NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
This document presents the review of the expert working group brought together by INSERM in the collective expertise procedure to answer the questions raised by the Director General of Health (DGH) concerning the position of vaccination in controlling tuberculosis in France. It is based on the scientific data available at the date of the first 6 months of 2004. The documentary base for this expert review consists of approximately 900 articles and documents.
The INSERM collective expertise centre co-ordinated this collective expertise with the Département animation et partenariat scientifique (DAPS, Department for Facilitation and Scientific Partnership) to prepare the dossier and with the documentation service of the Département de l'information scientifique et de la communication (DISC; the Department of Scientific Information and Communication) for the literature search.
Foreword
Combating tuberculosis is a priority for the World Health Organisation (WHO). One-third of the world population is infected by the Koch bacillus. It is currently estimated that there are 8 million new cases per year, and that the number of deaths throughout the world is 2 million. The situation is particularly serious in developing countries, in which 95% of the new cases are detected. The fall in the incidence of tuberculosis seen over the last three decades in most industrialised countries has been attributed to the reduction in the risk of infection in an improved socio-economic environment.
Considerable diversity is seen in the tuberculosis control programmes in industrialised countries. Some, such as the United States and the Netherlands, have never included generalised vaccination in their programme. Others, such as Sweden, have more recently adopted a targeted vaccination strategy on groups at risk. Vaccination with BCG in France remains a legal obligation for children entering the community (day nurseries, school, etc.). The secular trend toward the fall in tuberculosis in industrialised countries has led these countries intermittently to re-evaluate the relevance and organisation of the programme to combat tuberculosis.
The Director General of Health (DGH) has requested through the collective expertise procedure that INSERM conduct a review of recent knowledge on tuberculosis and the methods of combating this disease and to evaluate the consequences of any change in vaccination policy in France in epidemiological terms.
To respond to this request, INSERM has set up a multi-disciplinary group of specialist experts in the fields of epidemiological monitoring, childhood infectious diseases, bacteriology, vaccinology, molecular biology, and public health.
The expert group worked from a question grid, the major questions of which are shown below:
What are the risk factors for infection and tuberculosis disease in children and adults?
What are the different expressions of tuberculosis disease in children and adults?
What are the advances brought by the molecular approach to tuberculosis to improve treatment and prevention?
What are the instruments, devices, and programmes for screening and diagnosing the infection and the disease?
What are the conditions for treatment of the disease and the infection in adults and children to be effective?
What are the systems implemented on an international level to control tuberculosis?
What are the prevalence, incidence, and mortality data for the different forms of tuberculosis in France?
What are the variations by region, age, and population groups?
How is and how was BCG vaccination organised in France?
What are the outcome data on the clinical efficacy of the BCG vaccine in children and in adults?
What are the pharmacovigilance data on the different undesirable effects seen after BCG vaccination?
What is the status of research into new vaccines against tuberculosis and what are the future perspectives to increase the efficacy of the BCG vaccine?
What are the different vaccine policies in industrialised countries and recent changes in these?
What are the advantages and disadvantages of different vaccination strategies?
What would be the economic impact of restricting or stopping vaccination in France?
The expert group analysed almost 900 articles and documents on the different aspects of the questions raised during six working parties organised between the months of January and June 2004. The experts presented a review of the most recent data and analysed the epidemiological impact of the different vaccination options in France.
Expert advisory group and authors
Expert group and authors
Roland brosch, Bacterial Molecular Genetics Unit, Pasteur Institute, Paris.
Didier che and Bénédicte decludt, Department of Infectious Diseases, Health Monitoring Institute, Saint-Maurice
Pierre durieux, Public Health Unit, Georges Pompidou Hospital, Paris
Joël gaudelus, Department of Paediatrics, Jean Verdier Hospital, Bondy
Brigitte gicquel, Mycobacterial Genetics Unit, Pasteur Institute, Paris
Nicole guérin, Vaccinations Technical Committee
Thomas hanslik, Department of Internal Medicine and Nephrology, Amboise Paré Hospital, Boulogne-Billancourt
Andrea infuso, Department of Infectious Diseases, Health Monitoring Institute, Saint-Maurice
Vincent jarlier, Department of Bacteriology and Hygiene, Pitié-Salpêtrière Hospital, Paris
Philippe-Henry lagrange, Microbiology Laboratory, Saint-Louis Hospital, Paris
Daniel lévy-bruhl, Department of Infectious Diseases, Institute for Health Monitoring, Saint-Maurice
Gilles marchal, National Reference Centre for Mycobacteria, Pasteur Institute, Paris
Arnaud trebucq, Tuberculosis Division, International Union against Tuberculosis and Respiratory Diseases, Paris
Patrick zylberman, Centre for Medicine, Sciences, Health and Society, Villejuif
The following presented communications
Zina bessa, Populations, Underprivileged and Exclusion Health Office, General Directorate for Health, Paris
Jean-Laurent casanova, Department of Paediatric Immunology and Haematology, Necker Sick Children's Hospital; Human Genetics Laboratory for Infectious Diseases, UMR, René Descartes University-INSERM U550, Faculty of Medicine, Paris
Victoria romanus, Swedish Institute for Infectious Disease Control, Solna, Sweden
Scientific and editorial coordination
Elisabeth alimi, Senior Expert Reviewer, INSERM Collective Expertise Centre, Xavier-Bichat Faculty of Medicine, Paris, Reader, Paris XII University
Fabienne bonnin, Scientific Assistant, INSERM Collective Expertise Centre, Xavier-Bichat Faculty of Medicine, Paris
Catherine chenu, Scientific Assistant, INSERM Collective Expertise Centre, Xavier-Bichat Faculty of Medicine, Paris
Jeanne étiemble, Director, INSERM Collective Expertise Centre, Xavier-Bichat Faculty of Medicine, Paris
Catherine pouzat, Scientific Assistant, INSERM Collective Expertise Centre, Xavier-Bichat Faculty of Medicine, Paris
Literature Assistance
Chantal rondet-grellier, documentalist, INSERM Collective Expertise Centre, Xavier-Bichat Faculty of Medicine, Paris
Summary
Tuberculosis, which is a major public health problem throughout the world, has led the WHO to encourage governments to promote a national programme to combat the disease. In developing countries where tuberculosis is highly endemic, BCG vaccination of neonates is a key action in this programme, and improving the efficacy of the vaccine is considered to be a world-wide priority.
The existence of vaccination policies within the programme varies in industrialised countries. Some countries have prioritised early case detection, treatment of infectious cases and preventative therapy for infected people more than vaccination. In France, a country in which there is a strong tradition of vaccination, BCG vaccination is obligatory for children entering the community, and take-up of the vaccine is high (95% at 6 years old). By application of a statute and decree published in July 2004, a single vaccination is recommended from now on, and tuberculin tests are no longer performed to control the vaccination.
It is difficult to distinguish the specific role of vaccination in the overall impact of programmes to control tuberculosis. It is, however, universally accepted that vaccination prevents young children against the severe forms of the disease (meningitis and miliary). In addition, the effectiveness of BCG vaccination has been evaluated in some countries in which stopping vaccination programmes resulted in an increase in the number of cases of tuberculosis.
Several European countries that have a low incidence of tuberculosis have stopped primary vaccination and performed targeted vaccination on groups at risk. The effectiveness of selective vaccination strategy depends on the take-up of vaccination obtained in the target groups and therefore on the ability to identify and vaccinate these groups.
The mean incidence of declared cases in France, which is in the region of 11 per 100,000, is equivalent to the mean incidence in Western Europe. This overall figure masks a large geographical disparity but particularly disparity between native and foreign populations. The incidence of declared cases in Paris is 5 times higher than the mean national incidence. In foreign populations within France coming from countries with high endemic rates, the incidence is approximately 10 times greater than in the native French populations and is increasing (by almost 20% per year in the 15–24-year-old population).
Before envisaging a change in vaccination strategy, it appears essential firstly to study the consequences of this in epidemiological terms based on current data (and considering any change in the latter) and from experiences of other countries under the same conditions. This analysis is only a first stage and will need to be accompanied by an evaluation of the availability and effectiveness of other factors in the system to combat tuberculosis, which are extremely important: control of patients liable to transmit the disease; preventative chemotherapy for infected patients. These systems should therefore be improved in France before any change in the vaccination strategy.
The very considerable advances in knowledge achieved through the molecular approach to tuberculosis and from improved understanding of immune response mechanisms will have more or less short-term applications in screening treatment and prevention and, in particular, in vaccination. These changes will clearly need to be taken into consideration in the context of evolution of the programme to combat tuberculosis.
Tuberculosis in a child always indicates recent infection from an adult
Mycobacterium tuberculosis, discovered in 1882 by Robert Koch, is the agent responsible for tuberculosis (TB). Inhalation of bacteria suspended in air is practically the only method of infection, particularly following close contact. The bacteria lodge in the pulmonary alveoli and are phagocytosed by macrophages in which they die, remain quiescent, or multiply. In the last of these three cases, the macrophages are destroyed and the bacteria are released. They are then phagocytosed again by other macrophages and by dendritic cells. The bacteria ingested by dendritic cells are transported in the lymph ducts toward the regional lymph nodes. In the nodes, the infected dendritic cells are then able to induce selection and clonal expansion of specific T (thymus-dependent) lymphocytes. After a period ranging from a few days to several weeks, these specific T lymphocytes leave the initial draining lymph node and migrate toward the initial focus or foci of infection, where they produce an inflammatory reaction by recognising the living or dead tuberculosis bacterial antigens. A local focus of infection called a tubercle therefore forms gradually, containing living, degenerating, or fused (giant cell) macrophages, bacteria, and lymphocytes. This tubercle may become a granuloma with central necrosis and fibrosis.
In most situations, the development of specific cellular immunity in which various categories of T lymphocytes play a major role limits multiplication of bacilli, and the person remains asymptomatic. This state is defined as tuberculosis infection (TB infection), also called latent tuberculosis infection or primary infection, indicating the encounter with M. tuberculosis. It is characterised by a delayed type hypersensitivity reaction to tuberculin or to partially purified proteins derived from tuberculin (PPD). This involved "positive turning" of the tuberculin reactions, which are accompanied by a normal clinical examination, normal chest x-ray, and negative bacteriology.
In some cases, multiplication of the bacilli is poorly controlled, and active tuberculosis develops. This is the tuberculosis, also called tuberculosis disease (TB disease) or tuberculosis. It may develop either in the immediate period after infection or after several years. The disease is accompanied by clinical and/or radiological signs and/or positive bacteriology. The shift from TB infection to TB disease depends on many factors. Outside of any context of immune suppression, approximately 5% of patients who have developed tuberculosis infection developed tuberculosis within 2 years after infection and an additional 5% will develop tuberculosis during their lives. The risk of progressing to TB disease is increased in immunosuppressed people, particularly in the presence of HIV infection.
Children are infected by adults with the pulmonary form of the disease. Childhood tuberculosis therefore always indicates recent infection from an adult. It is also therefore an indicator that the tuberculous bacillus is in circulation and an indicator of failed screening and management of tuberculosis in adults. In children, TB disease develops in the immediate period after infection. The number of mycobacteria is relatively low (and bacteriological proof is therefore uncommon). Of the 73 cases of childhood TB disease reported in a study conducted in Île-de-France in 1997 1 , 10 (14%) had a positive microscopic examination, and 25 (34%) had a positive culture. The child is therefore far less often infectious than the adult.
The risk of progressing from TB infection to TB disease increases with the younger age of the child: 43% before the age of 1 year old, 24% between 1 and 5 years old, and 16% in adolescents between 14 and 15 years old. The risk of developing a severe form (disseminated form, military disease, or meningitis) is particularly high in infants. TB infection is only indicated by positive tuberculin reactions. Depending on the series, between 20 to 60% of cases of TB disease in children are asymptomatic. When they are symptomatic, the signs are non-specific. It is therefore essential to consider tuberculosis in the presence of any situation at risk (families of migrants from high prevalence countries, adverse social environments, families with health care access difficulties, families with people who have HIV infection) and in the presence of any clinical situation, particularly respiratory, that does not respond normally when treated correctly. It is essential to search for an infectious person among the close contacts and to test this person for the clinical signs that are indicative of the disease; this is a very important factor for diagnosing childhood tuberculosis and managing all children within the close contacts. It is estimated that, on average, a person with positive sputum on direct microscopic examination infects 10 people in 1 year.
Tuberculosis located in the thorax, mostly pulmonary tuberculoses (approximately 70% of cases), is the most common form in adults in France. Of the 30% of so-called extra-pulmonary tuberculoses, approximately 10% are associated with pulmonary involvement. Active tuberculosis (positive direct microscopic examination) is responsible for dissemination of the infection and of the disease. The survey conducted in Île-de-France in 1997 in children reported the site of the disease to be pulmonary in 49% of cases, isolated extra-pulmonary in 37% of cases, and both pulmonary and extra-pulmonary in 14% of cases. In the localised forms, lymph node, osteo-articular, and urogenital tuberculoses are the most common. Neuromeningeal tuberculosis included tuberculous meningitis, with some 10 cases declared in France between 1998 and 2000 in children under 15 years old—more than half of which were in children under 5 years old—and cerebral tuberculoma. Miliary tuberculosis represents haematogenous spread of the tuberculous bacillus responsible for diffuse disease.
Lymphadenitis is the most common expression of non-tuberculous mycobacteria in children
Of the 50 or so species of mycobacteria that have been described, around a dozen appear to be responsible for human infections. Apart from the mycobacteria with clearly established pathogenic potential in human beings, M. leprae, M. ulcerans and species relating to the M. tuberculosis complex (M. tuberculosis, M. bovis, M. africanum), atypical mycobacteria exist (currently grouped under the term non-tuberculous mycobacteria (NTM)) that are very widely present in our environment and are responsible for localised or disseminated diseases.
The NTM contained in water or dust infects human beings through the skin, lung, or gastrointestinal tract, usually from spray. The mycobacterium avium-intracellulare complex contains two genetically different species, M. avium and M. intracellulare. The number of mycobacterioses (the term used for diseases produced by atypical mycobacteria), mostly due to M. avium, was increasing until 1996. Patients infected with HIV contributed greatly to this increase. Disseminated mycobacterioses due to M. avium-intracellulare have become frequent in people suffering from AIDS. The major risk factor is the extent of the immunosuppression. The general incidence of M. avium-intracellulare mycobacterioses was 11% in a series of 196 HIV-infected children, rising to 24% in those who had a CD4+ lymphocyte count of less than 100/mm3. However, since the use of the major anti-retroviral agents, immunosuppression is better controlled, and the number of mycobacterioses is falling markedly.
The NTM are responsible for various types of diseases. Chronic pulmonary diseases are due, above all, to the M. avium-intracellulare complex. This disease is extremely rare in children, except in those with cystic fibrosis or AIDS. Between the ages of 1 and 5 years of age, the most common localisation of NTM infections is lymphadenitis. These are superficial lymphadenopathies, usually cervical, sub-maxillary, or pretragial, or even axillary. Approximately 60% of cases proven by culture are due to M. avium. 90% of cervical lymphadenopathies in children under 12 years of age are caused by mycobacteria and are due to atypical mycobacteria and 10% due to M. tuberculosis. The ratio reverses in children over 12 years of age and in adults.
Skin disease occurs classically at the site of inoculation of the mycobacterium in the absence of immunosuppression. The most common forms are due to M. marinum (swimming pool granuloma and aquarium disease). Multiple skin lesions may be present in immunosuppressed patients.
The proximity of contact with an adult with tuberculosis (sputum-positive) is the key factor in the risk of M. tuberculosis infection
Several factors can influence the risk of exposure to M. tuberculosis but also the risk of becoming infected in an exposed person and the risk of developing disease from an infection.
The risk of exposure depends on the prevalence of active pulmonary tuberculosis in the population considered. The level of infectiousness increases with the number of bacteria present in sputum and therefore when the infector is sputum positive, i.e., his/her sputum contains acid alcohol fast bacilli (AAFB) on microscopic examination. Not being a carrier positive on microscopic examination of the bacillus in an adult does not exclude the adult from being infectious, although the likelihood, however, is far lower. The risk of infection is also influenced by several factors, the most important of which is close contact (population density and proximity of contacts).
Several factors determine the risk of being infected. Firstly, risk depends on the infectiousness of the source person, which depends on the frequency of cough and density of bacilli in sputum. It is possible that the level of intrinsic pathogenicity of the strain also plays an important role. The level of exposure that is determined by the proximity of contact between the exposed person and the person with tuberculosis is also an important factor in the risk of infection.
There are several risk factors for developing TB disease once infected (age, recent nature of the infection, gender, etc.), although the most important at present is the existence of immune deficiency and in particular immune deficiency secondary to HIV infection. Tuberculosis in adults usually occurs as a result of recent (re) infection, when it occurs in a population at high risk of transmission, whereas it usually occurs as a result of reactivation in populations at low risk of transmission.
Genetic susceptibility factors to tuberculosis exist
Expression of the disease occurs as a result of the host-pathogen relationship and on environmental factors. The great majority of infected people do not develop the disease. In the tragic episode of Lübeck in 1930, in which more than 250 babies were infected with virulent M. tuberculosis injected instead of BCG, some 180 children survived. This suggests that genetic susceptibility or resistance factors exist. In addition, a particularly high incidence of tuberculosis was seen in epidemics in the American Indian populations which did not have a long history of exposure to the bacillus, suggesting that genetic selection of people resistant to tuberculosis had already occurred in other populations. Finally, twin studies have demonstrated a higher level of concordance for the disease in monozygotic than in dizygotic twins.
Identifications of the genes predisposing to a multifactorial disease such as tuberculosis in human beings are based on the use of genetic linkage analysis and association studies.
Linkage analyses are used to locate a chromosomal region containing one or more genes of interest. The following stages involve testing the role of polymorphism of candidate genes located in the areas localised, directly, using association studies. The general principle of association studies is to compare the frequency of polymorphisms between sick and healthy subjects (infected and non-infected). The role of a polymorphism can only be validated in any situation by functional studies, highlighting the essential complementarity of genetic epidemiology and molecular genetic studies.
Not all of the resistance mechanisms to tuberculosis are known with certainty. All the mechanisms associated with innate and acquired immunity may be involved at different stages of the infection and of the disease; this therefore involves antigen presentation by the class II major histocompatibility complex, activation of macrophages, cytokine secretion by T lymphocytes, and granuloma formation.
1,25-Dihydroxyvitamin D3 (1,25 (OH)2 D3) is an immunomodulating hormone. It has long been suspected to have a regulating effect on immunity toward tuberculosis. There are epidemiological links between tuberculosis and vitamin D deficiency. It has been shown that the prevalences of vitamin D deficiency and of tuberculosis are high in migrants from Asia (Gujarat) to the United Kingdom. Vitamin D acts by binding to a receptor expressed on monocytes and on activated B and T lymphocytes. Polymorphisms of the gene for this receptor (tt genotype in homozygotes) have been positively correlated with serum 25 (OH) D3 concentrations and with bone mineral density. From case control studies, the tt genotype was over-represented in tuberculous patients from Gambia and in Gujarat suffering from tuberculosis in London.
Association studies between tuberculosis resistance and polymorphism of the "natural resistance-associated macrophage protein 1" gene or NRAMP1 appears to indicate that this is a susceptibility gene to tuberculosis in human beings, although its contribution to overall genetic susceptibility to tuberculosis in human beings is low.
An association has been reported between tuberculosis and polymorphisms of the interleukin 1β (1L-1β) gene and its receptor antagonist (1L-1Ra) gene in Gujarat-origin patients living in England, although the alleles predisposing to tuberculosis had only modest effects.
Several recent studies have identified an association between tuberculosis and a polymorphism of the interferon γ gene. Conversely, data disagree for the tumour necrosis factor α gene and the interleukin 10 gene.
Finally, susceptibility loci have been identified on chromosomes 15q and Xq in families from Gambia and from South Africa.
All these findings indicate that genetic susceptibility to tuberculosis does exist and that this is polygenic. Ongoing studies should lead to improved understanding of this effect.
New tools are adding to our understanding of the pathogenic activity of M. tuberculosis and of the protective action of the vaccine
Development of new genetic tools and the unravelling of the M. tuberculosis H37Rv genomic sequence have considerably advanced our knowledge of this bacterium both in terms of its genetic organisation, on the pathogenic molecular mechanisms used by M. tuberculosis to infect the host, and on the protective effect of the BCG vaccine.
In contrast to other pathogenic bacteria, M. tuberculosis does not have a classical toxin type of virulence factor. Several approaches have been used to determine the molecular bases of its pathogenic activity. Molecular genetics using the STM method (signature-tagged transposon mutagenesis) have been used to conduct direct research on strains attenuated for pathogenicity in the mouse.
Genomics, i.e., the systematic identification of all the genes of a cell by DNA sequencing by computerised analysis, has led to the finding that the M. tuberculosis chromosome is circular and that it contains 4,411,529 base pairs coding for approximately 4,000 genes. The complete sequence of the genome has revealed that a very large number of genes are dedicated to the synthesis, modification, and degradation of lipids and has also led to the identification of new genes coding for proteins belonging to a new family (PE and PPE proteins, characterised respectively by proline-glutamic acid and proline-proline-glutamic acid groups in the amino-terminal part), which are present in very large numbers in M. tuberculosis.
Comparative studies have revealed interesting polymorphisms. Several regions of difference (RD1-RD14), coding for approximately 140 proteins, are absent in M. bovis BCG; the vaccine strain compared to the virulent strain, M. tuberculosis H37Rv. RD1, is the only region absent in attenuated strains of M. bovis BCG and M. microti (harmless to human beings, like BCG), although this is present in all other members of the M. tuberculosis complex. When the RD1 region is re-introduced by complementation into BCG and into M. microti, the pathogenicity of these two strains is partially restored. Conversely, reintroduction of five other regions of difference (RD3, RD4, RD5, RD7, RD9), which are suspected to be involved in pathogenicity does not appear to affect the pathogenic potential of these two strains. Because the RD1 region also contains the gene coding for the protein antigen ESAT-6, which is strongly recognised by human T lymphocytes, it is now clear that all strains of vaccines used on a large scale in the history of vaccination against tuberculosis did not contain this important antigen. From this point on, proteins belonging to the RD1 region have been considered to be potentially very useful targets for prevention (protective antigen diagnosis), replacing tuberculin and therapy (the drug target).
In addition, studies testing for the presence or absence of RD regions in a larger number of strains from the M. tuberculosis complex have helped to define the phylogenetic relationships between the different members of the complex and allowed a new plan of the evolution of the tuberculosis bacilli to be proposed, casting doubt on the often-stated hypothesis according to which M. bovis is believed to be the ancestor of M. tuberculosis. The sequencing of M. tuberculosis H37Rv supports this doubt. All this information is therefore available to identify new potential targets for anti-tuberculous drugs.
A comparison of the pathogenicity of different strains of M. tuberculosis has shown that strain HN878, belonging to the "Beijing" family of tuberculosis, was highly pathogenic. Based on epidemiological observations it has been suggested, although the experimental proof has not been obtained to date, that the protective effect of BCG against strains from the Beijing family appears to be less than against other strains of M. tuberculosis.
Current strains of BCG are all derived from the Calmette and Guérin attenuated strain of M. bovis obtained at the beginning of the 20th century. Following distribution of this strain to different laboratories across the world, subsequent passages on culture medium performed in these laboratories (before to the introduction of lyophilisation of strains) led to additional genetic modifications such as deletions, point mutations, or duplications. An analysis of the genetic changes combined with historical data on the distribution of strains of BCG now allows the strains of BCG to be distinguished precisely between each other and molecular quality control to be established for the vaccine production. This could also help to determine whether certain strains of BCG have greater protective power.
The epidemiological analysis of cases of tuberculosis has long been based on description of cases, evaluation of their level of infectiousness, and screening for infection in close contacts of the cases, particularly using tuberculin tests. The poor discriminatory power of the different phenotypic markers (lysotype and resistance profile to anti-tuberculosis drugs) led to the development of several genetic techniques, allowing strains of M. tuberculosis to be compared on the basis of their genome to establish their similarity. The aim of this is to add to the evaluation of tuberculosis transmission in institutions (hospitals, prisons, places for people of no fixed abode, etc.) and in the community, as a complement to traditional epidemiological methods (contact tracing), which remain essential.
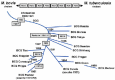
Figure
Genealogical trees of the different substrains of BCG based on the date of their distribution and molecular characteristics (from Behr and Small, 1999; Oettinger et al., 1999) .
Restriction fragment length polymorphism (RFLP) analysis is the reference method. This involves determining the number and size of restriction fragments obtained from chromosomal DNA that carry the insertion sequences IS6110, which are specific to the M. tuberculosis complex. The numbers and locations of the IS6110 copies vary, depending on the strains. These have many applications: laboratory study of cross-infection, studying strains of tuberculous bacilli isolated in succession from the same patient suffering several episodes of tuberculosis, studying tuberculosis transmission in closed communities, and studying tuberculosis transmission in the general population. If the fingerprints are different, this indicates that different strains are involved and excludes an epidemiological link between the cases. If the fingerprints are identical, this indicates that the strains are not different (but not necessarily identical), and neither excludes nor proves an epidemiological relationship between the cases. Other methods (spoligotyping, MIRU-VNTR, RD-analysis) are also used for the molecular characterisation of clinical isolates of M. tuberculosis.
General genomics, comparative or functional genomics of mycobacteria, and the associated disciplines have provided a bulk of new information that undoubtedly will contribute to the development of new anti-tuberculous and preventative agents.
Nevertheless, despite these impressive advances, it will still be several years before the current vaccine can be replaced with new vaccines. Improvement in our understanding of the molecular bases of M. tuberculosis and of BCG is therefore essential for the quality control of BCG, identification and typing of strains of M. tuberculosis, and of BCG and to diagnose infections.
Microscopic examination of sputum and culturing remain the key methods to diagnose tuberculosis
Microscopic examination of a pathological substance is the initial stage and often the only available option in many developing countries to make the bacteriological diagnosis of tuberculosis. To identify mycobacteria, their acid alcohol fast property is used. The microscopic examination of sputum allows rapid detection of the most patients carrying the bacillus and therefore the most infectious to their close contacts, often in less than an hour. Because it may be used in the least well equipped countries, this is the examination recommended as a priority by the World Health Organisation (WHO) to diagnose infectious tuberculosis in symptomatic patients. This examination, however, is negative in one of two patients with pulmonary tuberculosis.
Culture of the pathological substance is far more sensitive (70 to 90%) than microscopic examination and allows the isolated mycobacterium to be identified and its antibiotic sensitivity to be assessed. Because of the nutrient requirements and slow growth of the tuberculosis bacilli, enriched culture media must be used. Growth may be detected in 1 to 2 weeks using liquid media and automated detection systems. The speed of growth is directly proportional to the number of bacteria present when cultured and is slower when the microscopic examination is negative. Isolation of the bacillus is required to make a certain diagnosis of the species of bacterium and to provide an antibiotic sensitivity pattern. Gene amplification tests are useful to rapidly identify mycobacteria seen on microscopic examination but do not allow reliable medical decisions to be taken in cases of negative microscopy, particularly in cases of meningitis.
The tuberculin test is not used to diagnose symptomatic tuberculosis. The test is frequently positive in healthy people from a population at risk because of the high prevalence of infections (50% or more). It must therefore be noted that it does not contribute to the diagnosis of active tuberculosis in these populations.
Because M. tuberculosis is the only species of bacterium responsible for meningitis that may release tuberculo-stearic acid (a long chain fatty acid that forms the wall of these bacteria), identifying this fatty acid in cerebrospinal fluid is highly indicative of tuberculosis. However, although this technique is highly sensitive, the equipment required for it to be performed is very expensive and does not yet allow its routine use.
Measurement of gamma interferon (IFN-γ) production after in vitro stimulation of blood lymphocytes does not allow people with TB disease and those with TB infection to be identified correctly nor to distinguish between vaccinated patients when the stimulation is performed with PPD tuberculin (QuantiFERON®-TB test).
Microscopic examination and culture of sputum remain the fundamental methods for diagnosing the disease in France. Microscopic examination allows the most infectious patients to be identified. There is an urgent need to develop new tools providing rapid high-performance diagnosis to combat epidemics, particularly in a context where effective treatment exists.
Intradermal tuberculin reaction can identify tuberculous infection
The tuberculin skin test (TST, intradermal reaction-IDR- to tuberculin) has considerable diagnostic value which no longer needs to be stressed in the context of TB latent infection (LTBI). It is sensitive and specific, although some variability associated with the test itself (intrinsic) and with the host tested (extrinsic) does exist.
There are many intrinsic variability factors for the tuberculin test, including those associated with its poor biological standardisation of the tuberculin produced, the conditions under which the test is performed, and the methods by which the tuberculin skin reaction is read. Quality of reading (only the swelling should be only measured) and its reproducibility depend firstly on the reading technique and secondly on the training of the reader. Quantitative measurement of the diameter or diameters of swelling must be demanded and not an assessment based on interpretation from the outset (values read to the closest thresholds: 5, 10, 15 mm). This latter approach has been demonstrated to be the greatest contributor to false-positive and false-negative results.
There are two orders of extrinsic variability factors for the tuberculin test: those associated with the environment of the infected patients and those specific to the patients tested. The former consists of previous sensitisation by NTM or after BCG vaccination. People then produce false-positive reactions, which are due to common antigens present in tuberculin. Host-related factors are represented by individual capacity to respond to the test, depending on underlying diseases, recent past history of infection and, where applicable, immunosuppressant drugs being used.
A major concern of the tuberculin test is that its interpretation depends on delayed reading with respect to injection: a not insignificant number of people tested are lost to follow up before the reading is performed. This must be 48 to 72 hours after the injection. In addition, delayed reading requires very significant resources, which hinders correct use of the test.
New tests based on in vitro immunological methods that help to differentiate responses due to LTBI from responses due to BCG vaccination are still being developed. Stimulation of blood lymphocytes with specific M. tuberculosis antigens (ESAT-6, CFP-10, etc.) may help to differentiate people with LTBI from those immunised with BCG.
Active screening involves the close contacts of a case of tuberculosis, those belonging to an at-risk group, or those at individual risk
According to the English concept of "case finding", the term screening means the identification of people suffering from TB disease and people with LTBI.
Two methods of screening are used to identify TB disease:
- passive identification involving an entire population. The diagnosis is made following a consultation with the general practitioner for symptoms, generally drawn out pulmonary symptoms associated with deterioration in general health. The diagnosis is confirmed with radiological and bacteriological examinations.
- active screening in which a clinical examination, chest X-ray, and (IDR) to tuberculin are performed in the case of regulatory measures for at-risk people and in the follow up investigation from a case of TB disease. If suspected, microbiological examinations are requested to confirm the diagnosis.
To identify LTBI, a single screening method is used: the IDR to the tuberculin test. In the context of national programmes to combat tuberculosis, this is the only test used to define populations at risk of developing TB disease and therefore to provide the indication for chemoprophylaxis. In cases of LTBI infection, the quantitative values for swelling diameters from the IDR must be interpreted following the recommendations of the working party from the Higher Public Hygiene Counsel for France. These values are difficult to interpret in people vaccinated with BCG, and in the medium term, use of more specific antigens should be considered.
Use of results of the intradermal reaction (IDR) in treatment decision-making in investigations following a case in a child under 15 years old* (from the Higher Public Hygiene Counsel for France 2003)
| IDR swelling | BCG <10 years previously | BCG >10 years previously | No BCG |
|---|---|---|---|
| <5 mm | No treatment | IDR negative | No treatment |
| No treatment | |||
| Between 5 and 9 mm | In favour of reaction due to BCG | IDR positive | In favour of LTBI treatment |
| No treatment | In favour of reaction due to BCG or LTBI | ||
| Specialist advice | |||
| Between 10 and 14 mm | In favour of reaction due to BCG or LTBI | IDR positive | In favour of LTBI treatment |
| Specialist advice | In favour of LTBI treatment | ||
| >15 mm | In favour of recent LTBI treatment | IDR positive | In favour of recent LTBI treatment |
| In favour of recent LTBI Treatment |
* This is treatment of TB infection after excluding TB disease.
Passive screening is not effective in identifying TB disease. This is due to the fact that the general practitioners involved have to think about the diagnosis, which poses problems in a country with a low endemic rate. Information for general practitioners should be increased to reduce delays in diagnosis and increase their involvement in declaration measures. Active screening is more effective if decision-making algorithms (construction of clinical-radiological scores for the indication to treat) are correctly integrated into the different components of management.
The populations at risk of developing TB disease are defined according to the incidence of tuberculosis disease following geographical (at-risk regions), occupational (at-risk establishments), or individual (at-risk people) susceptibility criteria. The screening methods generally involve those described for active screening.
Active screening involves three clearly identified situations: investigation following a new case of TB disease, populations at risk dependent on a regulatory system, and individual populations at risk.
Use of results of the intradermal reaction (IDR) in treatment decision-making for investigations following a case in a child under 15 years old or above* (from the Higher Public Hygiene Counsel for France 2003)
| IDR swelling | In the situation of an investigation following a case | Exposed occupation (initial and follow-up) |
|---|---|---|
| <5 mm | Monitor at 3 months | IDR negative |
| Old or recent LTBI unlikely | ||
| No treatment | ||
| Monitor according to the risk of the occupational sector** | ||
| Between 5 and 9 mm | Monitor at 3 months | IDR positive |
| Reaction due to BCG or LTBI but in favour of recent LTBI | ||
| No treatment | ||
| Monitor according to the risk of the occupational sector** | ||
| Between 10 and 14 mm | Monitor at 3 months | IDR positive |
| Probable LTBI | ||
| The context will help to define the length of history. | ||
| If context favours recent LTBI | ||
| Treat if not | ||
| Monitor according to the risk of the occupational sector** | ||
| >15 mm | IDR positive | |
| Probable recent LTBI | ||
| treatment |
* This is treatment of TB infection after excluding TB disease; ** Opinion of the CSHPF (Higher Public Hygiene Counsel for France) dated 15/11/2002. For immunosuppressed people in whom the IDR may be falsely negative, the decision is taken according to the type, degree, and duration of the immunosuppression.
Screening of case contacts forms part of the anti-tuberculosis service actions under the responsibility of the general councils. The methods whereby this screening is applied have been fully described. The active screening must be a high priority, as an essential complement to measures to combat TB disease. Active screening may not be completely effective for several reasons: patient (index case) refusing to identify his/her contacts, contacts difficult to trace, delay in starting the investigation, delayed reading of the IDR. A one-stage test equivalent to the IDR could help to partly resolve these defects.
One problem inherent to the test itself is that it does not in any way differentiate people at risk of developing active tuberculosis from those not at risk. The assessment of people belonging to the former group should perhaps be the subject of a consensus opinion widely disseminated to the professionals who perform the screening. People detected as positive to IDR in groups at risk could be considered to be one of the indicating factors for supervised treatment. The development and validation of predictive immunological tests for uncontrolled bacterial replication (quantitative measurement of circulating specific B lymphocytes) could perhaps resolve this problem.
However, in terms of the application of this approach (investigation following a case) several conditions must be satisfied, including effective co-ordination of the different parties involved, for the investigation to be effectively conducted as quickly as possible after the report of any new case of tuberculosis, together with compulsory recording of the methods of the investigation and its results (positive and negative) with official notification of detected LTBI.
In the active regulatory-driven screening of populations at risk, the populations concerned identified by the competent authorities are subject to obligatory screening. These are foreigners permitted to stay and work in France, persons imprisoned for the first time, populations exposed to occupational risk of tuberculosis, and students of foreign origin. French children coming from foreign families who have stayed (for several weeks) in the country of their family where the endemic rate of tuberculosis is high, or who have received members of their family from a country with a high endemic rate, should undergo appropriate monitoring through school and university medical services.
For the active screening recommended for at-risk people, one new recommendation may affect certain specific at risk people who do not fall into the above groups. These are migrants of undetermined status with respect to their stay in France, people in situations of social disadvantage, and patients with predisposing diseases. For these people, centres (for example those managing drug abusers, alcohol-dependent people and people with mental disorders) must be trained to conduct this screening and to refer people with recent LTBI to a doctor who will manage their treatment.
Several conditions must be established to apply these last two screening methods. Firstly the definition of the groups at risk must be clear and accepted, particularly when the screening is recommended and non-regulatory driven. Secondly, appropriate education, supervision and effective follow up of chemoprophylaxis must be provided, with records of annual results and the consequences of the treatments. Finally, it is also important that the residential centres or homes for migrants are associated with the screening of the populations they house. Nevertheless, the methods and agreed relationships between these places and the managing organisations must be established.
Common conditions must be respected for all the three methods of screening for asymptomatic LTBI:
- if the screening is positive, this must be followed by management to eliminate the tuberculosis in the person who has a positive IDR
- positive screening must be linked to prescription of prophylactic chemotherapy, and it must be ensured that this is effective; this requires supervision and effective follow up of the chemoprophylaxis, with records of annual results and the consequences of the treatment
- it must be ensured that the management of people screened is entirely free of charge
Removal of the obligation for revaccination and the possible removal of generalised primary BCG vaccination may have the indirect benefit (but not until a period of 12 to 20 years after the event) of removing the constraints of quantitative interpretation of the results of the TST in the population born in France. However, the problem will continue to arise for high-risk populations coming from countries with high endemic rates who continue to be vaccinated with BCG. This must be considered practically and not theoretically, because the proportion of at-risk patients from these populations to be screened is significant. It would then be worthwhile to envisage and conduct clinical trials to validate alternative immunological methods to the current TST and to propose that this be applied after describing their true indications, limits, and advantages, depending on the situation encountered.
Compliance with treatment is a major factor in strategies to control tuberculosis
The standardised treatment of tuberculosis involves an intensive 2-month phase with rifampicin (R), isoniazide (H), pyrazinamide (Z), and ethambutol (E) and a 4-month continuation phase with an association of rifampicin and isoniazide. This treatment regimen, which lasts for 6 months, is recommended on an international level and applies to any form of tuberculosis (pulmonary or extra-pulmonary). The same standardised regimen is recommended in children over 5 years old, taking the same precautions as for adults. In younger children who may not be able to express themselves, care must be taken to identify visual disturbance due to ethambutol, although this phenomenon appears to be rare.
If the prescribed regimen conforms to these recommendations and the bacillus is sensitive to the antibiotics prescribed, recovery of the tuberculous patient will depend mostly on compliance with treatment, i.e., the inclination of the patient to follow his/her treatment and the organisation of health care. Some populations at risk of poor compliance are easy to identify: people of no fixed abode, the alcohol-dependent, drug addicts, people with mental disorders, etc. For people who do not belong to these groups at risk it is impossible to predict whether the person will follow the treatment regularly. The quality of receiving services and the relationship between the patient and the care team and taking the social, occupational, family and cultural context into account are key factors for good compliance with treatment. To improve compliance, directly observed treatment (DOT) is the technique recommended by the international bodies. This involves a trained supervised person watching the patient taking his/her drugs throughout the treatment. DOT is very rarely used in France and only in certain very specific cases (experience of social Samu, outpatient care, in people of no fixed abode).
People among the close contacts of patients suffering from active infectious tuberculosis are the most exposed to the risk of tuberculosis. When these people are infected, it is during the period immediately following the infection when they are at the greatest risk of developing TB disease. There is at present no consensus on the best treatment to be used in cases of tuberculosis infection. The proposed regimens include either isoniazide alone, rifampicin and isoniazide, rifampicin and pyrazinamide, or rifampicin alone.
The prevalence of anti-tuberculous resistance in new cases of tuberculosis (primary resistance) is low in France
Multi-drug resistance (MDR) is defined as resistance of M. tuberculosis to at least isoniazide and rifampicin, the two most potent anti-tuberculous drugs. It is said to be primary when it is seen in strains of M. tuberculosis isolated from patients who have never been treated, or who have been treated for less than 4 weeks. It is said to be acquired or secondary when the patients have received previous antibiotic treatment for 4 weeks or more. The finding of multi-drug resistance seriously compromises the patient's recovery because the second-line drugs are relatively ineffective, toxic, and expensive and have to be given for 18 to 24 months. It appears, however, that new fluoroquinolones offer true hope to enrich the range of therapies available and improve treatments of MDR forms of the disease.
Since 1992, annual surveys conducted by the National Reference Centre for resistance of mycobacteria to anti-tuberculous drugs have enabled the great majority of cases of culture-positive MDR tuberculosis to be identified at the French National and overseas laboratories. The mean incidence of MDR bacilli is approximately 50 cases per year (prevalence rate, 0.7%) and has increased slightly since 1997. Most of these cases are male (70%), born in foreign countries (56%), and already treated for long periods of time before the discovery of their MDR bacilli (66%). HIV co-infection is present in 21% of cases and is associated with primary MDR tuberculosis. Of these MDR cases, 16% have been the subject of repeated annual declarations suggesting that they remain sources of MDR infection for a long time. The fact that 16% of MDR cases have remained culture positive for several years indicates that specific actions must be taken to improve the treatment of these MDR patients.
The low number of these multi-resistant cases (approximately 50 per year) that has been relatively stable over time and the low rate of primary isoniazide resistance (<5%) are indirect indicators of compliance with treatments in France. However, only a regular evaluation of the outcome of patients started on treatment would provide a continuous direct indicator of the quality of patient management in accordance with European recommendations.
Annual prevalence of cases of tuberculosis due to multi-drug resistant bacilli (MDR) among culture-positive cases in France since 1992 (from Robert et al., 2003)
| Year of declaration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | |
| Cases of MDR tuberculosis | 48 | 40 | 58 | 40 | 29 | 26 | 39 | 49 | 51 | 48 | 79 |
| Total culture positive | 8,441 | 8,539 | 7,751 | 7,119 | 6,441 | 5,917 | 5,766 | 5,597 | 5,569 | 5,445 | 5,609 |
| Cases (%) | 0.6 | 0.5 | 0.7 | 0.6 | 0.5 | 0.4 | 0.7 | 0.9 | 0.9 | 0.9 | 1.4 |
On an international scale, the recrudescence in the number of cases of tuberculosis at the end of the 1980s in industrialised countries (particularly in the United States), improved evaluation of the situation in low-income countries, the development of methods to combat the disease, and the excellent cost-effectiveness ratio led the WHO to promote the DOTS strategy (directly observed treatment-short course) to control the disease. This strategy involves:
- involvement of government in supporting all the activities to combat tuberculosis
- detection of infectious cases by microscopic examination of sputum smears from symptomatic patients presenting to health services
- the use of standardised short-term chemotherapy (6 to 8 months) in at least all patients in whom sputum smears are positive on direct microscopy
- regular uninterrupted supply of all the essential anti-tuberculous drugs
- a standardised system for recording and notifying cases and the evaluation of treatment result for in each patient
Control of tuberculosis in industrialised countries depends greatly on the control in low income countries, because the total number of cases of tuberculosis in several western European countries (Denmark, Luxembourg, Holland, Norway, Sweden, Switzerland) is higher in people born in other countries than in their own. Systems have been put in place in different countries to rapidly diagnose, declare, and treat tuberculosis in immigrant populations.
The mean incidence of tuberculosis in mainland France is 10 times higher in people of foreign nationality
Between 1972 and 1988, the number of cases of tuberculosis notified in mainland France fell by 71% (from 31,167 to 9,191 cases). This change slowed to −2.5% per year between 1988 and 1991. At the start of the 1990s, a reversal of the trend was seen with an increase in the number of notified cases of 11% between 1991 and 1993. The incidence subsequently fell again by an average of 9% per year until 1997. Since then it has been stable, with approximately 11 cases per 100 000 inhabitants on mainland France.
In 2002, 6,322 cases of tuberculosis were notified in France (mainland France: 6,162 cases; overseas departments: 160 cases) i.e., an incidence of 10.5 cases per 100,000 inhabitants in mainland France. The incidence in the Île-de-France region (including Paris and its suburbs) was 4 times higher than the national mean outside of Île-de-France (27.1/105 versus 6.7/105). This rate has remained stable since 1997.
The incidence increases with age, rising to 19.7 per 100,000 people over 75 years of age and above in mainland France. The median age is 42 years of age, and 62% of cases are male. In 2002, 277 children under 15 years of age were affected by tuberculosis.
Nationality was completed in 2002 for 5,346 cases (84.6%), people of foreign nationality making up 40.6% of cases of notified tuberculosis (2,170/5,346), whereas this group makes up less than 6% of the total population.
The mean incidence in mainland France is 5.6 cases per 100,000 people of French nationality and 64.9 cases per 100,000 people of foreign nationality. People of foreign nationality between 25 and 39 years of age are the most affected, with an incidence of 111.3 cases per 100,000. This has increased very greatly compared to previous years. The incidence in young 15–24- and 25–39-year-olds of foreign nationality is 23 times higher than the rate seen in people of French nationality of the same age (88.6/105 versus 3.8/105 and 111.3/105 versus 4.7/105, respectively). The mean annual rate of change between 1997 and 2002 is −6% in people of French nationality and +8% in people of foreign nationality. This increases to +19% in people of foreign nationality between 15 and 24 years of age.
The incidence of notified cases of tuberculosis in those under 15 years of age is 1.6 cases per 100,000 in 2002 in children of French nationality and 13.6 per 100,000 in children of foreign nationality, with an incidence of 2.7 between 0 and 4 years of age and 1.2 between 5 and 14 years of age in children of French nationality compared with an incidence of 20.4 between 0 and 4 years of age and 10.7 between 5 and 14 years of age in children of foreign nationality.
In 2002, isolated or associated pulmonary forms of the disease made up 72.2% of cases, and extra-pulmonary forms made up 26.7% (no information was available for 1.1% of cases). Between 1992 and 2002, 62 cases of tuberculous meningitis were declared in children under 15 years of age, i.e., an average of 6 cases per year (2 to 9 cases) or 0.03 to 0.1% of all cases of tuberculosis, all forms combined. Of the 62 cases, 36 (58%) were under 5 years of age and 26 (42%) were between 5 and 14 years of age. Of the 47 children who suffered from meningitis in whom vaccination status was known without knowing the vaccine technique used, 28 had been vaccinated.
The proportion of HIV-infected people among all cases of tuberculosis is 5.9% (5.8% in mainland France, 8.3% in Île-de-France, and 10% in the overseas departments). Those born in foreign countries are more frequently seropositive for HIV than those born in France (9.9% versus 3.3%). Of the 42% of cases in whom serological status was known, the proportion of HIV-infected people (French plus foreigners) is 14.1% in 2002 (12.6% in 1997).
The observed trends in declared cases are also reflected in mortality rates. The number of deaths from a primary cause of tuberculosis fell on average by 7% per year from 1971 (3,666 deaths) to 1992 (816 deaths). A +13% rise in the number of deaths was seen in 1993 compared to the previous year. This peak did not last, and the number of deaths has continued to fall after 1994. In 1999, 695 deaths due to tuberculosis as the principal cause were recorded (source: INSERM-CépiDc), i.e., 11.9 deaths per million people.
Incidence according to age and nationality, mainland France, 1997–2002 (from Che et al., 2004)
| French nationality | Foreign nationality | |||||||
|---|---|---|---|---|---|---|---|---|
| 1997 | 2002 | 1997 | 2002 | |||||
| Age (yrs.) | N | Incidence/105 | N | Incidence/105 | N | Incidence/105 | N | Incidence/105 |
| 0–14 | 175 | 1.7 | 161 | 1.6 | 51 | 6.8 | 59 | 13.6 |
| 15–24 | 290 | 3.6 | 274 | 3.8 | 186 | 36.5 | 319 | 88.6 |
| 25–39 | 857 | 7.2 | 560 | 4.7 | 667 | 69.6 | 973 | 111.3 |
| 40–59 | 1,157 | 9.4 | 809 | 5.7 | 444 | 46.5 | 511 | 48.7 |
| > 60 | 1,812 | 16.8 | 1,272 | 10.7 | 236 | 58.1 | 257 | 47.9 |
| Total | 4,291 | 8.1 | 3,076 | 5.6 | 1,584 | 44.2 | 2,119 | 64.9 |
Clinical forms according to age, whole of France, 2002 (from Che et al., 2004)
| Age (years) | Isolated pulmonary form | Isolated extra pulmonary form | Mixed form | Total (including unknown form) |
|---|---|---|---|---|
| 0–4 | 69 (56.6%) | 29 (23.8%) | 17 (13.9%) | 122 |
| 5–14 | 74 (47.7%) | 55 (35.5%) | 21 (13.5%) | 155 |
| 15–24 | 444 (59.4%) | 208 (27.8%) | 87 (11.6%) | 747 |
| 25–39 | 1,123 (58.9%) | 514 (27.0%) | 254 (13.3%) | 1,906 |
| 40–59 | 1,025 (63.4%) | 406 (25.1%) | 161 (10.0%) | 1,617 |
| > 60 | 1,091 (62.1%) | 467 (26.6%) | 186 (10.6%) | 1,756 |
| Total | 3,835 (60.1%) | 1,686 (26.7%) | 728 (11.5%) | 6,322 |
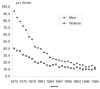
Figure
Change in mortality due to tuberculosis between 1972 and 1999 (from INSERM-CépiDc) .
Number of deaths by age in 1999 due to tuberculosis, all forms combined (from INSERM-CépiDc)
| <1 | 1–4 | 5–14 | 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–84 | 85–84 | 85–94 | ≥95 | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 0 | 0 | 0 | 0 | 2 | 18 | 30 | 27 | 71 | 118 | 94 | 6 | 366 |
| F | 0 | 0 | 1 | 2 | 2 | 3 | 6 | 9 | 42 | 127 | 125 | 12 | 329 |
| T | 0 | 0 | 1 | 2 | 4 | 21 | 36 | 36 | 113 | 245 | 219 | 18 | 695 |
M: Male F: Female T: Total
BCG vaccinations protect against meningitis and miliary disease in infants and young children
In the years 1925 to 1926, one of three infants died from tuberculosis (meningitis, severe tuberculosis, miliary disease) if one of the patients had tuberculosis. It was noted that the mortality rate was far lower in infants who had been vaccinated, although no statistical study was performed at this period because doctors and researchers did not dare to conduct trials with an unvaccinated control group.
One of the first controlled trials was the trial conducted between 1936 and 1939 in 8 Indian tribes in which tuberculosis was common (90% of the under 20-year-olds had a positive tuberculin reaction). The young people who had a negative reaction to tuberculin either were or were not vaccinated with BCG: 1,551 received the vaccine and 1,457 received a placebo. Systematic medical examinations were performed until 1944 and then every 2 years thereafter until 1956. By 1944, 4.1% of the vaccinated people had developed tuberculosis compared to 16.4% of the controls, including 10 miliary cases. By 1956, there had been 13 deaths due to tuberculosis in the vaccinated group and 68 in the control group. After a review of the tuberculosis status of all the participants in 1992, it was demonstrated that the vaccinated people were better protected against the disease than the non-vaccinated. The protective effect of BCG vaccination should therefore last for more than 20 years.
Another controlled trial was conducted in England in 1950 on 14–15½-year-old adolescents who had a negative tuberculin reaction. At that time, tuberculosis was common in this age group among the unvaccinated, 40.3% having a positive tuberculin reaction. The adolescents were followed up medically every 14 months for 10 years and every 3 to 5 years thereafter (total follow-up period, 20 years). 213 cases of tuberculosis (including one death and 5 miliary cases) were recorded at 10 years in the 12,867 control subjects and 48 cases in the 13,598 vaccinated subjects.
In France, a trial started in 1948 compared the frequency of tuberculosis in a population of 4- to 16-year-old children who had been offered BCG vaccination compared to a non-vaccinated control group made up of the children who had not been vaccinated because they were absent on the day of vaccination or because of parental refusal. Results at 20 years demonstrated 54% protection against pulmonary forms and 85% against other forms of tuberculosis, and in particular there were no cases of meningitis among the 15,618 vaccinated subjects compared to 3 among the 3,169 unvaccinated subjects.
Two meta-analyses have reviewed the major results published on infants or children and demonstrated that protection provided by BCG is slightly greater in infants than in children, in the region of 80% for severe forms (miliary and meningitis) and 55% for pulmonary forms.
Alongside these trials assessing the effect of vaccination in the same population, to demonstrate the efficacy of the vaccine we must also consider trials examining stopping vaccination in one or more regions or over time in the same country.
One study compared the frequency of meningitis between the Federal Republic of Germany (FRG) and the German Democratic Republic (GDR) between 1971 and 1978. The FRG stopped BCG vaccination in June 1975, whereas this was continued in the GDR. During the period in question, there were 57 cases of tuberculous meningitis per 2.1 million neonates in the FRG and no cases of meningitis in the GDR per 0.8 million neonates.
Generalised BCG vaccination was stopped in Sweden in 1975. The cumulative incidence of tuberculosis in children between 0 and 4 years of age born between 1969 and 1974 was 0.8 per 100,000. This increased to 8.1 per 100,000 in children born between 1975 and the end of 1980, the period during which vaccination recommendations for at-risk children were not followed. Of children born between 1984 and 1989, improvement in vaccine coverage in at-risk children (born abroad or with foreign parents) reduced the cumulative incidence between 0 and 4 years of age to 2.7 per 100,000 without, however, reducing this to the level of the cohorts born before 1975.
These studies show that BCG is an effective vaccine, and that stopping vaccination results in an increase in cases of tuberculosis.
An efficacy gradient exists depending on the geographical location of the trials with respect to the equator: the vaccine is more effective in the north of North America and in Northern Europe than in tropical regions. Attempts have been made to offer explanations for this difference. The design of the trials, strain, and dose of vaccine used have not been found to be contributing factors to variability. Similarly, the route of administration of the BCG (intradermal or multipuncture), the age of children and start year of the trial has very little or no impact on the observed variability of the vaccine efficacy. Conversely, the frequency of prevaccination sensitisation to tuberculin may explain the differences in vaccine efficacy. There are differences in exposure to environmental non-tuberculous mycobacteria (NTM) between people living in hot and cold climates. Pre-existing immunity to these NTM may interfere with the viability of BCG in the host, thereby reducing the immune response to the vaccine.
The vaccine also appears to be effective against environmental mycobacteria, because observations from Northern Europe show that since BCG vaccination was stopped, there have been more cases of mycobacterioses, particularly lymph node, in children. The increase in cervical lymphadenitis in particular (generally benign) represents a high financial and human cost, up to the point where the diagnosis is established.
The efficacy studies conclude that vaccinating infants with BCG does not prevent M. tuberculosis infection and has no effect on transmission of tuberculosis. Conversely, it confers high protection against tuberculosis meningitis and disseminated tuberculosis in infants and young children. Greater protection rates (72 to 83%) are seen in laboratory-confirmed cases, providing the most accurate estimates of the efficacy of BCG. According to the findings of a study published in 2004, it would appear that the vaccine provides protection for a period of more than 20 years. However, most available studies were conducted over shorter periods, and it is accepted, therefore, that the vaccine protects for at least 10 to 15 years, with an efficacy that ranges from 50 to 60% against pulmonary forms of the disease.
Two methods of administration of the vaccine exist at present in France (intradermal and multipuncture); multipuncture is the most frequently used method
All of the vaccine-producing strains originated from the strain prepared between 1908 and 1921 by Calmette and Guérin. This is a live strain of M. bovis attenuated by 231 passages in culture medium. This strain has been distributed throughout different laboratories across the world. The culture support and maintenance conditions vary between the producing laboratories, and several differentiated strains emerged up to the years 1960–1965. From that time onward, lyophilisation techniques, allowing the live bacteria to be stored for very long periods of time, were developed, and protocols defined the production of vials of lyophilised bacteria forming the batch for secondary insemination, which itself came from a vial of the primary stock insemination. For a given producer the BCG currently produced is identical to that which was made over 50 years ago. According to the WHO, vaccines were produced in 2001 by 18 manufacturers, and 7 strains are currently used in this population. Depending on the strain used, the concentration ranges between 50,000 and 3,000,000 bacilli per dose for the intradermal vaccination.
The administration technique recommended by the WHO is the intradermal (ID) route with a reconstituted lyophilised vaccine. Vaccination by the intradermal route in France is performed with a lyophilised BCG vaccine (Pasteur intradermal BCG vaccine) that is presented in 10-dose bottles to be reconstituted with 1-ml of solvent. The injection is administered using a 1-ml syringe graduated in one-hundredths of a millilitre and a special needle. The volume to be injected is 0.1 ml in children over 1 year of age. The vaccinating dose contains 800,000 to 3,200,000 bacilli. A half dose (0.05 ml) is used in children under 1 year of age. Because this is a living vaccine, the injection must be administered without local anaesthesia. The recommended site is the postero-external part of the arm at the junction of the middle and upper third. It is technically problematic for inexperienced care workers to administer an intradermal injection of vaccine in children under 1 year of age and for this reason in pediatric practice, it is accepted in France that the BCG vaccine is administered by multipuncture in children under 3 years of age.
The multipuncture technique (Monovax device, liquid vaccine) consists of a plastic device with 9 spikes covered with a cap containing the liquid vaccine. The shelf life of the vaccine is relatively short, approximately 1 month. The concentration of bacilli is between 50 and 250 million per dose, and the number of organisms introduced into the body varies depending on age. It is recommended that the number of impacts is increased as a function of age. The amount introduced is imprecise, however, and the method cannot be considered to be quantitative.
A study of delayed hypersensitivity induced by the BCG vaccination performed by the multipuncture technique demonstrates that the percentage of positive tuberculin reactions measured by a 10-unit IDR at the age of 6 years of age in children vaccinated during the neonatal period is 75%, compared to 95% after intradermal vaccination. The post-vaccination tuberculin test positivity rate in children of school age (average, 11.8 years of age) in the United Kingdom was lower in those vaccinated by multipuncture (27.2% negative) than in those vaccinated by the intradermal route (6.8% negative). Indirect efficacy data, however, suggest that both administration routes of the vaccine offer equivalent efficacy to protect against the severe forms of tuberculosis.
The short-term future plans for manufacturers in France are to stop marketing the multipuncture vaccine and to replace the Pasteur-Mérieux intradermal BCG vaccine with the Danish Statens Serum Institute of Copenhagen (SSI) vaccine. The supply of appropriate injection equipment, reminding users to adapt the vaccine dose according to age, and above all training the vaccine administrators, are essential factors for good vaccine tolerability: the only French study published in the last 10 years on the adverse effects of BCG concerns incorrect use, overdose, or technical errors.
Adverse effects of vaccination are principally local reactions, and very rarely, systemic infections
Adverse effects are expected from a vaccine with live attenuated organisms administered to infants using a difficult technique, the intradermal route. Although rare, the adverse effects of BCG may be serious.
Minor reactions are above all local and regional. These may involve cold abscesses at the injection site. The most common side effect is lymphadenitis in the lymph chain draining the injection site. This occurs most frequently in very young children and may remain silent or progress toward chronic suppuration. Because neonates are at higher risk of lymphadenitis than older children, infants under 1 year of age must receive a reduced dose of the vaccine.
The published incidence of lymphadenitis ranges from 0.1 to more than 1 case per 100 vaccinations; this threshold appears to be the maximum acceptable for generalised vaccination programmes. Less than 1 case of suppurative lymphadenitis is seen of every 1,000 vaccinations.
BCG vaccination may be complicated by osteitis. A few cases of osteitis have been described, mostly in Scandinavian countries, and appear to be associated with the Gothenburg strain. These are very rare nowadays. Tuberculous meningitis due to BCG is also extremely rare.
Systemic infections due to the BCG vaccination (systemic BCGitis) have also been reported, some of these being fatal. One study of systemic infections concluded that the incidence was 2.19 per million vaccinated people. No exhaustive notification scheme for systemic or localised BCGitis exists to date. These complications are associated either with severe immune abnormalities (severe combined immunodeficiency syndrome (SCID) being the great majority) or to an immunodeficiency affecting the interleukin 12-interferon γ access, which is specific for genetic susceptibility to mycobacteria, or in 25% of cases with no current genetic explanation.
An analysis of French pharmacovigilance (safety monitoring) data recorded during the last 5 years confirms the safety-of-use profile of the two vaccines used in France (intradermal and multipuncture), i.e., a predominance of local post-vaccine effects most of which involve abscesses at the injection site (more than 60% of all local effects, reported after administration of these two vaccines). No case of disseminated BCGitis was reported during this analytical period, which tends to demonstrate the gaps in the declaration system and should indicate the need for this to be tightened.
Estimate of the incidence of adverse effects from BCG vaccination; retrospective study in Europe (from Lotte et al. 1988)
| Incidence per 1 million vaccinations | ||
|---|---|---|
| Complications | Age < 1 year | Age 1–20 years |
| Subcutaneous abscess at the injection site, regional lymphadenitis | 387 | 25 |
| Musculo-skeletal problem | 0.39–0.89 | 0.06 |
| Multiple lymphadenitis, non-fatal disseminated lesions | 0.31–0.39 | 0.36 |
| Fatal disseminated lesions | 0.19–1.56 | 0.06–0.72 |
A 4-year review of spontaneous notifications of adverse effects due to the Pasteur intradermal BCG vaccine reported that in almost one-third of cases, the effects were due to incorrect use (BCG administered instead of a tuberculin test) or to overdose. The intradermal injections must be administered by trained staff using a standardised lyophilised vaccine, and with appropriate injection material and individual doses depending on the age of the person being vaccinated.
Adverse effects reported after overdose in a 4-year period (from Benamar and Loupi, 2001)
| Overdose Factor (Number of times the recommended dose) | Adverse effects | Number of cases |
|---|---|---|
| 2 times | Axilliary lymphadenopathy | 1 |
| 5 times | Abscess at the injection site | 1 |
| Painful itching reaction | 1 | |
| 8 times | Local inflammation | 1 |
| 10 times | Abscess at the injection site | 4 |
| Local necrosis | 2 | |
| Local necrosis and lymphadenopathy | 21 | |
| Total | 11* |
*Of the 13 cases of overdose notified, 2 had no effect.
The major current concern is with HIV infection. A recent study confirmed that there were no serious adverse events in asymptomatic HIV-infected children vaccinated at birth. To avoid any risk of systemic BCG infection in these children, the WHO (Special programme to combat AIDS and extended vaccination programme 1987) recommends that neonates be vaccinated as early as possible after birth in countries where tuberculosis is an important public health problem, and that those who have clinical signs of AIDS not be vaccinated.
Research into new vaccines has been directed toward increasing the efficacy and inducing new immune responses
Thanks to the extensive mobilisation of many teams, research into new vaccines against tuberculosis has accelerated considerably during the last 10 years. The immune responses induced by BCG have been studied in animal models such as the mouse, guinea pig, or macaque monkey before clinical trials start in human beings.
It has long been known that the humoral response alone does not protect against tuberculosis. Conversely, cell responses play a major role. The Th1 cell response controlled by the MHC II (major histocompatibility complex class II) cell response is essential in the protection. Cytotoxic responses controlled by MHC I also play an important role. The other responses, known until now as the unconventional responses such as Tγδ cell responses and responses controlled by CD1 molecules induced and/or directed against mycobacterial antigens, exist after infection or vaccination with BCG. Their roles in protection against tuberculosis are currently being studied. 2
Antigens that induce a Th1 cell response have therefore been researched. Those that were recognised by tuberculous patients or contact subjects have been copied and then tested in animal models. New vaccines more effective than BCG in animal models are now available for clinical trials.
Subunit, protein or recombinant poxvirus vaccines may be used as a complement to BCG. Superior protection to BCG vaccination was found in pre-clinical studies when a protocol was used that included an initial vaccination with BCG followed by vaccination with one of these new vaccines. This type of protocol is important because BCG vaccination will be continued in regions that are endemic for tuberculosis.
Attenuated strains of M. tuberculosis or recombinant strains of BCG, which are more effective than BCG have also been obtained over the last 10 years. An evaluation of the safety of these new live vaccines is currently been conducted in several animal models, including models mimicking immunosuppression. These live vaccines, which are more effective in BCG in the pre-clinical trials conducted up until now, may be used if the subunit vaccines are not found to be promising at the end of the clinical trials.
Classical vaccination with BCG may be continued to avoid serious cases of tuberculosis in children, such as meningitis. The new vaccines will act as a complement to BCG to increase vaccine efficacy, and it would be possible to design stimulation protocols using recombinant proteins with a suitable adjuvant, or with recombinant viruses obtained from the vaccine virus. For populations who are not vaccinated with BCG, direct vaccination with recombinant virus or recombinant proteins could be envisaged.
In France, a vaccine policy has developed from the 1920s, although coverage rates remained low until the beginning of the 1970s
From the end of the 1920s, Calmette advanced the Pasteur doctrine of vaccinating neonates with BCG, based on two principles. The first principle was that prevention is better than treatment. In this period, it was not compulsory to declare tuberculosis, and the dispensary was the major instrument for screening neonates and caring for at-risk infants.
The second principle, which has been constantly upheld by the Académie de Médecine and the Tuberculosis Commission of the Permanent Social Hygiene Council, was based on the view that prophylactic separation of neonates, even when vaccinated with their mother or parents who had tuberculosis, is an essential factor in the vaccine strategy.
In 1940, despite a fall in mortality of approximately 45% since the end of the First World War, 254 male French people between 15 and 40 years of age compared to 100 Dutch and 181 female French people per 100 female Dutch people still died from tuberculosis. Infant mortality from tuberculosis also fell very considerable during the first half of the 20th century. This ranged from a fall of between 74 and 83% between 1901 and 1936 in Parisian children under 4 years of age. At the eve of the Second World War, tuberculous meningitis was responsible for no less than 60% of deaths from tuberculosis between the ages of 1 and 4 years of age.
The first, oral, BCG vaccination took place on 18th July 1921 at the Hôpital de la Charité Maternity Unit Crèche in Paris. From July 1924, practitioners and above all dispensaries began to vaccinate with the help of the Pasteur Institute. The children came in general from underprivileged social environments, and the number of vaccinations only increased slowly. In the middle of the 1930s, the proportion of children vaccinated had risen to one-third of all births.
The legal obligation, which was voted in after the war (1950), had little immediate effect. Until 1960, outside of private consulting rooms, little more than half of the generation of children were vaccinated each year with BCG. Ten years later, the number of immunised children had doubled.
Frequency of BCG vaccination in France (from Debré and Bernard, 1939)
| Year | Number of births | Number of infants vaccinated with BCG | BCG/Births (%) |
|---|---|---|---|
| 1925 | 770,060 | 4,628 | 0.56 |
| 1934 | 677,365 | 189,909 | 28.1 |
| 1935 | 640,527 | 210,668 | 32.9 |
| 1936 | 630,059 | 194,905 | 31.0 |
Although the legal obligation did not lead to rapid generalisation of vaccination, it did, nevertheless, lead parents to have this performed increasingly early, both in Paris and in the provinces. In 1976 in Paris the vaccination rate reached 97.6%, exceeding the coverage for smallpox (88% at 2 years of age) and for diphtheria-poliomyelitis (84%). However, possibly because of the overly frequent choice of the Monovax multipuncture inoculation method in preference to the intradermal injection, more than one of two children at the start of the 1980s still had a negative tuberculin reaction at the age of 6.
The factors responsible for the slow advance in vaccination include:
- a certain trend within the medical profession toward scepticism and not using BCG at least before the Second World War
- under-administration of the control system. In 1932, it was impossible to know precisely the subsequent outcome of vaccinated children. Most observations made by doctors and even by the public hygiene authorities were incomplete and isolated. This "light-handedness" of the health authorities with respect to mandatory verification of the skin reaction was further highlighted in 1982.
- the birth of anti-vaccinationism. After the vote on the law dated 5 January 1950 (compulsory BCG), Catholic doctors and family defence associations rose up against the "vaccine invasion". BCG was the subject of a formal question to the National Assembly in May 1951.
- intransigence of the legislative body. The law dated January 1950 and decree of application dated 9 July 1951 trampled over certain rules protecting individual liberties (free choice of doctor, freedom of medical prescription, paternal rights).
The principle of prophylactic separation of vaccinated neonates with their mothers or parents who had tuberculosis, a key factor in the vaccine strategy, provoked anger from the family associations and Catholic doctors. When questioned, the Conseil d'État (1953) maintained the legality of this. According to the High Courts, the separation was considered as an integral part of the vaccination methodology.
The fall in the incidence of tuberculosis can be explained by vaccination but also by the improvement in housing and living conditions and by the increase in the beds/deaths from tuberculosis ratio. Recent historical records place the emphasis on the socio-economic and institutional measures: increase in food consumption as a result of real increase in income, resulting from industrialisation and isolation of patients in the advanced stages of the disease. In France, however, the inflexion of the specific mortality curve compared to some neighbouring countries occurred late, explained by the many cumbersome administration processes of the medical care establishments and lack of tuberculosis beds, combined with the exacerbating slow spread of vaccination.
In conclusion, although a number of lives were saved by BCG thanks to pediatric activity, mandatory vaccination in France came in too late and coverage rates remained too low for too long for vaccination to have a real impact on the decline in incidence before the 1960s.
Vaccine policies differ from one country to another in industrialised countries
In low-income countries, BCG vaccination is part of the expanded programme on immunisation (EPI): its objective is to prevent serious forms of tuberculosis in children. In these countries, the WHO recommends a single dose of BCG per child as soon as possible after birth unless clinical signs of immunodeficiency are present. Adult vaccination is not recommended. Efforts to control tuberculosis are based on early case detection and treatment using directly observed treatment (DOT). Improvement in the vaccine against tuberculosis is considered to be a major leading priority on a worldwide scale, particularly with the hope that improved vaccine efficacy will help to break the chain of transmission of the disease.
In industrialised countries, vaccine policies differ enormously from one country to another. In the United Stated (incidence 6/105), BCG vaccination has never been recommended and the strategy to combat tuberculosis is based on early detection and treatment of infectious cases and on preventative therapy for infected people. In Japan (incidence 26/105) tuberculin tests after BCG and BCG revaccination have recently been abandoned; continuation of generalised vaccination of children is being discussed. In New Zealand (incidence 9/105), the generalised vaccination policy for adolescents has been replaced in recent years by a selective approach (vaccination of neonates in high-risk regions). However, to judge the differences in the epidemiological situation after changing the vaccine, there is a lack of historical data for Japan and no data for New Zealand.
Summary of BCG vaccine policies in the United States, Japan, and New Zealand
| Country | Current BCG vaccine policy | Recent changes made to the BCG vaccine policy | Incidence of tuberculosis |
|---|---|---|---|
| United States | BCG vaccination not recommended: to be considered only for very high risk groups | BCG to be considered for people in contact with patients who have had multiresistant tuberculosis in 1996 | 6 cases per 100,000 |
| Japan | Generalised vaccination in those under 4 years of age (recommended between 3 and 12 months) | Tuberculin tests and BCG revaccinations in schools stopped in 2002 | 26 cases per 100,000 |
| New Zealand | Vaccination of neonates in certain groups at risk since the 1970s. | Generalised vaccination of adolescents stopped in1990 | 9 cases per 100,000 |
| Vaccination of children and adults in certain specific conditions |
In Western Europe, the overall incidence of tuberculosis was 11 cases per 100,000 in 2001. Rates ranged between 5 and 13 cases per 100,000 population in most countries and were higher in Spain and in Portugal. In most of these countries, the incidence is falling in the native population, whereas it is stable or rising in populations originating from high-incidence countries. Several studies have shown that the incidence of tuberculosis remains high in migrants during the first years after their arrival into the host country.
BCG vaccination policies are very variable in Western Europe. In some countries, such as Germany and Austria, vaccination is not recommended. Around 10 countries (Belgium, Denmark, Spain, Italy, Sweden, etc.) recommend vaccination for groups at risk. A few countries still practice generalised vaccination.
Since the 1970s in Western Europe, generalised vaccination has gradually been abandoned in young children. This has occurred as a result of the epidemiological trends toward a lower incidence of tuberculosis, the frequency of side effects (partly associated with specific strains of BCG), the belief that BCG offers low efficacy, and for cost-benefit reasons.
BCG vaccine policies and incidence of tuberculosis in Western Europe
| Vaccine policy | Overall incidence/105 | Paediatric incidence/105 | |
|---|---|---|---|
| Austria | none | 13.3 | 5.0 |
| Germany | none | 9.2 | 2.4 |
| Belgium | <5 years targeted | 12.9 | 4.3 |
| Denmark | children targeted | 9.6 | 5.0 |
| Spain | children targeted * | 18.7 | 8.5 |
| Italy | children targeted | 7.8 | 1.9 |
| Norway | infants targeted: generalised at 12–14 years of age | 6.4 | 2.7 |
| Holland | <12 years targeted | 9 | 2.2 |
| United Kingdom | infants targeted: generalised at 12–14 years of age | 11.8 | 4.4 |
| Sweden | children targeted >6 months | 4.8 | 0.8 |
| Switzerland | children <12 months targeted | 8.5 | 1.4 |
| Finland | generalised in neonates | 9.5 | 0.7 |
| France | generalised before 6 years of age | 10.6 | 2.4 |
| Greece | generalised at 6 years of age | 5.8 | 2.9 |
| Ireland | generalised in neonates | 10.6 | 2.0 |
| Portugal | generalised in neonates | 43.8 | 7.0 |
*Generalised vaccination in the Basque country.
The policy of targeted vaccination in children at risk is motivated by the need to avoid serious forms of the disease in the most exposed people. The definition of groups of children at risk varies depending on the country. Vaccine coverage in the target populations has not always been evaluated and can vary from one country to another: 97–100% in Norway; more than 80% in Sweden; 42–77% in the United Kingdom (local studies).
The association between vaccine policy and notification data on pediatric tuberculosis must be interpreted with caution. The incidence of pediatric tuberculosis (2,250 cases in Europe in 2001) ranges less than 1 per 100,000 in Finland and Sweden up to 8.5 per 100,000 in Spain. This is always higher in children born in foreign countries. However, the notification data for pediatric cases are difficult to compare between countries because of large differences in diagnostic and reporting practices.
France is one of the few European countries to maintain generalised BCG vaccination under the age of 6 years of age. The regulation dating from 1950 and adapted in 1965 was modified in 1996 by decree 96–775 dated 5 September. The changes involved reducing the frequency of post-vaccine control tuberculin reactions and certain modifications relating to occupational risks. Decree 2004-635 dated 30 June 2004 removed revaccination of children, and the decree dated 13 July 2004 put an end to the practice of control tuberculin tests after vaccination.
Vaccine policy in France
| Vaccination from the first month of age for children at risk |
| Obligatory vaccination on entering the community or at 6 years of age at the latest due to compulsory school attendance at this age |
| Vaccination of specific occupational categories (health and social occupations) |
Studies on BCG vaccine coverage in France are based principally on children up to 6 years of age. Coverage at 3 and 9 months is estimated to be 38% and 55%, respectively. Since 1994, vaccine coverage has oscillated between 81 and 83% in 2-year-old children, and 95% of children have received BCG by the age of 6.
The number of cases of tuberculosis avoided in France through vaccination can be estimated
The effectiveness of a vaccination programme against an infectious disease transmitted between people is due partly to its direct action in protecting vaccinated subjects and secondly from its indirect action in reducing the risk of disease for unvaccinated people due to a reduction in the number of cases liable to infect them. BCG prevents above all, however, the non-infectious extrapulmonary cases of the disease, and its impact on the risk of infection is extremely low. Even assuming BCG to be effective in preventing pulmonary forms of tuberculosis, its impact on transmission of the disease would remain low, because these forms are exceptionally infectious in children. BCG therefore has principally a direct individual protective effect, and the number of cases of meningitis and miliary disease, and even of pulmonary tuberculosis avoided in vaccinated children, is the principle benefit of vaccination. The number of cases of tuberculosis avoided as a result of vaccination can be estimated from effectiveness and vaccine coverage data and from the observed incidence of tuberculosis.
In the most favourable vaccination scenario (assuming the effectiveness of BCG to last until the age of 15 years of age, and to be 85% on meningitis and miliary disease and 75% for other sites) the number of cases of tuberculosis avoided each year by vaccination would be 802, including 16 cases of meningitis/miliary disease and 786 other localisations of the disease in children between 0 and 14 years of age. Using average efficacy values for BCG (75% for meningitis and miliary disease and 50% for other localisations), this figure would be an annual number of 318 cases.
Estimate of the annual number of cases of tuberculosis avoided in children of less than 15 years of age by BCG vaccination – mainland France – Compulsory Declaration 1997–2002
| Age vaccine coverage | Sites of tuberculosis | Cases observed annual mean | Cases expected without vaccination | Cases avoided by vaccination |
|---|---|---|---|---|
| 0–4 years | Meningitis/miliary | 4.1 | 13 | 9 |
| 80% | Other forms | 177 | 441 | 264 |
| All forms | 454 | 273 | ||
| 5–14 years | Meningitis/miliary | 1.6 | 9 | 7 |
| 95% | Other forms | 210 | 732 | 522 |
| All forms | 741 | 529 | ||
| Total | Meningitis/miliary | 5.7 | 22 | 16 |
| 0–14 years | Other forms | 387 | 1,173 | 786 |
| All forms | 1,195 | 802 |
Calculations were performed assuming the efficacy of the vaccine against meningitis/miliary disease to be 85% and 75% against other sites (the most favourable vaccination scenario) and correcting for the incompletely exhaustive nature of tuberculosis case notifications.
It is likely that the epidemiological impact of BCG vaccination is overestimated, because some children who develop tuberculosis may have been infected before they arrived in France. Almost one-third of cases of tuberculosis notified through the compulsory declaration concern children born outside of France. Nevertheless, the delay between tuberculosis-infection and tuberculosis-disease is relatively short in children, and it may be considered that a large proportion of these children have probably been infected after they arrived in France.
In Europe, stopping generalised vaccination has led to an increase in the incidence of pediatric tuberculosis and mycobacterioses
Over the last decade, several European countries stopped generalised primary BCG vaccination on a national or regional scale. The epidemiological consequences of such a measure provide important information about the efficacy of vaccination. Published data on the impact of vaccinating neonates on the incidence of pediatric tuberculosis are available for the Czech Republic, Germany, Ireland, and Sweden. In the first three of these countries, comparisons between areas that did or did not practice BCG showed pediatric TB rates to be at least 4 times higher in regions without BCG.
Incidence of pediatric tuberculosis in three countries practising different BCG vaccination policies depending on the regions
| Regions without BCG | Regions with BCG | |||
|---|---|---|---|---|
| Country | N | Rate/105 | N | Rate/105 |
| Czech Republic | ||||
| Pediatric tuberculosis, children born and followed up between 1986 and 1992 | 31 | 7.1 | 24 | 1.2 |
| Germany* | ||||
| Pediatric meningeal tuberculosis between 1975–1980 (born between 1975 and 1978) | 57 | 2.7 | 0 | 0 |
| Ireland | ||||
| Pediatric tuberculosis in 1981–1989 (declarations) | 132 | 5.45 | 96 | 1.4 |
| Paediatric tuberculosis in 1991 (survey) | 38 | 13.4 | 23 | 3.4 |
*Regions without BCG = Federal Republic of Germany; Regions with BCG = Democratic Republic of Germany.
In Sweden, after the discontinuation of generalised vaccination in 1975, targeted vaccination initially had a poor coverage in the groups at risk, and there was a large increase in the incidence of tuberculosis in children born in Sweden to foreign parents (incidence multiplied by a factor of 15 between 1975 and 1980). The coverage had improved by 1984, and the incidence in these children has fallen. In the cohort of children born in 2000, the BCG coverage of children at risk was 87.1% by age 25–35 months.
Cumulative incidence of tuberculosis in children between 0 and 4 years of age born in Sweden between 1969 and 1989 (from Romanus, 1995)
| Total | Number born to Swedish parents | Number born to foreign parents | |||||
|---|---|---|---|---|---|---|---|
| Period of birth | BCG coverage (%) | N | Rate/105 | N | Rate/105 | N | Rate/105 |
| 1969–1974 | >95 | 7 | 1 | 5 | 0.8 | 2 | 2.6 |
| 1975–1980 | 2–4 | 45 | 8.1 | 19 | 3.9 | 26 | 39.5 |
| 1981–1983 | 6–16 | 15 | 5.4 | 10 | 4.1 | 5 | 15.5 |
| 1984–1989 | 13–14 | 17 | 2.7 | 7 | 1.3** | 10 | 14.5 |
* BCG coverage at the age of 2 years of age in the general population. ** Difference with 1969–1974 not statistically significant.
A very high incidence of atypical mycobacteriosis in unvaccinated children was seen compared to vaccinated children in Sweden and the Czech Republic. The incidence of lymph node mycobacteriosis due to M. avium complex increased from 0.02 to 2.1/105 (x100) in Sweden and 0 to 3.6/105 in the Czech Republic.
Number and incidence of extra-pulmonary infections due to atypical mycobacteria in children under 15 years of age before and after discontinuation of BCG vaccination in Sweden (from Romanus et al. 1995)
| Total | Number born to Swedish parents | Number born to foreign parents | |||||
|---|---|---|---|---|---|---|---|
| Period of birth | BCG coverage (%) | N | Rate/105 | N | Rate/105 | N | Rate/105 |
| 1969–1974 | >95 | 2 | 0.02 | 1 | 1 | ||
| 1975–1980 | 2–4 | 83 | 0.8 | 79 | 0.8 | 4 | 1.2 |
| 1981–1983 | 6–16 | 160 | 2.1 | 156 | 2.1 | 4 | 1.4 |
| 1984–1989 | 13–14 | 145 | 1.9 | 144 | 2 | 1 | 0.3 |
* BCG coverage at the age of 2 years old in the general population. ** Annual mean per 100,000 children under 15 years of age.
Evaluation of the advantages and disadvantages of different vaccination options should incorporate medico-economic data
An analysis of the advantages and disadvantages of BCG vaccination firstly requires that reliable estimates of many parameters, such as the efficacy of the vaccine, vaccine coverage, duration of protection conferred by vaccination, incidence of tuberculosis and of non-tuberculous mycobacterioses, frequency and severity of vaccine side effects, cost of the vaccine (including the cost of managing its adverse effects) and alternatively the cost of treating a case of tuberculosis or non-tuberculous mycobacteriosis are available. These data are in fact often imprecise and fragmented, and an evaluation of the advantages and disadvantages of different possible vaccine strategies will therefore be incomplete.
A cost-effectiveness analysis of generalised vaccination of infants compared to selective vaccination of "groups at risk" conducted in Finland, a country in which the epidemiological conditions are similar to those in France, demonstrated the importance attached to the definition of "groups at risk". If it is impossible to correctly identify populations in which the incidence is at least 30 or even 50 times higher than that of the rest of the population, a selective vaccination strategy risks not working (despite a reduction in the cost of vaccination due to avoided cases).
In Japan, a country in which the incidence of tuberculosis is higher than in France, generalised vaccination was compared to non-vaccination in a cost-benefit analysis in a hypothetical cohort of 1,207 million children born in 1996 and followed up for 10 years. This model demonstrated that, using an assumed vaccine efficacy of 80%, the number of people needing to be vaccinated to avoid one case was 2,125; this rose to 10,399 when the assumed vaccine efficacy was 40%. The cost per case avoided was US $ 35,950 for a vaccine efficacy of 80% and US $ 175,862 for a vaccine efficacy of 40%. If an analogous calculation is performed with lower incidence rates, the conclusions are less favourable still. For example if this Japanese model is used with the Finnish data, 2,830 infants would have to be vaccinated to avoid one case, at a cost of US $ 49,722 per case avoided.
Cost-effectiveness ratio (per case of tuberculosis avoided) of BCG vaccination of infants followed up to the age of 15 years in Finland (taken from data from Hersh et al., 2003)
| Number of cases/100,000 | Cost effectiveness ratio (US $) per case avoided | |||
|---|---|---|---|---|
| Expected | Avoided | Selective versus none | Generalised versus selective | |
| No vaccination | 42 | |||
| Selective vaccination: | ||||
| population at x 2 risk | 36 | 6 | 7,146 | 5,089 |
| population at x 5 risk | 30 | 12 | 2,525 | 7,104 |
| population at x 10 risk | 24 | 18 | 937 | 10,537 |
| population at x 20 risk | 19 | 23 | 164 | 17,153 |
| population at x 30 risk | 16 | 26 | benefits | 24,045 |
| population at x 50 risk | 13 | 29 | benefits | 38,311 |
| Generalised vaccination | 8 | 34 | ||
A cost-benefit analysis of relaxing the BCG vaccination policy in France demonstrated in 1996 that regardless of the envisaged strategy, the savings obtained by relaxing the vaccination strategy were greater than the resultant costs of treating the additional cases occurring following such a relaxation. These ranged from approximately 22 to 45 million Euros after 20 years, depending on the strategy.
Published data show that BCG vaccination is an intervention that offers similar cost-effectiveness and cost-benefit ratios to those of other very expensive treatments in terms of direct medical costs. Removal of tuberculin controls and revaccinations would already provide substantial savings without increasing the number of cases of tuberculosis. Targeted vaccination of groups at risk could be less expensive than generalised vaccination but would be less effective in terms of the number of cases avoided. In addition, a targeted strategy would require an operating definition of the groups at risk, which remains to be established.
The consequences of different vaccine options in France can be evaluated in epidemiological terms
A detailed analysis of compulsory declaration data reveals considerable heterogeneity in the epidemiology of tuberculosis in France according to two variables collected in the notification form: nationality and region of domicile of the case
Mean incidence rates of smear-positive cases (between 2000 and 2002) by nationality and by mainland France region (corrected on the basis of an exhaustivity rate of 80% identical for both populations)
| Nationality | |||
|---|---|---|---|
| Regions | French | Foreign | Total |
| Île-de-France | 7.9 | 57.2 | 13.5 |
| Provence-Alpes-Côte-d'Azur | 4.1 | 25.9 | 5.7 |
| Bretagne | 4.9 | 25.1 | 5.2 |
| Languedoc-Roussillon | 3.9 | 19.6 | 4.8 |
| Haute-Normandie | 3.6 | 34.2 | 4.5 |
| Alsace | 3.0 | 21.6 | 4.4 |
| Basse-Normandie | 3.8 | 20.6 | 4.1 |
| Centre | 3.1 | 23.4 | 3.9 |
| Corsica | 1.6 | 24.1 | 3.8 |
| Rhône-Alpes | 3.0 | 15.9 | 3.8 |
| Nord-Pas-de-Calais | 3.1 | 20.5 | 3.7 |
| Auvergne | 3.4 | 12.6 | 3.7 |
| Picardy | 3.0 | 20.3 | 3.6 |
| Franche-Comté | 2.5 | 20.8 | 3.4 |
| Champagne-Ardennes | 2.8 | 15.1 | 3.3 |
| Bourgogne | 3.0 | 9.4 | 3.2 |
| Pays de la Loire | 2.8 | 25.3 | 3.1 |
| Poitou-Charentes | 2.9 | 15.2 | 3.1 |
| Aquitaine | 2.6 | 12.0 | 3.0 |
| Lorraine | 2.5 | 8.1 | 2.8 |
| Midi-Pyrénées | 2.3 | 13.9 | 2.8 |
| Limousin | 2.2 | 14.5 | 2.5 |
| Mainland France | 4.1 | 33.0 | 5.7 |
* Per 100,000 people
Three regions (Île-de-France, Paca, and Bretagne) have higher incidence rates of smear-positive cases than the threshold proposed by IUATLD to consider stopping generalised BCG vaccination of children (5 cases per 100,000 inhabitants). In addition, in all regions of France the incidence in people of foreign nationality is far greater than that of people of French nationality, the ratios between the two incidence figures ranging between 3 and 15.
Criteria of the International Union Against Tuberculosis and Lung Diseases (IUATLD) to consider stopping generalised BCG vaccination in children
| The mean annual incidence over the last 3 years of cases of smear-positive pulmonary cases must be less than 5 cases per 100,000 inhabitants, or |
| The mean annual incidence over the last 5 years of cases of meningitis in children under 5 years old must be less than 1 case per 10 million inhabitants or |
| The annual risk of tuberculosis infection must be less than 0.1% |
The epidemiological consequences of reducing or stopping vaccination can be evaluated.
Taking into account the data from the incidence table of smear-positive cases could lead to a proposal to stop BCG vaccination, except in the three regions that do not satisfy the first IUATLD criterion. This strategy would leave children at risk of tuberculosis living outside of these three regions without vaccine protection. In addition, this would be difficult to apply in practice (people moving home and moving about) and to justify (particularly in the regions very close to the 5/105 threshold).
Another approach could be a targeted vaccination strategy on populations at risk defined on the basis of recent trends in changing epidemiology of tuberculosis in France. The overall incidence of tuberculosis continues to fall annually, whereas the incidence in populations at risk continues to grow. The incidence in subjects of foreign nationality is very much higher than the threshold proposed by IUATLD to consider stopping vaccination.
In view of the data available today, it may be estimated that the number of children to be vaccinated living in an environment at risk would be approximately 100,000 per generation. The three major risk factors identified are: being born in a country with high prevalence of tuberculosis, being born in the family coming from such a country, and the presence of a past history of tuberculosis in the family. The countries of high prevalence are African and Asian countries (except for Japan), Central and South America, and countries of the former USSR.
Using the hypothesis that vaccination coverage would be maintained in the populations at risk defined above at its current level (95% at 6 years of age), there would be approximately 200 additional cases occurring in the low-risk, unvaccinated population, of which approximately 4 would be severe forms (meningitis and miliary cases). Vaccination of fewer than 15% of children would therefore enable 75% of these cases currently avoided by generalised vaccination to be avoided. However, these estimates are made under the most favourable conditions for vaccination (vaccine efficacy 85% for meningitis and miliary cases and 75% for the other sites). Using mean efficacy values for BCG (of 75% on meningitis and miliary disease and 50% for the other sites), the figure would be 80 additional cases.
In addition, a fall in vaccine coverage in the target population could result in an increase in the number of cases in this population: 286 additional cases for a vaccine coverage of 50% of these children and 539 additional cases for a vaccine coverage of 10%.
Limiting vaccination to the populations at risk defined above would result in 308 new cases of atypical mycobacterial infections in children, or even more if the vaccine coverage (95% at 6 years of age) was not maintained.
Conversely, such a strategy targeted on populations at risk would result in a reduction in the number of adverse effects due to vaccination, because it would reduce the size of the vaccinated population. This would lead to 40 cases of lymphadenitis per year instead of some 300, currently estimated with generalised vaccination. It can also be estimated that vaccination of populations at risk defined above reduce the mean number of the most severe cases of BCGitis (associated with severe combined immunodeficiency) to one case per year.
Finally, the last approach of totally stopping vaccination could be proposed on the basis of current epidemiological data in France (mean incidence of positive-sputum cases, mean incidence of tuberculous meningitis in children) which are close to the threshold (proposed by IUATLD) to consider discontinuing vaccination.
Totally discontinuing vaccination would result in an increase in cases of tuberculosis, which could rise to up to 800 additional cases: 16 would be serious cases of meningitis or miliary tuberculosis in children between 0 and 14 years of age, 12 of whom would belong to the at-risk group. If the calculations are performed using mean vaccine efficacy figures (75% for meningitis and miliary cases and 50% for other sites), the number of additional cases of tuberculosis would be 318.
In addition, stopping vaccination would result in approximately 343 additional cases of atypical mycobacterial infections in France.
On the other hand, totally stopping vaccination would allow 12 disseminated BCGitis to be avoided each year, approximately 4 of which would be associated with severe combined immunodeficiencies. It would also eliminate slightly less than 300 cases of local or regional lymphadenitis per year associated with the injection.
Epidemiological impact of reducing and stopping vaccination compared to the current strategy of generalised vaccination
| Generalised vaccination | Vaccination of populations at risk | Stopping vaccination | |
|---|---|---|---|
| Size of cohort to be vaccinated | 720 000 | 100 000 | 0 |
| Number of expected cases | |||
| Meningitis and miliary | 6 | 10 | 22 |
| Tuberculosis, all cases | 393 | 593 | 1,195 |
| Mycobacterioses | 60** | 368** | 403** |
| Adverse effects | |||
| Localised effects (lymphadenitis) | < 300 | 40 | 0 |
| Severe BCGitis | 12 | 1–2 | 0 |
* Defined on the basis of country of birth and past history of tuberculosis in the family; estimates presented for vaccination targeted on at-risk groups are made using a vaccine coverage of 95%.
** The incidence of atypical mycobacteriosis in France is estimated from Finnish data for children between 0–15 years of age.
An epidemiological comparison of different vaccination options is only a first stage in defining a vaccination policy
If it is assumed that the declaration of sputum-positive cases is 80% exhaustive, the mean rate of sputum-positive cases forms would therefore be 5.7 cases per 100 000 inhabitants, slightly above the threshold recommended by IUATLD to consider stopping generalised vaccination of children. This argues more in favour of continuing generalised vaccination, which would enable up to 200 cases of tuberculosis including at least 4 cases of meningitis or miliary tuberculosis, to be avoided compared to targeted vaccination and up to 800 cases of tuberculosis including, at least 16 cases of meningitis or miliary disease, compared to stopping vaccination. Conversely, continuing generalised vaccination of children would result in the continued annual development of several cases of disseminated BCGitis and lymphadenitis. It is not therefore possible to confirm whether the benefit/risk balance of BCG vaccination is tipped unequivocally in favour of a policy of generalised vaccination in low-risk populations. If it were decided to continue generalised vaccination consideration could be given to delaying the age of vaccination for children at low risk of infection to after 6 months, the age at which the very great majority of children suffering from severe combined immunodeficiency have been identified.
The alternative of targeting BCG vaccination toward children living in a situation at risk would continue to allow approximately three-quarters of the cases of tuberculosis, which are currently avoided by generalised vaccination, to be avoided. However, using the most favourable vaccination hypothesis, restricting vaccination to children living in situations at risk would result in at least 4 additional cases of very severe cases of tuberculosis (meningitis and miliary disease) per year. These cases, particularly tuberculous meningitis, are characterised by a complication-free recovery rate of less than 50% in most of the published series of cases. In addition, the actual impact of such a vaccination option would depend on the ability to maintain the current level of coverage in the population of at-risk children (between 81 and 84% for 2-year-old children and 95% for 6-year-old children). Finally, the estimate that 100,000 children living in a situation at risk are to be vaccinated each year must be interpreted with caution. Doubt could be cast on this if the population of immigrant children or children from immigrant families within the population of children living in France were to increase significantly.
The analysis of the epidemiological impact of vaccination is based on a certain number of assumptions, in particular relating to the completeness of tuberculosis declarations in France, the efficacy of vaccination, and the proportion of cases of tuberculosis occurring in children with a risk factor. The estimates that come from these must be interpreted as orders of magnitude of the actual or future impact of different vaccination options. However, the conclusions relating to the relevancy of the different options considered does not appear to be affected by the uncertainty about these parameters.
Finally, it is important to note two new factors that could have an important influence on vaccine coverage: the outcome of consideration currently being given to questioning the principle of compulsory vaccination–removal of this obligation could lead to a fall in vaccine coverage; the decision by the only producer of the multipuncture BCG vaccine to stop production of this product which is used in France for more than 90% of the primary BCG vaccinations. The intradermal route is a delicate administration technique to which the very great majority of French vaccinating doctors are not accustomed. It also leads to a higher rate of local-regional side effects than multipuncture. A fall in vaccine coverage could be seen after the stocks of multipuncture BCG vaccine have been exhausted (end of 2005).
The analysis of the epidemiological consequences of a change in the vaccine strategy in France, which has been conducted taking account of all the available data reported in this work, only represents a first, essential but insufficient, stage to enlighten the decision-making process. This must be accompanied by an operational assessment of the availability and efficacy of other components of the system to control tuberculosis in France, which will take on even more importance if vaccination is restricted: effective management of patients liable to transmit the disease; preventative chemotherapy for infected patients.
Footnotes
- 1
This survey included all children of less than 15 years of age seen in public hospitals from the Paris region in 1997 and who were started on anti-tuberculous treatment (for tuberculosis disease or infection).
- 2
See address www
.tb-vac.org; research project planned by the TB-VAC Consortium of the European Commission.
Created: 2004.
- NLM CatalogRelated NLM Catalog Entries
- Review Deficiencies and handicaps of perinatal origin: Screening and management[ 2004]Review Deficiencies and handicaps of perinatal origin: Screening and managementINSERM Collective Expertise Centre. 2004
- Review Psychotherapy: Three approaches evaluated[ 2004]Review Psychotherapy: Three approaches evaluatedINSERM Collective Expertise Centre. 2004
- Review Tobacco: Understand dependence in order to act[ 2004]Review Tobacco: Understand dependence in order to actINSERM Collective Expertise Centre. 2004
- Review Cancer: A methodological approach for studying the link between cancer and the environment[ 2005]Review Cancer: A methodological approach for studying the link between cancer and the environmentINSERM Collective Expertise Centre. 2005
- Review Conduct: Disorder in children and adolescents[ 2005]Review Conduct: Disorder in children and adolescentsINSERM Collective Expertise Centre. 2005
- Tuberculosis: Place of vaccination in control of the diseaseTuberculosis: Place of vaccination in control of the disease
- Peperomia cubugonana (1959)Nucleotide
- Salmonella enterica subsp. enterica serovar Reading strain CVM N16S036, whole ge...Salmonella enterica subsp. enterica serovar Reading strain CVM N16S036, whole genome shotgun sequencing projectgi|1820309717|gb|AAPIMI000000000.1| I010000000Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
