NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Talati R, Scholle JM, Phung OJ, et al. Effectiveness and Safety of Antiepileptic Medications in Patients With Epilepsy [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Dec. (Comparative Effectiveness Reviews, No. 40.)

Effectiveness and Safety of Antiepileptic Medications in Patients With Epilepsy [Internet].
Show detailsBackground
Seizures are single or paroxysmal events arising from abnormal, excessive, hypersynchronous discharges from central nervous system neurons, and range in severity from symptoms not readily apparent to an observer to dramatic convulsions.1 Epilepsy describes a clinical phenomenon in which a person has recurrent seizures due to a chronic, underlying process.1,2
Over a lifetime, approximately 10 percent of people in the United States will suffer a seizure, with 1 percent to 3 percent developing epilepsy.2-4 The annual incidence of epilepsy is about 50 per 100,000 with a prevalence of 5–10 per 1,000.4,5
Antiepileptic drug therapy is the mainstay of treatment for most patients with epilepsy. Seizure classification is an important element in designing the treatment plan, since some antiepileptic drugs have different activities against various seizure types. The International League Against Epilepsy classifies the three main types of seizures: partial, generalized, and unclassified. The main subtypes are given in Table 1.1 Partial seizure activity is restricted, at least initially, to discrete areas of the cerebral cortex while generalized seizure activity occurs in diffuse regions of the brain simultaneously. If consciousness is fully preserved during the partial seizure, it is termed a simple partial seizure. If consciousness is impaired during the partial seizure, it is termed a complex partial seizure. If a seizure begins as a partial seizure and then spreads diffusely throughout the cortex, it is termed a partial seizure with secondary generalization. Because of the focused nature of a partial seizure, only a specific area of the body is usually involved, at least initially. Generalized seizures are termed absence seizures if they are characterized by sudden, brief lapses of consciousness without loss of postural control. Absence seizures usually begin in childhood (ages 4–8) or early adolescence and are the main seizure type in 15–20 percent of children with epilepsy. Generalized seizures are termed generalized tonic–clonic seizures if they are characterized by generalized muscle contraction for a period followed by intermittent muscle contraction and relaxation. There is usually a postictal phase with confusion that accompanies the end of convulsions. Generalized seizures are the main seizure type in approximately 10 percent of people with epilepsy. Generalized seizures are termed atonic seizures if sudden loss and then regaining of postural muscle tone characterize them. While consciousness is briefly impaired, there is usually no postictal confusion in people with atonic seizures. Generalized seizures are termed myoclonus seizures if a sudden jerking movement of the skeletal muscle characterizes it. A patient with epilepsy may experience more than one subtype of seizure over their lifetime.1
Table 1
Classification of seizure types.
Epilepsy syndromes are disorders in which epilepsy is a predominant feature, and there is sufficient evidence to suggest a common underlying mechanism.1 Three main epilepsy syndromes have been classified; one is associated with partial seizures (Mesial Temporal Lobe Epilepsy Syndrome) and the others are associated with generalized seizures (Juvenile Myoclonic Epilepsy Syndrome and Lennox-Gastaut Epilepsy Syndrome). Mesial Temporal Lobe Epilepsy Syndrome is associated with complex partial epilepsy and has distinctive clinical, electroencephalographic, and pathologic findings. High-resolution magnetic resonance imaging can detect the characteristic hippocampal sclerosis that appears to be essential in the pathophysiology of the syndrome. Epilepsy in people with this syndrome tends to be refractory to treatment with anticonvulsants but responds well to surgical intervention. Juvenile Myoclonic Epilepsy Syndrome is a generalized seizure disorder that appears in early adolescence. While most of the seizures the patient experiences consist of bilateral myoclonic jerks, people may also experience tonic–clonic or absence seizures. The condition is otherwise benign, and although complete remission is uncommon, the seizures respond well to anticonvulsant medication. Lennox-Gastaut Epilepsy Syndrome occurs in children and is defined by the following triad: multiple seizure types (generalized tonic–clonic, atonic, and atypical absence), specific electroencephalographic findings (<3 Hz spike-and-wave discharges), and impaired cognitive function. Lennox-Gastaut Epilepsy Syndrome is associated with central nervous system delays or dysfunction from a variety of causes, including developmental abnormalities, perinatal hypoxia/ischemia, trauma, infection, and other acquired lesions. The multifactorial nature of this syndrome suggests that it is a nonspecific response of the brain to diffuse neural injury. Unfortunately, many patients have a poor prognosis due to the underlying central nervous system pathology and the consequences of severe, poorly controlled epilepsy.1
The incidence of new-onset epilepsy is high during the first 9 years of life and then plateaus over the next 30 years.1 The incidence drops in 40–59 year olds and then rises again in the elderly.4,5 The age of epilepsy onset is marked by different underlying causes as depicted in Table 2.1,2,5 Childhood marks the age at which many of the well-defined epilepsy syndromes present. During adolescence and early adulthood, there is a transition away from idiopathic or genetically based epilepsy to more cases secondary to acquired central nervous system lesions (e.g., head trauma, infections, brain tumors). A patient with a penetrating head wound, depressed skull fracture, intracranial hemorrhage, or prolonged posttraumatic coma or amnesia has a 40–50 percent risk of developing epilepsy, while a patient with a closed head injury and cerebral contusion has a 5–25 percent risk. The causes of seizures in older adults include cerebrovascular disease, trauma (e.g. blunt trauma and subdural hematoma), brain tumors, and degenerative diseases such as Alzheimer's disease. Cerebrovascular disease may account for approximately 50 percent of new cases of epilepsy in patients older than 65 years.1
Table 2
Epilepsy etiology based on age.
The overall goals of antiepileptic therapy are to prevent seizures and avoid untoward side effects with a regimen that is convenient and easy to follow. People with epilepsy usually initiate treatment with one antiepileptic drug at the time of diagnosis, but 30 percent of patients will be refractory to this medication.6 While control of seizures is the overriding goal of therapy, selecting an effective drug with the least potential for side effects becomes a crucial decision for clinicians.
Table 3 identifies approved medications for the treatment of epilepsy, their known or suspected mechanism of action, type of seizures principally treated, adverse effects, drug interaction potential, and availability of a generic product.1,2,7-9 This is meant to be a brief overview, not an exhaustive review, of the mechanisms or characteristics.
Table 3
Important characteristics of antiepileptic medications.
Since 1993, the Food and Drug Administration (FDA) has approved several newer antiepileptic drugs (felbamate, gabapentin, lacosamide, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, rufinamide, tiagabine, topiramate, vigabatrin, zonisamide) for the treatment of epilepsy. 6 This offered clinicians and patients many new options over the older antiepileptic medications approved between 1953 and 1983 (phenytoin, 1953; primidone, 1954; ethosuximide, 1960; carbamazepine, 1968; clonazepam, 1975; divalproex, 1978; valproic acid, 1983). While most newer antiepileptic drugs are approved as second-line agents for the treatment of refractory seizures, topiramate, oxcarbazepine, and lamotrigine are also approved for monotherapy in certain situations.6
According to the Biopharmaceutics Classification System (BCS), drugs are relegated into four classes; high solubility/high permeability (Class I, optimal class with lowest risk of absorption variability), low solubility/high permeability (Class II), high solubility/low permeability (Class III) and low solubility/low permeability (Class IV).10 A drug is considered to have high solubility when the highest dose strength is soluble in 250 mL or less of aqueous media over a pH range of 1 to 7.5 at 37 degrees Celsius.11,12 A drug is considered to be highly permeable when the extent of absorption (bioavailability) is ≥ 90 percent.11,12 In 2000, the FDA started using the BCS to grant a waiver of in vivo bioavailability and bioequivalence testing of immediate release solid dosage forms for Class I drugs.13 The BCS classification for older and newer epilepsy medications is given in Table 4.11,14-27
Table 4
Biopharmaceutics Classification System of antiepileptic medications.
The comparative benefits and harms of older antiepileptic drugs (pre-1993 FDA approved medications: phenytoin, carbamazepine, carbamazepine valproic acid, clonazepam, phenobarbital, ethosuximide, primidone) versus newer antiepileptic drugs (1993 or newer FDA approved medications) have been assessed in numerous randomized controlled trials (RCTs), with varying results. In the Standard And New Antiepileptic Drugs (SANAD) study, there were two treatment arms. In Arm A, carbamazepine was compared with other newer antiepileptic treatments (i.e. gabapentin, lamotrigine, topiramate, oxcarbazepine), while in Arm B, valproate was compared with newer antiepileptic agents (i.e., lamotrigine and topiramate). The efficacy, tolerability, and safety of newer antiepileptic agents were compared with their older counterparts. In this RCT, lamotrigine significantly extended the time to treatment failure versus carbamazepine in patients with partial seizures, but the time to treatment failure was similar between lamotrigine and valproate in patients with generalized seizures. However, other newer antiepileptic agents demonstrated similar or inferior 12-month remission rates compared with older antiepileptics.28,29 Many older and newer antiepileptic medications share mechanisms related to sodium channels and gamma-amino-butyric-acid and are used either alone or in combination to control siezures. As such, it is possible to determine their comparative effectiveness.
Another important issue in the management of epilepsy surrounds generic substitution of innovator antiepileptic medications. The American Academy of Neurology has issued two position papers stating that there is concern with generic antiepileptic medication substitution and that physicians should specifically approve all generic substitutions.30,31 The International League Against Epilepsy established a working group on generic products in epilepsy treatment. They concluded that generic medications offer a valuable and cost-effective choice in the management of epilepsy but that generic substitution is not recommended in patients who achieve seizure remission on an innovator product.32 The FDA and the American Society of Health-System Pharmacists do not share the view that antiepileptic medications, or other narrow therapeutic index medications (medications where the difference between the minimum effective and minimum toxic concentrations are close together), should be treated differently with respect to generic substitution.33-36 However, their responses have been related to the process of determining bioequivalence and therapeutic equivalence rather than specifically providing an evaluation of comparative effectiveness. As such, several states including Hawaii, Illinois, Tennessee, and Utah prevent automatic generic substitution for innovator antiepileptic medications and another 24 state legislatures (including California and New York) have discussed or are considering legislation preventing generic substitution.33,37-40 A common example of legislation includes: “Would prohibit a pharmacist from substituting or interchanging any antiepileptic drug, innovator or generic, without notification to both the prescribing physician and the patient or the patient's representative.”40 Variations include written consent from the prescriber and/or patient before substitution can occur.
Opponents of generic substitution of antiepileptic medications oppose it on one or more of the following reasons: bioequivalence studies mandated by FDA are in normal volunteers and not in patients with epilepsy; bioequivalence may occur in the fasting but not the fed state (unless food is known to affect absorption when both are required); the acceptable limit for variance (90 percent confidence interval for the Cmax and area under the curve for the generic falls within 0.80 and 1.25 (i.e., 20 percent over or under) of the innovator medication) is not narrow enough; generics may be close enough to the innovator to be bioequivalent but not to another generic medication (if one generic consistently but predictably achieves higher concentrations than the innovator and another consistently but predictably achieves lower concentrations than the two generic medications may not be bioequivalent); and bioequivalence may be seen for a generic and innovator medication within a group of patients but not necessarily within each individual patient with epilepsy.33,41
Due to the inconclusive results of the SANAD study and other currently available studies, a comparative effectiveness review of the efficacy, tolerability, and safety of older versus newer antiepileptic treatments is needed. Similarly, given the controversy surrounding generic substitution of antiepileptic medications, a comparative effectiveness review of the efficacy, tolerability, and safety is needed.
Objective
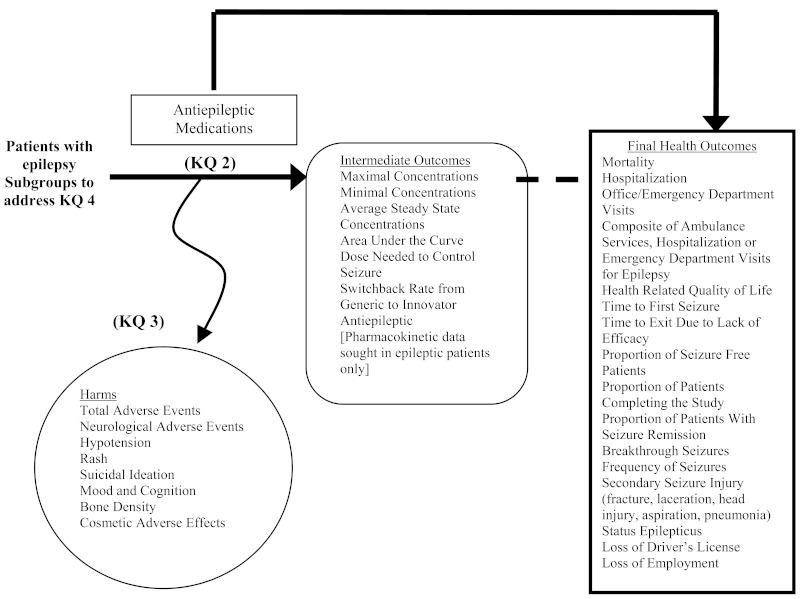
The objective of this study was to perform a comparative effectiveness review of the efficacy, safety, and tolerability of antiepileptic medications and to address the issue of generic substitution by qualitatively and/or quantitatively comparing older versus newer antiepileptic medications and comparing innovator antiepileptic medications to their generic counterparts. The analytic framework for the evaluation of effectiveness and safety of antiepileptic medication in patients with epilepsy is located in Figure 1.
Key Questions
- Key Question 1: In patients with epilepsy, what is the comparative effectiveness/efficacy of antiepileptic medications on health outcomes: mortality, hospitalizations, office/emergency department visits, composite endpoint (ambulance services, hospitalizations, or emergency department visits for epilepsy), health-related quality of life, seizures (time to first seizure, time to exit for trial due to lack of efficacy, proportion of seizure-free patients, proportion of patients with seizure remission, breakthrough seizures, frequency of seizures), secondary seizure injury (fracture, laceration, head trauma, aspiration pneumonia), status epilepticus, loss of driver's license, and loss of employment?
- Key Question 2: In patients with epilepsy, what is the comparative effectiveness/efficacy of antiepileptic medications on intermediate outcomes: pharmacokinetics, the comparative dose of medication needed to control seizures, and switchback rates?
- Key Question 3: In patients with epilepsy, what is the comparative impact of antiepileptic medications on serious adverse events such as neurological adverse effects, hypotension, rash, suicidal ideation, mood and cognition, bone density, and cosmetic adverse effects?
- Key Question 4: In patients with epilepsy, what are the comparative benefits or harms for antiepileptic medications in subgroups of patients differentiated by seizure etiology (partial, generalized, specific epilepsy syndrome), seizure type (new onset disease, chronic disease), gender, ethnicity, patient age, and patient pharmacogenetic profile; and by types of antiepileptic medication (medication classes, individual medications and medications meeting the definition of having a narrow therapeutic index [BCS class II-IV])?
- Introduction - Effectiveness and Safety of Antiepileptic Medications in Patients...Introduction - Effectiveness and Safety of Antiepileptic Medications in Patients With Epilepsy
- MBOAT7 [Bubalus bubalis]MBOAT7 [Bubalus bubalis]Gene ID:102401164Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...