NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The neurodegeneration in Alzheimer's disease (AD) may be caused by deposition of amyloid β peptide (Αβ) in plaques in brain tissue (amyloid hypothesis). Mechanisms of Αβ production in the brain have been the subject of considerable interest. Several factors regulate processing of amyloid precursor protein (APP), which is cleaved by three types of membrane-bound proteases designated α-, β-, and γ-secretases. Although we are only beginning to understand their functions, the discovery of nonamyloidogenic α-secretase has already provided some answers to long-standing questions of Alzheimer drug development. Here we summarize recent advances in the identification of APP α-secretases. Pharmacological upregulation γ-secretases should provide rational drug design for Alzheimer's disease.
Introduction
Dementia can be produced by a large number of pathological processes. Alzheimer's disease is the most common form of senile dementia and is characterized by the progressive impairment of cognitive domains caused by a loss of neurons from particular regions of the cerebral cortex, accompanied by the presence of amyloid deposition. A major component of senile plaques is amyloid β-protein (Ab) and this 39-43 amino acid peptide plays a crucial role in the pathogenesis of Alzheimer's disease. Αβ is derived by proteolysis of the amyloid precursor protein (APP)1-4 a ubiquitously expressed type I transmembrane protein. But in normal brain, Αβ deposition can not be found even in aged people. APP undergoes endoproteolytic processing within the Αβ domain in the trans-Golgi network (TGN) and at the cell surface (Fig. 3.1), and the generation of Αβ is inhibited. The α-secretase cuts the extracellular domain closer to the membrane, leaving 12 amino acids on the external surface. This results in the “constitutive” secretion of the large extracellular domain of APP (sAPP) into the medium. It has been shown that the fraction of sAPP can be increased by activating protein kinase C (PKC) cascade (“regulated” α-cleavage). Stimulation of α-cleavage of APP leads to a significant decrease in Αβ formation. Three related metalloproteases seem to exert an α-secretase activity. Therefore, stimulation of α-secretase as well as inhibition of amyloidogenic enzymes (β- and γ-secretases) have been suggested to theoretically reduce Αβ production.5,6
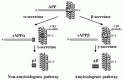
Figure 1
Proteolysis of APP.
ADAMs and α-Secretase
Several lines of evidence suggest that α-secretase activity is modulated by metal ions and metalloprotease inhibitors. Three members of ADAM (a distintegrin and metalloprotease) family (Table 3.1), ADAM9 (meltrin γ MDC9), ADAM10 (MADM) and ADAM17 (TNFα-converting enzyme or TACE), are reported to be candidate α-secretases (Table 3.2). These have the consensus sequence of zinc-dependent metalloprotease “HEXGHXXGXXHD” and seem to be active metalloprotease. In 1998, TACE was reported to play a central role in regulated α-cleavage by the use of gene disruption.7 Primary embryonic fibroblasts derived from TACE-knockout mice lost regulated sAPP secretion, while basal or constitutive secretion was not affected. Recent results of Slack et al demonstrate that constitutive sAPP secretion was also increased in HEK293 cells transiently transfected with TACE cDNA.8 These indicate that TACE is capable of catalyzing constitutive α-secretory cleavage of APP, but additional members of the metalloprotease, possibly ADAMs, mediate endogenous constitutive cleavage of APP.
Table 1
ADAM family.
Table 2
Comparison Among ADAMs 9, 10 and 17.
In 1999, Lammich et al reported that transfection of ADAM10 cDNA into HEK293 cells increased basal and PKC-stimulated α-secretase activity.9 Several investigators confirm the results.10-13 We independently demonstrated in 1999 that ADAM9 has an α-secretase activity in COS cells.14 ADAM9 and 10 were localized by immunostaining and cell surface biotinylation in the plasma membrane and Golgi apparatus. These results support the view that APP is cleaved both at the cell surface and along the secretory pathway. Hooper et al suggested that constitutive α-cleavage of APP occurred in a post-TGN,6 while it is primarily an intracellular source that is cleaved in the regulated pathway. One possible explanation for the uncertainty about the identity of α-secretase is that there may be more than one enzyme, depending on the cell type and on the cellular localization.
ADAM9 (MDC9) has been suggested to participate in the shedding of the ectodomain of the heparin-binding epidermal growth factor (HBEGF) in response to phorbol esters. We found that coexpression of mouse ADAM9 (mADAM9) and APP cDNA into COS cells enhanced both constitutive and regulated α-cleavage of APP.15 When we transfected APP695LAA in which HHQK of Αβ1316 is replaced with LHHAA to confer greater resistance to α-secretory cleavage, phorbol ester failed to activate α-cleavage, indicating that regulated cleavage is conducted by ADAM9. We also found that a general metalloprotease inhibitor SI27 inhibited the phorbol ester-induced increase in sAPPα secretion together with concomitant increase in sAPPβ secretion. Increasing concentration of the inhibitor had reciprocal effects on sAPPα and sAPPβ secretion.14 Purified recombinant mADAM9 could cleave APP in vitro at α-cleavage site.
To clone human counterpart of mADAM9, total RNA was prepared from human glioblastoma cell line A172 which shows a high endogenous α-secretase activity. cDNA was synthesized with the Thermoscript RT-PCR System (Life Technologies). One clone had a divergent carboxyl terminus and was designated hADAM9s.16 A full-length human ADAM9 (hADAM9) consists of an open reading frame of 2460 nt encoding 819 amino acid residues. The former lacks 106 bp, resulting in premature termination (total, 655 amino acids) and the deletion of the C-terminal transmembrane domain. We isolated this truncated hADAM9s protein from the culture medium of cDNA-transfected COS cells and showed that hADAM9s was capable of processing APP at asite. All these results strongly suggest that α-secretory cleavage occurs at the cell surface and modulation of APP metabolism can be achieved from extracellular space.
Some members of the ADAM family (ADAMs 11, 12, 28) have alternative splicing forms and have been secreted. ADAM12s is expressed in some tumor cell lines and placenta, while full-length ADAM12 is expressed in every tissue. ADAM12s is found in pregnancy serum and cleaves the major IGF-binding protein in human serum, IGFBP3. These results suggest that the splicing form of ADAM has tissue-specific unique functions.
Recently, mice lacking ADAM9 were generated.17 They developed normally, are viable and fertile, and did not have any pathological phenotypes. Interestingly, there were no differences in the production of the Αβ and p3 (APP α- γ-secretase product as well as HBEGF shedding. This argues against an essential role for ADAM9 as an α-secretase in mice (see Future Perspectives).
Relations between α- and Other Secretases
Αβ peptide is generated through the proteolytic cleavage of the APP molecule by two proteases, termed β- and γ-secretases. β-secretase the N-terminus of Αβ to produce a soluble form of APP (sAPPβ) and a 99-residue, C-terminal membrane-bound fragment C99. C99 is substrate for membrane-bound γ-secretase, which clips in the middle of the transmembrane region. Candidate β-secretase was identified as BACE1, a membrane-bound aspartic protease.
Recent results from Selkoe laboratory suggest that presenilins 1 and 2, which are endoplasmic reticulum (ER) or Golgi protein and whose dysfuction is a cause of early-onset autosomal dominant cases of familial Alzheimer's disease, are atypical aspartic proteases involved in the processing of APP and Notch.18 Since presenilins do not seem to fit into any known family of protease, some investigators cast doubts on the proteolytic nature of presenilins19-21 and they argue that presenilins are simply important components of a high-molecular-weight γ-secretase complex and act like a cofactor or a molecular chaperone of γ-secretase. Purification of γ-secretase complex to homogeneity could solve this problem.
Future Perspectives
To identify the endogenous α-secretase in A172 cells, RNA interference (RNAi) was induced by double-stranded RNA (dsRNA) encoding ADAM sequences. Recently, it was been reported that specific gene silencing with 21-nucleotide dsRNAs in mammalian cells provides a useful and reasonable tool. A172 cells are exceptional human glioblastoma cells with an endogenous potent α-secretase activity. A single application of RNAi decreased the amount of sAPPα in the medium by 25% compared with the control, and double RNAi and triple RNAi caused a 63–77% and 87% suppression of α-secretase activity, respectively (Asai M et al, manuscript in preparation). The results indicate that ADAM9, ADAM10 and ADAM17 catalyze α-secretory cleavage and therefore act as α-secretases in A172 cells.
Despite our lack of full knowledge concerning the identity of APP α-secretase, it is clear that the activation of these proteases may offer new therapeutic methods. Inhibitors of β- and γ-secretases may prevent the deposition of Αβ in brain, whereas an activator of α-secretase (and Αβ-degrading enzymes) may inhibit the accumulation of Αβ with the same efficiency.
There is strong evidence to suggest that NSAIDs may prevent or delay the onset of Alzheimer's disease. Indomethacin at 25–50 mM promoted non-amyloidogenic α-secretase in A172 cells.22 Therefore NSAIDs may provide an avenue to prevention of the Alzheimer's disease.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (C) Advanced Brain Science Project from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. [PubMed: 12130773]
- 2.
- Mills J, Reiner PB. Regulation of amyloid precursor protein cleavage. J Neurochem. 1999;72:443–460. [PubMed: 9930716]
- 3.
- Brown MB, Ye J, Rawson RB. et al. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria and humans. Cell. 2000;100:391–398. [PubMed: 10693756]
- 4.
- Nunan J, Small DH. Regulation of APP cleavage by α-, β-, and γ-secretases. FEBS Lett. 2000;483:6–10. [PubMed: 11033346]
- 5.
- Ishiura S, Hotoda N, Szabo B. et al. Alzheimer's amyloid α-secretase: A special target for drug development. Psychogeriatrics. 2001;1:273–276.
- 6.
- Hooper NM, Turner AJ. The search for α-secretase and its potential as a therapeutic approach to Alzheimer's disease. Curr Med Chem. 2002;9:1107–1119. [PubMed: 12052175]
- 7.
- Buxbaum JD, Liu KN, Luo Y. et al. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–27767. [PubMed: 9774383]
- 8.
- Slack BE, Ma LK, Seah CC. Constitutive shedding of the amyloid precursor protein ectodomain is up-regulated by tumour necrosis factor-α converting enzyme. Biochem J. 2001;357:787–794. [PMC free article: PMC1222008] [PubMed: 11463349]
- 9.
- Lammich S, Kojro E, Postina R. et al. Constitutive and regulated α-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA. 1999;96:3922–3927. [PMC free article: PMC22396] [PubMed: 10097139]
- 10.
- Lopez-Perez E, Zhang Y, Frank SJ. et al. Constitutive α-secretase cleavage of the β-amyloid precursor protein in the furin-deficient LoVo cell line: involvement of the pro-hormone convertase 7 and the disintegrin metalloprotease ADAM10. J Neurochem. 2001;76:1532–1539. [PubMed: 11238737]
- 11.
- Anders A, Gilbert S, Garten W. et al. Regulation of the α-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15:1837–1839. [PubMed: 11481247]
- 12.
- Marcinkiewicz M, Seidah NG. Coordinated expression of β-amyloid precursor protein and the putative β-secretase BACE and α-secretase ADAM10 in mouse and human brain. J Neurochem. 2000;75:2133–2143. [PubMed: 11032903]
- 13.
- Kojro E, Gimpl G, Lammich S. et al. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. [PMC free article: PMC33296] [PubMed: 11309494]
- 14.
- Koike H, Tomioka S, Sorimachi H. et al. Membrane-anchored metalloprotease MDC9 has an α-secretase activity responsible for processing the amyloid precursor protein. Biochem J. 1999;343:371–375. [PMC free article: PMC1220563] [PubMed: 10510302]
- 15.
- Hotoda N, Koike H, Sasagawa N. et al. Amyloid precursor protein is secreted by membraneanchored metalloprotease ADAM9In: Mizutani S, ed. Cell Surface Aminopeptidases.Elsevier Science B.V 2001401–406.
- 16.
- Hotoda N, Koike H, Sasagawa N. et al. A secreted form of human ADAM9 has an α-secretase activity for APP. Biochem Biophys Res Commun. 2002;293:800–805. [PubMed: 12054541]
- 17.
- Weskamp G, Cai H, Brodie TA. et al. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol Cell Biol. 2002;22:1537–1544. [PMC free article: PMC134708] [PubMed: 11839819]
- 18.
- Wolfe MS, Xia W, Ostaszewski BL. et al. Two transmembrane aspartates in presenilin-1 required for presenilin proteolysis and γ—secretase activity. Nature. 1999;398:513–517. [PubMed: 10206644]
- 19.
- Steiner H, Haass C. Intramembrane proteolysis by presenilins. Nat Rev Mol Cell Biol. 2000;1:217–224. [PubMed: 11252897]
- 20.
- Small DH. The role of presenilins in γ-secretase activity: catalyst or cofactor? J Neurochem. 2001;76:1612–1614. [PubMed: 11259477]
- 21.
- Checler F. The multiple paradoxes of presenilins. J Neurochem. 2001;76:1621–1627. [PubMed: 11259479]
- 22.
- Kinouchi T, Ono Y, Sorimachi H. et al. Arachidonate metabolites affect the secretion of an N-terminal fragment of Alzheimer's disease amyloid precursor protein. Biochem Biophys Res Commun. 1995;209:841–849. [PubMed: 7733976]
- APP α-Secretase, a Novel Target for Alzheimer Drug Therapy - Madame Curie Biosci...APP α-Secretase, a Novel Target for Alzheimer Drug Therapy - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...
