NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
General Concepts
Rickettsiae
The rickettsiae are a diverse collection of obligately intracellular Gram-negative bacteria found in ticks, lice, fleas, mites, chiggers, and mammals. They include the genera Rickettsiae, Ehrlichia, Orientia, and Coxiella. These zoonotic pathogens cause infections that disseminate in the blood to many organs.
Rickettsia
Clinical Manifestations
Rickettsia species cause Rocky Mountain spotted fever, rickettsialpox, other spotted fevers, epidemic typhus, and murine typhus. Orientia (formerly Rickettsia) tsutsugamushi causes scrub typhus. Patients present with febrile exanthems and visceral involvement; symptoms may include nausea, vomiting, abdominal pain, encephalitis, hypotension, acute renal failure, and respiratory distress.
Structure, Classification, and Antigenic Types
Rickettsia species are small, Gram-negative bacilli that are obligate intracellular parasites of eukaryotic cells. This genus consists of two antigenically defined groups: spotted fever group and typhus group, which are related; scrub typhus rickettsiae differ in lacking lipopolysaccharide, peptidoglycan, and a slime layer, and belong in the separate, although related, genus Orientia.
Pathogenesis
Rickettsia and Orientia species are transmitted by the bite of infected ticks or mites or by the feces of infected lice or fleas. From the portal of entry in the skin, rickettsiae spread via the bloodstream to infect the endothelium and sometimes the vascular smooth muscle cells. Rickettsia species enter their target cells, multiply by binary fission in the cytosol, and damage heavily parasitized cells directly.
Host Defenses
T-lymphocyte-mediated immune mechanisms and cytokines, including gamma interferon and tumor necrosis factor alpha, play a more important role than antibodies.
Epidemiology
The geographic distribution of these zoonoses is determined by that of the infected arthropod, which for most rickettsial species is the reservoir host.
Diagnosis
Rickettsioses are difficult to diagnose both clinically and in the laboratory. Cultivation requires viable eukaryotic host cells, such as antibiotic-free cell cultures, embryonated eggs, and susceptible animals. Confirmation of the diagnosis requires comparison of acute- and convalescent-phase serum antibody titers.
Control
Rickettsia species are susceptible to the broad-spectrum antibiotics, doxycycline, tetracycline, and chloramphenicol. Prevention of exposure to infected arthropods offers some protection. A vaccine exists for epidemic typhus but is not readily available.
Ehrlichia
Clinical Manifestations
Ehrlichia species cause ehrlichioses that vary in severity from a life-threatening febrile disease that resembles Rocky Mountain spotted fever, except for less frequent rash, to an infectious mononucleosis-like syndrome.
Classification and Antigenic Types
Ehrlichia sennetsu, E chaffeensis, and the human granulocytic ehrlichia are genetically distinct and are easily distinguished antigenically.
Pathogenesis
A reservoir of E chaffeensis is deer, and for both human monocytic and granulocytic ehrlichiosis are transmitted when ticks bite human skin and inoculate organisms, which then spread by the bloodstream. Macrophages or neutrophils have cytoplasmic vacuoles that contain ehrlichiae dividing by binary fission in each of these ehrlichioses.
Host Defenses
Host defenses against E chaffeensis include cytokine-mediated restriction of iron supplies to the ehrlichiae.
Epidemiology
Sennetsu ehrlichiosis has been documented in Japan and Malaysia. Human infections with E chaffeensis- and E phagocytophila- like organisms have been found recently. Human monocytic ehrlichiosis originates in most of the Atlantic, southeastern, and south central states from New Jersey to Texas. Human granulocytic ehrlichiosis has been identified in the upper midwest and New England thus far.
Diagnosis
Clinical and laboratory clues must be confirmed serologically or by polymerase chain reaction detection of specific ehrlichial DNA.
Coxiella
Clinical Manifestations
Coxiella burnetii causes Q fever, which may present as an acute febrile illness with pneumonia or as a chronic infection with endocarditis.
Structure, Classification, and Antigenic Types
Coxiella burnetii varies in size and has an endospore-like form. This species has lipopolysaccharide and phage type diversity.
Pathogenesis
Coxiella burnetii organisms are transmitted to the human lungs by aerosol from heavily infected placentas of sheep and other mammals and disseminate in the bloodstream to the liver and bone marrow, where they are phagocytosed by macrophages. Growth within phagolysosomes is followed by formation of T-lymphocyte-mediated granulomas. In the few patients who develop serious chronic Q fever, heart valves contain organisms within macrophages.
Host Defenses
Host defense depends on T lymphocytes and gamma interferon.
Epidemiology
Q fever is found worldwide. It is associated mainly with exposure to infected placentas and birth fluids of sheep and other mammals.
Diagnosis
The disease is difficult to diagnose clinically, and cultivation poses a biohazard. Therefore, serology is the mainstay of laboratory diagnosis.
Control
Antibiotics are effective against acute Q fever. A vaccine containing killed phase I organism shows promise in protecting against infection.
Bartonella
Bartonella (Rochalimaea) quintana, the agent of trench fever, was formerly considered as a rickettsial agent. It can be cultured outside of eukaryotic cells and is transmitted to humans via lice. Trench fever was a significant medical problem during World War I and has reappeared among homeless and alcoholic persons. Recently, cat scratch disease and bacillary angiomatosis and peliosis were discovered to be caused in most cases by a related organism, B henselae. Bartonella bacilliformis has long been known to cause a sand fly-transmitted acute infection in South America that destroys the red blood cells and a chronic infection that causes a vascular tumor-like lesion similar to those of B henselae.
Introduction
Rickettsiae are small, Gram-negative bacilli that have evolved in such close association with arthropod hosts that they are adapted to survive within the host cells. They represent a rather diverse collection of bacteria, and therefore listing characteristics that apply to the entire group is difficult. The common threads that hold the rickettsiae into a group are their epidemiology, their obligate intracellular lifestyle, and the laboratory technology required to work with them. In the laboratory, rickettsiae cannot be cultivated on agar plates or in broth, but only in viable eukaryotic host cells (e.g., in cell culture, embryonated eggs, or susceptible animals). The exception, which shows the artificial nature of using obligate intracellular parasitism as a defining phenotypic characteristic, is Bartonella (Rochalimaea) quintana, which is cultivable axenically, but was traditionally considered as a rickettsia. The diversity of rickettsiae is demonstrated in the variety of specific intracellular locations where they live and the remarkable differences in their major outer membrane proteins and genetic relatedness (Table 38-1). An example of extreme adaptation is that the metabolic activity of Coxiella burnetii is greatly increased in the acidic environment of the phagolysosome, which is a harsh location for survival for most other organisms. Obligate intracellular parasitism among bacteria is not unique to rickettsiae. Chlamydiae also have evolved to occupy an intracellular niche, and numerous bacteria (e.g., Mycobacteria, Legionella, Salmonella, Shigella, Francisella, and Brucella) are facultative intracellular parasites. In contrast with chlamydiae, all rickettsiae can synthesize ATP. Coxiella burnetii is the only rickettsia that appears to have a developmental cycle.
Table 38-1
Properties of Selected Rickettsial Organisms.
Some organisms in the family Rickettsiaceae are closely related genetically (e.g., Rickettsia rickettsii, R akari, R prowazekii, and R typhi); others are related less closely to Rickettsia species (e.g., Ehrlichia and Bartonella); and others not related to Rickettsia species (e.g., C burnetii). The phenotypic traits of the medically important organism Orientia (Rickettsia) tsutsugamushi suggest that the species may be an example of convergent evolution in a similar ecologic niche.
Rickettsioses are zoonoses that, except for Q fever, are usually transmitted to humans by arthropods (tick, mite, flea, louse, or chigger) (Table 38-2). Therefore, their geographic distribution is determined by that of the infected arthropod, which for most rickettsial species is the reservoir host. Rickettsiae are important causes of human diseases in the United States (Rocky Mountain spotted fever, Q fever, murine typhus, sylvatic typhus, human monocytic ehrlichiosis, human granulocytic ehrlichiosis, and rickettsialpox) and around the world (Q fever, murine typhus, scrub typhus, epidemic typhus, boutonneuse fever, and other spotted fevers) (Table 38-2).
Table 38-2
Distinguishing Characteristics Of Rickettsial Diseases.
Rickettsiae of the Spotted Fever and Typhus Groups
The rickettsial diseases are arranged into several major categories (Table 38-2), the first two of which are the spotted fever and typhus fever groups.
Clinical Manifestations
Rocky Mountain Spotted Fever
Rocky Mountain spotted fever is among the most severe of human infectious diseases, with a mortality of 20 to 25 percent unless treated with an appropriate antibiotic. The severity and mortality are greater for men, elderly persons, and black men with glucose-6-phosphate dehydrogenase deficiency. Although, in theory, the disease is always curable by early, appropriate treatment, the case fatality rate is still 4 percent. The incidence of disease parallels the geographic distribution of infected Dermacentor variabilis ticks in the eastern United States and D andersoni in the Rocky Mountain states, where the infection was first recognized. Rocky Mountain spotted fever was subsequently recognized in the eastern United States. The incidence has declined in the Rocky Mountain states and increased dramatically in the southeastern United States and Oklahoma. Currently most cases actually occur in the Atlantic states from Maryland to Georgia, as well as in Oklahoma, Missouri, Kansas, Ohio, Tennessee, Arkansas, and Texas, although cases are reported in nearly every state. In the southeastern states, the disease occurs during the seasonal activity of D variabilis ticks (April through September) and affects children more frequently than adults. Significant changes in incidence do occur. From a low of 199 cases reported in 1959, the annual number of cases rose steadily to a peak of 1,192 cases in 1981, with a subsequent decline and plateau of approximately 700 cases since 1985. The reasons for these fluctuations are unclear.
The rickettsiae are maintained in nature principally by transovarial transmission from infected female ticks to infected ova that hatch into infected larval offspring (Fig. 38-1). A low rate of acquisition of rickettsiae by uninfected ticks occurs when the ticks feed upon small mammals with enough rickettsiae in their blood to establish tick infection. This effect replenishes lines of infected ticks that are occasionally killed by massive rickettsial overgrowth. A recently observed factor of potential importance in this balance of nature is the interference phenomenon, by which infection of ticks with nonpathogenic spotted fever group rickettsiae prevents the establishment of infection by R rickettsii.
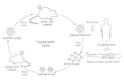
Figure 38-1
Transovarian passage of R rickettsii in the tick vector is an important cycle in maintaining the infection in nature from one generation of tick to another. Horizontal transmission (i.e., acquisition of the bacteria by uninfected ticks feeding on infected (more...)
The clinical gravity of Rocky Mountain spotted fever is due to severe damage to blood vessels by R rickettsii. This organism is unusual among rickettsiae in its ability to spread and invade vascular smooth muscle cells as well as endothelium. Damage to the blood vessels in the skin in locations of the rash leads to visible hemorrhages in one-half of all infected persons (Fig. 38-2). Attempted plugging of vascular wall destruction consumes platelets, with consequent thrombocytopenia also affecting approximately one-half of the patients.

Figure 38-2
Common clinical manifestations of the rickettsial diseases. .
Rickettsialpox and Other Spotted Fevers
In the 1940s an epidemic of disease characterized by fever, rash, and cutaneous necrosis appeared in one area of New York City. The etiology was traced to R akari transmitted by the bite of mites (Liponyssoides sanguineus) that infested the numerous mice in an apartment house in this area. The disease was named rickettsialpox because many patients had blister-like rashes resembling those of chickenpox. Epidemics were diagnosed in other cities, and R akari has been isolated in other countries (e.g., the Ukraine). Perhaps because this nonfatal disease is seldom considered by physicians, or its incidence is truly low, the diagnosis is rarely made. Transovarial transmission in the mite and periodic documentation of cases assure us that the etiologic agent is still with us.
Boutonneuse fever, so called because of the papular rash in some cases, has many synonyms, reflecting different geographic regions of occurrence (e.g., Mediterranean spotted fever, Kenya tick typhus, and South African tick bite fever). Cases are observed in the United States in travelers returning from endemic areas. The agent, R conorii, is closely related to R. rickettsii. Severe disease resembling Rocky Mountain spotted fever can cause death in high-risk groups (e.g., elderly, alcoholic, and glucose-6-phosphate dehydrogenase-deficient patients). Cutaneous necrosis caused by rickettsial vascular infection at the tick bite site of inoculation, known as an eschar or tache noire, is observed in only half the patients with boutonneuse fever. The curiously high prevalence of antibodies reactive with R conorii in healthy populations in endemic regions might be explained by missed diagnosis of prior illness, subclinical infection, infection with an antigenically related but less pathogenic rickettsia, or nonspecificity of the laboratory test.
Other spotted fevers occur in geographic distributions of little concern to many physicians in the United States. North Asian tick typhus caused by R sibirica, Queensland tick typhus caused by R australis, and the recently discovered oriental spotted fever caused by R japonica demonstrate that spotted fever group rickettsiae occur worldwide.
Epidemic Typhus and Brill-Zinsser Disease
Epidemics of louse-borne typhus fever have had important effects on the course of history; for example, typhus in one army but not in the opposing force has determined the outcome of wars. Populations have been decimated by epidemic typhus. During and immediately after World War I, 30 million cases occurred, with 3 million deaths. Unsanitary, crowded conditions in the wake of war, famine, flood, and other disasters and in poor countries today encourage human louse infestation and transmission of R prowazekii. Epidemics usually occur in cold months in poor highland areas, such as the Andes, Himalayas, Mexico, Central America, and Africa. Lice live in clothing, attach to the human host several times daily to take a blood meal, and become infected with R prowazekii if the host has rickettsiae circulating in the blood. If the infected louse infests another person, rickettsiae are deposited on the skin via the louse feces or in the crushed body of a louse. Scratching inoculates rickettsiae into the skin.
Between epidemics R prowazekii persists as a latent human infection. Years later, when immunity is diminished, some persons suffer recrudescent typhus fever (Brill-Zinsser disease). These milder sporadic cases can ignite further epidemics in a susceptible louse-infested population. In the United States Brill-Zinsser disease is seen in immigrants who suffered typhus fever before entering the country. In the eastern United States, sporadic human cases of R prowazekii infection have been traced to a zoonotic cycle involving flying squirrels and their own species of lice and fleas.
Murine Typhus
Murine typhus is prevalent throughout the world, particularly in ports, countries with warm climates, and other locations where rat populations are high. Rickettsia typhi is associated with rats and fleas, particularly the oriental rat flea, although other ecologic cycles (e.g., opossums and cat fleas) have been implicated. Fleas are infected by transovarian transmission or by feeding on an animal with rickettsiae circulating in the blood. Rickettsiae are shed from fleas in the feces, from which humans acquire the infection through the skin, respiratory tract, or conjunctiva. During the 1940s more than 4,000 cases of murine typhus occurred annually in the United States. The incidence declined coincident with increased utilization of the insecticide DDT. Although the infection and clinical involvement affects the brain, lungs, and other visceral organs in addition to the skin, mortality in humans is less than 1 percent.
Structure, Classification, and Antigenic Types
Rickettsia species include two antigenically defined groups that are closely related genetically but differ in their surface-exposed protein and lipopolysaccharide antigens. These are the spotted fever and typhus groups. The organisms in these groups are smaller (0.3 μm by 1.0 μm) than most Gram-negative bacilli that live in the extracellular environment (Fig. 38-3). They are surrounded by a poorly characterized structure that is observed as an electron-lucent zone by transmission electron microscopy and is considered to represent a polysaccharide-rich slime layer or capsule. The cell wall contains lipopolysaccharides, a major component that differs antigenically between the typhus group and the spotted fever group. These rickettsiae also contain major outer membrane proteins with both cross-reactive antigens and surface-exposed epitopes that are species specific and easily denatured by temperatures above 54°C. The major outer membrane protein of typhus group rickettsiae has an apparent molecular mass of 120,000 Da. Spotted fever group rickettsiae generally have a pair of analogous proteins with some diversity of their molecular masses. Rickettsia prowazekii has a transport mechanism that exchanges ATP for ADP in its intracellular environment, thus providing a means to usurp host cell energy sources under favorable circumstances. Rickettsiae also are able to synthesize ATP via metabolism of glutamate. Adaptation to the intracellular environment is further evidenced in a variety of transport mechanisms to obtain crucial substances such as particular amino acids from cytoplasmic pools in the host cell. These adaptations and the presence of numerous independent metabolic activities demonstrate that rickettsiae are not degenerate forms of bacteria, but rather have evolved successfully for survival with an intracellular life-style.
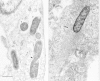
Figure 38-3
(A) Organisms of Rickettsia conorii(r) in a cultured human endothelial cell are located free in the cytosol. One rickettsia is dividing by binary fission (arrowhead). (B) These rickettsiae can move inside the cytoplasm of the host cell because of the (more...)
Pathogenesis
Rickettsiae are transmitted to humans by the bite of infected ticks and mites and by the feces of infected lice and fleas. They enter via the skin and spread through the bloodstream to infect vascular endothelium in the skin, brain, lungs, heart, kidneys, liver, gastrointestinal tract, and other organs (Fig. 38-1). Rickettsial attachment to the endothelial cell membrane induces phagocytosis, soon followed by escape from the phagosome into the cytosol (Fig. 38-4). Rickettsiae divide inside the cell. Rickettsia prowazekii remains inside the apparently healthy host cell until massive quantities of intracellular rickettsiae accumulate and the host cell bursts, releasing the organisms. In contrast, R rickettsii leaves the host cell via long, thin cell projections (filopodia) after a few cycles of binary fission. Hence, relatively few R rickettsii organisms accumulate inside any particular cell, and rickettsial infection spreads rapidly to involve many other cells. Perhaps because of the numerous times the host cell membrane is traversed, there is an influx of water that is initially sequestered in cisternae of cytopathically dilated rough endoplasmic reticulum in the cells more heavily infected with R rickettsii.
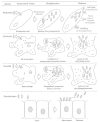
Figure 38-4
Pathogenesis of the rickettsial agents illustrating unique aspects of their interactions with eukaryotic cells.
The bursting of endothelial cells infected with R prowazekii is a dramatic pathologic event. The mechanism is unclear, although phospholipase activity, possibly of rickettsial origin, has been suggested. Injury to endothelium and vascular smooth muscle cells infected by R rickettsii seems to be caused directly by the rickettsiae, possibly through the activity of a rickettsial phospholipase or rickettsial protease or through free-radical peroxidation of host cell membranes. Host immune, inflammatory, and coagulation systems are activated and appear to benefit the patient. Cytokines and inflammatory mediators account for an undefined part of the clinical signs. Rickettsial lipopolysaccharide is biologically relatively nontoxic and does not appear to cause the pathogenic effects of these rickettsial diseases.
The pathologic effects of these rickettsial diseases originate from the multifocal areas of endothelial injury with loss of intravascular fluid into tissue spaces (edema), resultant low blood volume, reduced perfusion of the organs, and disordered function of the tissues with damaged blood vessels (e.g., encephalitis, pneumonitis, and hemorrhagic rash).
Diagnosis
Diagnosis of rickettsial infections is often difficult. The clinical signs and symptoms (e.g., fever, headache, nausea, vomiting, and muscle aches) resemble many other diseases during the early stages when antibiotic treatment is most effective. A history of exposure to the appropriate vector tick, louse, flea, or mite is helpful but cannot be relied upon. Observation of a rash, which usually appears on or after day 3 of illness, should suggest the possibility of a rickettsial infection but, of course, may occur in many other diseases also. Knowledge of the seasonal and geographic epidemiology of rickettsioses is useful, but is inconclusive for the individual patient. Except for epidemic louse-borne typhus, rickettsial diseases strike mostly as isolated single cases in any particular neighborhood. Therefore, clinico-epidemiologic diagnosis is ultimately a matter of suspicion, empirical treatment, and later laboratory confirmation of the specific diagnosis.
Because rickettsiae are both fastidious and hazardous, few laboratories undertake their isolation and diagnostic identification (Fig. 38-5). Some laboratories are able to identify rickettsiae by immunohistology in skin biopsies as a timely, acute diagnostic procedure, but to establish the diagnosis physicians usually rely on serologic demonstration of the development of antibodies to rickettsial antigens in serum collected after the patient has recovered. Currently, assays that demonstrate antibodies to rickettsial antigens themselves (e.g., the indirect fluorescence antibody test or latex agglutination) are preferable to the nonspecific, insensitive Weil-Felix test that is based on the cross-reactive antigens of OX-19 and OX-2 strains of Proteus vulgaris.

Figure 38-5
Laboratory methods used in confirming a diagnosis of rickettsial infection. These bacteria can be cultivated as indicated, but use of serology is more common.
Control
Although early treatment with doxycycline, tetracycline, or chloramphenicol is effective in controlling the infection in the individual patient, this action has no effect on rickettsiae in their natural ecologic niches (e.g., ticks). Human infections are prevented by control of the vector and reservoir hosts. Massive delousing with insecticide can abort an epidemic of typhus fever. Prevention of attachment of ticks and their removal before they have injected rickettsiae into the skin reduces the likelihood of a tick-borne spotted fever. Control of rodent populations and of the access of rats and mice to homes and other buildings may reduce human exposure to R typhi and R akari.
Vaccines against spotted fever and typhus group rickettsiae have been developed empirically by propagation of rickettsiae in ticks, lice, embryonated hen eggs, and cell culture. Vaccines containing killed organisms have provided incomplete protection. A live attenuated vaccine against epidemic typhus has proved successful, but is accompanied by a substantial incidence of side effects, including a mild form of typhus fever in some persons. The presence of strong immunity in convalescent subjects indicates that vaccine development is feasible, but it requires further study of rickettsial antigens and the effective anti-rickettsial immune response. T-lymphocyte-mediated immune mechanisms, including effects of the lymphokines, gamma interferon tumor necrosis factor, and interleukin-1, seem most important.
Orientia (Rickettsia) tsutsugamushi and Scrub Typhus
Although the agents of scrub typhus bear a single taxonomic name, Orientia (Rickettsia) tsutsugamushi, these interrelated organisms are somewhat heterogeneous and differ strikingly from Rickettsia species of the spotted fever and typhus groups.
Clinical Manifestations
Patients with scrub typhus often have only fever, headache, and swollen lymph nodes and in some cases myalgia, gastrointestinal complains, or cough beginning 6 to 21 days following exposure to the vector. Fewer than half of the patients have an eschar at the site where the larval mite fed and the classic rash. The mortality varies but averages 7 percent without anti-rickettsial treatment.
Structure, Classification, and Antigenic Types
Orientia (Rickettsia) tsutsugamushi is a very labile rickettsia that is particularly difficult to propagate and separate from the host cells in which it grows. In contrast with spotted fever group and typhus group rickettsiae, O tsutsugamushi does not seem to possess lipopolysaccharides, peptidoglycan, a slime layer, or other T-independent antigens. The rickettsial cell wall consists of proteins linked by disulfide bonds. Antigenically distinguishable strains represent only part of what seems to be a great antigenic mosaic. Immunity to infection with the homologous strain wanes within a few years; cross-protective immunity to heterologous strains disappears within a few months. The reasons for this lack of long-term immunity are unclear.
Pathogenesis
Orientia (Rickettsia) tsutsugamushi is injected into the skin during feeding by a larval trombiculid mite (chigger). An eschar often forms at this location. Rickettsiae spread via the bloodstream and damage the microcirculation of the skin (rash), lungs (pneumonitis), brain (encephalitis), and other organs. The generalized enlargement of lymph nodes is unique among rickettsial diseases. Orientia (Rickettsia) tsutsugamushi is phagocytosed by the host cell, escapes from the phagosome into the cytosol, divides by binary fission, and is released from projections of the cell membrane (Fig. 38-4). The pathogenic mechanism of O tsutsugamushi is not known.
Epidemiology
Scrub typhus occurs where chiggers infected with virulent rickettsial strains feed upon humans. Leptotrombidium deliense and other mites are found particularly in areas where regrowth of scrub vegetation harbors the Rattus species that are hosts for the mites. Some of these foci are quite small and have been referred to as mite islands. Because O tsutsugamushi is transmitted transovarially from one generation of mites to the next, these dangerous areas tend to persist for as long as the ecologic conditions, including scrub vegetation, persist. Truly one of the neglected diseases, scrub typhus occurs over a vast area, including Japan, China, the Philippines, New Guinea, Indonesia, other islands of the southwest Pacific Ocean, southeastern Asia, northern Australia, India, Sri Lanka, Pakistan, Russia, and Korea. Recognized in western countries mainly because of large numbers of infections of military personnel during World War II and the Vietnam War, scrub typhus perennially affects native populations. Reinfection and undiagnosed infections are highly prevalent. Mortality ranges from 0 to 35 percent and has not been correlated with any specific factor.
Diagnosis
Classic textbook cases with fever, headache, eschar, and rash are far outnumbered by cases that lack rash or eschar. Such cases are usually misdiagnosed. Laboratory diagnosis is unavailable in many areas where scrub typhus occurs. Isolation of rickettsiae requires inoculation of mice or cell culture. Serologic diagnosis is made by specific methods (indirect fluorescence antibody test or enzyme immunoassay) or by the older method of demonstrating cross-reactive antibodies that agglutinate the OXK strain of P mirabilis.
Control
Scrub typhus can be treated with doxycycline, tetracycline, or chloramphenicol. Chigger repellents may prevent exposure. Prophylaxis with weekly doses of doxycycline during and for 6 weeks after exposure protects against scrub typhus. Attempts to develop a safe, effective vaccine have failed.
Ehrlichia
According to the evolutionary scheme suggested by 16S rRNA sequence homology, ehrlichiae are genetically related to Rickettsia species. The genus Ehrlichia contains Gram-negative bacteria that reside in a cluster (morula) within membrane-bound cytoplasmic vacuoles of monocytes and macrophages, or polymorphonuclear leukocytes. Ehrlichiae have been implicated as the agents of diseases of horses (E risticii and E equi), dogs (E canis, E ewingii and E platys, a platelet pathogen), and other animals. Ehrlichia sennetsu causes a human disease in Japan resembling infectious mononucleosis. Ehrlichiae are unusual in their cell wall structure and they can establish persistent infections.
In 1987 the first case of human ehrlichiosis was reported in the United States. A severely ill man with multiorgan system involvement had morula inclusions demonstrated in peripheral blood leukocytes. Subsequently, cases of human monocytic ehrlichiosis have been documented mainly in eastern and southern states between New Jersey and Texas. The infection has varied from severe and sometimes fatal, mimicking Rocky Mountain spotted fever, to oligosymptomatic and asymptomatic forms. A history of tick bite and the seasonal and geographic occurrence correlate with the predominant tick vector, Amblyomma americanum. Illness is often accompanied by leukopenia, thrombocytopenia, and damage to the liver. Lesions include perivasculitis in the central nervous system, kidney, heart, and lungs and granulomas in the bone marrow and liver. Clinical diagnosis is difficult. Laboratory diagnosis by indirect fluorescence antibody assay or polymerase chain reaction is not widely available. Ehrlichia chaffeensis morulae are difficult to detect in peripheral blood leukocytes.
In 1994 another serious new infectious disease, human granulocytic ehrlichiosis, was reported. Ehrlichiae seen within morulae in neutrophils in smears of peripheral blood were identified as very closely related to E phagocytophila (a European tick-transmitted infection of sheep, cattle, goats, and deer) and E equi. The causative organism, like other granulocytic ehrlichiae, has never been cultivated. Human granulocytic ehrlichiosis has been associated with the deer tick, Ixodes scapularis, and thus is found as far north as Minnesota, Wisconsin, and New England. Laboratory diagnosis is practically achieved by visualizing morulae in neutrophils, as serology and polymerase chain reaction for the agent are presently research procedures. Sometimes fatal, human granulocytic ehrlichiosis, like E chaffeensis infection, can be treated effectively with doxycycline.
Coxiella burnetii and Q Fever
Coxiella burnetii is sufficiently different genetically from the other rickettsial agents that it is placed in a separate group. Unlike the other agents, it is very resistant to chemicals and dehydration. Additionally, its transmission to humans is by the aerosol route, although a tick vector is involved in spread of the bacteria among the reservoir animal hosts.
Clinical Manifestations
Q fever is a highly variable disease, ranging from asymptomatic infection to fatal chronic infective endocarditis (Fig. 38-6). Some patients develop an acute febrile disease that is a nonspecific influenza-like illness or an atypical pneumonia. Other patients are diagnosed after identification of granulomas in their liver or bone marrow. The most serious clinical conditions are chronic C burnetii infections, which may involve cardiac valves, the central nervous system, and bone.

Figure 38-6
Clinical manifestations of Q fever. .
Structure, Classification, and Antigenic Type
Coxiella burnetii is an obligately intracellular bacterium with some peculiar characteristics. It is small, generally 0.25 μm by 0.5 to 1.25 μm. However, there is considerable ultrastructural pleomorphism, including small- and large-cell variants and possible endospore-like forms, suggesting a hypothetical developmental cycle. Among rickettsiae, C burnetii is the most resistant to environmental conditions, is the only species that resides in the phagolysosome, is activated metabolically by low pH, and has a plasmid. The extensive metabolic capacity of C burnetii suggests that its obligate intracellular parasitism is a highly evolved state rather than a degenerate condition. The cell wall is typical of Gram-negative bacteria and contains peptidoglycan, proteins, and lipopolysaccharide. When propagated under laboratory conditions in embryonated eggs or cell culture, C burnetii undergoes phase variation analogous to the smooth to rough lipopolysaccharide variation of members of the Enterobacteriaceae. Phase I is the form found in nature and in human infections. The phase II variant contains truncated lipopolysaccharide, is avirulent, and is a poor vaccine.
Pathogenesis
Human Q fever follows inhalation of aerosol particles derived from heavily infected placentas of sheep, goats, cattle, and other mammals. Coxiella burnetii proliferates in the lungs, causing atypical pneumonia in some patients. Hematogenous spread occurs, particularly to the liver, bone marrow, and spleen. The disease varies widely in severity, including asymptomatic, acute, subacute, or chronic febrile disease, granulomatous liver disease, and chronic infection of the heart valves. The target cells are macrophages in the lungs, liver, bone marrow, spleen, heart valves, and other organs. Coxiella burnetii is phagocytosed by Kupffer cells and other macrophages and divides by binary fission within phagolysosomes (Fig. 38-3). Apparently it is minimally harmful to the infected macrophages. Different strains have genetic and phenotypic diversity. The lipopolysaccharides are relatively nonendotoxic. Host-mediated pathogenic mechanisms appear to be important, especially immune and inflammatory reactions, such as T-lymphocyte-mediated granuloma formation.
Epidemiology
Coxiella burnetii infects a wide variety of ticks, domestic livestock, and other wild and domestic mammals and birds throughout the world. Most human infections follow exposure to heavily infected birth products of sheep, goats, and cattle, as occurs on farms, in research laboratories, and in abattoirs. Coxiella burnetii is also shed in milk, urine, and feces of infected animals. Animals probably become infected by aerosol and by the bite of any of the 40 species of ticks that carry the organisms.
Diagnosis
Clinical diagnosis depends upon a high index of suspicion, careful evaluation of epidemiologic factors, and ultimately, confirmation by serologic testing. Although C burnetii can be isolated by inoculation of animals, embryonated hen eggs, and cell culture, very few laboratories undertake this biohazardous approach. Likewise, the diagnosis is seldom made by visualization of the organisms in infected tissues. Acute Q fever is diagnosed by demonstration of the development of antibodies to protein antigens of C burnetii phase II organisms. Chronic Q fever endocarditis is diagnosed by demonstration of a high titer of antibodies, particularly IgG and IgA, against the lipopolysaccharide antigens of C burnetii phase I organisms in patients with signs of endocarditis whose routine blood cultures contain no organisms.
Control
Antibiotic treatment is more successful in ameliorating acute, self-limited Q fever than in curing life-threatening chronic endocarditis. Reduction in exposure to these widespread organisms is difficult because some serologically screened animals that have no detectable antibodies to C burnetii still shed organisms at parturition. Persons with known occupational hazards (e.g., Australian abattoir workers) have benefitted from a vaccine composed of killed phase I organisms. This vaccine is not readily available, but offers promise for development of safe, effective immunization.
Bartonella
It has been recognized recently that organisms thought to be closely related to rickettsiae such as the louse-borne causative agent of trench fever, Bartonella (formerly Rochalimaea)quintana, in fact, belong in the genus Bartonella. These bacteria can be cultivated in cell-free medium and hence do not fit the criterion of definition of rickettsiae as obligately intracellular bacteria. Bartonella quintana infections were a serious medical problem during World War I. Soldiers in the trenches were infested with body lice that passed B quintana in their feces onto the skin. Individuals who have recovered from trench fever continue to have R quintana circulating in this stage of infection and may serve as sources of infection for lice, which can transmit the infection to others.
In association with the AIDS epidemic, another species B henselae (in addition to B quintana) has been discovered to be the cause of opportunistic infections often masquerading as hemangioma-like lesions of skin and visceral organs, bacillary angiomatosis. Bartonella henselae was recognized subsequently to be the long sought after cause of cat scratch disease, which usually manifested as a self-limited enlargement and inflammation of lymph nodes of several months duration in the regional drainage of a cat scratch or bite.
Bartonella bacilliformis transmitted by the sandfly in certain regions of Western South America invades human red blood cells, causing acute, often severe, hemolytic anemia. In chronic infections, there are skin lesions known as verruga peruana (Peruvian warts) that are similar to those of bacillary angiomatosis. A Peruvian medical student, Daniel Carrion, proved these lesions to be caused by an infectious agent in 1885 when he fatally inoculated himself with material from a verruga peruana. He died of the acute infectious hemolytic anemia known today as Oroya fever or, in his memory, Carrion's disease.
References
- Adal KA, Cockerell CJ, Petri WA. Cat scratch disease, bacillary angiomatosis, and other infections due to Rochalimaea. N Engl J Med. 1994;330:1509–1515. [PubMed: 8164704]
- Audy JR (ed): Red Mites and Typhus. University Press, New York, 1968 .
- Bakken JS, Dumler JS, Chen S-M. et al. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA. 1994;272:212–218. [PubMed: 8022040]
- Brouqui P, Dumler JS, Raoult D. Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med. 1994;97:451–453. [PubMed: 7977434]
- Drancourt M, Mainardi JL, Brouqui P. et al. Bartonella (Rochalimaea)quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. [PubMed: 7529894]
- Fishbein DB, Dawson JE, Robinson LE. Human ehrlichiosis in the United States, 1985 to 1990. Ann Int Med. 1994;120:736–743. [PubMed: 8147546]
- Hechemy KE, Paretsky D, Walker DH, Mallavia (eds): Rickettsiology: Current Issues and Perspectives. Vol 590. NY Acad Sci, New York, 1990 .
- Helmick CG, Bernard KW, D'Angelo LJ. Rocky Mountain spotted fever: clinical, laboratory and epidemiological features of 262 cases. J Infect Dis. 1984;150:480. [PubMed: 6491365]
- Kaplowitz LG, Fischer JJ, Sparling PF. Rocky Mountain spotted fever: a clinical dilemma. Curr Clin Top Infect Dis. 1981;2:89.
- Marrie TJ: Q Fever. Vol I. The Disease. CRC Press, Boca Raton, FL. 1990 .
- McDade JE. Natural history of Rickettsia rickettsii. Annu Rev Microbiol. 1986;40:287. [PubMed: 3096192]
- McDade JE, Shepard CC, Redus MA. et al. Evidence of Rickettsia prowazekii infections in the United States. Am J Trop Med Hyg. 1980;29:277. [PubMed: 6154428]
- Moulder JW (ed): Intracellular Parasitism. CRC Press, Boca Raton, FL. 1989 .
- Walker DH (ed): Biology of Rickettsial Disease. Vols I and II. CRC Press, Boca Raton, FL, 1988 .
- Williams WJ, Radulovic S, Dasch GA. et al. Identification of Rickettsia conorii infection by polymerase chain reaction in a soldier returning from Somalia. Clin Infec Dis. 1994;19:93–99. [PubMed: 7948564]
- Wolbach SB, Todd JL, Palfrey FW: Etiology and Pathology of Typhus. The League of Red Cross Societies Harvard Press, Cambridge, Mass, 1922 .
- Zinsser H (ed): Rats, Lice, and History: Little, Brown, New York, 1935 .
- PubMedLinks to PubMed
- Review Epidemiology of rickettsial diseases.[Eur J Epidemiol. 1991]Review Epidemiology of rickettsial diseases.Walker DH, Fishbein DB. Eur J Epidemiol. 1991 May; 7(3):237-45.
- Review Urban zoonoses caused by Bartonella, Coxiella, Ehrlichia, and Rickettsia species.[Vector Borne Zoonotic Dis. 2001]Review Urban zoonoses caused by Bartonella, Coxiella, Ehrlichia, and Rickettsia species.Comer JA, Paddock CD, Childs JE. Vector Borne Zoonotic Dis. 2001 Summer; 1(2):91-118.
- Review The biology of rickettsiae.[Infect Agents Dis. 1996]Review The biology of rickettsiae.Hackstadt T. Infect Agents Dis. 1996 Jun; 5(3):127-43.
- Structural determination of Rickettsia lipid A without chemical extraction confirms shorter acyl chains in later-evolving spotted fever group pathogens.[mSphere. 2024]Structural determination of Rickettsia lipid A without chemical extraction confirms shorter acyl chains in later-evolving spotted fever group pathogens.Yang H, Verhoeve VI, Chandler CE, Nallar S, Snyder GA, Ernst RK, Gillespie JJ. mSphere. 2024 Feb 28; 9(2):e0060923. Epub 2024 Jan 23.
- Review Rickettsioses of the spotted fever group around the world.[J Dermatol. 1989]Review Rickettsioses of the spotted fever group around the world.Walker DH. J Dermatol. 1989 Jun; 16(3):169-77.
- Rickettsiae - Medical MicrobiologyRickettsiae - Medical Microbiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
