NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Center for Substance Abuse Treatment. Managing Chronic Pain in Adults With or in Recovery From Substance Use Disorders. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2012. (Treatment Improvement Protocol (TIP) Series, No. 54.)

Managing Chronic Pain in Adults With or in Recovery From Substance Use Disorders.
Show detailsOverview of Pain Management
Chronic noncancer pain (CNCP) is a major challenge for clinicians as well as for the patients who suffer from it. The complete elimination of pain is rarely obtainable for any substantial period. Therefore, patients and clinicians should discuss treatment goals that include reducing pain, maximizing function, and improving quality of life. The best outcomes can be achieved when chronic pain management addresses co-occurring mental disorders (e.g., depression, anxiety) and when it incorporates suitable nonpharmacologic and complementary therapies for symptom management. Exhibit 3-1 presents the consensus panel’s recommended strategy for treating CNCP in adults who have or are in recovery from a substance use disorder (SUD).
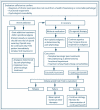
Exhibit 3-1
Algorithm for Managing Chronic Pain in Patients With SUD.
The Treatment Team
Chronic pain management is often complex and time consuming. It can be particularly challenging and stressful for clinicians working without input from other clinicians. The effectiveness of multiple interventions is augmented when all medical and behavioral healthcare professionals involved collaborate as a team (Sanders, Harden, & Vicente, 2005). A multidisciplinary team approach provides a breadth of perspectives and skills that can enhance outcomes and reduce stress on individual providers. Although it is ideal when all relevant providers work within the same system and under the same roof, often a collaborative team must be coordinated across a community. This combined effort requires identification of a designated lead care coordinator and a good system of communication among team members and the patient. A treatment team can include the following professionals:
- Primary care provider
- Addiction specialist
- Pain clinician
- Nurse
- Pharmacist
- Psychiatrist
- Psychologist
- Other behavioral health treatment specialists (e.g., social worker, marriage and family therapist, counselor)
- Physical or occupational therapists
Addiction specialists, in particular, can make significant contributions to the management of chronic pain in patients who have SUDs. They can:
- Put safeguards in place to help patients take opioids appropriately.
- Reinforce behavioral and self-care components of pain management.
- Work with patients to reduce stress.
- Assess patients’ recovery support system.
- Identify relapse.
When the addiction specialist is the prescriber of analgesics, medical responsibilities (e.g., prescribing of analgesics, physical therapy, orthotics) should be coordinated with the clinician responsible for other components of pain treatment. In some States, consultation with an addiction specialist is required before scheduled medications can be prescribed on a long-term basis to patients who have SUD histories. State laws, regulations, and policies are available at http://www.painpolicy.wisc.edu/.
The more complicated the case, the more beneficial a team approach becomes. However, many clinicians will have to treat complex patients who have little or no outside resources.
Treating Patients in Recovery
A thorough patient assessment (see Chapter 2) provides information that allows the clinician to judge the stability of a patient’s recovery from an SUD. Goals for treating CNCP in patients who are in long-term recovery or whose SUD is in the distant past are as follows:
- Treat CNCP with non-opioid analgesics as determined by pathophysiology.
- Recommend or prescribe nonpharmacological therapies (e.g., cognitive–behavioral therapy [CBT], exercises to decrease pain and improve function).
- Treat comorbidities.
- Assess treatment outcomes.
- Initiate opioid therapy only if the potential benefits outweigh risk and only for as long as it is unequivocally beneficial to the patient.
Non-Opioid Analgesics
Non-opioid pharmacological options include acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), as well as adjuvant medications—so called because they originally were developed for other purposes but have analgesic properties for certain conditions. The primary adjuvant analgesics are antidepressants and anticonvulsants. Exhibit 3-2 presents a summary of these analgesics as they pertain to patients who have SUDs.
Exhibit 3-2
Summary of Non-Opioid Analgesics.
Benzodiazepines
Researchers disagree on the beneficial and harmful effects of benzodiazepines and benzodiazepine receptor agonists on chronic pain. Several studies demonstrate increased pain with benzodiazepines or reduced pain following benzodiazepine antagonist use (Ciccone et al., 2000; Gear et al., 1997; Nemmani & Mogil, 2003; Pakulska & Czarnecka, 2001). All benzodiazepines have side effects, including impaired coordination, reduced memory, and addiction liability. For the following reasons, the consensus panel concludes that benzodiazepines have no role in the treatment of CNCP in patients who have comorbid SUD, beyond very short-term, closely supervised treatment of acute anxiety states:
- Guidelines from the American Psychiatric Association (2006) and the United Kingdom’s National Institute for Health and Clinical Excellence (Hughes et al., 2004) caution that benzodiazepines are not first-line medications.
- Excellent options to benzodiazepines for treating anxiety exist (see Treating Psychiatric Comorbidities, below).
- Anxiolytic use in adults with CNCP is often protracted.
- Benzodiazepines pose significant risk for addiction relapse and functional impairment.
The consensus panel recommends that clinicians treat comorbid anxiety and insomnia with antidepressants or anticonvulsants. Some antidepressants (e.g., trazodone, mirtazapine, amitriptyline, doxepin) may be useful sleep aids. Benzodiazepine weaning can be done in consultation with a psychiatrist or SUD treatment provider (see Center for Substance Abuse Treatment [CSAT], 2006).
Cannabinoids
At least two types of cannabinoid receptors are present in the human nervous system, and they interact with systems relevant to pain perception, including the serotonergic and dopaminergic systems. Cannabinoids are anti-inflammatory and increase levels of endogenous opioids. They inhibit glutamatergic transmission and antagonize the N-methyl-D-aspartate (NMDA) glutamate receptor, both of which actions would be expected to inhibit pain (Burns & Ineck, 2006; McCarberg, 2006).
The primary psychoactive chemical in marijuana responsible for its abuse potential is 9 tetrahydrocannabinol (THC). Synthetic THC (Marinol) is approved in the United States for chemotherapy-induced nausea and AIDS-induced anorexia. Sativex, a mixture of THC and cannabidiol, is an oromucosal spray that spares the lungs the toxicity of drugs and smoke. It is analgesic in neuropathic pain and is approved in Canada for the pain of multiple sclerosis. Nabilone is a synthetic drug similar to THC. Its reported analgesic effects were determined to be weaker than codeine in a controlled study of neuropathic pain (Frank, Serpell, Hughes, Matthews, & Kapur, 2008).
Although it is reasonable to conclude that modulating the human cannabinoid system shows promise for treating pain, there is no reason to believe that inhaled smoke is an acceptable delivery mode. The consensus panel does not recommend smoked marijuana for treating CNCP.
Nonpharmacological Treatments
An approach to pain management that integrates evidence-based pharmacological and nonpharmacological treatments can ease pain and reduce reliance on medication.
Nonpharmacological treatments for CNCP (Hart, 2008; Simpson, 2006):
- Pose no risk of relapse.
- May be more consistent with the recovering patient’s values and preferences than pharmacological treatments, especially opioid interventions.
- May reduce pain and improve quality of life in some patients who have CNCP.
- Should be included in most pain treatment plans.
Common nonpharmacological therapies for CNCP include:
- Therapeutic exercise.
- Physical therapy (PT).
- Cognitive–behavioral therapy (CBT).
- Complementary and alternative medicine (CAM; e.g., chiropractic therapy, massage therapy, acupuncture, mind–body therapies, relaxation strategies).
Appendix D provides information on how to find qualified practitioners who provide CAM.
Therapeutic Exercise
A number of practitioners, including physicians, chiropractors, and physical therapists, frequently include exercise instruction and supervised exercise components in CNCP treatment. Therapeutic exercise can increase strength, aerobic capacity, balance, and flexibility; improve posture; and enhance general well-being. Fitness can be an antidote to the sense of helplessness and personal fragility experienced by many people with CNCP. Moderate evidence shows that exercise alleviates low back pain, neck pain, fibromyalgia, and other conditions. Furthermore, exercise reduces anxiety and depression. Limited evidence suggests that exercise benefits individuals undergoing SUD treatment (Weinstock, Barry, & Petry, 2008).
Physical Therapy
PT facilitates recovery from a large variety of medical conditions, including cardiopulmonary, geriatric, pediatric, integumentary, neurologic, and orthopedic. Neurologic PT and orthopedic PT are most likely to be used to treat chronic pain. Physical therapists use various hands-on approaches to help patients increase their range of motion, strength, and functioning. They also offer training in movement and exercises that help patients feel and function better.
Many widely used interventions by physical therapists lack definitive evidence. For example, several Cochrane Collaboration reviews of a commonly used PT modality—transcutaneous electrical nerve stimulation—found inconsistent evidence of effectiveness in a variety of chronic and acute pain conditions. Despite this lack of an evidence base, PT interventions have the advantages of being nonsurgical, bringing low risk of injury or dependence, and encouraging patients’ involvement in their own recovery.
Cognitive–Behavioral Therapy
Several studies have shown that CBT can help patients who have CNCP reduce pain and associated distress, disability, depression, anxiety, and catastrophizing, as well as improve coping, functioning, and sleep (McCracken, MacKichan, & Eccleston, 2007; Thorn et al., 2007; Turner, Mancl, & Aaron, 2006; Vitiello, Rybarczyk, Von Korff, & Stepanski, 2009). In addition to its salutary effects on pain syndromes, CBT also benefits people who have SUDs. In a meta-analysis of 53 controlled trials of CBT for alcohol or illicit drug disorders, CBT was found to produce a small but significant benefit (Magill & Ray, 2009).
Complementary and Alternative Medicine
CAM includes health systems, practices, and products that are not necessarily considered part of conventional medicine (National Center for Complementary and Alternative Medicine, 2007). Surveys show that 27–60 percent of chronic pain patients use CAM (Fleming, Rabago, Mundt, & Fleming, 2007; McEachrane-Gross, Liebschutz, & Berlowitz, 2006; Nayak, Matheis, Agostinelli, & Shifleft, 2001). Clinicians are urged to learn about these approaches to pain treatment not only because of their therapeutic promise, but also because many patients use CAM, raising the possibility of interactions with conventional treatments (Simpson, 2006). Exhibit 3-3 presents one way to ask patients about their use of CAM.
Exhibit 3-3
Talking With Patients About Complementary and Alternative Medicine.
The evidence supporting CAM interventions for adults with comorbid CNCP and SUD is ambiguous. These conditions are complex and multifactorial and, therefore, difficult to study. Many systematic reviews of CAM research note generally poor-quality reporting and heterogeneous methodology that precludes definitive evidence-based conclusions (e.g., Gagnier, van Tulder, Berman, & Bombardier, 2006). Of the CAM interventions, manual therapies are the most widely used and the most studied (Simpson, 2006). Chiropractic and massage therapies are often covered by health insurance, making these therapies accessible and compatible with conventional therapies.
Treating Psychiatric Comorbidities
Research shows well-established associations among chronic pain, SUDs, and mental disorders (e.g., depression, anxiety, post-traumatic stress disorder [PTSD], somatoform disorders) (Chelminski et al., 2005; Covington, 2007; Manchikanti et al., 2007; Saffier, Colombo, Brown, Mundt, & Fleming, 2007; Wasan et al., 2007). Psychiatric comorbidity is of special significance for two reasons. First, it is often occult. Second, untreated psychopathology is associated with poor pain treatment outcomes (Edwards et al., 2007; Williams, Jones, Shen, Robinson, & Kroenke, 2004). Therefore, management of patients who have CNCP must include intervention for co-occurring psychopathology.
Because psychiatric comorbid disorders might be preexisting, or they may develop or worsen with chronic pain or SUDs, it is important to determine the onset of psychiatric symptoms during the screening and assessment process (see Chapter 2). The psychiatric disorder needs to be included in the comprehensive treatment plan that is developed for the patient in consultation with the patient’s treatment team (e.g., primary healthcare provider, substance abuse treatment counselor, pain management provider, mental health professional). CSAT (2005b) provides detailed information on treatment strategies and models for working with individuals with a wide spectrum of psychiatric co-occurring disorders.
Benzodiazepines are generally indicated for short-term treatment of anxiety; however, anxiety associated with chronic pain commonly persists for years. Effective options include (Van Ameringen, Mancini, Pipe, & Bennett, 2004):
- Psychological and behavioral treatments.
- Selective serotonin reuptake inhibitors (SSRIs).
- SNRIs.
- Tricyclic antidepressants.
- Several anticonvulsants.
The anxiety that is often comorbid with CNCP can often be managed satisfactorily with adjuvants prescribed for the pain syndrome. Several anticonvulsants that are used for CNCP are strongly anxiolytic. In a review, Van Ameringen and colleagues (2004) found that the strongest evidence was for pregabalin (for social phobia and generalized anxiety disorder), gabapentin (for social phobia), lamotrigine (for PTSD), and valproic acid (for panic disorder). In addition, many anti-depressants are effective for chronic pain and may be used to treat comorbid anxiety and depression, and both duloxetine and venlafaxine have been approved by the Food and Drug Administration for treatment of generalized anxiety disorder. Most tricyclic antidepressants are anxiolytic. Trazodone has also been found to be anxiolytic and is often used as a sedative in patients for whom benzodiazepine-like agents are undesirable. Treating comorbidities with medications that also alleviate pain can reduce polypharmacy, drug interactions, non-adherence, and, at times, financial costs.
The person who somatizes extensively may present a plethora of complaints. This situation may lead to the clinician’s inappropriate discounting of all the patient’s symptoms as trivial or imaginary. Clinicians should take the following steps in treating such a patient:
- Complete an inventory of all the patient’s complaints.
- Emphasize history and physical examination in the evaluation.
- Validate the patient’s symptoms while assuring him or her about the absence of worrisome pathology.
- Minimize expensive or invasive tests and treatments.
- Minimize use of medications with abuse liability, especially short-acting medications used as needed (PRN).
- Minimize use of passive modalities of therapy.
- Schedule regular appointments rather than PRN visits.
- Adequately treat comorbid Axis I (i.e., major psychiatric) disorders.
- Refer patients for counseling or relaxation training, as available.
Opioid Therapy
Limitations
Opioids are potent analgesics that may provide relief for many types of CNCP. However, even when effective, they have limitations, such as diminished efficacy over time (Ballantyne, 2006; Noble, Tregear, Treadwell, & Schoelles, 2008). Opioids also have adverse effects that many patients cannot tolerate (e.g., nausea, sedation, constipation). Other drawbacks include risk of addiction or addiction relapse, opioid-induced hyperalgesia (OIH), and many potential drug interactions. Serotonin syndrome is a potential adverse effect of both opioids and some medications used to treat depression, obsessive-compulsive disorder, or other behavioral health disorders. Serotonin syndrome can cause agitation, confusion, fever, and seizures, and it can be lethal if undetected or untreated. Patients who take SSRIs, SNRIs, St. John’s Wort, monoamine oxidase inhibitors, lithium, or HIV medications are at increased risk of serotonin syndrome (U.S. Food and Drug Administration, 2006). In addition, patients who take opioids chronically are at increased risk of serotonin syndrome if medications such as fentanyl, meperidine, or pentazocaine are needed in emergency or surgical care settings.
Although opioids are an important treatment component for many patients, they are rarely sufficient. Chronic opioid therapy rarely shows more than one-third pain reduction in studies extending beyond 18 months, indicating that opioids are best used as one part of a multidimensional approach for most patients.
When an SUD co-occurs with CNCP, the benefits of opioids are not well established and risk of relapse is increased (Reid et al., 2002). Studies indicate that most patients who are currently addicted to prescription opioids had a prior SUD, suggesting that people in recovery are at increased risk for relapse (Potter et al., 2004; Rosenblum et al., 2003). This may be especially true when the prior SUD involved opioids, because one of the most powerful triggers for relapse is exposure to the former drug of choice (Daley et al., 2003; Gardner, 2000). Trescot and colleagues (2008) provide a detailed review.
Before Initiating Opioid Treatment
Exhibit 3-4 shows steps to take before initiating opioid therapy. Information about patient education, informed consent, and treatment plans is provided in Chapter 5.
Exhibit 3-4
Steps To Take If Opioid Therapy Is Indicated. Department of Veterans Affairs & Department of Defense, 2010.
Opioid Selection
For patients who have histories of SUDs, it is essential to minimize exposure to the euphoric effects of opioids. To reduce the likelihood of such effects, clinicians should:
- Select opioids with minimal rewarding properties (e.g., tramadol, codeine), when effective.
- Avoid prescribing supratherapeutic doses (usually demonstrated by presence of sedation, lethargy, functional impairment).
- If higher potency opioids are required, prescribe slow-onset opioids with prolonged duration of action (Mironer, Brown, Satterthwaite, Haasis, & LaTourette, 2000).
Short-acting medications have been recommended to be used preemptively before activities known to cause pain, such as PT, or for pain limited to certain times of day. There is controversy regarding the appropriateness of this suggestion for patients who have CNCP (Devulder, Jacobs, Richarz, & Wigget, 2009), and the practice is especially hazardous in people with current or past SUDs.
The route of administration may influence addiction risk, so medications that are injected or easily convertible to forms that can be injected, smoked, or snorted are often avoided in patients who have SUDs. Some clinicians favor transdermal medication, with an agreement that refills are contingent on the patient’s returning the used patches to demonstrate that they were not punctured, cut, or diverted.
Dose Finding
Dose finding for the patient with an SUD, especially a history of abuse of or dependence on opioids, can be complicated because of existing or rapidly developing tolerance to opioids. Also, analgesics affect individuals differently. A person who states that a particular opioid “doesn’t work for me,” whereas another opioid does, may be accurately reporting analgesic response.
Titration schedules appropriate for the patient with no SUD history may expose the patient in SUD recovery to a protracted period of inadequate relief. Although no schedule can be applied to everyone, a general guide is that, if low doses of opioids (other than methadone) are initiated for severe pain, they should be titrated rapidly to avoid subjecting the patient to a prolonged period of dose finding. However, if relatively high doses are initiated, titration should be slower and determined to a great extent by the half-life of the drug. For some patients, increasing the dose may lead to decreased functioning. It is essential that clinicians understand that dose finding for methadone can be dangerous (see Exhibit 3-5).
Exhibit 3-5
Methadone Titration. The titration of methadone for chronic pain is complex and potentially dangerous because methadone levels increase during the first few days of treatment. This risk is compounded by the variable half-life among individuals and the (more...)
When an effective dose for a given patient has been determined, total opioid dose should thereafter be escalated very slowly, if at all, as tolerance develops. No study has ever shown that opioids eliminate chronic pain, other than in the very short term, so efforts to achieve a zero pain level with opioids will fail, while subjecting the patient to potentially intoxicating doses of the medication.
Relapse
For patients on chronic opioid therapy who have minor relapses and quickly regain stability, provision of substance abuse counseling, either in the medical setting or through a formal addiction program, may suffice. Opioids, if their continuation is deemed safe, must be very closely monitored, with short dispensing intervals and frequent urine drug testing. Unfortunately, many addiction treatment programs are unwilling to admit patients who are taking opioid pain medications, interpreting their prescription opioid use as a sign of active addiction.
Clinicians prescribing opioids need to establish relationships with substance abuse treatment providers who are willing to provide services for patients who need additional support in their recovery but do not require extensive services. For clinicians who treat a population with high levels of comorbid addiction, the development of onsite chemical dependence counseling services can be extremely helpful.
For relapse in patients for whom opioid addiction is a serious problem, referral to an opioid treatment program (OTP) for methadone maintenance therapy (MMT) may be the best choice. Such programs will not generally accept patients whose primary problem is pain because they do not have the resources to provide comprehensive pain management services. Patients who have chronic pain likely will not obtain adequate pain control through the single daily dose of methadone that can be provided through an OTP. Such programs may, however, be willing to collaborate in the management of patients, providing addiction treatment and allowing the prescription of additional opioids for pain management through a medical provider. Such arrangements require close communication between the OTP and the prescribing clinician so that patients who do not respond to SUD treatment can be safely withdrawn from opioids prescribed for pain. CSAT (2005a) provides more information about OTPs.
Another option for patients who have comorbid active addiction and CNCP is replacement of full agonist opioids with the partial opioid agonist buprenorphine (Heit, Covington, & Good, 2004; Heit & Gourlay, 2008). Benefits of this treatment include that dose escalation does not provide reinforcement and that the effects of other opioid substances may be attenuated. Buprenorphine can prevent withdrawal symptoms, allowing patients to stabilize and facilitating their progression into non-opioid and nonpharmacologic forms of pain treatment. However, buprenorphine prescribed specifically for pain is currently an off-label use (see Treating Patients in Medication-Assisted Recovery).
Opioid Discontinuation
Opioids should be discontinued if patient harm and public safety outweigh benefit. This situation may be apparent early in therapy, for example, if function is impaired by doses necessary to achieve useful analgesia. Harm also may outweigh benefit after a long period of successful treatment. Discontinuation of opioid therapy is addressed in Chapter 4.
Treating Patients in Medication-Assisted Recovery
Goals for treating CNCP in patients who are in medication-assisted recovery are the same as for patients who are in recovery without medications: reduce pain and craving and improve function. As with other patients:
- Start with recommending or prescribing nonpharmacological and non-opioid therapies.
- Treat comorbidities.
- Closely monitor treatment outcomes for evidence of benefit and harm.
Patients receiving opioid agonist treatment for addiction require special consideration when being treated for chronic pain. In these patients, the schedule and doses of opioid agonists sufficient to block withdrawal and craving are unlikely to provide adequate analgesia. Because of tolerance, a higher-than-usual dose of opioids may be needed (in addition to the maintenance dose) to provide pain relief.
Buprenorphine
Patients who have CNCP and are using sublingual buprenorphine treatment of opioid addiction pose special challenges. The drug is a partial mu agonist that binds tightly to the receptor. Because it is a partial agonist, its dose–response curve plateaus or even declines as the dose is increased. Thus, a ceiling dose limits both the available analgesia and the toxicity produced by overdose. Nevertheless, buprenorphine is an effective analgesic, and some patients who have addiction and CNCP may receive benefit for both conditions from it. To optimize analgesic efficacy, the drug should be given three times a day when pain reduction is a goal. High doses of buprenorphine can attenuate the effects of pure mu agonists given in addition to it. High doses tend to reduce the reinforcing effects of inappropriately consumed opioids but, at the same time, may reduce the effectiveness of opioids given for additional analgesia in the case of trauma or acute illness (Alford, Compton, & Samet, 2006).
Because buprenorphine has such high affinity for the mu receptor, it displaces full agonists and can induce acute opioid withdrawal; for example, if a patient on chronic methadone is given a dose of buprenorphine, acute opioid withdrawal may be precipitated (see CSAT [2004] for more information).
The use of buprenorphine for pain is off-label, albeit legal. Whereas clinicians must obtain a waiver to prescribe buprenorphine for an SUD, only a Drug Enforcement Administration (DEA) registration is required to prescribe buprenorphine for pain. To clarify (for pharmacists) that a prescription does not require the special DEA number, it is useful to specify on the prescription that the drug is “for pain.”
Methadone
Patients who have chronic pain do not obtain adequate pain control through a single daily dose of methadone because the analgesic effects of methadone are short acting in comparison with its half-life. The dosing schedule for the treatment of opioid addiction does not effectively treat pain, although the single dose often provides transient analgesia.
Methadone effects vary significantly from patient to patient, and finding a safe dose is difficult. Methadone’s analgesic effects last approximately 6 hours. However, its half-life is variable and may be up to 36 hours in some patients. Pain patients may take 10 days or longer to stabilize on methadone, so the clinician must titrate very slowly and balance the risk of insufficient dosing with the life-threatening dangers of overdosing (Heit & Gourlay, 2008) (Exhibit 3-5). It is critical for the clinician to advise patients to stop methadone treatment if they become sedated.
Methadone is an especially desirable analgesic for chronic use because of its low cost and its relatively slow development of analgesic tolerance; however, it is also especially toxic because of issues of accumulation, drug interaction, and QT prolongation. For these reasons, it should be prescribed only by providers who are thoroughly familiar with it.
It is critical that patients starting methadone receive a thorough education in the dangers of inadvertent overdose with this medication. They must understand that a dose that seems initially inadequate can be toxic a few days later because of accumulation. They should be advised to keep the medication out of reach so that they cannot take a dose when sedated. Furthermore, they must be informed of the extreme danger if a child or nontolerant adult ingests their medication. Chapter 5 provides more patient education information, and CSAT (2009b) describes emerging issues in the use of methadone.
Naltrexone
Patients taking naltrexone should not be prescribed outpatient opioids for any reason. Naltrexone is a long-acting oral or injectable mu antagonist that blocks the effects of opioids. It also reduces alcohol consumption by impeding its rewarding effects. Because naltrexone displaces opioid agonists from their binding sites, opioid analgesics will not be effective in patients on naltrexone. Increasing the dose of opioids to overcome the blockade puts the patient at risk of respiratory arrest. Pain relief for these patients requires non-opioid modalities.
If patients on naltrexone require emergency opioids for acute pain, higher doses are required, which, if continued, can become toxic as naltrexone levels wane. In this situation, inpatient or prolonged emergency department monitoring is required (Covington, 2008).
Tolerance and Hyperalgesia
Tolerance develops rapidly to the sedating, euphoric, and anxiolytic effects of opioids. It develops more slowly to their analgesic effects and seldom develops to their constipating effects. Tolerance can be characterized as decreased sensitivity to opioids, whereas OIH is increased sensitivity to pain resulting from opioid use. In a clinical setting, it may be impossible to distinguish between the two conditions, and they may coexist (Angst & Clark, 2006). Tolerance can develop in chronic opioid therapy regardless of opioid type, dose, route of administration, and administration schedules (DuPen, Shen, & Ersek, 2007). Hyperalgesia has been found to result from the use of those opioids thus far studied (i.e., methadone, buprenorphine, sufentanyl, fentanyl, morphine, heroin). Patients in MMT experience analgesic tolerance and OIH. Clinical implications of these findings are unclear, as studies indicate that OIH may develop to some measures of pain (e.g., cold pressor test) and not to others (e.g., pressure) (Mao, 2002).
When patients develop tolerance to the analgesic effects of a particular opioid, either dose escalation or opioid rotation may be useful (Exhibit 3-6). Opioid rotation, switching from one opioid to another, is a way to exploit incomplete cross-tolerance to achieve improved analgesia without an increase in (equivalent) doses.
Exhibit 3-6
Opioid Rotation. When an opioid is ineffective, becomes ineffective, or produces intolerable side effects, it is common practice to rotate opioids. This practice is based on the observation that particular opioids affect people differently, primarily (more...)
If a patient requests an increase in opioid dose, it is important for the clinician to try to discern whether the patient is experiencing increased pain or analgesic tolerance or is seeking some other effect (e.g., sedation, reduced anxiety). In the patient seeking sedation or reduced anxiety, a larger opioid dose provides temporary anxiolytic or sedative effects, but tolerance soon develops, necessitating another dose increase. To avoid a cycle of dose increases, the clinician should evaluate the patient’s request. When nonanalgesic effects seem to be the basis for the request, alternative non-opioid medications should be provided and opioid doses should not be increased.
As with tolerance, OIH appears to require increased doses of opioids to achieve previous levels of analgesia. However, with OIH, increased doses could exacerbate pain. Treating pain with a multimodal approach—in addition to analgesics—may reduce the need for opioids, thereby decreasing the risk of tolerance and OIH.
Treating Pain in Patients Who Have Active Addiction
The presence of active addiction—whether to alcohol, opioids, or other substances—makes successful treatment of chronic pain improbable (Covington, 2008; Weaver & Schnoll, 2007). For patients who have active addiction and CNCP, it may be impossible for clinicians in the primary care setting to provide the comprehensive services necessary to treat both conditions. Specifically, an active SUD indicates that the patient should be referred for formal addiction treatment. The clinician should work closely with the patient’s SUD treatment provider.
If the patient refuses the SUD referral, the clinician can use motivational interviewing techniques. CSAT (1999b) provides more information on motivational interviewing. If the patient still does not consent to addiction treatment, he or she should not be prescribed scheduled medications, except for acute pain or detoxification. CSAT (2006) provides more information on detoxification.
Once the patient’s SUD recovery is stable, the likelihood of managing his or her pain increases. The need for formal addiction treatment often necessitates a change in the plan for opioids, by discontinuing them or by changing the treatment setting through which they are provided.
Acute Pain Episodes
When patients who have CNCP and an SUD require acute pain management, such as for postoperative pain, precautionary steps can minimize risk of relapse.
Patients in recovery may benefit from non-pharmacological pain control. Some patients in recovery from SUDs may prefer to avoid the use of any medication. Evidence shows that stress management, CBT, manual therapies, and acupuncture offer effective relief for certain types of acute pain (Hurwitz et al., 2008; Vernon, Humphreys, & Hagino, 2007).
Patients in recovery may benefit from being switched from short- to long-acting medications as quickly as appropriate (to minimize reinforcing effects). They may also benefit from bolstered recovery support during postoperative periods (Covington, 2008).
Patients on agonist therapy for addiction or pain may be continued on their current opioid or on an equivalent dose of an alternative opioid; however, this should not be expected to control acute pain, which requires supplementation with (often greater-than-usual doses of ) additional opioids. In this situation, adjuvant NSAIDs may allow clinicians to provide pain relief with a reduction in opioid dosage (Mehta & Langford, 2006), and multimodal analgesia should be considered (Maheshwari, Boutary, Yun, Sirianni, & Dorr, 2006).
Patients on buprenorphine for opioid addiction may have reduced benefit from full agonist opioids used for acute pain, because the full agonist will be somewhat blocked. Non-opioid analgesics can be used, but in some cases buprenorphine will need to be discontinued so that full agonist opioids for pain can be used (Alford et al., 2006).
Patient-controlled analgesia should have relatively high bolus doses and short lockout intervals (specified intervals during which pressing the administration button results in no drug delivery), and patients should be closely monitored by medical staff. Pulse oximetry or end-tidal CO2 monitoring may provide an additional margin of safety when high doses of opioids are required.
Patients who are dependent on opioids or sedatives (including benzodiazepines) should not be withdrawn from these medications while undergoing acute medical interventions.
Exhibit 3-7 provides a discussion of treating patients who have sickle cell disease (SCD), which brings recurring acute pain, often against a backdrop of persistent pain and hyperalgesia.
Exhibit 3-7
Treating Patients Who Have Sickle Cell Disease. SCD is characterized by crises of acute pain, attributed to vasoocclusion, that is typically nociceptive but can be neuropathic as well. Opioids are the mainstay of treatment, although parenteral ketorolac (more...)
Other comorbidities that can complicate pain treatment result from other chronic illnesses. Exhibit 3-8 offers suggestions for providers for treating CNCP in patients who have HIV/AIDS.
Exhibit 3-8
Treating Patients Who Have HIV/AIDS. A vast range of pain syndromes are common in patients who have HIV/AIDS. Some are the result of HIV infection, others result from immunosuppression, and others are unrelated but comorbid with AIDS. Pain commonly results (more...)
Assessing Treatment Outcomes
Treatment of chronic pain is usually an evolving process, with medication and adjunctive therapies attempted, monitored, and adjusted or abandoned as indicated by patient response. Chapter 2 provides information about ongoing assessments.
Key Points
- Pain treatment goals should include improved functioning and pain reduction.
- Treatment for pain and comorbidities should be integrated.
- Non-opioid pharmacological and nonpharmacological therapies, including CAM, should be considered routine before opioid treatment is initiated.
- Opioids may be necessary and should not be ruled out based on an individual’s having an SUD history.
- The decision to treat pain with opioids should be based on a careful consideration of benefits and risks.
- Addiction specialists should be part of the treatment team and should be consulted in the development of the pain treatment plan, when possible.
- A substantial percentage of patients with and without SUDs will fail to benefit from prolonged opioid therapy, in which case it should be discontinued, as with any other failed treatment.
- Overview of Pain Management
- The Treatment Team
- Treating Patients in Recovery
- Nonpharmacological Treatments
- Treating Psychiatric Comorbidities
- Opioid Therapy
- Treating Patients in Medication-Assisted Recovery
- Tolerance and Hyperalgesia
- Treating Pain in Patients Who Have Active Addiction
- Acute Pain Episodes
- Assessing Treatment Outcomes
- Key Points
- Chronic Pain Management - Managing Chronic Pain in Adults With or in Recovery Fr...Chronic Pain Management - Managing Chronic Pain in Adults With or in Recovery From Substance Use Disorders
Your browsing activity is empty.
Activity recording is turned off.
See more...