- Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of an Aloe andongensis extract, Aloe Andongensis leaf juice. Int J Toxicol. 2007;26:1–50. [PubMed: 17613130]
- Dagne E, Bisrat D, Viljoen A, Van Wyk B.E. Chemistry of Aloe species. Curr Org Chem. 2000;4:1055–78.
- Danhof I.E. Remarkable Aloe: Aloe through the Ages. Grand Prairie, TX: Omnimedicus Press; 1987.
- Davis R.H. Aloe vera: A Scientific Approach. New York: Vantage Press; 1997.
- Davis K, Philpott S, Kumar D, Mendall M. Randomised double-blind placebo-controlled trial of Aloe vera for irritable bowel syndrome. Int J Clin Pract. 2006;60:1080–86. [PubMed: 16749917]
- de Witte P. Metabolism and pharmacokinetics of anthranoids. Pharmacology. 1993;47 1:86–97. [PubMed: 8234447]
- Ernst E. Adverse effects of herbal drugs in dermatology. Br J Dermatol. 2000;143:923–29. [PubMed: 11069498]
- Eshun K, He Q. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries—A review. Crit Rev Food Sci Nutr. 2004;44:91–6. [PubMed: 15116756]
- Esua M.F, Rauwald J.W. Novel bioactive maloyl glucans from Aloe vera gel: Isolation, structure elucidation and in vitro bioassays. Carbohydr Res. 2006;341:355–64. [PubMed: 16343466]
- Evangelos C, Spyros K, Spyros D. Henoch-Schonlein purpura associated with Aloe vera administration. Eur J Intern Med. 2005;16:59–60. [PubMed: 15733825]
- Femenia A, Garcia-Pascual P, Simal S, Rosello C. Effects of heat treatment and dehydration on bioactive polysaccharide acemannan and cell wall polymers from Aloe barbadensis Miller. Carbohydr Polym. 2003;51:397–405.
- Femenia A, Sànchez E.S, Simal S, Rossello C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr Polym. 1999;39:109–17.
- Fulton J.E. The stimulation of postdermabrasion wound healing with stabilized Aloe vera gel-polyethylene oxide dressing. J Dermatol Surg Oncol. 1990;16:460–67. [PubMed: 2341661]
- Ghannam N, Kingston M, Al-Meshaal I.A, Tariq M, Parman N.S, Woodhouse N. The antidiabetic activity of aloes: Preliminary clinical and experimental observations. Horm Res. 1986;24:288–94. [PubMed: 3096865]
- Grindlay D, Reynolds T. The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol. 1986;16:117–51. [PubMed: 3528673]
- Hamman J.H. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–616. [PMC free article: PMC6245421] [PubMed: 18794775]
- Heber D. Physicians' Desk Reference for Herbal Medicines. 4th ed. Montvale, NJ: Thomson; 2007.
- Heck E, Head M, Nowak D, Helm P, Baxter C. Aloe vera (gel) cream as a topical treatment for outpatient burns. Burns. 1981;7:291–94.
- Heggie S, Bryant G.P, Tripcony L, Keller J, Rose P, Glendenning M, Health J.A. Phase III study on the efficacy of topical Aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002;25:442–51. [PubMed: 12464836]
- Ikeno Y, Hubbard G, Lee S, Yu B.P, Herlihy J.T. The influence of long-term Aloe vera ingestion on age-related disease in male Fischer 344 rats. Phytother Res. 2002;16:712–18. [PubMed: 12458471]
- International Aloe Science Council. How Large is the Aloe Market? http://www.iasc.org/aloemarket.html (accessed August 18, 2009)
- Ishii Y, Tanizawa H, Takino Y. Studies of aloe. III. Mechanism of cathartic effect. Chem Pharm Bull (Tokyo). 1990;38:197–200. [PubMed: 2159853]
- Kanat O, Ozet A, Ataergin S. Aloe vera-induced acute toxic hepatitis in a healthy young man. Eur J Intern Med. 2006;17:589. [PubMed: 17142185]
- Kim K, Kim H, Kwon J, editors. et al. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009;16:856–63. [PubMed: 19303272]
- Koo M.W.L. Aloe vera: Antiulcer and antidiabetic effects. Phytother Res. 1994;8:461–64.
- Langmead L, Feakins R.M, Goldthorpe S, editors. Randomised, double-blind, placebo-controlled trial of oral Aloe vera gel for active ulcerative colitis. Aliment Pharmacol Ther. 2004;19:739–47. [PubMed: 15043514]
- Lans C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2006;2:45–55. [PMC free article: PMC1624823] [PubMed: 17040567]
- Lee A, Chui P.T, Aun C.S, Gin T, Lau A.S. Possible interaction between sevoflurane and Aloe vera. Ann Pharmacother. 2004;38:1651–54. [PubMed: 15292490]
- Lissoni P, Giani L, Zerbini S, Trabattoni P, Rovelli F. Biotherapy with the pineal immunomodulating hormone melatonin versus melatonin plus Aloe vera in untreatable advanced solid neoplasms. Nat Immun. 1998;16:27–33. [PubMed: 9789122]
- Loveman A.B. Leaf of Aloe vera in treatment of roentgen ray ulcers: Report of 2 additional cases. Arch Derm Syphilol. 1937;36:838–43.
- Luyckx V.A, Ballantine R, Claeys M. Herbal remedy-associated acute renal failure secondary to Cape aloes. Am J Kidney Dis. 2002;39:E13. [PubMed: 11877593]
- Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of Aloe vera used for burn wound healing: A systematic review. Burns. 2007;33:713–18. [PubMed: 17499928]
- Manderville F. Aloe vera in the treatment of radiation ulcers and mucous membranes. Radiology. 1939;32:598–9.
- Matsuda Y, Yokohira M, Suzuki S, editors. One-year chronic toxicity study of Aloe arborescens Miller var. natalensis Berger in Wistar Hannover rats. A pilot study. Food Chem Toxicol. 2007;46:733–39. [PubMed: 17981383]
- Merchant T.E, Bosley C, Smith J, editors. A Phase III trial comparing an anionic phospholipid-based cream and Aloe vera -based gel in the prevention of radiation dermatitis in pediatric patients. Radiat Oncol. 2007;2:45–52. [PMC free article: PMC2238757] [PubMed: 18093332]
- Moghadasian M.H, Frohlich J.J. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: Clinical and experimental evidence. Am J Med. 1999;107:588–94. [PubMed: 10625028]
- Morrow D.M, Rapaport M.J, Strick R.A. Hypersensitivity to aloe. Arch Dermatol. 1980;116:1064–65. [PubMed: 7416761]
- Nasiff H.A, Fajardo F.R, Velez F. Effect of aloe on hyperlipidemia in patients with negative response to diet. Revista Cubana de Med Gen Integral. 1993;9:43–51.
- Ni Y, Turner D, Yates K.M, Tizard I. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int immunopharmacol. 2004;4:1745–55. [PubMed: 15531291]
- Nusko G, Schneider B, Schneider I, Wittekind C, Hahn E.G. Anthranoid laxative use is not a risk factor for colorectal neoplasia: Results of a prospective case control study. Gut. 2000;46:651–55. [PMC free article: PMC1727932] [PubMed: 10764708]
- Odes H.S, Madar Z. A double-blind trial of celandine, aloevera and psyllium laxative preparation in adult patients with constipation. Digestion. 1991;49:65–71. [PubMed: 1800188]
- Olsen D.L, Raub W, Bradley C. The effect of Aloe vera gel/mild soap versus mild soap alone in preventing skin reactions in patients undergoing radiation therapy. Oncol Nurs Forum. 2001;28:543–47. [PubMed: 11338761]
- Park Y, Lee S. New Perspectives on Aloe. New York: Springer Verlag; 2006.
- Park J.Y, Mitrou P.N, Luben R, Khaw K.T, Bingham S.A. Is bowel habit linked to colorectal cancer? Results from the EPIC-Norfolk study. Eur J Cancer. 2009;45:139–45. [PubMed: 19013785]
- Paulsen E, Korsholm L, Brandrup F. A double-blind, placebo-controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2005;19:326–31. [PubMed: 15857459]
- Payne J.M. 1970. Tissue response to Aloe vera gel following periodontal surgery. Master's thesis. (accessed October 5, 2009)
- Pigatto P, Guzzi G. Aloe linked to thyroid dysfunction. Arch Med Res. 2005;36:608. [PubMed: 16099348]
- Rabe C, Musch A, Schirmacher P, Kruis W, Hoffmann R. Acute hepatitis induced by an Aloe vera preparation: A case report. World J Gastroenterol. 2005;11:303–4. [PMC free article: PMC4205424] [PubMed: 15633238]
- Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S. Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33:232–37. [PubMed: 16487267]
- Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food. 2004;7:61–6. [PubMed: 15117555]
- Ralph A, Provan G.J. Phytoprotectants. In: Garrow J.S, James W.P.T, Ralph A, editors. In Human Nutrition and Dietetics. Edinburgh: Churchill Livingstone; 2000. pp. 417–26.
- Ross S.A, Elsohly M.A, Wilkins S.P. Quantitative analysis of Aloe vera mucilaginous polysaccharide in commercial Aloe vera products. J AOAC Int. 1997;80:455–57.
- Rowe T.D. Effect of fresh Aloe vera gel in the treatment of third-degree roentgen reactions on white rats. J Am Pharm Assoc. 1940;29:348–50.
- Saccu D, Bogoni P, Procida G. Aloe exudate: Characterization by reversed phase HPLC and head-space GC-MS. J Agric Food Chem. 2001;49:4526–30. [PubMed: 11599983]
- Samman S. Lipid metabolism. In: Kuchel P.W, Ralston G.B, editors. In Schaum’s Outlines of Theory and Problems of Biochemistry. New York: McGraw Hill Book Company; 1998. pp. 362–401.
- Schmidt J.M, Greenspoon J.S. Aloe vera dermal wound gel is associated with a delay in wound healing. Obstet Gynecol. 1991;1:115–17. [PubMed: 2047051]
- Shah A.H, Qureshi S, Tariq M, Ageel A.M. Toxicity studieson six plants used in the traditional Arab system of medicine. Phytother Res. 1989;3:125–29.
- Siegers C.P, von Hertzberg-Lottin E, Otte M, Schneider B. Anthranoid laxative abuse-a risk for colorectal cancer? Gut. 1993;34:1099–101. [PMC free article: PMC1374362] [PubMed: 8174962]
- Steenkamp V, Stewart M.J. Medicinal applications and toxicological activities of Aloe products. Pharm Biol. 2007;45:411–20.
- Su C.K, Mehta V, Ravikumar L, editors. et al. Phase II double-blind randomized study comparing oral Aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms. Int J Radiat Oncol Biol Phys. 2004;60:171–77. [PubMed: 15337553]
- Sun J.H, Chen X.G, Jin R.T, Li T.N, Bian Y.X. People’s Liberation Army medicine information. Med J Chin Army. 1994;8:191–92.
- Syed T.A, Ahmad S.A, Holt A.H, Ahmad S.A, Ahmad S.H, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: A placebo-controlled, double-blind study. Trop Med Int Health. 1996;1:505–09. [PubMed: 8765459]
- Tanaka M, Misawa E, Ito Y, editors. et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006;29:1418–22. [PubMed: 16819181]
- Thamlikitkul V, Bunyapraphatsara N, Riewpaiboon W, editors. et al. Controlled trial of Aloe vera Linn. for treatment of minor burns. Siriraj Hosp Gaz. 1991;43:313–16.
- t'Hart L.A, van den Berg A.J, Kuis L, van Dijk H, Labadie R.P. An anti-complementary poly-saccharide with immunological adjuvant activity from the leaf parenchyma gel of Aloe vera. Planta Med. 1989;55:509–12. [PubMed: 2616669]
- Turner C, Williamson D.A, Stroud P.A, Talley D.J. Evaluation and comparison of commercially available Aloe vera L. products using size exclusion chromatography with refractive index and multiangle laser light scattering detection. Int Immunopharmacol. 2004;4:1727–37. [PubMed: 15531289]
- Ulbricht C, Armstrong J, Basch E. et al. An evidence-based systematic review of Aloe vera by the Natural Standard Research Collaboration. J Herb Pharmacother. 2008;7:279–323. [PubMed: 18928148]
- Vinson J.A, Al Kharrat H, Andreoli L. Effect of Aloe vera preparations on the human bioavailability of vitamins C and E. Phytomedicine. 2005;12:760–65. [PubMed: 16323295]
- Visuthikosol V, Sukwanarat Y, Chowchuen B, Sriurairatana S, Boonpucknavig V. Effect of Aloe vera gel to healing of burn wound a clinical and histologic study. J Med Assoc Thai. 1995;78:403–8. [PubMed: 7561562]
- Vogler B, Ernst E. Aloe vera: A systematic review of its clinical effectiveness. Br J Gen Pract. 1999;49:823–28. [PMC free article: PMC1313538] [PubMed: 10885091]
- Yeh G.Y, Eisenberg D.M, Kaptchuk T.J, Phillips R.S. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–94. [PubMed: 12663610]
- Yokohira M, Matsuda Y, Suzuki S. et al. Equivocal colonic carcinogenicity of Aloe arborescens Miller var. natalensis Berger at high-dose level in a Wistar Hannover rat 2-y study. J Food Sci. 2009;74:T24–30. [PubMed: 19323775]
- Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine. 1996;3:241–43. [PubMed: 23195077]
- Wang W, Cuyckens F, Van den Heuvel H. Structural characterization of chromone C-glucosides in a toxic herbal remedy. Rapid Commun Mass Spectrom. 2003;17:49–55. [PubMed: 12478554]
- Wang Y, Strong K. A two-year study monitoring several physical and chemical properties of field-grown Aloe barbadensis Miller leaves. Subtropical Plant Sci. 1995;47:34–8.
- Williams M.S, Burk M, Loprinzi C.L. Phase III double-blind evaluation of an Aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol Biol Phys. 1996;36:345–49. [PubMed: 8892458]
- World Health Organization. WHO Monographs on Selected Medicinal Plants. Vol. 1. Geneva: World Health Organization; 1999.
- Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats.[J Med Assoc Thai. 2000]Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats.Somboonwong J, Thanamittramanee S, Jariyapongskul A, Patumraj S. J Med Assoc Thai. 2000 Apr; 83(4):417-25.
- Review The therapeutic efficacy and properties of topical Aloe vera in thermal burns.[J Med Assoc Thai. 2004]Review The therapeutic efficacy and properties of topical Aloe vera in thermal burns.Somboonwong J, Duansak N. J Med Assoc Thai. 2004 Oct; 87 Suppl 4:S69-78.
- Review Aloe vera as an herbal medicine in the treatment of metabolic syndrome: A review.[Phytother Res. 2019]Review Aloe vera as an herbal medicine in the treatment of metabolic syndrome: A review.Shakib Z, Shahraki N, Razavi BM, Hosseinzadeh H. Phytother Res. 2019 Oct; 33(10):2649-2660. Epub 2019 Aug 28.
- Transforming growth factor-β (TGF-β) activation in cutaneous wounds after topical application of aloe vera gel.[Can J Physiol Pharmacol. 2016]Transforming growth factor-β (TGF-β) activation in cutaneous wounds after topical application of aloe vera gel.Takzaree N, Hadjiakhondi A, Hassanzadeh G, Rouini MR, Manayi A, Zolbin MM. Can J Physiol Pharmacol. 2016 Dec; 94(12):1285-1290. Epub 2016 Jul 14.
- Review Aloe vera: a systematic review of its clinical effectiveness.[Br J Gen Pract. 1999]Review Aloe vera: a systematic review of its clinical effectiveness.Vogler BK, Ernst E. Br J Gen Pract. 1999 Oct; 49(447):823-8.
- Evaluation of the Nutritional and Metabolic Effects of Aloe vera - Herbal Medici...Evaluation of the Nutritional and Metabolic Effects of Aloe vera - Herbal Medicine
Your browsing activity is empty.
Activity recording is turned off.
See more...