NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kansagara D, Dyer E, Englander H, et al. Treatment of Anemia in Patients with Heart Disease: A Systematic Review [Internet]. Washington (DC): Department of Veterans Affairs (US); 2011 Oct.

Treatment of Anemia in Patients with Heart Disease: A Systematic Review [Internet].
Show detailsLITERATURE FLOW
We reviewed 1,546 titles and abstracts from the electronic search, and identified an additional 83 from reviewing reference lists, and performing manual searches for recently published studies, and unpublished or ongoing studies.
After applying inclusion/exclusion criteria at the abstract level, 320 full-text articles were reviewed, as shown in Figure 2. Of the full-text articles, we rejected 266 that did not meet our inclusion criteria.
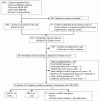
Figure 2
Literature Flow – Anemia and CHF.
KEY QUESTION #1. In patients with CHF or CHD, what are the health outcome benefits and harms of treating anemia with ESAs?
Summary
Sixteen randomized, controlled trials evaluated the impact of ESAs in patients with heart disease (Table 1).12-27 We excluded one study28 whose patient population was included in a subsequent publication.21, 28 Eleven trials enrolled patients with CHF, and in the 10 trials reporting systolic function, the mean ejection fraction was ≤ 35%. Most patients had comorbid CHD. Two trials included roughly even proportions of patients with CHD and CHF,14, 26 and only one trial focused exclusively on patients with CHD.27 The most commonly reported health outcomes were exercise tolerance measures such as NYHA (nine trials), and exercise duration as measured by the six-minute walk test (five trials) or the Naughton protocol (two trials). Nine trials reported mortality and seven trials reported hospitalizations. Two trials were primarily designed to assess the comparative effects of ESAs titrated to high or low hemoglobin targets in anemic patients with chronic kidney disease, but included a large proportion of patients with heart disease for whom adequate subgroup data are reported.13, 14
Table 1
Characteristics of randomized controlled trials of ESA therapy in patients with CHF or CHD.
Overall, we found little good quality evidence that ESA use consistently improves health outcomes. Some studies found ESA use improved exercise tolerance and duration, but this body of evidence is limited by inconsistency of findings and important methodologic weaknesses. The potential benefits of ESA use seen in some studies may be further tempered by the finding that ESA use is associated with serious harms such as mortality and vascular thrombosis, especially in patients with comorbid chronic kidney disease.
Methodologic Considerations
We characterized the quality of each of the included studies according to the impact methodologic flaws could have on an outcome of interest. For example, flaws such as the lack of patient and/or outcome assessor blinding could lead to biased results for subjective outcomes such as exercise tolerance. Five trials contained serious methodologic flaws which could have biased key findings,12, 15, 21, 24, 25 and unclear reporting made it difficult to assess the risk of bias in one trial.17 Additionally, there was some evidence for multiple publication bias: in two cases we found multiple publications reporting results for apparently overlapping populations.16, 18, 21, 28 The methodologic characteristics of each study are detailed in Appendix C, Table 1.
Exercise Tolerance and Duration
Overall, though there is some data that ESAs may improve exercise tolerance, the body of evidence is limited by inconsistent results and the methodologic weaknesses of some studies. Pooled results from nine trials reporting change in NYHA scores were highly heterogeneous and found a decline in NYHA scores in ESA-treated patients while control patients generally maintained stable scores or worsened (mean difference in NYHA scores treatment vs. control, -0.77, 95% CI -1.21 to -0.32, I2=96.0%, Figure 3). However, this improvement was significantly attenuated when we limited the analysis to the four methodologically stronger trials (mean difference in NYHA scores -0.15; 95% CI -0.36 to 0.06; I2=62.1%, Figure 4). The largest of these trials randomized 319 patients to twice-monthly darbepoietin or saline placebo, and measured exercise duration, tolerance and quality of life outcomes at 27 weeks.19 The authors found darbepoietin had no effect on any of the outcomes despite raising hemoglobin by 1.8 g/dL on average.
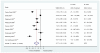
Figure 3
Change in NYHA scores in CHF patients: mean difference comparing ESA to control group.

Figure 4
Change in NYHA scores in CHF patients – studies with low risk of bias, and excluding studies with duplicate patient populations: mean difference comparing ESA to control group.
Six trials reported exercise distance or duration. Four of these trials reported the mean change in six-minute walk distance and found ESA use was associated with a marginally significant increase in distance walked, though results were quite different among the trials (mean change in meters walked: 74.4; 95% CI -0.16 to 149.0; I2=88.7%) (Figure 5). Two trials reported change in exercise treadmill time using the Naughton protocol; the larger trial found no improvement associated with ESA use,19 while a smaller trial found ESA use was associated with a small increase in exercise duration.28 Exclusion of poorer quality studies did not alter results substantially, but such analyses are limited by the very small number of studies.

Figure 5
Change in six-minute walk distance (meters) in CHF patients: mean difference comparing ESA to control group.
Quality of Life
Five trials reported quality of life measures as a primary or secondary outcome,17, 19, 22-24 but analysis was limited by the variety of and inconsistency among specific instruments used. Most trials used several different methods for evaluating quality of life. Four trials evaluated change in the Patient Global Assessment scale.19, 22-24 In two of these studies, a significantly greater proportion of treatment patients reported improvement than controls, but one of these studies had several important methodologic flaws, including lack of blinding, that could bias these subjective results.24 The other trial found no improvement in two simultaneously measured QOL instruments including the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the Kansas City Cardiomyopathy Questionnaire (KCCQ).23 Four trials reported MLHFQ scores,19, 22-24 but only one trial with high risk of bias showed a significant improvement in scores associated with treatment.24 Kourea et al. found treatment was associated with improvement in the Duke Activity Status Index (DASI), Beck Depression Inventory (BDI) and Zung Self-rating Depression Scale (SDS).17
The KCCQ measures quality of life in multiple domains which can be combined into summary scores to facilitate interpretation. Three trials reported different types of KCCQ summary scores without defining which domains were used in each summation.17, 22, 23 One trial reported a significant difference between groups in mean change from baseline of a KCCQ “total symptom score”: 8.2 v 1.5, p=0.027.22 Another trial noted significant improvements in a KCCQ “functional score” (21 +/- 19 v 2 +/- 11, p=0.004), as well as a KCCQ “summary” score (20 +/- 20 v 6 +/-14, p=0.04).17
Mortality
Nine trials reporting at least one death in the treatment or control group found ESA use was associated with a marginally significant increased mortality risk (RR 1.11; 95% CI 0.99 – 1.24; I2=0.0%). An analysis of the six trials with low risk of bias found very similar results (Figure 6).

Figure 6
All-cause mortality in patients with CHF or CHD – studies with low risk of bias: ESA vs. control.
These findings are largely driven by two large trials with extended follow-up and very high event rates. Indeed, a sensitivity analysis without these two trials showed ESAs had a neutral effect on mortality (RR 0.79, 95% CI 0.51 – 1.22; I2=0.0%). One of the trials compared aggressive (goal hematocrit 42%) to less aggressive (goal hematocrit 30%) epoietin titration in patients with end-stage renal disease and heart failure and/or ischemic heart disease.26 After a prolonged follow-up of 29 months, the authors found a 20 percent increase in the risk of all-cause mortality, and most of the events were of cardiovascular origin. Another large trial compared darbepoietin to placebo in patients with type 2 diabetes and chronic kidney disease. A prespecified analysis of the large subgroup with comorbid heart disease showed a non-significant increased risk of death in the treatment group after a similarly long follow-up period.14, 29
Hospitalizations
Six trials found ESA treatment was associated with a reduction in hospitalizations (RR 0.70, 95% CI 0.57 – 0.87; I2=37.7%), but, again, this benefit largely disappeared when we included only the higher quality trials (Figure 7).

Figure 7
Risk of one or more hospitalizations in patients with CHF or CHD – studies with low risk of bias: ESA vs. Control.
Cardiovascular Events
ESAs had a neutral effect on the occurrence of cardiovascular events across seven trials (RR 0.96, 95% CI 0.85 – 1.08; I2=41.5%, Figure 8). The only trial showing a benefit focused on CHD patients, and had many significant methodologic weaknesses which threaten the validity of the results.27

Figure 8
Cardiovascular events in patients with CHF or CHD: ESA vs. control.
Cerebrovascular Events
There were very few cerebrovascular events among the four trials reporting this outcome (Figure 9). There was an increased risk of stroke associated with ESA use in the TREAT trial among patients with diabetes and chronic kidney disease (RR 1.92, 95% CI 1.38 – 2.68),30 but these data are not reported separately for the large subgroup of heart disease patients.
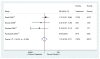
Figure 9
Cerebrovascular events in patients with CHF or CHD: ESA vs. control.
Other Harms
Combined results from seven trials suggest ESA use may be associated with excess risk of hypertension (RR 1.11, 95% CI 1.00 – 1.24; I2= 0.0%), though the findings are again dominated by one large trial.14 The finding of excess risk became non-significant when we excluded this trial (RR 1.25, 95 % CI 0.65 – 2.38; I2=0.0%). Reported hypertension events in the other trials were rare, but the quality of adverse event reporting was unclear and the definitions used varied widely (Figure 10).

Figure 10
Hypertension events in patients with CHF or CHD: ESA vs. control.
One large trial in end-stage renal disease patients found an increase in the risk of thrombosis – mainly of vascular access sites – associated with aggressive epoietin titration (RR 1.37, 95% CI 1.17 – 1.61).26 The risk of venous thromboembolism was similarly increased in another trial of patients with chronic kidney disease and diabetes (RR 1.80, 95% CI 1.08 – 2.98), though data for the cardiac disease subgroup were not reported separately.14 On the other hand, only two other studies reported the occurrence of venous thromboembolic events with no difference seen between groups.19, 22
Hemoglobin Target
We were not able to determine how anemia severity and hemoglobin change influenced outcomes in the placebo-controlled trials. Almost all the small trials comparing ESAs to placebo in heart failure patients included patients with moderate anemia and a mean baseline hemoglobin within the narrow 10 – 12 g/dL range. In all cases, ESA use was associated with a significant increase in hemoglobin (mean increase range 1.6 – 2.8 g/dL). In order to better understand the influence of baseline hemoglobin and change in hemoglobin on outcomes, we conducted the two following sensitivity analyses for all outcomes and found no substantive difference in results: 1) exclusion of studies in which the mean baseline hemoglobin < 11 g/dL; and 2) exclusion of studies in which the mean increase in hemoglobin associated with ESA use was < 2 g/dL. However, the utility of such subgroup analysis is limited by the relatively small number of trials, and also by concurrent characteristics which could influence results. For instance, exclusion of studies with mean baseline hemoglobin < 11 g/dL examining change in NYHA scores left only the poorer quality studies. Furthermore, there may not have been enough variation in mean baseline hemoglobin and change in hemoglobin across studies given the relatively small sample of trials.
The best evidence evaluating the influence of hemoglobin targets comes from the three trials (or subgroups of trials) of patients with comorbid chronic kidney and heart disease, in which ESAs titrated to normal or near-normal targets were compared to ESAs titrated to lower targets (hemoglobin 9 – 11.3 g/dL).13, 14, 20 None of the trials found a benefit from aggressive ESA use and, in fact, two of the trials found a significant increase in venous thromboembolic risk and a near-significant increase in mortality.14, 20
No trials in heart disease patients have evaluated the effects of more moderate hemoglobin targets (e.g. hemoglobin 10 – 12 g/dL) compared to lower targets.
In Progress Trials
Two trials of ESAs in heart failure are ongoing. The Reduction of Events with Darbepoetin alfa in Heart Failure (RED-HF) study is an international, multicenter, randomized and placebo-controlled trial.31 The intent is to recruit ∼2600 optimally treated patients with low ejection fraction (∼40%) and symptomatic CHF with a Hgb concentration 9.0 – 12.0 g/dL. Patients will be administered darbepoetin every two weeks, titrated to a goal Hgb of >= 13.0 g/dL, with oral iron repletion as needed. The primary outcome is time to death from any cause or first hospital admission for worsening CHF. The secondary outcomes include mean change in KCCQ scores at six months. Started in June 2006, this event-driven, industry-sponsored trial is estimated to finish in 2014.
Also expected are results from the Anemia in Heart Failure With a Preserved Ejection Fraction trial.32 This randomized, placebo-controlled trial is examining the effects of weekly erythropoietin, also titrated to a target hemoglobin of 13 g/dL, in 80 patients with anemia and heart failure and a preserved ejection fraction. They will evaluate the primary outcomes of left ventricular end diastolic volume atsix months, as well as secondary outcomes of peak oxygen consumption, six-minute walk duration, KCCQ scores, hospitalization, and others. Started in July 2007, it is anticipated to be completed by March 2012.
KEY QUESTION #2. In patients with CHF or CHD, what are the health outcome benefits and harms of using iron to treat iron deficiency with or without anemia?
Summary
Two small and one large, well-conducted multicenter trials show that IV iron can improve short-term exercise tolerance and quality of life in patients with symptomatic systolic heart failure and iron deficiency, with or without anemia. The impact on distal health outcomes such as mortality and cardiovascular events remains undertested, as do the long-term effects of such treatment. The evidence supporting symptomatic benefit most closely applies to patients with NYHA III heart failure and evidence of low iron stores.
Details
We included three trials of IV iron in patients with iron deficiency. Results are largely dominated by one recent trial that studied the effect of iron infusion on patients with iron deficiency with or without anemia.33
The FAIR-HF (Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure) trial is a randomized, double-blind, multicenter trial that evaluated the efficacy of intravenous-iron infusion on symptoms and submaximal exercise capacity in a cohort of patients with chronic mild or moderate heart failure due to left ventricular systolic dysfunction. The study enrolled 459 stable outpatients with NYHA class II or III heart failure, low ejection fraction, and iron deficiency as defined by a ferritin < 100 μg/dL or between 100 – 299 μg/dL if the transferrin saturation was < 20 percent. Pre-specified primary endpoints included self-reported Patient Global Assessment and NYHA functional class after 24 weeks of therapy. Secondary endpoints included distance walked in six minutes and health-related quality of life. Patients receiving IV iron received 200 mg infusion of ferric carboxymaltose with repeat dosing until iron repletion was achieved (correction phase) and then every four weeks during the maintenance phase, which started at week eight or week twelve, depending on the required iron-repletion dose. Control patients received an IV saline placebo with the same dosing schedule.
Patient characteristics, which were well-matched between the two groups at baseline, are detailed in Table 2. Most patients had NYHA Class III symptoms and moderate to severe systolic dysfunction. Only half the patients were anemic (HHgb ≤ 12g/dL), but most had ferritin levels < 100.
Table 2
Characteristics of randomized controlled trials of iron therapy in patients with CHF or CHD.
Patients in the treatment group were more likely to report they were much or moderately improved on the Patient Global Assessment compared with control patients (50 v 28%, OR 2.51; 95% CI 1.75 – 3.61). Iron treated patients also showed improvement in NYHA functional class (OR for improvement by one class, 2.40; 95% CI 1.55 – 3.71). Improvements in Patient Global Assessment and NYHA scores were observed in both prespecified subgroups of patients with and without anemia (Hgb ≤ 12g/dL). Significant improvements were also seen in secondary endpoints, including an increased distance on the six-minute walk test (313 meters compared with 277 meters) and quality of life assessments (EQ5-D, where higher score is better, of 63 vs. 57).
The FAIR-HF trial was large and well-conducted, but there are several limitations of note. It relied on subjective primary endpoints, though the strong study design should minimize the risk of biased results. The size of the study and relatively short follow-up period limit its ability to examine intervention effects on more distal health outcomes such as mortality. Finally, there were too few patients with NYHA class II heart failure to meaningfully apply results to this group of patients.
We also included two smaller trials of iron therapy. The first randomized 40 patients with iron deficiency, anemia, chronic heart failure and chronic kidney disease to receive 200 mg of intravenous iron sucrose or saline weekly for five weeks.34 Investigators found that after six months, participants who received iron sucrose had significant improvement in MLHFQ score, decreased levels of N-terminal pro-brain natriuretic peptide (117.5 +/- 87.4 pg/ml vs. 450.9 +/-248.8 pg/ml, p<0.01) and C-reactive protein (2.3 +/- 0.8 mg/l vs. 6.5 +/-3.7 mg/l, p <0.01), an increase in left ventricular ejection fraction percentage (35.7 +/- 4.7 vs. 28.8 +/- 2.4), and distance on the six-minute walk test.
The FERRIC-HF (Ferric Iron Sucrose in Heart Failure) trial randomized 35 patients and measured the effect of 200 mg of intravenous iron sucrose compared with placebo on exercise tolerance and QOL.35 The lack of blinding contributes to a high risk of bias given the subjective nature of the functional status and QOL outcomes. Also, substantially more patients in the intervention group dropped out of the study (16 v 9%).
KEY QUESTION #3. In patients with CHF or CHD, what are the health outcome benefits and harms of treating anemia with red blood cell transfusions?
Summary
We found 35 studies that examine the association between red blood cell transfusion and clinical outcomes in patients with CHD or CHF. Ten of these studies evaluated transfusion use in the perioperative period; the remaining reports, all but one published since 2001, focused on the non-surgical population. Three of these studies were subgroup analyses of the same registry;36-38 thus, in the end, we found 23 unique studies of the potential benefits and harms of transfusion outside of the perioperative period in patients with ischemic heart disease and/or CHF.
Outside of the surgical setting, red blood cell transfusion has been evaluated as a treatment for anemia in heart disease in two controlled trials: one found no difference in survival from more aggressive transfusion above a threshold hemoglobin 10 g/dL,39 while the other found a higher incidence of heart failure in patients transfused to that level, without a difference in survival.40
Twenty-one additional observational studies have been conducted in patients undergoing percutaneous coronary intervention (PCI) or admitted with acute coronary syndrome, myocardial infarction, or decompensated heart failure. Inconsistency of findings and methodological weaknesses complicate the interpretation of results, but several themes emerge: 1) the evidence strongly suggests that transfusion has no benefit and may be harmful in patients with heart disease and hemoglobin >10 g/dL, with the possible exception of those with ST-elevation myocardial infarction; 2) outcomes do not appear to improve with transfusion in non-ST-elevation ACS patients with hemoglobin levels down to the 8 – 9 g/dL range; 3) transfusion is consistently associated with higher mortality risk in the unselected PCI population, across multiple studies with mean nadir hemoglobin of 8 – 9 g/dL; and 4) the elevated risk in the PCI population is seen in patients with anemia related or unrelated to bleeding but may be higher in the non-bleeding anemic population. There is no evidence to guide decision-making in the stable coronary disease population, and the two studies in decompensated heart failure have conflicting results.
The literature evaluating the use of perioperative transfusion in patients with heart disease is concentrated primarily on the cardiac surgery population but does include several studies in vascular and orthopedic surgery and one in the general non-cardiac surgery population. Seven perioperative randomized controlled trials have been conducted, and each found no difference in survival or cardiovascular complications between patients transfused to a higher versus lower target hemoglobin. In the observational cohorts, transfusion did not appear to offer any protection; and in one study in the vascular surgery setting, mortality and myocardial infarction rates were higher overall in the transfused group, a harm in subgroup analysis limited to those transfused at a hemoglobin ≥ 9 g/dL.41
Non-operative Setting
Randomized Controlled Trials
Only 2 of the 23 studies in nonsurgical populations were randomized controlled trials (Table 3). 39, 40 The TRICC trial, published in 1999, remains the only large controlled trial of transfusion strategies in hospitalized patients.42 This landmark study randomized 838 euvolemic, non-bleeding, critically ill patients with hemoglobin < 9 g/dL to one of two transfusion thresholds: hemoglobin of 7 g/dL (restrictive transfusion strategy) or 10 g/dL (liberal strategy). They found no significant difference between the two groups in mortality in the hospital or at 30 days, or in other clinical outcomes including cardiac events, pulmonary or infectious complications, organ dysfunction scores, and length of stay. Importantly, the trend suggested the potential for higher mortality and more cardiac events in patients treated to the higher hemoglobin level. In the subgroups of patients younger than 55 years of age or with APACHE II scores of 20 or lower, the mortality rate was statistically significantly higher in the liberally transfused group.
Table 3
Randomized controlled trials of red blood cell transfusion for anemia in patients with CHD or CHF, stratified by patient setting.
In 2001, the TRICC authors published a post-hoc subgroup analysis focusing on patients with cardiovascular disease in general and ischemic heart disease in particular.39 Once again, there were no significant differences in any clinical outcome. However, the trend toward improved survival with a restrictive transfusion strategy disappeared in the general cardiovascular disease population, and in the ischemic heart disease subgroup, there was a higher mortality rate in the restrictive group, though the difference was nonsignificant (30 day mortality 21.1% versus 26.1% with liberal and restrictive strategies, respectively; p=0.38). Like the TRICC trial population overall, the ischemic heart disease subgroup was severely ill with multiple comorbidities (mean APACHE II scores of 23, 87% requiring mechanical ventilation).
Cooper et al. performed a pilot trial (CRIT) designed to evaluate conservative versus liberal transfusion strategies specifically in patients with acute myocardial infarction.40 They randomized 45 patients with hematocrit under 30 percent to a transfusion trigger of 24 percent (conservative strategy), with a target hematocrit 24 – 27 percent, or a trigger of 30 percent (liberal strategy), with a target of 30 – 33 percent. They found a higher rate of the primary endpoint, a composite of in-hospital death, recurrent MI, or new/worsening heart failure, in the liberally transfused group compared to the conservative group (38% versus 13%; p=0.046). The difference was explained entirely by a higher incidence of new or worsening CHF.
An additional study, the Myocardial Ischemia and Transfusion trial, began two years ago and is now collecting final outcomes data.43 This multicenter, randomized, controlled trial aimed to enroll 200 anemic patients hospitalized with acute coronary syndrome, including both STEMI and NSTE-ACS, or stable CAD undergoing cardiac catheterization during the index hospitalization. Like the TRICC and CRIT trials, patients were assigned to a restrictive (< 8 g/dL) or liberal (< 10 g/dL) transfusion threshold and were observed for clinical outcomes including mortality, myocardial ischemia, stroke, heart failure, infectious complications, and readmission.
Observational Studies
Given the sparse and the inconsistent data from the trial literature, clinical decision-making appears largely guided by an imperfect body of evidence characterized by conflicting observational data. We reviewed these observational studies, in part to clarify their utility in guiding transfusion treatment decisions (Table 4).
Table 4
Observational studies of red blood cell transfusion for anemia in patients with CHD/CHF.
Because of the observational nature of these studies, the decision to transfuse patients was based on clinical judgment which, in turn, would be naturally influenced by severity of illness, symptoms, and observation of bleeding. All the included observational studies suffer from the possibility of residual confounding and are all, therefore, of lower quality than the evidence provided by randomized controlled trials. However, there were methodologic differences amongst observational studies. For instance, some accounted for bleeding and conducted propensity to transfuse adjustments, while others did not. These factors are summarized in Appendix C, Table 3.
Percutaneous Coronary Intervention
Nine observational studies looked exclusively at populations undergoing percutaneous coronary intervention; six included all indications,36-38, 44-48 and three examined PCI solely in the setting of acute MI.49-51
In those studies that recorded it, 1.8 – 6.7 percent of unselected patients undergoing PCI received PRBC transfusion; rates were higher in those studies for which anemia was an inclusion criterion. A substantial proportion of patients who received transfusions did so because of major bleeding (22 – 100%), and median nadir hematocrit prior to transfusion ranged across studies from 24 to 30 percent. After adjustment for potential confounding factors in multivariable analyses, transfusion was associated with worse survival in all studies but one;36-38 it found no significant relationship between transfusion exposure and death or MI, both in cohorts with hematocrit < 27 percent and 24 – 30 percent. The association between transfusion and increased mortality appeared stronger in non-bleeding patients,44, 51 but was also noted in several studies that examined patients with major bleeding.44-46
Acute Coronary Syndrome/Myocardial Infarction
Twelve observational studies evaluated transfusion in the setting of acute coronary syndrome or myocardial infarction; four included only patients with non-ST-elevation ACS,52-55 two included patients exclusively with ST-elevation MI,50, 56 and six examined mixed ST/non-ST elevation ACS populations. Of these, three included predominately STEMI patients,51, 57, 58 two had a majority NSTE-ACS population,59, 60 and one did not record the breakdown.49 PCI rates ranged from 10 to 100 percent and transfusion rates from 4 to 30 percent across cohorts. Nadir hematocrit among patients who were transfused averaged from 25 to 29 percent in those studies that recorded it.
Eight of the ACS/AMI studies did find an association between transfusion and higher risk of death, including the three studies that focused exclusively on AMI patients undergoing PCI;49-51 three involving patients with high-risk non-ST-elevation ACS,52-54 and two examining patients primarily with ST-elevation myocardial infarction.56, 58 One additional study found no relationship between transfusion status and in-hospital mortality in a mixed ST/non-ST-elevation ACS population, regardless of whether the transfusion was bleeding-related or for non-specific anemia.60
Six studies examined whether mortality risk varies according to hemoglobin level, but they had varied results and used different thresholds for their stratified analyses, making it difficult to draw firm conclusions.52, 54, 55, 57-59
Wu et al. examined a cohort of nearly 79,000 Medicare beneficiaries 65 years of age or older who were hospitalized with confirmed acute MI and were not actively bleeding.59 They found a consistent association between transfusion and improved survival in patients with hematocrit values on admission of 30 percent or less, stronger in each successively lower hematocrit category. This benefit was lost in patients with hematocrit above 33 percent, and risk of death at 30 days was statistically significantly higher with transfusion once hematocrit rose above 36 percent.
Meanwhile, Rao et al. analyzed 24,112 patients who had been enrolled in three large international ACS trials (GUSTO IIB, PURSUIT, PARAGON B).52 They found that receipt of transfusion predicted increased risk of death and death/MI at 30 days. After stratifying results by nadir hematocrit, they noted no association between transfusion exposure and mortality with a hematocrit of 25 percent or below, but they found a highly increased probability of death at 30 days with transfusion at a nadir hematocrit of 30 percent or higher.
Sabatine et al. performed a post-hoc meta-analysis of 16 prior TIMI cardiac trials, finding that transfusion appeared to confer a protective effect in terms of cardiovascular mortality in patients with STEMI and admission hemoglobin less than 12 g/dL.57 Meanwhile, there was a non-significant trend towards worse outcomes in STEMI with transfusion at hemoglobin level greater than 12 g/dL; and in the NSTE-ACS population, they noted an association between transfusion and higher risk for a combined mortality and cardiovascular event endpoint at all hemoglobin levels.
Of the three remaining studies that performed stratified analyses, transfusion was found to be of potential benefit in acute MI patients with nadir hemoglobin 8 g/dL or less,58 and nonsignificant trends toward improved outcomes were noted in NSTE-ACS patients with hemoglobin at presentation less than 9 g/dL,54 and NSTE-ACS patients with hematocrit 24 percent or less.55 In each case, transfusion at hemoglobin or hematocrit levels higher than these thresholds was associated with increased mortality.
Heart Failure
Two observational studies evaluated patients with acute decompensated heart failure,61, 62 with conflicting results. Garty et al. evaluated 2,335 patients admitted for acute decompensated heart failure to public hospitals in Israel.61 They found that transfusion appeared to confer lower risk of death at 30 days, with trends toward benefit in-hospital, at one year and at four years. Meanwhile, Kao et al. noted higher adjusted in-hospital mortality with transfusion in a large cohort of patients hospitalized for heart failure in California.62 This association was noted in both anemic and non-anemic patients but was much stronger in the non-anemic cohort.
Perioperative Setting
Cardiac Surgery
There were four randomized controlled trials of which two enrolled fewer than 40 patients total and were designed to evaluate primarily hemodynamic and lab parameters, while two were larger, enrolling 400 to 500 patients with primary clinical endpoints (Table 3). All were consistent in finding no difference in survival or cardiovascular complications with a conservative compared to a liberal transfusion strategy.63-66
Non-cardiac Surgery
Six studies, including three controlled trials and three observational studies, have reported outcomes based on transfusion status in patients with heart disease undergoing non-cardiac surgery. In the three controlled trials, performed in hip fracture and vascular surgery populations, there was no apparent benefit or harm from a more versus less aggressive transfusion strategy (Table 3).67-69 By far, the largest of these studies, the FOCUS trial that enrolled over 2,000 patients undergoing hip fracture surgery, has only reported results in abstract form, with full publication expected in the very near future. The authors report no difference in mortality and functional status outcomes between the liberal and conservative transfusion groups. In the observational cohorts, transfusion did not appear to offer any protection, and in one study in the vascular surgery setting, mortality and myocardial infarction rates were higher overall in the transfused group, a harm in subgroup analysis limited to those transfused at a hemoglobin ≥ 9 g/dL (Table 3).41, 68, 70
- LITERATURE FLOW
- In patients with CHF or CHD, what are the health outcome benefits and harms of treating anemia with ESAs?
- In patients with CHF or CHD, what are the health outcome benefits and harms of using iron to treat iron deficiency with or without anemia?
- In patients with CHF or CHD, what are the health outcome benefits and harms of treating anemia with red blood cell transfusions?
- RESULTS - Treatment of Anemia in Patients with Heart Disease: A Systematic Revie...RESULTS - Treatment of Anemia in Patients with Heart Disease: A Systematic Review
Your browsing activity is empty.
Activity recording is turned off.
See more...