NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The Key Role of Ca2+ Channels in Cellular Signaling
The Stretch Reflex Exemplifies the Importance of Electrical-to-Chemical Transduction
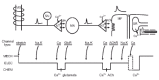
The stretch reflex represents the best-known and longest studied neural circuit. Unlike sensory circuits, for example in the visual system, the stretch reflex connects an external input to a behavioral output, both mechanical in nature, and is thus easily demonstrable without technical equipment. It provides an extremely reliable input—output relationship —hence, our view of the “knee-jerk” reaction as the epitome of automatic behavior. Yet, the underlying physiology of this stretch reflex is not so simple. Indeed, it consists of an extensive chain of events, characterized by many conversions between mechanical, electrical and chemical forms of energy, complex enough to rival a contraption by Rube Goldberg (fig. 1). The stretch reflex invokes the burgeoning fields of excitation—secretion coupling and excitation —contraction coupling. Fortunately, after decades of study (and several Nobel Prizes, the most recent in 2003), the chain of causality between a tap on the tendon and the subsequent knee jerk is now understood in considerable detail. At three stages—two electro-chemical synapses and one electro-mechanical transducer—electrical impulse spread is linked to the flow of calcium ions, and, in one way or another, that flow of Ca2+ is controlled by Ca2+ channels. These Ca2+ channels do not function in isolation—rather, they work in coordination with other varieties of ion channels, notably, voltage-gated sodium and potassium channels, as well as ligand-gated channels controlled by the neurotransmitters glutamate and acetylcholine. But the critical and specific role of Ca2+ channels in signal transduction is unique: in every instance, the conversion of an electrical signal to a chemical message requires the activation of Ca2+ channels. This is a nearly universal rule in excitable cells.
Why Are Calcium Channels So Powerful, Pervasive and Fascinating?
Let us begin with the issue of how Ca2+ channels are generically well suited to their unique task, and return later to the issue of Ca2+ channel types and their specialization for specific roles. The chemistry of calcium puts it into a different category than alkali metal elements such as sodium or potassium, or other alkaline earth elements such as magnesium.1-4 It has been suggested that once cells opted to use high-energy phosphate compounds as metabolic currency, they faced great evolutionary pressure to maintain an unusually low intracellular Ca2+ concentration ([Ca2+]i < 100 nM). Otherwise, salts of calcium and phosphate would precipitate, turning the cytosol into a bone-like solid.1 Accordingly, special homeostatic mechanisms such as calcium pumps evolved. In turn, the low [Ca2+]i made it possible to produce a significant change in concentration with only a small Ca2+ flux across the cell membrane; this kind of leverage for signaling is not shared by sodium or potassium ions. In a typical cell, the concentrations of Na+ and K+ on both sides of the membrane are in the millimolar range, so a much larger flux of ions would be needed to produce a proportional change in concentration; thus, the ion fluxes that generate Na+ and K+ spikes are not sufficient to deliver an ion-encoded message. Moreover, calcium has an additional advantage for signaling, in that receptor proteins that respond efficiently to Ca2+ ions could be readily utilized because calcium's divalent charge provides the energy needed to help drive large conformational changes. Finally, inasmuch as Ca2+ is a “hard” ion, it satisfies a more sharply defined set of requirements for high-affinity binding than a soft ion such as Mg2+, thereby favoring highly specific interactions.
Why is it so significant that Ca2+ channels are highly sensitive to membrane voltage? Coupling membrane potential to second-messenger signaling greatly expands cellular capability. Electrical events have intrinsic advantages in cell signaling, including the ability of action potentials to spread quickly and faithfully, and the possibility of summation of synaptic inputs. Finally, voltage-dependence offers a means of linking the activity of one channel (e.g., Na+) to that of another (e.g., K+ or Ca2+ channels), even if the channels are not in close physical proximity.
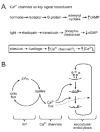
Combining the ideas of Ca2+ as a chemical messenger and change in membrane as an initiator of Ca2+ influx brings us to the essence of the advantages offered by voltage-gated Ca2+ channels. The opening of a voltage-gated Ca2+ channel is a powerful mechanism for delivering the second messenger (Ca2+) very quickly, as there is no lack of Ca2+ in the external milieu, and the concentration gradient for generating a large net flux is provided by the same processes that keep cytosolic Ca2+ low. Consequently, large fluxes translate to a rapid rise in intracellular Ca2+. In addition, Ca2+ channels in the plasma membrane have an added advantage relative to channels in intracellular membranes, in that they have access to the plasma membrane's voltage potential, a global indicator of cellular activity. A rapid, voltage-dependent closing of the channel allows for dissipation of the message by diffusion and strong, rapid local buffering, thereby achieving spatio-temporally precise signaling. In fact, Ca2+ channels can be thought of as playing much the same role as other sources of second messengers in signaling cascades. Thus, Ca2+ channel activity can be likened to renowned signaling systems like G protein—coupled receptors and adenylyl cyclase (fig. 2A). In contrast, such a comparison would not be appropriate for Na+, K+ and Cl- channels.
To capitalize on all of these advantages, Ca2+ channels must satisfy a number of structural and biochemical requirements. First, they should be highly voltage-dependent, making them quick to both open and close in response to changes in membrane potential (e.g., action potentials and excitatory postsynaptic potentials). Second, they must be highly permeable to Ca2+, but not to other ions such as Na+, or K+. Calcium channels must also be properly localized near the relevant targets of Ca2+ regulation, allowing for local increases in Ca2+ concentration. Finally, they must be subject to modulation, and diverse enough to allow for selective regulation of different classes of calcium channels. All of these requirements seem to have been fulfilled by the Ca2+ channels one finds in excitable cells. It is thus perhaps no wonder that calcium channels are critical for so many cellular processes.
Nevertheless, Ca2+ Channels Were Relative Latecomers to Cellular Electrophysiology
Considering the pivotal role of Ca2+ channels in so many cellular processes, as well as the near-ubiquitous presence of difference types of Ca2+ channels in excitable cells, it would seem that they would have been intensely studied from the very beginning of the era of intracellular recording and channel biophysics. But this was hardly the case. Why was the calcium channel nearly overlooked at first, and why did it receive relatively little attention even after it was first discovered? Neural circuits like the stretch reflex were very much on the minds of Alan Hodgkin, Andrew Huxley, and John Eccles, the winners of the first Nobel Prize in modern electrophysiology. However, they focused primarily on electrogenesis by sodium channels and the postsynaptic manifestations of neurotransmission, indisputably important elements of neuronal communication. In the case of Hodgkin, Huxley and Bernard Katz, a sensible preference for large preparations and large currents, attributes suitable for propagation over long distances, led them to study the squid giant axon. Here, the electrophysiological study of excitability became rightfully synonymous with the Sodium Theory. In the case of Eccles, the focus was on postsynaptic recordings and the nature of excitatory and inhibitory transmission, questions passed down from Charles Sherrington.
One glimpse of the central focus of the era can be obtained by revisiting a thick compendium of classical literature, gathered in 1972 by Ian Cooke and Mack Lipkin. Their Cellular Neurophysiology5 offered a generation of neuroscience students easy access to the original classic studies. Reflective of the ethos of the time, there was a huge disparity in Cooke and Lipkin's choice between amounts of space devoted to Na+ versus Ca2+ entry; indeed, not a single paper is devoted to Ca2+ channels as such. Today, however, it is apparent that Ca2+ channels have reached the forefront of the field of ion channel research, as judged by the number of abstracts, meetings and publications devoted to Ca2+ channels as opposed to Na+ channels. Indeed, entire international symposia are now dedicated exclusively to current research on calcium channels; due to their vital role in cellular signaling, their diversity, and great susceptibility to modulation, Ca2+ channels have attracted considerable interest. It may be satisfying for some of us to see such fervent attention to the molecular object of our affections, but the question still remains: how did we get from there to here?
The Winding Road of Calcium Channel Discovery
Bernard Katz and Colleagues: Early Beginnings at University College London
Where did the notion of a calcium channel first start? Clearly, it was in the Biophysics Department at University College London, founded by A.V. Hill and later led by Bernard Katz (fig. 3A). Most investigators would credit Katz, Paul Fatt and Bernard Ginsborg for pioneering the calcium channel field with their work in large muscle cells of crab6 and crayfish.7 These three were charter members of a small group of electrophysiologists who clarified many aspects of nerve propagation and synaptic transmission. But despite the pedigree of these investigators, calcium channels were not a major source of attention in the early 1950s, despite their now clearly critical role in even the simplest neuromuscular circuit. Indeed, the discovery of Ca2+ spikes aroused no great excitement in the 1950s, nor even many years later, when the accumulated triumphs of electrophysiology were reviewed.

Figure 3
Three of the principal founders of the field of voltage-gated calcium channels. A, Sir Bernard Katz. B, Susumu Hagiwara. C, Harald Reuter.
Given their functional importance, why were Ca2+ channels so slow to catch on? Several reasons come to mind.
The First Paper Drew Little Attention Because the Investigators Themselves Were Unsure of What They Had Found
Fatt and Katz entitled their 1953 paper “The Electrical Properties of Crustacean Muscle Fibres”, not exactly a title destined to be a Citation Classic! In fact, the discovery itself was merely an accident. Fatt and Katz were after bigger game—the electrical properties of inhibitory transmission—and were proceeding through a checklist of resting potential, membrane resistance and capacitance, the current—voltage relationship, and the basis of electrogenesis. They were not expecting to find electrical responses that persisted in sodium-free media, for example when Na+ was replaced by tetrabutylammonium (TBA). The data at hand were limited, so their conclusions were suitably cautious. They discussed in considerable detail the “choline action potential” and the “tetrabutylammonium action potential”, but were quite circumspect about concluding that Ca2+ was the charge carrier: “The mechanism of the action potential, and the species of ions involved in the movement of charge across the membrane, remain a puzzling problem...(i) it may be that TBA remains adsorbed to the fibre surface, but is mobilized during excitation and temporarily transferred into the cell interior; (ii) alternatively, influx of calcium or magnesium, or outflux of some internal anion may be responsible for transport of charge.”
The Next Advance in Understanding Was Slow in Coming
It took a full five years before Fatt, now working with Ginsborg, published a follow-up study, showing that full-blown action potentials could be generated in the absence of Na+, even without quaternary ammonium ions, provided the external solution contained strontium or barium ions.7 After soaking the preparation in tetraethylammonium-containing solution and subsequent washout of TEA, Ca2+ could serve as a charge carrier (later, it was found that the quaternary ammonium compounds entered the fiber and served to dampen outward potassium currents). This led to a cautious but clear statement that the movement of Sr2+, Ba2+ or even Ca2+ itself could support action potentials across the membrane.
Interest in Excitability Revolved around Proposed Mechanisms in Squid Axons
At the time, the results in crustacean muscle were of interest mainly because they seemed so unusual. The “Sodium Theory” had already been proven in myelinated nerve,8 skeletal muscle9 and heart tissue.10 The behavior of all tissues was judged against the standard of squid axons, where the recordings were most reliable. Exceptions were therefore of growing importance, if for no other reason than an ongoing debate with Ichiji Tasaki, who opposed the Sodium Theory of Hodgkin and colleagues. Since most of the 1953 Fatt & Katz paper was devoted to nailing down the fact that the action potential was not due to sodium entry, spikes dependent on Ca2+ entry were seen mainly as a curious variant on the dominant Na+ entry mechanism.
The Studies in Crustacean Muscle Focused on Electrogenesis Only, without Reference to Muscle Contraction
A direct relationship between Ca2+ entry and muscle contraction was not mentioned. In those days, the role of Ca2+ in contractile activation was not fully appreciated. This was despite Heilbrunn and Wiercinski's experiments with Ca2+ injection,11 as well as the much earlier work of Sydney Ringer, who showed the critical dependence of cardiac contraction on external Ca2+. Even the discovery of troponin-C as a Ca2+ sensor for muscle contraction 12 didn't bring to full light the importance of Ca2+ in contractile activation.
The Uncovering of Ca2+ Spikes Appeared Amidst a Crowded Field of Discovery
Even at its publication, Fatt and Katz's paper on the electrical properties of crab muscle was unfortunately but understandably overshadowed by its companion paper,13 their classic work on inhibitory transmission, a study establishing that inhibitory impulses can increase the post-junctional membrane conductance, thereby shunting excitatory neurotransmission whether or not the membrane actually hyperpolarizes. Bernard Katz himself was partway along a marvelous intellectual trajectory, ranging from excitability14 to mechanisms of excitatory transmission15 and the quantal basis of transmitter release.16
Each of the above factors contributed to the slow entrance of calcium channels into the burgeoning field of ion channel electrophysiology. However, despite their relative lateness entering the field, once their importance became clear, calcium channels quickly assumed a prominent position in the arena of ion channel electrophysiology.
The Inescapable Influence of Alan Hodgkin and Squid Axons
As an aside to the story of Katz and his associates in the Department of Biophysics, it is interesting to ask the question of whether Alan Hodgkin and Andrew Huxley, the principal pioneers in the field of membrane excitability, ever actually studied Ca2+ channels. As co-founders of the Sodium Theory (together with Katz), Hodgkin and Huxley never directly worked on calcium channels, although they came much closer than people usually think. Limitations on availability of squid to certain seasons and certain locations, and perhaps restlessness while the rest of the world caught up to the 1952 papers, spurred their interest in solving other problems. Thus, Hodgkin and Huxley turned eventually to excitation—contraction coupling, the terminal step in the stretch reflex, in vertebrate skeletal muscle. In classic experiments performed with Bob Taylor, Huxley used focal stimulation to demonstrate that voltage-dependence of contraction arose at regularly spaced “hot-spots” within the Z-line of frog skeletal muscle, corresponding to the openings of transverse tubules. Concurrently, working with Paul Horowicz, Hodgkin applied K+-rich solutions to depolarize single muscle fibers and described the very steep voltage-dependence of contraction. We now know that E—C coupling is critically dependent on voltage-gated Ca2+ channels (comprised of the α1S (Cav1.1) subunit along with ancillary subunits) in the transverse tubules. So in fact Hodgkin and Huxley both implicitly studied Ca2+ channels.
While we are on the subject of Ca2+ channels and muscle contraction, it is worth noting that several scientific heirs of Hodgkin and Huxley's have made vital contributions to the field of E—C coupling, some directly or indirectly involving Ca2+ channels. Knox Chandler (a postdoc of Hodgkin's) and Martin Schneider (a graduate student with Horowicz, thereby Hodgkin's scientific “grandson”) are good examples. They co-discovered a charge movement in the transverse tubules that mediated the voltage-dependence of contraction.17 That charge movement was really a form of gating current, now known to be critical for controlling the gating of almost all voltage-dependent channels. What wasn't clear then, and was only shown much later by Eduardo Rios, a postdoc of Schneider's, was that the charge movement observed by Schneider and Chandler actually originates in the skeletal L-type (α1S) Ca2+ channel.18
Certainly one of the most fascinating parts of the story of Alan Hodgkin and calcium channels is as follows. Hodgkin and Keynes used squid axons to make the first measurements of activity-dependent 45Ca2+ flux.19 They found that the net entry of tracer calcium was 600-fold less than the entry of sodium, making Ca2+ unlikely to contribute to the spike. However, they included this prophetic statement in their discussion:
“The finding of a greater uptake of calcium by stimulated nerve may be relevant in considering the mode of release of chemical transmitters by nerve endings. In addition to its action in dispersing squid axoplasm, calcium has been found to have a disruptive effect on other intrac- ellular structures, such as the sarcosomes of heart muscle, and it is interesting to speculate whether a penetration of calcium at the nerve ending might not be one of the factors involved in breaking up the intracellular vesicles near the membrane20 and releasing acetylcholine from them. There is some indication that Mg2+ ions do not have the same action as Ca2+ on intracellular structures, so that a competition between them to enter the nerve terminals, together with a failure of Mg2+ to disrupt the vesicles, might help to explain the inhibitory effect of magnesium.” This was a clear (and accurate) assessment of the importance of Ca2+ entry in mediating many cellular processes, such as transmitter release and E—C coupling.
Katz's group was also very familiar with the importance of Ca2+ in transmission21 and the antagonistic effect of Mg2+.22 Although in Katz's department, these authors did not mention Fatt and Katz's 1953 paper; after all, at that time the working hypothesis, (del Castillo and Katz and later, Jenkinson)23,24 was to envision Ca2+ and Mg2+ competing for an unidentified surface receptor X, which upon binding ligand converted to an active form X', allowing release of acetylcholine. So Hodgkin and Keynes were breaking new ground by suggesting that neurosecretion might be triggered by Ca2+ influx. They further suggested that Fatt and Katz might have been studying excitability that depended on some kind of divalent cation influx. So the first insight into the notion of the calcium channel's role in excitation-response coupling may actually have originated with Hodgkin and Keynes in 1957; the follow year, Fatt and Ginsborg nicely credited this paper in their study of divalent cation spikes,7 thereby adding favor to the notion of Ca2+-dependent excitability.
Katz and Miledi Re-Enter the Fray by Focusing on the Squid Giant Synapse
So when did it become accepted that Ca2+ entry through voltage-gated calcium channels triggers transmitter release? Katz's classic text Nerve, Muscle, and Synapse 25 provided a retrospective snapshot of his views more than a dozen years after his 1953 paper on crab muscle electrophysiology. Katz quoted Fatt and Ginsborg's evidence in crustacean muscle for “entry of a divalent cation like Ca”, but only as a curious exception to the generalization of Na+ influx as the inward current during excitation. With characteristic modesty, there was no reference to Fatt and Katz.6 The birth of calcium channels was tucked away in a short paragraph alongside the efflux of an internal anion in the impulse-conducting plant cell Nitella, both deemed illustrative exceptions. It appears that the provocation from Hodgkin and Keynes19 about synaptic transmission had not yet taken effect. However, something was clearly brewing, because there is a forward-looking statement in the very last paragraph of Katz's book:
It is interesting to note certain features that the initiation of muscle contraction shares with the initiation of an action potential and with the release of a transmitter substance at a nerve terminal. In all three cases, the primary event is a depolarization of the cell membrane. Depolarization, however, is not a sufficient stimulus; it becomes effective in producing its diverse results only if calcium is present, whether the final result be increase of sodium permeability,26 the facilitated release of acetylcholine quanta,27 or the activation of myosin molecules.28
The germ of an idea about a messenger role for Ca2+ was there, but Katz continued to focus on the presence of calcium, not on Ca2+ flux per se.
Of course, in the long run, Katz made further giant contributions to understanding the significance of Ca2+ influx for triggering downstream cellular events. This was revisited in 1967, after the momentous period of clarifying the quantal nature of neurotransmission and the key role of calcium. Working with Ricardo Miledi at the Marine Station in Naples, Katz returned to the subject of Ca2+ entry, but now looking in nerve terminals. Using the squid giant synapse, Katz and Miledi showed: that transmission required membrane depolarization, but not sodium channels; that Ca2+ spikes could be generated at the terminal if outward K+ currents were blocked; and that transmitter release required repolarization when initiated by strong depolarization, consistent with the requirement for both channel opening and a strong electrical driving force for Ca2+ influx. Interestingly, in his papers describing his work on the squid synapse, Katz refers back to the work on crustacean muscle, but once again only to the paper of Fatt & Ginsborg,7 and not his own earlier work.6 Nevertheless, the studies of Katz and Miledi were to provide a foundation for elegant studies in the 1970s by Llinαs and colleagues29 and later by Augustine, Charlton and Smith.30
Susumu Hagiwara: Ca2+-Based Electrogenesis in Full Bloom
Another significant contributor to the birth of the field of calcium channels was Susumu Hagiwara (fig. 3B). Known affectionately as “Hagi”, Hagiwara was a zoologist whose roots were in Japan, but who worked mainly in the States. Although he held an M.D. (as well as a Ph.D.), Hagiwara was not particularly obsessed about working strictly with mammals or even vertebrates; nowadays he might be classified a comparative neuroethologist. His interests were extremely broad, ranging from the singing of cicadas to echolocation in bats, from the electrical properties of eggs to taste sensation in cats. Within this expansive range of topics, Hagiwara is best known for his studies on “calcium spikes”, first in barnacle giant muscle fibers, then later in many other preparations.
Interestingly, the Chilean barnacle was actually a substitute for the squid at the Monte Mar marine station on the Chilean coast. The squid were displaced from their usual territory by a large storm, forcing Hagi and his colleagues Alan Grinnell and Jared Diamond to find another preparation. As described earlier by Hoyle, the giant muscle fibers of the barnacle were up to a millimeter in diameter and five centimeters in length. So here was a large preparation in which Ca2+ channels could be brought to center stage under appropriate experimental conditions (even though these fibers don't have full-blown spikes under physiological conditions).
Hagiwara is often credited with helping to establish the ubiquity of Ca2+ channels because of his work on so many different preparations. Ted Bullock and Alan Grinnell, two of Hagiwara's long-time colleagues, provided this quote from Bertil Hille:31 “Hagi [was] a research scientist of peerless distinction... He is remembered as the champion who brought the calcium channel to its rightful respected place and in the process discovered blocking ions, flux saturation, inactivation dependent on internal calcium, and many other unanticipated phenomena.”
This well-deserved praise for Hagiwara does not change the history of the calcium channel itself, or its slow emergence from obscurity. Once again, Bullock and Grinnell on Hagiwara: “He recounts, with characteristically self-deprecating humor, how, during this period when the calcium channel was only found in miserable animals like crustaceans and was thought to play no important function in the mammalian brain... I suffered tremendously from a minority [sic] complex.”
Along with its humble position in the phylogenetic tree, the barnacle had an additional handicap: the complicated morphological cable properties of its giant muscle fiber made it very difficult to study in a rigorously biophysical way. As Hagiwara and Byerly stated, “At first it appeared that barnacle giant muscle fibers might become the ‘squid axon’ of Ca currents... however, there are deep, long invaginations of the sarcolemma in all crustacean muscle fibers...[which] cause barnacle muscle to fail to satisfy criteria about reliability and speed of voltage control.” In his classic book on ion channels,32 Hille judiciously skips over these cable complications, but chooses instead to introduce the gigaohm seal (patch-clamp) recording methods of Neher and Sakmann and Sigworth,33 right in the middle of the chapter on calcium channels. This seems fair enough, as this was one field that benefited enormously from patch-clamp techniques.
Despite the obscure idiosyncrasies of the invertebrate preparations of those early days, the interactions between investigators were typified by collegiality. Alan Grinnell described the relationship between Katz and Hagiwara as follows: “Hagi and BK met several times, and I know they admired each others' work. One of the reasons I went to UCLA after my postdoc in London was Katz's strong recommendation of Hagi. And in 1967, when Hagi and I went to Chile to study the giant synapse in squids there—which proved abortive because a major storm came up, following which the squid were unobtainable for several years, so we worked on giant barnacles instead—we were later grateful for the storm, since that fall Katz & Miledi published their beautiful J Physiol paper on Naples squid establishing the role of Ca2+ in synaptic transmission. As Hagi said at the time, we would have been scooped by the best.”
Around the same time, Hagiwara and Nakajima34 published a fundamental paper describing the pharmacology of Ca2+ spikes, neatly combining work on both barnacle muscle and heart muscle. The agents tetrodotoxin (TTX) and procaine, normally effective on Na+ spikes of other tissues, failed to block the barnacle Ca2+ spike. On the other hand, manganese acted as an inhibitor, competing with Ca2+ to block the Ca2+ spike. In heart, TTX and procaine suppressed the initial rate of the rising phase, but spared the plateau; in contrast, manganese acted primarily on the plateau phase. As Hagiwara & Nakajima recognized, the heart provides a clear example of a setting where Ca2+ channels are absolutely essential for maintaining life; we know now that all heart cells use Ca2+ channels for one or more aspects of their electrical activity. Ca2+ channels are critical for pacemaker activity in the sino-atrial node, whose depolarization is aided in its last stages by an increasing permeability to Ca2+ ions. Slow propagation in the atrio-ventricular node absolutely requires Ca2+ channels. Finally, in the atria and ventricles, where the pumping action of the heart takes place, both the action potential plateau and contraction rely on Ca2+ channels.
Harald Reuter: First Ca2+ Currents Under Voltage Clamp
Enter the next major father figure in this saga, Harald Reuter (fig. 3C). Born in Germany, Reuter has spent the bulk of his illustrious scientific career in Bern, Switzerland, but with brief and sometimes nearly annual peregrinations to the States. Reuter first came to calcium channel research as an M.D., with a non-quantitative training in cardiac pharmacology and an abiding interest in how adrenaline affects the heart. Reuter's voltage-clamp recordings of shortened Purkinje fibers, using the method of Trautwein and associates, were the first to provide evidence for a Ca2+ current.35 Interestingly, the same Journal of Physiology volume that featured Reuter's paper on the putative Ca2+ current also contained Katz and Miledi's study of synaptic transmission in the absence of nerve impulses, using TTX to block Na+ channels.36 One year following his presentation of Ca2+ currents, Reuter, together with Seitz, found evidence linking external Na+ to Ca2+ efflux, now known as the Na+—Ca2+ exchange.37
The work in cardiac Purkinje fibers was later followed by recordings from bundles of ventricular or atrial heart muscle,38,39 which also showed inward currents supported by Ca2+ influx. In magnitude and kinetics, the putative Ca2+ channel currents were smaller and slower than sodium currents. This conclusion soon came under attack on technical grounds, mainly from Ted Johnson and Mel Lieberman, who suggested that the “calcium current” or “slow inward current” might be an artifact arising from the methodologies that needed to be applied to try controlling the membrane potential while the membrane current was generated.40 The multi-cellular cardiac preparations were regarded as far inferior to experimental systems such as the squid giant axon, where techniques for uniformly controlling the membrane potential were more rigorously applicable. For example, narrow extracellular spaces in the cardiac tissue could support unwanted and immeasurable voltage drops in series with the excitable membrane of interest, invalidating the voltage clamp. The next few years were particularly confusing for the study of cardiac Ca2+ channels, which was a world unto itself for a while, with little crossover from those interested in nerve or skeletal muscle. The controversy about the very existence of Ca2+ currents began to resolve with cardiac preparations with more favorable geometries, such as the rabbit Purkinje fiber (wide clefts)41 combined with three-microelectrode voltage clamp, which measured and capitalized on cable non-uniformity but nevertheless verified the existence of bona fide Ca2+ currents.42 The ultimate resolution came with the advent of methods for studying single cells, first with large suction electrodes and later with modern patch-clamp methodology. The ability to alter the intracellular ionic composition, first tediously developed in multicellular preparations,43 became much easier at the level of single cells.44 Interestingly, Harald Reuter was very active in the use of both suction and patch-clamp electrodes, and made many additional contributions to the field of cardiac electrophysiology and Ca2+ channels using these newer techniques.
In hindsight, most would agree that the first sets of voltage-clamp experiments had not paid sufficient attention to possible technical difficulties, but were nevertheless correct in uncovering a new component of inward excitatory current. It is clear now that the problems of spatio-temporal control of membrane potential were actually less serious than originally thought, whereas issues of overlapping currents were more severe. An experiment in which extracellular [Ca2+] varied could undoubtedly evoke changes in Ca2+-dependent currents as well as in the flux through Ca2+ channels themselves.
The mid-1960s also marked the advent of pharmacological approaches to identifying Ca2+ currents using channel blockers such as verapamil, nifedipine and diltiazem, thanks to the efforts of Albrecht Fleckenstein and others (for review, see ref. 45). Application of such agents buttressed emergent ideas about the distinct nature of sodium and calcium channels in cardiac cells. Today, Ca2+ channel blockers are effective in a number of cardiovascular syndromes and represent a multibillion-dollar business; who would have thought that good could result from channel blockade that could in principle reduce the strength of cardiac contraction? In fact, the main therapeutic target for the Ca2+ channel blockers is the L-type Ca2+ channel in vascular smooth muscle, where relaxation favors blood flow and lowers blood pressure; this arises from the ability of the blockers to act more potently on vascular smooth muscle than on heart (a piece of good fortune for both the patient and the drug industry).
The Diversity of Native Ca2+ Channel Currents
Early Studies on the Diversity of Ca2+ Channel Types
In the early days of Ca2+ channel electrophysiology using morphologically simple preparations, the original presumption (with or without credit to Gertrude Stein) was that “a Ca2+ channel is a Ca2+ channel is a Ca2+ channel”, with little regard for the possibility of multiple Ca2+ channel types. Peter Baker, one of the leading scientists in the study of squid axon excitability, was an example of someone with little patience for studies of multiple Ca2+ channels. To him, the important issues were whether Ca2+ channels truly existed, how they were linked to the process of vesicle exocytosis, and how vesicle fusion itself took place. This was fair enough, and perhaps fitting for someone who began his career at a time when the very nature of excitability and the role of Na+ influx were still under fire.
However, Hagiwara was ahead of his time in recognizing the multiplicity of channels and their possible importance in excitability. Working with starfish eggs, he and his colleagues Ozawa and Sand described two inward currents, labeled I and II, both supported by Ca2+ influx;46 (see also Fox and Krasne47 for their work on egg cells of a marine worm). Today, their “I” and “II” would be more descriptively termed low-voltage activated (LVA) and high-voltage activated (HVA), respectively. As Hagiwara and Byerly stated in 1981: “...at present it seems most objective to give up the prejudice that the Ca channel is like the Na channel and allow that there may be various types of Ca channels.” But not everyone was convinced: Lux's group48 summarized their patch-clamp recordings of unitary Ca2+ channel activity from bird, snail and rat by saying “...we conclude that Ca2+ channels everywhere are basically the same.”
The distinction between LVA and HVA channels gained momentum through several studies in the early 1980s, mostly in biological systems less obscure than starfish eggs. In beautiful recordings of action potentials in inferior olivary neurons, Llinαs and Yarom49 discovered low-threshold Ca2+ spikes that depended on an underlying conductance; this conductance required strongly negative potentials to become de-inactivated. This was the current-clamp equivalent of LVA channels, later termed T-type channels. Carbone and Lux,50 as well as Fedulova, Kostyuk and colleagues,51 provided further evidence of LVA Ca2+ channels in the form of single-channel recordings of chick and rat sensory neurons. Finally, Matteson and Armstrong52,53 demonstrated slow deactivation of T-type channels upon sudden repolarization, a key property of these channels. In 1985, Bean obtained similar evidence for the distinctiveness of T-type channels in atrial myocytes, showing that only the HVA population of channels was responsive to Ca2+ channel blockers and β-adrenergic stimulation.54
In that period, the evidence supporting the separation of voltage-gated Ca2+ channels into LVA and HVA channels was mostly biophysical. This evidence included voltage-dependence of gating (LVA channels require a more negative holding potential, and activate at much more negative test potentials than HVA channels), speed of deactivation (LVA channels deactivate slowly compared to HVA channels), resistance to run-down (loss of activity) following patch excision or dialysis of the cytosol (LVA channels are more resistant to run-down), and differential sensitivity to Cd2+ (LVA channels are much less sensitive to Cd2+). Taken together, it became increasingly evident that voltage-gated calcium channels could be accurately separated into two broad categories on the basis of biophysical properties.
A Tripartite Classification of Native Ca2+ Channels
Our group's own interest in multiple types of Ca2+ channels took an interesting turn when Martha Nowycky and Aaron Fox teamed up to examine the properties of single Ca2+ channels in cell bodies of chick sensory neurons.55 Based on published research, for example Aaron's previous work on marine worm egg Ca2+ channels, we fully expected to find two categories of Ca2+ channels, LVA and HVA. However, the observed pattern of unitary channel properties— their slope conductances and activation and inactivation properties—fit more neatly into three distinct categories, leading Martha to postulate that there must be a third category. Upon closer examination of our whole-cell and single-channel recordings, it became clear that she was correct, and we soon found other criteria for distinguishing the three channel types, which we then called T-type (LVA) and N- and L-type (both HVA). The L-type channels were so named because they had a large unitary conductance to Ba2+, supported a long-lasting Ba2+ current (different properties were found with Ca2+ as the charge carrier, but the Ba2+ currents provided the most distinctive channel profiles) and were similar to single channels found in heart cells.56 The T-type channels generated tiny unitary Ba2+ currents, gave rise to a transient average current, showed characteristically slow deactivation following sudden repolarization,57 and were also found in heart cells.54,58 Finally, the N-type channels were largely specific to neurons, had an intermediate conductance to Ba2+, and, although they required negative holding potentials to be available for opening, they were activated at high voltages, indicating that they were neither T- nor L-type. Contrary to recurrent rumors, the N-type channel was not named by or for Martha Nowycky, although there would have been some justice had this been the case.
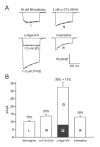
In coming up with this tripartite classification, the trickiest distinction was between L-type and N-type channels. Their profiles of unitary Ba2+ conductance and voltage-dependent gating were distinctive enough, but the most decisive evidence came from experiments with the drug Bay K8644; this dihydropyridine compound acts as a stimulator (agonist) of L-type Ca2+ channels, greatly increasing divalent cation influx by promoting a mode of activity with very long openings (fig. 4A). Indeed, the behavior and pharmacology of neuronal L-type channels59 were very similar to those of cardiac L-type channels.60 In contrast, N-type channel activity was not affected by Bay K8644 (fig.4B). Once again, pharmacology provided essential tools for studying Ca2+ channels, and confirmed distinctions derived from biophysical comparisons.
Shortly after the first presentation of the tripartite classification of voltage-gated calcium channels, a peptide toxin derived from marine snail venom, ω-conotoxin-GVIA, became available through the excellent work of Baldomero Olivera and Doju Yoshikami. Working together with them, we found that this peptide blocks HVA channel activity, particularly that of N-type channels.61 We then used this peptide in collaboration with Richard Miller's group to demonstrate that N-type channels play an important role in the release of norepinephrine from sympathetic neurons.62 Soon thereafter, the groups of Peter Hess and Bruce Bean showed that ω-conotoxin-GVIA was actually quite selective for N-type channels and thus completely spared L-type channels,63,64 providing further evidence to support distinctions between N- and L-type Ca2+ channels.
The Tripartite Classification of Neuronal Ca2+ Channels Was Not Universally Accepted
Some of the experts in the field, including Richard Miller and Harald Reuter, embraced the distinction between T-, N-, and L-type channels, noting that it resolved many puzzling aspects of findings on neurotransmitter release.65,66 However, the idea of three categories of voltage-gated Ca2+ channels was slow to catch on with the groups that had contributed to the original LVA— HVA distinction. The greatest resistance to the tripartite classification came from Swandulla and Armstrong,67 who found the separation between L-type and N-type channels unconvincing, based on their favorite criterion, the speed of tail deactivation.67 Carbone and Lux also found it difficult initially to accept the pharmacological separation between L- and N-type channels, in part because they were unable to elicit convincing responses to dihydropyridine antagonists such as nifedipine, and even the agonist Bay K8644, despite these compounds' carefully-verified effectiveness in heart cells.68 In contrast, the Kiev group of Platon Kostyuk and colleagues were rather receptive to the idea that the HVA channels could indeed be split into two groups; they confirmed the specificity of Bay K8644 for one of those groups, and even proposed a distinction between high-threshold, inactivating (HTI) and high-threshold, non-inactivating (HTN) channels, in keeping with the N- and L-type subdivisions, respectively.
It is interesting to look back over the rapid progression of developments in the study of the N-type channel (Table 1). In the decade following its initial discovery, we witnessed the emergence of a specific blocker (ω-conotoxin-GVIA) as well as the cloning of the underlying α1 subunit, α1B (Cav2.2; for a review of voltage-gated calcium channel nomenclature) (see refs. 69 and 70). Other highlights include the recognition of the key role of N-type channels in neurotransmitter release in peripheral and central neurons, and their susceptibility to modulation by G-protein—coupled receptors. More recently, the N-type channel has been examined as a possible therapeutic target for treatment of intractable pain, though this has yet to receive full FDA approval.
Table 1
A few significant events in the brief history of N-type Ca2+ channels.
Beyond T, N and L: The Identification of P/Q-Type Channels
Not long after the debut of N-type channels, Rodolfo Llin´s and colleagues discovered a non-inactivating, DHP-insensitive current in the cell bodies of cerebellar Purkinje neurons; because of this localization, they named these channels P-type Ca2+ channels.71,72 Bruce Bean, Michael Adams and colleagues then demonstrated that these P-type channels were exquisitely sensitive to the funnel web spider toxin peptides ω-Aga-IVA and ω-Aga-IVB. Around the same time, a full-length brain Ca2+ channel gene called BI (for brain-1) was cloned and expressed by Yasuo Mori and Shoshaku Numa in Kyoto.73 Many call this α1A, following the general classification of brain Ca2+ channels from Terry Snutch, Henry Lester and Norman Davidson. Because the message for this Ca2+ channel was abundant in cerebellum, and specifically in cerebellar Purkinje cells, Rudolfo Llin´s felt sure that it encoded the P-type channel. However, Numa was reluctant to commit to this, as the properties of the cloned α1A subunits, when expressed in Xenopus oocytes, deviated greatly from those of native P-type channels recorded from cerebellar Purkinje neurons. Rather, the behavior of the cloned α1A subunit in oocytes was much more similar to a current component labeled Q-type by our group.74 Several lines of evidence, including antibodies, antisense techniques75 and gene knockout have now convincingly established that both P- and Q-type currents both arise from α1A. For example, both current types are completely eliminated in cerebellar Purkinje and granule neurons of α1A-/- mice.76 Thus, the currently accepted terminology of “P/Q-type” channel seems appropriate, as the original distinctions between the P and Q components have been mostly accounted for by splice variation in the α1A gene.77
This is an appropriate point to acknowledge the enormous contributions of those who have provided the molecular tools that played a critical role in identifying diverse Ca2+ channels and dissecting their contributions to neuronal function (see later chapters in this book). Baldomero Olivera has been widely recognized as a pioneer in discovering the ω-conotoxins, but what is not commonly known is that he came to the field as a molecular biologist and adapted to the limited resources available in his native Philippines by studying a local menace, the deadly marine snails. His careful and thorough examination of these snails led to the discovery of a large number of invaluable neurotoxins.78 Michael Adams, who discovered the peptide ω-Aga-IVA, began his work as an entomologist, not a neuroscientist. The availability of ω-conotoxin GVIA and ω-Aga-IVA helped settle debates over the distinctiveness of N- and P/Q-type channels, clarified their relationship to their respective α1 subunits, and greatly accelerated our examination of these channels' roles in neuronal processes such as Ca2+ signaling and neurotransmission.
The Uncovering of Yet another Kind of HVA Neuronal Ca2+ Channel: R-Type
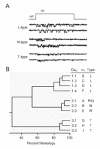
By the early 1990s, the actions and specificity of the newly available toxins and pharmacological compounds were understood sufficiently to allow a separation of current components based primarily on pharmacology. Andy Randall used a combination of nimodipine (L-type— specific), ω-conotoxin-GVIA (N-type—specific) and ω-Aga-IVA (P/Q-type—specific) to tease apart the various Ca2+ current types in cerebellar granule neurons.74 Because of the homogeneous nature of the neurons in this preparation, as well as their favorable passive properties, a clean, reproducible dissection of HVA Ca2+ current types was obtained (fig. 5). Present were currents carried by the three usual suspects, L-, N- and P/Q-type channels. However, a fourth current type, displaying properties distinct from the other three, was revealed upon application of inhibitors of the three known channel types. Because this current was resistant to the three inhibitors (making it residual after inhibition of the other three current types), it was named R-type. This current type showed unique properties, including very rapid decay and an unusual sensitivity to block by Ni2+. Moreover, there was a strong resemblance to the properties of a novel α1 subunit (doe-1) cloned from a marine ray by our group in collaboration with Tom Schwarz and his colleagues.79
The designation of another channel type seemed problematic to several experts in the field, who initially dismissed the notion of a fourth HVA Ca2+ channel type, suggesting that the residual current might result from incomplete block of L-, N- or P/Q-type channels. Even the distinct kinetics of this R-type current might have been explained by alternative splicing of one of the other channel type's message (as was the case with P- and Q-type channels), or perhaps by associating with a different accessory subunit. Lacking a mammalian homolog for the α1 gene encoding this current, the smoking gun we needed was a specific inhibitor of this residual current, ideally one selective enough to spare L-, N- and P/Q-type currents. Fortunately, this was provided by Robert Newcomb, George Miljanich and colleagues, who isolated another spider toxin peptide, designated SNX-482.80 This inhibitor blocks R-type currents in several neuronal systems.
Issues Surrounding α1E and Its Initial Identification with T-Type Channels
Another interesting episode in the unveiling of the Ca2+ channel family occurred when matching α1E, the most recent member of the Cav2 subfamily to be cloned, with a Ca2+ channel phenotype defined by electrophysiological properties. When Tuck-Wah Soong, Terry Snutch, and their colleagues first isolated α1E, they suggested somewhat provocatively that this gene might encode low-voltage—activated T-type channels.81,82 Of all the voltage-gated Ca2+ channels, T-type currents were the easiest to isolate biophysically, yet the hardest to study biochemically (perhaps their role in generating relatively slow pacemaker depolarizations required only small currents and thus low levels of protein in the membrane); therefore, successfully cloning this channel would represent a major step forward in the study of T-type calcium channels. On the other hand, our group74,83 favored the notion that α1E encodes the R-type current that remained after blockade of neuronal L-, N-, and P/Q-type channels. This controversy was ultimately laid to rest by the cloning of novel α1 subunits by Edward Perez-Reyes, LeAnn Cribbs and their colleagues.84-86 These genes were classified α1G, α1H and α1I, in keeping with the convention of the time (these genes comprise their own subfamily, called Cav3). The isolation of these clones stands as a testament to the persistence of the researchers and their willingness to try in silico approaches. When these three genes were expressed and recorded, the currents matched nicely with native T-type (LVA) currents; none of these three clones gave rise to R-type currents. On the other hand, it is now generally accepted that α1E is the major contributor to R-type current. Recently, the interest in R-type channels has sharply increased due to their ability to support neurotransmitter release87,88 and their contribution to dendritic Ca2+ homeostasis89 and synaptic plasticity.90 In the discovery of the R-type channel, one sees repeated the familiar history of an initial distinction of a Ca2+ channel type based on biophysical and pharmacological criteria, followed by an emerging consensus about its molecular basis, driven in part by cloning. What began as a curiosity or anomaly (and to many investigators, an artifact) rapidly transitioned from controversy to general acceptance, proving to be more interesting than its discoverers could have anticipated.
The Current State of the Three-Branched Calcium Channel Family Tree
A new set of questions comes to mind now that the diverse nature of the Ca2+ channel family, first proposed on the basis of patch-clamp recordings, has received corroboration from molecular biology. Sequence analysis has confirmed and extended our original tripartite classification (fig. 6). The members of the Cav2 subfamily, first typified by N-type channels (N-type = α1B; P/Q-type = α1A; R-type = α1E), appear functionally quite distinct from the Cav1 subfamily of L-type channels (α1S, α1C, α1D and α1F) and the Cav3 subfamily of T-type channels (α1G, α1H and α1I). Each of the cloned channels shows a steep voltage-dependence, highly similar to a first approximation, and a strong selectivity for Ca2+ and Ba2+ rather than Na+. In this regard, truly “a Ca2+ channel is a Ca2+ channel is a Ca2+ channel”. If so, why might such diversity have evolved? The most obvious answer is that the individual family members evolved to fill distinct physiological or cell biological niches. The employment of more than one type of channel allows greater flexibility with regard to time- and voltage-dependence, cellular localization, and responsiveness to different forms of modulatory regulation. Diverse as they are, Ca2+. channel types are far less numerous than the signaling roles for voltage-gated Ca2+ entry itself.
Conclusions: Some Lessons and Ironies
The history of calcium channel discovery offers many insights and a few interesting ironies. Although first identified in the heyday of classical neurophysiology, Ca2+ channels did not receive much initial attention, despite their now-evident importance in processes such as chemical neurotransmission and excitation-contraction coupling. The timeline of Ca2+ channel discovery is remarkable when one considers the extraordinarily long gestation period from first sighting (1953/1958) to full-blown focus (1970s for multicellular approaches, 1980s for patch-clamp recordings). The circuitous path to understanding how Ca2+ entry triggers neurosecretion, reviewed in this chapter, is indicative of a more general disconnect between the participation of Ca2+ in electrogenesis and the role of Ca2+ as a second messenger. Squid giant axons, the preeminent preparation for studying axonal conduction, do not express high levels of Ca2+ channels. Despite pioneering work by Reuter and others, this paucity of Ca2+ channels in squid axons had a strong influence: Johnson and Lieberman were not alone in asking why the heart should use calcium channels to make action potentials when the reigning preparation, the squid giant axon, did not. The course of scientific discovery might have been quite different if physiologists had focused on crustacean muscles, which appear to lack sodium channels, rather than squid giant axons. Another irony is that full-blown Ca2+ spikes, the first indication of the presence of Ca2+ channels, are not actually critical for excitation—contraction coupling in the muscle fibers of crab, crayfish or barnacle, and are evident only when K+ channels are blocked or Ca2+ channel inactivation is suppressed.
In mammalian preparations, the sino-atrial and atrio-ventricular nodes offer the clearest examples of Ca2+ channel—dependent electrogenesis, but these systems are rather difficult to study, even today. On the other hand, skeletal muscle is one of the richest sources of homogeneous Ca2+ channels, and thereby facilitated the early biochemical characterization of Cav1.1 (α1S) subunits by William Catterall, Kevin Campbell, Michel Lazdunski and others, as well as the first cloning of a Ca2+ channel by Shoshaku Numa and colleagues. Yet, despite much elegant work,91 the steps between Ca2+ channel activation and subsequent internal Ca2+ release from the sarcoplasmic reticulum remain incompletely understood even a decade later.
In recent years, rapid progress in the fields of Ca2+ channel structure, function, diversity and regulation can be attributed to several key factors. First, investigators have approached the study of calcium channels from many angles, ranging from hardcore biophysics and biochemistry to clinical perspectives—recall that Harald Reuter and Albrecht Fleckenstein both began their careers as cardiac pharmacologists. Second, even with the advent of improved patch-clamp technologies, and the availability of a veritable cornucopia of potent, selective Ca2+ channel drugs, the field would not have experienced such a rapid rate of progress without a shift in the prevailing attitudes of the individual investigators. The sharing of information and resources between scientists has greatly contributed to the speed of new discoveries. In earlier times, compartmentalization stood as a barrier to collaborative discovery. For example, an early paper about Ca2+ channels in cardiac preparations would credit Hagiwara and Nakajima as well as Niedergerke and Orkand for circumstantial evidence of such channels in heart, but not cite Fatt and Katz or Fatt and Ginsborg's work! On the other hand, working from an invertebrate vantage point, Hagiwara and Byerly entitled their 1981 review “Calcium Channel”, but stated disarmingly that they were not familiar with the literature in vertebrate heart or smooth muscle. As Rodolfo Llinαs put it in a 1983 letter: “We need to unify our fields a bit more. Nature seems to be trying to tell us something and we continue stubbornly to think that muscles and nerve cells are not next of kin.” Thankfully, this wish for unification has now largely been realized, and we now understand a great deal more about what nature can tell us about Ca2+ channels, their diversity, and their critical roles in cellular processes.
Acknowledgments
We are grateful to Alan Grinnell for sharing reminiscences about Susumu Hagiwara and for commenting on an early version of this chapter. This work was supported by NIH (NS24067).
References
- 1.
- Williams RJ. Cation distributions and the energy status of cells. J Bioenerg. 1970;1:215–225. [PubMed: 4274114]
- 2.
- Falke JJ, Drake SK, Hazard AL. et al. Molecular tuning of ion binding to calcium signaling proteins. Q Rev Biophys. 1994;27:219–290. [PubMed: 7899550]
- 3.
- Carafoli E, Santella L, Branca D. et al. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. [PubMed: 11370791]
- 4.
- Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–1122. [PMC free article: PMC122154] [PubMed: 11830654]
- 5.
- Cooke IM, Lipkin M. Cellular Neurophysiology. New York: Holt, Rinehart and Winston. 1972
- 6.
- Fatt P, Katz B. The electrical properties of crustacean muscle fibres. J Physiol-London. 1953a;120:171–204. [PMC free article: PMC1366030] [PubMed: 13062231]
- 7.
- Fatt P, Ginsborg BL. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol-London. 1958;142:516–543. [PMC free article: PMC1356760] [PubMed: 13576452]
- 8.
- Huxley AF, Stampfli R. Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibres. J Physiol-London. 1951;112:496–508. [PMC free article: PMC1393028] [PubMed: 14825229]
- 9.
- Nastuk WL, Hodgkin AL. The electrical activity of single muscle fibres. J Cell Comp Physl. 1950;35:39–73.
- 10.
- Draper MH, Weidmann S. Cardica resting and action potentials recorded with an intracellular electrode. J Physiol-London. 1951;115:74–79. [PMC free article: PMC1392010] [PubMed: 14889431]
- 11.
- Heilbrunn LV, Wiercinski FJ. The action of various cations on muscle protoplasm. J Cell Compar Physl. 1947;29:15–32. [PubMed: 20285919]
- 12.
- Ebashi S, Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Bio. 1968;18:123–183. [PubMed: 4894870]
- 13.
- Fatt P, Katz B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol-London. 1953b;121 [PMC free article: PMC1366081] [PubMed: 13085341]
- 14.
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol-London. 1949;108:37–77. [PMC free article: PMC1392331] [PubMed: 18128147]
- 15.
- Fatt P, Katz V. An analysis of the end-plate potential recorded with an intra-cellular electrode. J Physiol-London. 1951;115
- 16.
- del CastilloJ, Katz B. Quantal components of the end plate potential. J Physiol-London. 1954b:560–573. [PMC free article: PMC1366292] [PubMed: 13175199]
- 17.
- Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242:244–246. [PubMed: 4540479]
- 18.
- Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. [PubMed: 2434854]
- 19.
- Hodgkin AL, Keynes RD. Movements of labelled calcium in squid giant axons. J Physiol-London. 1957;138:253–281. [PMC free article: PMC1363043] [PubMed: 13526124]
- 20.
- del CastilloJ, Katz B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Mol Bio. 1956;6:122–. [PubMed: 13420190]
- 21.
- del CastilloJ, Stark L. The effect of calcium ions on the motor end-plate potentials. J Physiol-London. 1952;116:507–515. [PMC free article: PMC1392217] [PubMed: 14946716]
- 22.
- del CastilloJ, Engbaek L. The nature of the neuromuscular block produced by magnesium. J Physiol-London. 1954;124:370–384. [PMC free article: PMC1366273] [PubMed: 13175138]
- 23.
- del CastilloJ, Katz B. The effect of magnesium on the activity of motor nerve endings. J Physiol-London. 1954a;124:553–559. [PMC free article: PMC1366291] [PubMed: 13175198]
- 24.
- Jenkinson DH. The nature of the antagonism betwen calcium and magnesium ions at the neuromuscular junction. J Physiol-London. 1957;138:434–444. [PMC free article: PMC1363054] [PubMed: 13481883]
- 25.
- Katz B. Nerve, Muscle, and SynapseNew York: McGraw-Hill,1966.
- 26.
- Hodgkin AL. The Croonian Lecture: Ionic movements and electrical activity in giant nerve fibres. Proc Roy Soc (London) ser B. 1958;148:1–37. [PubMed: 13494473]
- 27.
- Katz B. The Croonian Lecture: The transmission of impulses from nerve to muscle, and the subcellular unit of synaptic action. Proc Roy Soc (London) ser B. 1962;155:455–477.
- 28.
- Huxley AF. Muscle. Annu Rev Physiol. 1964;26:131–152. [PubMed: 14145317]
- 29.
- Llinás R, Steinberg IZ, Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. P Natl Acad Sci USA. 1976;73:2913–2922. [PMC free article: PMC430802] [PubMed: 183215]
- 30.
- Augustine GJ, Charlton MP, Smith SJ. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. [PubMed: 2436546]
- 31.
- Bullock TH, Grinnell AD. Susumu Hagiwara - November 6, 1922-April 1, 1989. Biogr Mem Natl Acad Sci. 1996;69:59–85. [PubMed: 11616259]
- 32.
- Hille B. Ionic Channels of Excitable Membranes, Second Edition. Sunderland, MA: Sinauer Associates. 1992
- 33.
- Hamill OP, Marty A, Neher E. et al. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. [PubMed: 6270629]
- 34.
- Hagiwara S, Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966;49:793–806. [PMC free article: PMC2195507] [PubMed: 5943615]
- 35.
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol-London. 1967;192:479–492. [PMC free article: PMC1365567] [PubMed: 6050160]
- 36.
- Katz B, Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol-London. 1967;192:407–436. [PMC free article: PMC1365564] [PubMed: 4383089]
- 37.
- Reuter H, Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol-London. 1968;195:451–470. [PMC free article: PMC1351672] [PubMed: 5647333]
- 38.
- Rougier O, Vassort G, Garnier D. et al. Existence and role of a slow inward current during the frog atrial action potential. PflüArch. 1969;308:91–110. [PubMed: 4976798]
- 39.
- Beeler Jr. GW, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol-London. 1970;207:191–209. [PMC free article: PMC1348700] [PubMed: 5503869]
- 40.
- Johnson EA, Lieberman M. Heart: excitation and contraction. Ann Rev Physiol. 1971;33:479–532. [PubMed: 4951054]
- 41.
- Colatsky TJ, Tsien RW. Electrical properties associated with wide intercellular clefts in rabbit Purkinje fibres. J Physiol-London. 1979;290:227–252. [PMC free article: PMC1278833] [PubMed: 469754]
- 42.
- Kass RS, Siegelbaum SA, Tsien RW. Three-micro-electrode voltage clamp experiments in calf cardiac Purkinje fibres: is slow inward current adequately measured? J Physiol-London. 1979;290:201–225. [PMC free article: PMC1278832] [PubMed: 469751]
- 43.
- Marban E, Tsien RW. Effects of nystatin-mediated intracellular ion substitution on membrane currents in calf purkinje fibres. J Physiol-London. 1982;329:569–587. [PMC free article: PMC1224797] [PubMed: 6292409]
- 44.
- Lee KS, Akaike N, Brown AM. The suction pipette method for internal perfusion and voltage clamp of small excitable cells. J Neurosci Meth. 1980;2:51–78. [PubMed: 7329091]
- 45.
- Fleckenstein A. History of calcium antagonists. Circ Res. 1983;52:I3–16. [PubMed: 6339106]
- 46.
- Hagiwara S, Ozawa S, Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975;65:617–644. [PMC free article: PMC2214882] [PubMed: 240906]
- 47.
- Fox AP, Krasne S. Two calcium currents in Neanthes arenaceodentatus egg cell membranes. J Physiol-London. 1984;356:491–505. [PMC free article: PMC1193178] [PubMed: 6097675]
- 48.
- Brown AM, Camerer H, Kunze DL. et al. Similarity of unitary Ca2+ currents in three different species. Nature. 1982;299:156–158. [PubMed: 6287284]
- 49.
- Llinαs R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol-London. 1981;315:549–567. [PMC free article: PMC1249398] [PubMed: 6273544]
- 50.
- Carbone E, Lux HD. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984;46:413–418. [PMC free article: PMC1434947] [PubMed: 6487739]
- 51.
- Fedulova SA, Kostyuk PG, Veselovsky NS. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol-London. 1985;359:431–446. [PMC free article: PMC1193384] [PubMed: 2582115]
- 52.
- Armstrong CM, Matteson DR. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985;4682:65–67. [PubMed: 2578071]
- 53.
- Matteson DR, Armstrong CM. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986;87:161–182. [PMC free article: PMC2217130] [PubMed: 2419479]
- 54.
- Bean BP. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985;86:1–30. [PMC free article: PMC2228774] [PubMed: 2411846]
- 55.
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. [PubMed: 2410796]
- 56.
- Reuter H, Stevens CF, Tsien RW. et al. Properties of single calcium channels in cardiac cell culture. Nature. 1982;297:501–504. [PubMed: 6283360]
- 57.
- Armstrong CM, Matteson DR. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985;227:65–67. [PubMed: 2578071]
- 58.
- Nilius B, Hess P, Lansman JB. et al. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985;316:443–446. [PubMed: 2410797]
- 59.
- Nowycky MC, Fox AP, Tsien RW. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. P Natl Acad Sci USA. 1985;82:2178–2182. [PMC free article: PMC397516] [PubMed: 2580308]
- 60.
- Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. [PubMed: 6207437]
- 61.
- McCleskey EW, Fox AP, Feldman DH. et al. ω-Conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. P Natl Acad Sci USA. 1987;84:4327–4331. [PMC free article: PMC305078] [PubMed: 2438698]
- 62.
- Hirning LD, Fox AP, McCleskey EW. et al. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61. [PubMed: 2447647]
- 63.
- Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. [PubMed: 2560643]
- 64.
- Boland LM, Morrill JA, Bean BP. ω-Conotoxin block of N-type calcium channels in frog and rat sympathetic neurons. J Neurosci. 1994;14:5011–5027. [PMC free article: PMC6577193] [PubMed: 8046465]
- 65.
- Reuter H. A variety of calcium channels. Nature. 1985;316:391. [PubMed: 2410795]
- 66.
- Miller RJ. Multiple calcium channels and neuronal function. Science. 1987;235:46–52. [PubMed: 2432656]
- 67.
- Swandulla D, Armstrong CM. Fast-deactivating calcium channels in chick sensory neurons. J Gen Physiol. 1988;92:197–218. [PMC free article: PMC2228895] [PubMed: 2844957]
- 68.
- Lux HD. Studies on the development of voltage-activated calcium channels in vertebrate neurons In: Grinnell AD, Armstrong D, Jackson MB, eds. Calcium and Ion Channel Modulation New York: Plenum Press, 1988313324. [PubMed: 2847210]
- 69.
- Birnbaumer L, Campbell KP, Catterall WA. et al. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. [PubMed: 7917287]
- 70.
- Ertel EA, Campbell KP, Harpold MM. et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. [PubMed: 10774722]
- 71.
- Llinás R, Sugimori M, Lin JW. et al. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. P Natl Acad Sci USA. 1989;86:1689–1693. [PMC free article: PMC286766] [PubMed: 2537980]
- 72.
- Llinás R, Sugimori M, Hillman DE. et al. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992;15:351–355. [PubMed: 1382335]
- 73.
- Mori Y, Friedrich T, Kim MS. et al. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. [PubMed: 1849233]
- 74.
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. [PMC free article: PMC6577783] [PubMed: 7722641]
- 75.
- Gillard SE, Volsen SG, Smith W. et al. Identification of pore-forming subunit of P-type calcium channels: an antisense study on rat cerebellar Purkinje cells in culture. Neuropharmacology. 1997;36:405–409. [PubMed: 9175621]
- 76.
- Jun K-S, Piedras-Rentería ES, Smith SM. et al. Ablation of P/Q-type Ca2+ channel currents and progressive, fatal ataxia in mice lacking the a1A subunit. Proc Natl Acad Sci USA. 1999 [PMC free article: PMC24805] [PubMed: 10611370]
- 77.
- Bourinet E, Soong TW, Sutton K. et al. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. [PubMed: 10321243]
- 78.
- Olivera BM, Rivier J, Scott JK. et al. Conotoxins. J Biol Chem. 1991;266:22067–22070. [PubMed: 1939227]
- 79.
- Ellinor PT, Zhang J-F, Randall AD. et al. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;63:455–458. [PubMed: 8389006]
- 80.
- Newcomb R, Szoke B, Palma A. et al. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. [PubMed: 9799496]
- 81.
- Soong TW, Stea A, Hodson CD. et al. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. [PubMed: 8388125]
- 82.
- Niidome T, Mori Y. Primary structure and tissue distribution of a novel calcium channel from rabbit brain. Ann NY Acad Sci. 1993;707:368–372. [PubMed: 9137571]
- 83.
- Zhang J-F, Randall AD, Ellinor PT. et al. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. [PubMed: 8107963]
- 84.
- Cribbs LL, Lee JH, Yang J. et al. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. [PubMed: 9670923]
- 85.
- Perez-Reyes E, Cribbs LL, Daud A. et al. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. [PubMed: 9495342]
- 86.
- Lee JH, Daud AN, Cribbs LL. et al. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999;19:1912–1921. [PMC free article: PMC6782566] [PubMed: 10066244]
- 87.
- Wu LG, Borst JG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA. 1998;95:4720–4725. [PMC free article: PMC22557] [PubMed: 9539805]
- 88.
- Albillos A, Neher E, Moser T. R-Type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci. 2000;20:8323–8330. [PMC free article: PMC6773200] [PubMed: 11069939]
- 89.
- Yasuda R, Sabatini BL, Svoboda K. Plasticity of calcium channels in dendritic spines. Nat Neurosci. 2003;6:948–955. [PubMed: 12937422]
- 90.
- Breustedt J, Vogt KE, Miller RJ. et al. Alpha1E-containing Ca2+ channels are involved in synaptic plasticity. P Natl Acad Sci USA. 2003;100:12450–12455. [PMC free article: PMC218778] [PubMed: 14519849]
- 91.
- Tanabe T, Beam KG, Adams BA. et al. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–569. [PubMed: 2165570]
- The Key Role of Ca2+ Channels in Cellular Signaling
- The Winding Road of Calcium Channel Discovery
- The First Paper Drew Little Attention Because the Investigators Themselves Were Unsure of What They Had Found
- The Next Advance in Understanding Was Slow in Coming
- Interest in Excitability Revolved around Proposed Mechanisms in Squid Axons
- The Studies in Crustacean Muscle Focused on Electrogenesis Only, without Reference to Muscle Contraction
- The Uncovering of Ca2+ Spikes Appeared Amidst a Crowded Field of Discovery
- The Diversity of Native Ca2+ Channel Currents
- Acknowledgments
- References
- A Brief History of Calcium Channel Discovery - Madame Curie Bioscience DatabaseA Brief History of Calcium Channel Discovery - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...