This publication is provided for historical reference only and the information may be out of date.
This chapter addresses the symptoms, complaints, and other medical sequelae commonly seen in persons using various forms of stimulants (e.g., cocaine, crack, amphetamines, methamphetamine [MA]) who appear at hospital emergency rooms (ERs) and other medical settings, or who need specialized medical care while participating in residential or outpatient substance use disorder programs. The purpose of the chapter is to assist medical personnel in recognizing and treating problems that may arise for stimulant users with acute or chronic intoxication or in various phases of withdrawal after protracted use of these drugs and differentiating these from similar presentations of other medical and psychiatric conditions. Another emphasis is the need for establishing and ensuring linkages between medical facilities and appropriate, comprehensive substance use disorder treatment/rehabilitation programs.
In a meta-analysis of 555 consecutive cocaine-related visits to hospital ERs in four cities between 1989 and 1992, Schrank concluded that catastrophic complications directly related to the use of this stimulant comprise only a small fraction of the sequelae (Schrank, 1993). Deaths were relatively rare, occurring in only four patients. In this sequence of cases, there were no reports of myocardial infarction, intracranial hemorrhage, ischemic stroke, infarcted bowel, or pulmonary barotrauma. The most common reasons for ER visits by cocaine users were cardiopulmonary symptoms (usually chest pains or palpitations); psychiatric complaints, ranging from altered mental states to suicidal ideation; and neurological problems, including seizures and delirium.
Most of the patients had multiple problems, many related to intravenous substance use. Rapid medical intervention was crucial for cocaine users with seizures, hyperthermia, potentially lethal arrhythmias, or toxic delirium. However, most patients responded well to simple evaluation, observation, and supportive care. Pharmacological intervention was required in less than one-fourth of the 555 cases. Schrank emphasized the importance of recognizing concurrent use of multiple substances and the presence of cocaine or other drugs in victims of traumatic injury and in obstetrical patients.
MA users are much less likely than individuals using cocaine to arrive at the ER with such acute medical problems as cerebrovascular accidents, acute cardiac ischemia and failure, hyperthermia, or seizures. The major presenting symptoms for MA users pertain primarily to altered mental status, including confusion, delusions, paranoid reactions, hallucinations, and suicidal ideation. The rapid development of tolerance to its physiological effects among chronic MA users may explain the relative infrequency of cardiac complications in this group (Heischobar and Miller, 1991).
Toxicity, Addiction, and Other Adverse Reactions
The precise clinical effects of cocaine and MA depend on a complex mixture of the pharmacological properties and purity of the drug used; the dose, frequency of use, and route of drug administration; the user's state of intoxication or withdrawal and previous experience with the drug; and other concomitant medical and psychiatric conditions, including simultaneous use of other substances as well as personality attributes and expectations regarding drug reactions. All of these factors not only mediate drug effects, but also influence the user's susceptibility to substance abuse or dependence (Ellinwood and Lee, 1989; Gold, 1997).
Route of Administration
The method by which stimulants are taken--the route of administration--determines the dosage and the rapidity and intensity of effects. Route of administration also affects the potential for adverse reactions and the likelihood of addiction. The principal routes used to administer cocaine and MA are oral ingestion, nasal insufflation (snorting), intravenous injection, and inhalation of smoke vapors (smoking/inhalation). These stimulants can also be taken vaginally, rectally, or sublingually.
In general, smoking and intravenous use rapidly evoke similarly intense responses, whereas oral ingestion and intranasal administration are slower delivery mechanisms, causing lower and more gradually rising blood levels and less intense subjective responses. Indeed, cocaine is seldom taken by mouth in this country because first-pass hepatic biotransformation metabolizes 70 to 80 percent of the dose and substantially diminishes the drug's effects (Gold and Miller, 1997). When crack cocaine is smoked, a highly concentrated dose is rapidly delivered to the brain. Several studies have reported a close correlation between subjects' plasma levels and the subjective effects from single doses given to relatively naive users who have not developed tolerance (Ellinwood and Lee, 1989; Gold and Miller, 1997; Volkow et al., 1997a). As the efficiency of the delivery system increases, so does the intensity of both the pleasurable and adverse effects. Figure 5-1 depicts these general variations in response times according to the different routes of administration for cocaine and MA.
Table
Figure 5-1: Effects of Route of Administration for Cocaine and MA.
To some extent, the dangerous consequences and addictive potential of stimulants also reflect the route of drug administration. Oral ingestion of MA is thought to protect the user from cardiotoxicity (Cook et al., 1993), and the lower and more sloped peak blood levels achieved by this route are also thought to be responsible for lower rates of addiction (Gold and Miller, 1997). Also, cocaine appears to be less addictive if doses remain small, peak plasma levels and the onset of drug effects are slow, and unpleasant withdrawal effects are absent or minimal. Oral ingestion and, to some extent, intranasal routes fulfill these criteria of slower, less hazardous consequences (Gold, 1997).
Intravenous use is more toxic than intranasal or oral routes, but inhalation is generally perceived as the quickest and, from the user's perspective, the most desirable delivery method because smoked crack cocaine and ice MA produce the highest peak blood levels and the most potent subjective impact without attendant hazards from syringe needle use (Cho, 1990; Cook, 1991; Gold, 1997). Other investigators report that smoked ice does not seem to produce the same intense "rush" as injection. There is some indication that in utero exposure alters cocaine reinforcement properties for adults or, at least, increases rates of cocaine self-administration in the laboratory (Gold and Miller, 1997).
Different routes of drug administration also produce different side effects. Intravenous users frequently develop illnesses associated with the preparation of drugs for use (i.e., mixing/making) and the use or sharing of unsterile needles, including HIV infection, hepatitis, tuberculosis, lung infections and pneumonia, bacterial or viral endocarditis, cellulitis, wound abscesses, sepsis, thrombosis, renal infarction, and thrombophlebitis (Sowder and Beschner, 1993; Gold, 1997).
Nasal insufflation is associated with sinusitis, loss of sense of smell, congestion, atrophy of nasal mucosa, nosebleeds, perforation or necrosis of the nasal septum, hoarseness, and problems with swallowing. Crack users complain of throat ailments and a productive cough with black sputum (Gold, 1997; Gold and Miller, 1997). Intravenous use of MA is associated with greater severity of medical and social problems compared with other routes of administration (Sowder and Beschner, 1993).
Figure 5-2 compares differences in use and consequent problems between intravenous and nonintravenous MA users. Apparently, MA users recognize these route-related effects and tend to vary the routes of administration because the drug causes irritation to nasal mucosa and lungs (Center for Substance Abuse Treatment [CSAT], 1997). Even prolonged use of amphetamine-containing diet pills has resulted in ischemic colitis and pulmonary edema (Sowder and Beschner, 1993).
Figure
Figure 5-2: Dose Frequency Escalation Patterns, Cocaine and Amphetamine.
Differences Between Cocaine and MA
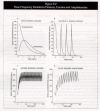
The major differences between cocaine and MA pertain to the rapidity of responses and the duration of their effects. The sought-after effects of MA can persist for hours, whereas those from cocaine are over in minutes. This has important consequences for the choice of drug and the patterns of administration adopted by users. The plasma levels from smoked crack cocaine both peak and decline rapidly, whereas those from smoked MA also peak relatively rapidly but decline more slowly because metabolism takes longer. Regularly repeated use may be more common among cocaine users trying to sustain the drug's effects, whereas withdrawal is more protracted for MA users (Cook, 1991; Gold and Miller, 1997). Figure 5-3 shows some of the differences between cocaine and MA.
Table
Figure 5-3: Differences Between Cocaine and MA.
The plasma levels of cocaine/crack peak and decline rapidly, with a terminal half-life of about 56 to 60 minutes. MA plasma concentrations also peak rapidly but remain high for much longer (Cho, 1990; Cook, 1991). In normal subjects, the plasma half-life of cocaine ranges from 40 to 90 minutes (Rowbotham, 1993).
Because the biological half-life of cocaine is relatively short, repeated dosing is necessary to sustain an effect (Gold and Miller, 1997). By contrast, repeated dosing with MA, before metabolism and elimination are complete, can result in substantial accumulation of the drug in the body with increased likelihood for addiction (Cho, 1990; Cook, 1991).
Other factors in the growing preference for smokable forms of MA, as well as crack cocaine, include availability and price. Crack is generally less expensive and more available than powdered cocaine hydrochloride and produces, in the initial smoker, a very intense but brief rush described by some as a "full body orgasm" (Gold, 1997). Because ice costs less than other forms of MA per dose, and because the euphoria attained may persist for several hours, this form of MA delivers the most "bang for the buck." Because abuse liability increases as time before onset of action decreases, and the concentrations of the drug that reach the brain and receptor sites increase (Cornish and O'Brien, 1996), the current concern about increased use of stimulants pertains to both the smokable preparations (crack and ice) and to continuing intravenous use of both drugs.
Dose
The incidence and severity of MA- and cocaine-induced side effects and toxic reactions are also dose-related. As the dose increases, the profile of side effects progresses from mild excitement to more intense reactions, even psychosis (CSAT, 1997). Because tolerance develops rapidly to the desired euphoric effects, stimulant users nearly always escalate dose size and frequency of drug use in pursuit of the vanishing rush. If initial use was by oral or intranasal routes, users also tend to switch to intravenous administration or inhalation, methods that promise more rapid response rates and peak plasma levels (Ellinwood and Lee, 1989).
Chronic MA users may consume as much as 15 grams of the drug per day in doses exceeding 1 gram every 4 hours over a 24-hour period. Because the conventional dose is 10 mg, doses of 150 mg to 1 g would ordinarily be highly toxic to naive users (Cho, 1990). There is, however, considerable individual variation in toxicity and overdose from stimulants. Although general ranges have been established for lethal doses and blood levels, reactions are unpredictable (Gold and Miller, 1997).
The lethal dose of cocaine for 50 percent of novice users (LD50) is 1.5 grams. The LD50 for MA has not specifically been established, and there is significant individual variability to its toxicity. For example, doses of 30 mg can produce severe reactions, yet doses of 400 to 500 mg are not necessarily fatal. Reported tissue levels of MA in fatalities, nonetheless, have ranged from 1µg/mL to over 14 µg/mL. Reported blood levels have also ranged from 27 µg/mL to only 0.6 µg/mL (Mori et al., 1992).
Purity of the Drug
The purity of the stimulant used also influences the rate and completeness of its absorption and effects. The purer the drug, the greater the effects. "Street" drugs, however, are seldom entirely pure. The purity of confiscated cocaine hydrochloride intended for oral or intranasal consumption generally ranges between 20 and 80 percent; the purity of intravenous cocaine preparations can vary between 7 and 100 percent; and for freebase or crack intended for smoking, from 40 to 100 percent (Gold and Miller, 1997). Most seized batches of MA have 40 to 70 percent purity (Burton, 1991; CSAT, 1997).
Adulterants are added to cocaine to increase its weight by cutting or substituting less expensive but similar-tasting and acting products that will maximize profits for the dealer while still satisfying the customer. In general, the adulterants in cocaine do not pose serious health-related problems, although these cannot be completely discounted (Schrank, 1993). Cocaine is most often cut with mannitol, lactose, quinine, glucose, or other inert compounds for weight, and with caffeine, lidocaine, other stimulants, anesthetics, or hallucinogens for taste and effect (Schrank, 1993; Gold, 1997).
The manufacturing processes for illicit MA and ice are often crude and can involve many impurities and contaminants that do pose serious health consequences. Until recently, most of the MA sold on the street was manufactured from phenyl-2-propanone (P2P), a method of synthesis in which lead acetate is used as a chemical reagent. The large quantities of lead in the final product can result in symptoms of hepatitis, nephritis, and encephalopathy (Allcott et al., 1987). Two outbreaks of lead poisoning in Oregon in 1977 and 1988 involving a total of 14 cases among intravenous MA users were blamed on the lead acetate used in the P2P manufacturing process. Testing revealed the presence of 60 percent lead by weight in one case (Irvine and Chin, 1991).
The typical clandestine manufacturing process for MA has changed over the last 12 years--from the P2P method--to an ephedrine-based method and, more recently, to pseudoephedrine and phenylpropanolamine processing (CSAT, 1997). The difference between these two primary synthesis methods is primarily the precursor chemicals used.
The newer and more popular ephedrine method, which accounted for 89 percent of production in 1995, makes it simpler to synthesize MA, uses less strictly controlled ingredients, produces less odor than chemical reactions involving P2P, and yields a more potent and psychoactive form of MA (with a higher percentage of the more active dextro steroisomer, rather than equal proportions of dextro and levo stereoisomers produced by the P2P method) (Burton, 1991; Cho, 1990; CSAT, 1997; Drug Enforcement Agency [DEA], 1996). In addition, dextro-MA is three to four times more potent to the central nervous system than levo-MA (Sowder and Beschner, 1993). Therefore, the MA currently being manufactured has especially potent effects.
Illicit MA is also likely to contain potentially toxic contaminants from unintended reaction byproducts and reagent residuals as well as processing errors. Many clandestine laboratories are operated by uneducated and unskilled chemists who get recipes from unpublished, handwritten sources or through the Internet. As with cocaine, most of the contaminants are intentional fillers used to dilute or cut the product and may include lactose, lidocaine, procaine, caffeine, quinine, or sodium bicarbonate.
Other impurities in illicit MA can cause dangerous toxic reactions. Some identified contaminants have been shown to have great potency for producing seizures in mice. Poisoning from other reagents and organic byproducts, including mercury, has also been suspected but not documented (Burton, 1991).
Patterns of Use
The effects of stimulant use also reflect the temporal pattern of drug administration and the user's experience history or chronicity. Users describe various motivations for initial experimentation with cocaine or MA, including a desire for heightening a sense of well-being and euphoria, increasing alertness and energy, boosting self-esteem, enhancing sexual desire and responsiveness, dispelling fatigue, improving performance, losing weight, or consuming more alcohol without feeling intoxicated (Hando and Hall, 1997; Sowder and Beschner, 1993). Some users only administer stimulants periodically, although most discover that tolerance builds rapidly to many of the desired effects, particularly euphoria, so that increasing doses are needed to achieve similar effects.
Although serious medical, psychological, and social consequences have followed experimental low-dose use of stimulants, two other patterns of self-administration are of greater concern. The fourth edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Press, 1994) characterizes these as (1) episodic use, separated by at least 2 days of nonuse, with gradually escalating doses and more potent routes of administration that often culminate in binge use; and (2) daily, or almost daily, use with no wide fluctuations in dose, but a gradual escalation. Actually, compulsive users probably represent only 5 to 10 percent of the total number of MA users and an even smaller percentage of all users of amphetamines (Cho, 1990).
Because of their different pharmacological properties, MA users typically administer the drug on a daily basis, whereas crack cocaine users binge on large amounts for a shorter period, interspersed by periods of nonuse (CSAT, 1997; King and Ellinwood, 1997). Figure 5-4 illustrates rapidly escalating and sustained plasma levels attained by different time patterns of drug administration during cocaine and MA binges. An extremely compulsive pattern of rapidly repeated injections or inhalations is required to sustain high plasma levels of cocaine, in contrast to amphetamine/MA, which has a longer half-life (Ellinwood and Lee, 1989).
Table
Figure 5-4: Common Signs and Symptoms of Acute Stimulant Intoxication.
The greatest behavioral pathology and most serious medical consequences usually follow compulsive bingeing with high doses of either smoked or injected MA or cocaine (Ellinwood and Lee, 1989). The following paragraphs describe the sequence of phases that typically occur in the establishment of this hazardous, "high-transition" pattern and some of their accompanying side effects (as depicted in Ellinwood and Lee, 1989; King and Ellinwood, 1997). Knowledge of these phases can be useful for the medical practitioner in taking a substance use history and understanding what effects are likely to accompany a particular stage of acute intoxication, withdrawal, or more chronic use patterns.
Intoxication
Stimulant use phases
- Initiation, single-dose phase. Early use of a single dose of stimulants results in euphoria and increased energy that correspond closely to stimulant plasma levels. Higher levels of euphoria are achieved by injection or inhalation routes of administration that evoke a rapid rise to peak drug concentrations. The rush experienced by persons who inhale (smoke) or inject stimulants is profoundly rewarding and reinforcing. Classical conditioning to the cues associated with drug use reputedly occurs during this initial phase.
- Consolidation, dose-frequency escalation phase. As tolerance develops to the euphorigenic effects, users tend to increase doses and frequency of stimulant administration in an attempt to recapture the original and most intense rush sensations. They may also switch the route of administration to get a more rapid response. During this phase, intermittent consumption is prolonged with the discovery that higher doses produce greater effects.
- Maintenance phase with bingeing. High-dose and frequent-use patterns often lead to even more compulsive bingeing over a few hours to days that ceases only when the user is totally exhausted or the stimulant supply runs out. Binges typically last 12 to 18 hours (but may last 2 to 3 days or longer) for cocaine users and much longer--from 3 to 15 days--for MA users. The high and sustained plasma levels achieved during binges can have considerable pathological effects. The binge is characterized by frequent mood swings as plasma levels of the stimulant fluctuate. Stereotypic behaviors and thinking exclude other concerns so that the user focuses exclusively on internal sensations and withdraws from social activities in pursuit of direct pharmacological effects. Almost all activity is directed to acquiring the drug and consuming it. Also, the settings in which drugs are consumed are progressively restricted.
"Crash" and withdrawal syndrome phases
- Early crash phase. The binge terminates with a "crash" that has several successive phases that follow each other in rapid succession over a relatively brief interval after a cocaine run, but are more prolonged and pronounced in MA use. Dysphoria, anxiety, and agitation begin a short time after cessation of stimulant use, followed by an intense drug craving that often leads to recidivism. Users may exhibit a repetitive cycle of bingeing, with an intervening crash, over a period of several months. The more protracted withdrawal from ice produces a particularly irritable and jittery state that coincides with the initial "come-down" period after binge use and is a dangerous time because the user is short-tempered and unpredictable. This "tweaking" period is exacerbated by the user's prolonged lack of sleep. At this point, the tweaker is extremely frustrated because no dose will reestablish euphoria. Although not apparently intoxicated, the tweaker may have rapid eye movements, concise but quivery speech, and brisk, somewhat jerky movements The tweaker's thinking seems scattered and subject to paranoid delusions.
- Middle crash phase. Anxiety and agitation are followed by a period of fatigue, increasing depression, and anhedonia with decreased mental and physical energy. An intense desire for sleep, often accompanied by insomnia, usually replaces the drug craving. During this part of the crash, users may use alcohol, benzodiazepines, or opiates to induce and prolong sleep. The middle crash phase terminates with a period of protracted sleep, often for 24 to 36 hours during which time any attempt at therapy or other intervention is inappropriate.
- Late crash phase. The period of hypersomnolence in the late crash phase is often followed upon wakening by intense hunger.
- Protracted withdrawal. Following the crash phase (or early withdrawal), the user experiences symptoms that are opposite to those of stimulant intoxication: fatigue, loss of physical and mental energy, depression, anhedonia, and a limited interest in his surroundings. These symptoms may increase in intensity over the 12 to 96 hours immediately following the crash, or they may wax and wane over several weeks. A severe and persisting depression in this phase can result in suicidal ideation or suicide attempts and is a major concern for the user. Anhedonia and dysphoria usually dissipate over a 6- to 18-week period for MA users. In the protracted withdrawal phase, periods of drug craving may reemerge. These cravings are often triggered by conditioned environmental cues and can only be extinguished by sustained abstinence.
Tolerance/Sensitization To Stimulant Effects
Chronic users of stimulants develop tolerance to many of the initial effects, often after only a few weeks of drug use. This means that a higher dose is required to achieve the same effects, or markedly diminished effects are attained if the same dose is continued (American Psychiatric Press, 1994). Most notably, tolerance develops rapidly to the euphorogenic effects of stimulants and is the ostensible cause for most dose escalation by stimulant users--although dose increases may also stem from a desire to experience more intense effects. Tolerance also develops to the anorectic effects of MA in humans because weight loss stops after several weeks. Tolerance also appears to develop to the cardiotoxic effects of large doses of MA that many users survive. In fact, many of the initial symptoms of stimulant intoxication disappear with chronic use: Blood pressure may be normal, and nausea and vomiting are seldom seen. This tolerance is not the result of increased MA metabolism because chronic users show metabolic patterns similar to naive users (Angrist, 1994).
Interestingly, chronic, high-dose stimulant users may also become sensitized to the drug, a unique phenomenon characteristic of psychomotor stimulants. Sensitization is essentially the reverse of tolerance and produces undesirable effects with lower doses of the drug than were required to yield these same reactions in an earlier phase of the addiction process. There appears to be some sensitization to the psychosis-inducing effects of stimulants in humans. After one psychotic episode is experienced following chronic, high-dose use, a lower minimal dose of cocaine or MA may induce another psychotic episode, with more rapid onset following drug intake and a longer duration than the initial psychosis. Many sensitized stimulant users experience an almost immediate return of paranoia, psychosis, and stereotyped thinking if drug use is resumed (Angrist, 1994; CSAT, 1997). The sensitization process in stimulant dependence is elaborated in the section on toxic psychosis in this chapter.
Clinical Manifestations And Medical Management
As already noted, the intensity and duration of acute manifestations of stimulant intoxication correlate generally with the rate of rise and the height of peak blood levels reflected in brain concentrations. Acute intoxication with stimulants resembles hypomania or a manic state. In low doses, the libido is stimulated and sexual performance is enhanced. In high doses, spontaneous ejaculation and orgasm can occur. With increasing doses, poor judgment, indiscretions, sexual acting-out, and other bizarre behaviors or mental alterations are more likely to be seen. Acute stimulant intoxication can result in seizures, confusion, dystonias, respiratory depression, chest pain, or cardiac arrhythmias (Gold and Miller, 1997) (see Figure 5-4).
Distinctive Characteristics Of MA Intoxication
- There may be a peculiar odor of ammonia or stale urine, especially among users who smoke MA that has been crudely synthesized in illicit laboratories. Smoked ice is, however, essentially odorless.
- The MA user may present with tachycardia (rapid heart rate), although not usually accompanied by arrhythmia (irregular heartbeat). Compared with cocaine intoxication, MA intoxication causes fewer heart, pulmonary, and circulatory problems, especially for users of newer forms of ephedrine-based dextro-MA that stimulate the heart, lungs, and blood vessels to a lesser degree than older forms containing equal parts of levo- and dextro-MA (Inaba et al., 1993).
- MA users are more likely to appear in the ER as a result of trauma from fighting or motor vehicle accidents than for physical complaints.
- Because of its longer lasting effects, MA abuse may lead to more frequent psychiatric impairment, more potent central nervous system (CNS) effects, and more overdoses. Chronic abuse of MA (beyond 2 weeks) is more hazardous than chronic cocaine abuse because of MA's sustained effects. Moreover, drug-induced psychoses in MA users are likely to last longer than those of cocaine users and, in addition, may not respond as readily to available treatments.
- MA users are more likely than cocaine users to be single substance rather than polysubstance users (although many also use marijuana).
- Stereotyped activity--persistent, repetitive, and compulsive activity such as vacuuming the same part of the floor over and over again, popping knuckles repeatedly, picking at scabs, or taking apart and reassembling mechanical devices--may appear in MA users.
Distinctive Characteristics Of Cocaine Intoxication
- Cocaine users are more likely than MA users to present with serious and potentially lethal physical complications (e.g., cardiac arrhythmia, chest pains, cerebrovascular accident [e.g., strokes], toxic seizures, hypertension crises, hyperthermia).
- Cocaine users are also more likely than MA users to use multiple substances, especially alcohol, benzodiazepines, or opiates.
Management of Stimulant Intoxication
Acute MA intoxication, unless delirium or psychosis is present, seldom comes to medical attention. Most cocaine users who come to an ER with drug-related complaints have not used the drug for several hours, and peak plasma levels have already subsided, especially if the cocaine was injected or smoked (Rowbotham, 1993).
Uncomplicated intoxication requires only observation and monitoring in a subdued environment until symptoms subside over several hours. General measures include monitoring of vital signs for rising pulse rate, temperature, or blood pressure; providing a quiet and cool environment that helps to diminish agitation and overreaction to external stimuli; and close observation. Physical exertion and an overheated room can potentiate adverse effects because stimulants affect the body's heat-regulating mechanism at the same time that blood vessel constriction conserves heat. Although verbal reassurance is usually sufficient for quieting the patient, indications that agitation is escalating and moving toward paranoia and potential psychosis, with increasing risk for violence, may warrant pharmacological intervention. Fast-acting benzodiazepines such as lorazepam (Ativan) or diazepam (Valium) are useful for calming an anxious, agitated patient (Ellinwood, 1975; Weis, 1997).
Stimulant Overdose
Toxic, fatal, or subfatal syndromes are seldom seen in chronic, high-dose, intravenous stimulant users, probably because tolerance develops rapidly. Most stimulant overdose fatalities occur in neophytes or persons who accidentally ingest large amounts, such as "bodypackers" or children (Ellinwood, 1975). (Bodypackers are individuals who have swallowed waterproof packets filled with cocaine, usually in South America, in an attempt to clear U.S. customs undetected and then pass the packets through the gastrointestinal tract.) It should be noted, however, that the toxic dose for stimulants has enormous variability and appears to be idiosyncratic and unpredictable, without a known relationship to body weight. Hence, the amount of cocaine or MA used is not a reliable predictor of the reaction (Weis, 1997).
The symptoms of a sublethal stimulant overdose may include dizziness, tremor, irritability, confusion, hostility, hallucinations, panic, headache, skin flushing, chest pain, palpitations, cardiac arrhythmias, hypertension, vomiting, cramps, and excessive sweating. High doses of stimulants may cause high fever, cardiac arrhythmias and arrest, irregular breathing, seizures, and stroke. Agitated states characterized by increased aggressiveness or psychotic features may also occur with intoxication, particularly for MA (Weis, 1997). The development of hyperpyrexia (excessively high fever), severe hypertension, convulsions, and cardiovascular collapse signal a life-threatening situation (Ellinwood, 1975).
Lethal doses of stimulants--administered to laboratory dogs by injection or observed in bodypackers when ingested packets leak or break--produce a predictable sequence of events culminating in generalized convulsions and death. Heart rate, blood pressure, cardiac output, and body temperature rise rapidly, and a toxic delirium is observed before generalized and terminal seizures begin (Ellinwood, 1975; Rowbotham, 1993; Wetli, 1993).
Management of a Potentially Lethal Overdose
Stimulant users who present with life-threatening medical conditions and toxic drug levels should be treated with standard life-saving techniques that respond to the presenting symptoms (Weis, 1997). Acute neurological symptoms such as seizures or rapidly elevating vital signs require immediate intervention. Nondrug causes of any symptoms should be carefully ruled out, and the patient should also be evaluated for multiple substance use. Stimulant overdose patients should be hospitalized, especially if treatment is threatened by polysubstance use (Gold, 1997).
No specific antidotes or antagonists to stimulant overdose are available--unlike naloxone (Narcan) for opiates and the benzodiazepine antagonist flumazenil (Romazicon). However, the following procedures are suggested:
- Request specialist consultations as needed.
- Manage hyperthermia by sedating to slow down and stop agitated movements and rapidly cooling the patient with body ice packs, mist and fan techniques, or cooling blankets (Ellinwood, 1975; Gold, 1997). Simple measures such as preventing or correcting elevated body temperature can be remarkably effective in preventing death from cocaine toxicity (Rowbotham, 1993). Although dantrolene (Dantrium) can be administered for very serious and escalating, uncontrolled hyperthermia (Weis, 1997), such drugs do not always enhance the cooling process for patients with life-threatening hyperthermia (Goldfrank and Hoffman, 1993).
- If restraints are required to start an intravenous administration, use mesh-type blankets only transiently to avoid interfering further with heat loss.
- Provide adequate ventilation and oxygenation.
- Uncontrolled hypertension can be managed by intravenous administration of phentolamine (Regitine) or dopamine (Intropin). Although clinical experience supports the use of labetalol (Normodyne) for treating hypertension, no controlled experimental studies support the efficacy of this drug as an alpha-adrenergic blocker (its beta-adrenergic effects are more potent). Rapidly acting and easily controlled antihypertensive agents such as the vasodilator nitroprusside or the alpha-adrenergic blocker phentolamine are usually preferable (Goldfrank and Hoffman, 1993).
- Treat seizures like status epilepticus with intravenous diazepam or other benzodiazepine. Diazepam is most effective if administered before or shortly after cocaine ingestion but is less effective after seizures begin (Rowbotham, 1993). Phenobarbitol or phenytoin (Dilantin) may be used if diazepam is ineffective (Schrank, 1993). Alternatively, 25 to 50 mg of intravenous pentobarbitol can be administered to control cocaine-induced seizures (Gold, 1997).
- Complaints of chest pain warrant evaluation for possible myocardial ischemia and infarction. Nitrates are indicated for cocaine-induced myocardial ischemia to alleviate coronary vasoconstriction. Beta-adrenergic blockers such as propranolol (Inderal) should not be used because they may enhance vasospasm. Aspirin should be administered, unless contraindicated, to reduce cocaine-mediated platelet aggregation (Goldfrank and Hoffman, 1993).
Use standard treatments for arrhythmias, including phenytoin. Atrial arrhythmias that do not respond to cooling and sedation may require cautious use of calcium channel blockers or mixed alpha-/beta-adrenergic blockers such as verapamil (Calan), esmolol (Brevibloc), and labetalol (Goldfrank and Hoffman, 1993). Lidocaine may be contraindicated for ventricular arrhythmias that begin immediately after cocaine use as a response to catecholamine excess but is appropriate for ventricular arrhythmias that indicate an ischemic myocardium. Sodium bicarbonate has proven useful for cocaine-induced wide-complex arrhythmias (Goldfrank and Hoffman, 1993). Also note that management of acute psychiatric manifestations of cocaine intoxication by sedation appears to have a salutary effect on emerging cardiovascular complications.
In general, phenothiazines, especially chlorpromazine (Thorazine, Mellerial), are contraindicated because these drugs lower the seizure threshold (Gold, 1997). Haloperidol (Haldol) has not proven efficacious in preclinical studies in protecting against cocaine-induced seizures or fatalities, but it may have utility for MA-induced psychoses. The serious difficulties encountered in using haloperidol for sedative-hypnotic withdrawal in humans when agitation and hyperthermia are present may also apply to its use for acutely agitated or psychotic stimulant users who already have deficits in thermoregulatory control. Haloperidol may precipitate or exacerbate acute dystonic reactions associated with cocaine use (Goldfrank and Hoffman, 1993).
Manifestations of Stimulant Withdrawal/Abstinence
A characteristic withdrawal-type syndrome usually develops within hours to days after cessation of prolonged and heavy stimulant use. The symptoms can follow long-term use or much shorter binges. Although "cocaine blues" were described as early as the turn of the century (Gawin and Kleber, 1986), more recent investigators note that stimulant withdrawal is much less definitive than withdrawal from opiates or alcohol and has not been so well studied (Lago and Kosten, 1994; West and Gossop, 1994). (See Figure 5-5 for some common signs of stimulant withdrawal.)
Table
Figure 5-5: Common Signs and Symptoms of Stimulant Withdrawal/Abstinence Syndrome.
Some clinicians distinguish between stimulant withdrawal symptoms following a several-day binge and complaints that characterize withdrawal after more chronic high-dose use. Stimulant users who have binged for 2 to 3 days are dysphoric, exhausted, and sleep for 24 to 48 hours. Cocaine users in this category commonly use alcohol, marijuana, benzodiazepines, or heroin with cocaine to reduce irritability. Following more chronic and regular stimulant use, withdrawal symptoms that subside over 2 to 4 days include dysphoria, irritability, difficulty sleeping, and intense dreaming (CSAT, 1995d).
Other clinicians emphasize differences in severity between withdrawal from cocaine and withdrawal from MA. A substantial number of persons with cocaine dependence have no clinically evident withdrawal symptoms. For the minority of cocaine users who do complain, symptoms begin within hours to days of the last dose, the crash lasts for 3 to 4 days, withdrawal persists from 1 to 10 weeks, with waxing and waning of the drug craving. The mood state of the cocaine user may return to normal after several days to a month.
Withdrawal symptoms seem to be most severe in the initial days following cessation of use (Cornish and O'Brien, 1996; Gold and Miller, 1997). Although there are no physical manifestations of a withdrawal syndrome when MA use is stopped, there are several symptoms that occur when a chronic user stops taking the drug (National Institute on Drug Abuse [NIDA], 1998a). Symptoms begin 12 to 24 hours after binge use is terminated and may persist for 1 to 2 weeks. The client initially feels depressed and anxious, with an intense craving for MA.
This phase is followed by fatigue and sleepiness, although this may be mixed with insomnia. Upon awakening after prolonged sleep, the client may be very hungry, and there may be persisting anhedonia and dysphoria. Other symptoms include paranoia and aggression. Depression appears to be more severe and prolonged following MA use and is correlated with duration of use and size of the doses (Gold and Miller, 1997; Gawin and Ellinwood, 1988).
Management of Stimulant Withdrawal
Stimulant withdrawal is not medically life threatening and, unlike alcohol or barbiturate withdrawal, does not require pharmaceutical intervention. Although no consistent physiological disruptions requiring gradual withdrawal have been observed, some medications may be used to attenuate symptoms and provide support.
The greatest risk from the distinctive stimulant abstinence syndrome is of doing harm to self or others. Because withdrawal-related dysphoria and depression can be particularly severe in stimulant users, risk of suicide is intensified, and sensitive management is essential. Cocaine-induced depression usually dissipates fairly rapidly--in a matter of hours. The depression is agitated and often related to actual situations resulting from drug use (e.g., the client is disturbed that all of his money has been "blown" on the cocaine binge or that interpersonal relationships are jeopardized by his continuing drug dependence).
However, withdrawal-associated depression following high-dose MA use is more prolonged. During the tweaking phase of withdrawal, the high-dose MA user begins a rocky, jittery reaction characterized by agitated paranoia, extreme frustration, and the return of intense drug cravings. Suicidal ideation may be high, and violence is easily provoked.
Tweaking effects after persistent bingeing on ice are particularly dangerous. Clients may misinterpret caretakers' gestures and turn against them. Restraints and sedation in a secure facility may be necessary. Although stress reduction techniques and other approaches to preventing harm should be used standardly, medical personnel can also use benzodiazepines (e.g., diazepam) to control agitation and tachycardia (see further discussion of violence as a special issue).
For clients with preexisting diagnosed or unrecognized clinical depression, cocaine worsens symptomatology. These individuals are most likely to experience deepening dysphoria and/or paranoia after cocaine use. Treatment with selective serotonin reuptake inhibitors (SSRIs) may be of use (Gold, 1997).
Continuing agitation and persistent inability to fall asleep during the tweaking stage may also be treated symptomatically by using the antidepressant trazodone (Desyrel), whose dopaminergic properties help to sedate the client. Benadryl is also used for its sedating properties and for its effects on the dermatologic problems that often accompany MA use (e.g., itching and hypersensitivity of the skin). However, caution should be exercised in using any medications with high abuse/dependence potential. In general, prescriptions should not be written for use outside the treatment facility because use or resale of these drugs is very tempting to this population.
After the tweaking stage, MA abstainers usually "crash" and sleep several days at a time, depending on the dose and duration of the binge. This hypersomnolence may interfere with assessment of mental status and potential for dangerous behavior. Hence, clients should be evaluated immediately after wakening from this prolonged sleep for persisting dysphoria and other psychiatric symptoms of anxiety and depression (Weis, 1997). During this hypersomnolent state, and until sleep deprivation is overcome, active participation in therapy or followup of a referral to a treatment program by stimulant users is not a realistic expectation.
Drug craving during stimulant withdrawal has been treated with a variety of medications (e.g., bromocriptine, amantadine) without demonstrated efficacy in alleviating symptoms, getting clients "clean," or preventing relapse.
"Cocaine dreams" may occur during this period or as late as 8 or 9 months after termination of stimulant use during a protracted abstinence phase. They usually entail vivid recall of actually using and experiencing the high. The client may actually sweat and experience other symptoms of intoxication while dreaming. These intense dreams, which may sometimes contain vignettes in which the drug user loses or drops a supply or refuses to smoke crack/ice, can be used therapeutically to convince clients that they are making progress in treatment by making a subconscious choice not to use. Otherwise, the dreams may enhance drug cravings and intensify a vulnerability for relapse. These dreams are primarily experienced by users of injected cocaine/MA and smoked crack or ice.
Because stimulant users frequently self-medicate withdrawal symptoms with alcohol, benzodiazepines, or opiates, there may be symptoms of withdrawal from these drugs if they have been used continuously or at high doses. These require specific management and titration of substitute doses or other means of alleviating symptoms.
Manifestations of Chronic Stimulant Use Disorders
Although fatalities from stimulant overdose or acute myocardial infarction following administration of cocaine by inexperienced users have been documented, and other medical and psychiatric complications have been observed at all dose levels and routes of administration among naive users, the majority of serious stimulant-induced medical and psychological complications follows chronic, high-dose use.
Because tolerance develops rapidly to the subjective and cardiovascular effects of stimulants, the specification of complications following chronic use is complex (Rowbotham, 1993). However, cocaine toxicity affects nearly every organ system, with the most dramatic changes found in the cardiovascular system, the brain, the liver, and the pulmonary system (Majewska, 1996). Although there are some minor differences between the sequelae of chronic MA and cocaine use, the incidence of such side effects as chest pain, seizures, paranoid reactions, and suicidal thoughts is about the same for both drugs. Chronic MA users appear to have more headaches, severe depression, and hallucinations than counterpart cocaine users, but the evidence from community samples is not definitive (CSAT, 1997).
Figure 5-6 summarizes some of the more common symptoms and potentially serious complaints presented by chronic stimulant users. The following section contains a detailed description of stimulant-induced medical and psychiatric complications with limited comments about management of these conditions. Schrank specifies more detailed ER procedures for responding to some of the more frequently seen complications (Schrank, 1993). Figure 5-7 shows the distinctive indicators of chronic MA use and chronic cocaine use.
Table
Figure 5-6: Common Symptoms of Chronic Stimulant Abuse/Dependence.
Table
Figure 5-7: Distinctive Indicators of Chronic Abuse of Cocaine Versus MA.
Identification and Management of Medical Complications
Cardiovascular System Effects
Cardiotoxicity stemming from catecholamine excess is observed in both cocaine and MA users. Although the cardiac effects are more profound for MA users because this drug results in even greater elevation of catecholamines than cocaine, the incidence of fatalities following myocardial infarction has been less frequent until the recent increase in inhaling (smoking) or injecting MA (Cho, 1990; Cook et al., 1993; CSAT, 1997).
Stimulants, especially cocaine, have been linked to virtually every form of heart disease, including different forms of arrhythmias, coronary vasospasm, myocardial ischemia, myocardial infarction, and cardiomyopathy (Cornish and O'Brien, 1996; Gold, 1997). Case reports of fatalities from myocardial infarction and tachyarrhythmias document their occurrence at all dose levels and routes of administration in otherwise healthy young adults without the usual coronary risk factors, but preexisting coronary artery disease can exacerbate the response and increase the likelihood of sudden death, as can hyperthermia and agitation (Ellinwood and Lee, 1989; Gold, 1997; Schrank, 1993). Tachycardia, hypertension, ruptured blood vessels, arrhythmias, and arteriosclerotic lesions typically precede myocardial ischemia and infarction (Majewska, 1996). With prompt medical intervention, patients generally survive stimulant-induced cardiomyopathy with heart failure (CSAT, 1997). There is some controversy about optimal pharmacologic approaches to treating arrhythmias and other cardiac effects. Lidocaine was previously used but may be contraindicated for ventricular arrhythmias because it lowers the seizure threshold (Goldfrank and Hoffman, 1993). However, certain calcium channel blockers (e.g., Nifedipine, diltiazem, verapamil) seem promising (Gold, 1997; Schrank, 1993).
Respiratory/Pulmonary Effects
Cocaine crack smokers frequently seek medical attention for difficulties in breathing (dyspnea) or severe chest pain. This may result from cocaine-induced pulmonary hemorrhage, lung damage, pneumonia, pulmonary edema, asthma, pneumothorax, pneumomediastinum, or pneumopericardium (Cornish and O'Brien, 1996; Gold, 1997). Pulmonary barotrauma may result from spasmic coughing following smoke inhalation or odd mechanisms of drug delivery (mouth-to-mouth inhalation), with sudden increases in airway pressure that result in alveolar rupture and the induction of free air into the pleural cavity, mediastinum, or subcutaneous tissues. The amount of free air is usually small and resolves spontaneously under observation. The possibility of esophageal rupture should be considered in pneumomediastinum if vomiting is present (Schrank, 1993). Cocaine can also cause sudden death by respiratory failure from drug-induced inhibition of the medullary centers in the brain (Gold, 1997).
Tracheobronchitis with cough is a frequent accompaniment of crack smoking, as are lobar and nonlobar pneumonias. Bronchospasm is another complaint of these smokers, usually in clients with a history of asthma (Schrank, 1993). Crack lung is a new syndrome that manifests with symptoms of pneumonia--severe chest pains and breathing problems with high fever--but no substantiating lung X-ray evidence. The condition does not respond to standard treatment, although anti-inflammatory drugs may relieve symptoms. Clients with crack lung may suffer from oxygen starvation or loss of blood with potentially fatal results (Gold, 1997).
Pulmonary edema has been observed in both cocaine and MA fatalities and attributed variously to deep inhalation aggravation of preexisting conditions (Nestor et al., 1989) and granulomas formed in response to adulterants added to the drugs (CSAT, 1997). Chronic obstructive lung disease in MA users is thought to result from thrombosis of small pulmonary vessels with gradual reduction of pulmonary vascular bed, pulmonary fibrosis, and granuloma formation (CSAT, 1997).
Cerebrovascular Complications
An increasing amount of research has recently focused on neurological impairments apparently resulting from high-dose and chronic use of stimulants, particularly by more rapid routes of administration. Some of the more devastating cerebrovascular consequences of cocaine and MA use have been known for years--seizures, ischemic strokes, and subarachnoid and intracerebral hemorrhages. Other neurological complications include optic neuropathy, global brain ischemia, and edema following myocardial infarction. Newer brain-imaging techniques now demonstrate various degrees of previously undetected and unsuspected cerebral atrophy and brain lesions in many chronic cocaine users (Cornish and O'Brien, 1996; Schrank, 1993; Majewska, 1996).
Seizures are a well-known complication of cocaine use, occurring almost immediately after any of the more rapid delivery routes, but not always dose-related. Chronic use may sensitize (kindle) an individual's response, but this is not definitively proven (Daras, 1996; Gold, 1997). Most cocaine-induced seizures are of short duration and leave no residual effects, although prolonged seizures can be catastrophic (see earlier references under overdose management) (Schrank, 1993; Cornish and O'Brien, 1996).
Cerebral hemorrhage and ischemic strokes are relatively rare events for stimulant users but occur more frequently with users of crack and ice. At least half of those who suffer brain hemorrhages have such underlying abnormalities as arteriovenous malformations and cerebral aneurysms. Stimulant-induced hypertension probably leads to rupture of these abnormalities that will also require surgical intervention to correct. Cocaine-induced hypertension and vasospasm seem to be associated with other cases.
The toxic role of simultaneous alcohol and cocaine use that produces cocaethylene (see later discussion) is also under investigation (Schrank, 1993; Daras, 1996). Another unresolved issue is whether CNS vasculitis is a causal factor; MA-induced necrotizing vasculitis has been documented since 1970 (Miller et al., 1993). Cocaine-using clients who complain of headache while intoxicated should be evaluated for possible intracranial hemorrhage (Ellinwood and Lee, 1989; Daras, 1996).
The recently documented neurological deficiencies found in chronic cocaine users, particularly in the basal ganglia and frontal cortex, are similar to those found in a variety of neurological/psychiatric disorders, including bipolar disorder, schizophrenia, and frontal lobe degeneration from seizures, stroke, or injury that is accompanied by dementia, apathy, depression, and social disinhibition (Majewska, 1996). Animal studies have confirmed similar enduring, possibly permanent, CNS changes, associated with repeated high doses of MA. Neurotoxicity at an early age may predispose stimulant users to premature onset of movement disorders such as Parkinson's disease and other dystonic or choreoathetoid disorders involving undulating, involuntary, whole body movements that may appear at the end of a prolonged binge in chronic users and are not related to use of neuroleptic medications (CSAT, 1997; Gold and Miller, 1997).
An array of cognitive deficits is also observed in cocaine and other stimulant users that also characterizes brain aging and dementia and may indicate premature brain aging or possible cerebral atrophy in these drug-dependent individuals. These include problems in attention, concentration, problem-solving, abstraction, arithmetic performance, new learning, and short-term memory (Majewska, 1996; Cornish and O'Brien, 1996; Gold, 1997). Unfortunately, many of the studies documenting these deficits lack adequate data on respondents' premorbid performance (Daras, 1996).
Muscular and Renal Toxicity
Cocaine and MA may be direct muscle toxins because acute rhabdomyolysis--a condition that destroys skeletal muscle--has been diagnosed in users who did not have any of the previously associated risk factors (i.e., hyperthermia, agitation, seizures, hypotension, toxic delirium or coma, or acute renal failure). Muscle necrosis may occur after any route of drug administration, and the presence of rhabdomyolysis should be considered in stimulant-intoxicated clients, particularly those complaining of myalgia or muscle tenderness. One study found that one-fourth of clients presenting with cocaine-related complaints had evidence of mild, usually asymptomatic, rhabdomyolysis--defined as elevated creatine kinase levels that were five times higher than normal (Goldfrank and Hoffman, 1993; Schrank, 1993).
Although mild cases of rhabdomyolysis may not lead to renal complications, renal insult and failure is a distinct possibility for patients with concomitant hyperthermia, seizures, delirium, or coma (Schrank, 1993). Renal failure from rhabdomyolysis has been reported as an outcome of cocaine and MA use (Scandling and Spital, 1982). Accompanying hepatic damage is rare and probably an idiosyncratic response (CSAT, 1997).
Gastrointestinal Complaints
Abdominal pain, nausea, and vomiting are experienced by some stimulant users, probably indicating mild intestinal ischemia. Severe bowel infarction with elevated white blood cell counts, metabolic acidosis, and shock have also been observed. Occasionally, severe abdominal pain, bowel obstruction, or sudden onset of seizures are indicative of leakage or rupture of packets of cocaine ingested by "bodypackers" (Schrank, 1993). Another observed syndrome, "cocaine colitis," manifests as abdominal pain along with diarrhea and bloody stools, probably indicating diffuse gastrointestinal hemorrhage and tissue necrosis (Goldfrank and Hoffman, 1993).
Infections
As already noted, intravenous injection of cocaine or MA is associated with a variety of infectious diseases. Unsterile paraphernalia are particularly likely to result in blood-borne transmission of HIV/AIDS and hepatitis B, C, and D. Associated malnutrition in chronic users further lowers resistance to infection. Injection cocaine users are at greater risk of infectious endocarditis than other parenteral drug users (Daras, 1996).
Disinhibition and the initial aphrodisiac effects of stimulants are associated with participation in high-risk and unprotected sexual activity. Vigorous and prolonged sexual activity or anal intercourse is likely to damage tissues or protective condoms and thereby increase the likelihood of transmitting sexually contracted diseases (Cornish and O'Brien, 1996).
Effects on Reproductive Function And Fetus/Newborn
Use of stimulants by pregnant women has been related to poor obstetrical outcomes and adverse effects for the developing fetus, the newborn and the older child. An increased incidence of preecalmpsia, spontaneous abortions, and abruptio placentae has been observed among cocaine-using pregnant women. Toxic effects also result in fetal cerebral infarctions as well as low birth weight for gestational age and small head circumference. Recent studies in several separate locations have found similar rates of these complications in MA- and cocaine-using women and their prenatally exposed offspring (CSAT, 1997; Oro and Dixon, 1987).
Newborns exposed to stimulants in the womb may have poor feeding and sleep patterns, tremor, and hypertonia. Difficulties in consoling these "jittery" babies may inhibit close bonding with their mothers and contribute to developmental problems (Gold, 1997). Despite a spate of articles outlining probable behavioral and cognitive deficiencies in prenatally stimulant-exposed children, more recent meta-analyses have only confirmed lower birth weight compared with controls, but no clearly attributable effects on the fetus, infant, or child (Rabin and Little, 1994; Cornish and O'Brien, 1996).
A major problem with most of the earlier studies is that polysubstance use among pregnant women is ubiquitous, so that attribution of prenatal-exposure effects to any single drug is very difficult. Additional methodological confounds were introduced by differences in mothers' nutritional status, prenatal care, socioeconomic level, and trimester of maternal drug use, as well as a failure to account for the impact of the home environment in predicting IQ (CSAT, 1997; Cornish and O'Brien, 1996). Essentially, the long-term effects of maternal stimulant use on offspring are not known.
Chronic, high-dose stimulant use also affects reproductive and sexual functioning in both males and females. Men report gynecomastia (development of breasts), loss of sexual interest, impotence, and difficulty in maintaining an erection or ejaculating. Women have derangements of the menstrual cycle, including amenorrhea and infertility, as well as greater difficulty in achieving orgasm (Gold, 1997). Female stimulant users who have irregular or no menstrual periods often think they cannot get pregnant, although they are not always infertile and can have unwanted pregnancies. Testing for pregnancy and regular use of birth control should be encouraged.
HIV/AIDS and Hepatitis
Increased HIV and hepatitis B and C transmission are likely consequences of increased stimulant use, particularly in individuals who inject intravenously and share equipment. Infection with HIV and other infectious diseases is spread among injection drug users primarily through the reuse of contaminated syringes, needles, or other paraphernalia by more than one person. In nearly one-third of Americans infected with HIV, injection drug use is a risk factor, making substance abuse the fastest growing vector for the spread of HIV in the nation (NIDA, 1998a).
Research also indicates that MA and related psychomotor stimulants can increase libido in users, in contrast to opiates, which actually decrease libido. However, long-term cocaine use may be associated with decreased sexual functioning, at least in men (Rawson et al., 1998b). In addition, the use of MA seems to be associated with rougher sex, which may lead to bleeding and abrasions. The combination of injection and sexual risks may result in HIV becoming a greater problem among MA users than among opiate and other substance users, something that already seems to be happening in California (NIDA, 1998a).
Identification and Management of Psychological Complications
Toxic Psychosis
Initially described by Young and Scoville in 1938, amphetamine psychosis is a usually brief and spontaneously remitting paranoid state that is frequently accompanied by intense, fear- evoking delusions and hallucinations, but with clear consciousness and a relatively intact formal thought process (Angrist, 1994). Stimulant-induced psychosis occurs while the user is intoxicated, not in withdrawal after drug cessation (Tinklenberg, 1975). The condition is not rare or idiosyncratic, but typically follows chronic, high-dose administration of amphetamines, MA, or cocaine. However, this drug-induced psychosis is more prevalent among amphetamine and MA users than those who use cocaine, probably because the short half-life of cocaine makes it difficult to accumulate and sustain high plasma levels of that drug (Angrist, 1994; King and Ellinwood, 1997). Nonetheless, the condition has been reported after acute intoxication in relatively naive users and occasionally after low doses.
Original reports of the condition described a threshold dose for eliciting a psychotic response as chronic administration of 50 mg amphetamine daily, but at least 10 cases have been documented at lower doses, and there are also case studies of psychotic reactions after a single dose (usually high) or only brief exposure to the drug (Angrist, 1994). Because studies of stimulant users have found a surprising prevalence of coexisting, often premorbid, psychiatric disorders (one-fourth of participantsin one study had preexisting schizophrenia), low doses of stimulants may actually precipitate latent schizophrenia in some users whose psychosis is then mistakenly diagnosed as stimulant-induced (Angrist, 1994).
Amphetamine-induced psychosis
Several investigators claim that a toxic paranoid reaction or psychosis, usually accompanied by delusions and/or hallucinations, is a probable complication of high-dose MA use. Amphetamine-induced psychosis has been investigated prospectively under experimental conditions in at least two studies involving fewer than 50 clients. Griffith and colleagues successfully elicited psychotic symptoms in 25 of 31 experienced users after high-dose administration of MA and observed that 22 of the 25 were frankly psychotic (Griffith et al., 1972). Bell evoked similar amphetamine-induced psychosis in 11 of 13 subjects (Bell, 1973)--the remaining 2 were found to have preexisting schizophrenia (Angrist, 1994). Surveys of chronic cocaine users in treatment have also found that one-half to two-thirds had paranoid experiences that were not trivial (Angrist, 1994).
However, there are methodological problems with each of the investigative approaches to studying stimulant-induced psychosis that make the findings less than compelling. When this condition is studied prospectively, the drug-experienced volunteers who must be used for ethical reasons have unknown (unobserved) sensitivity and tolerance to stimulants; the numbers are, of necessity, quite small for drawing definitive conclusions, and at least some of the participants have managed to continue drug use, even under rigorous laboratory conditions. Data from case reports have other drawbacks: The premorbid history of drug use and psychiatric status is unknown and may not be accurately reported by respondents (Angrist, 1994; CSAT, 1997).
Development of toxic psychosis
Some researchers and clinicians describe the development of stimulant-induced psychosis as an evolving process. Panel members depicted MA users as having brief and transient psychotic episodes before a full-blown psychosis emerges after more extensive chronic use. MA users often recognize these early psychotic effects and try to stave them off by self-medicating with alcohol or decreasing drug use. In several articles, Ellinwood and colleagues describe the evolution of MA-induced psychosis as progressively abnormal behaviors--beginning at moderately high doses--with intense feelings of curiosity about the environment and patterns of exploration that result, for example, in examining the punctuation periods in a magazine text for evidence of a secret code (Ellinwood et al., 1973).
This first enthusiasm about "discoveries" moves over time and increasing doses from "watching the world" to feelings of being watched. Behaviors become more fixed and stereotyped, culminating with intense suspiciousness and, in psychotic reactions, paranoid delusions that misinterpret environmental cues. Visual hallucinations may be overreactions to barely glimpsed and recognizable objects in the client's peripheral vision; auditory hallucinations similarly begin with hearing simple noises. In later stages of the psychosis, the client loses all contact with reality and has delusions of persecution. If he is exhausted after a prolonged binge, the hyper-reactivity to stimuli and confusion can lead to panic, sudden violence, even homicide (King and Ellinwood, 1997).
Manifestations of toxic psychosis
The DSM-IV (American Psychiatric Press, 1994) distinguishes between cocaine intoxication with "perceptual disturbances" and cocaine-induced psychotic disorder with either delusions or hallucinations (depending on which is the prominent feature). In the former, the drug user has intact reality testing and is aware that auditory, visual, or tactile hallucinations are substance-induced, not actual representations of external reality. Another common manifestation, before paranoia is rampant and as deterioration develops, is stereotypy--persistent, repetitive acts such as disassembling and reassembling radios or other small gadgets that seems to offer some relief from agitation and anxiety. Even though the client seems to know that the behavior is meaningless, stopping it results in irritability and frustration (King and Ellinwood, 1997; Tinklenberg, 1975). As chronic, high-dose stimulant consumption continues, most users also withdraw from all social interactions and initiate other bizarre behaviors before the intensive drug use culminates in paranoid reactions or psychosis without any insight into activities.
Symptoms of stimulant-induced toxic psychosis usually abate spontaneously within a week (CSAT, 1997). Hallucinations stop within 24 to 48 hours of abstinence, and paranoia and delusions decrease over the next week to 15 days. The client may sleep after the first 24 hours for as long as 3 days, with extensive dreaming during this phase (Ellinwood, 1975). Clinicians also report that drug-induced psychosis dissipates more quickly for cocaine users--usually in 1 to 3 days--compared with up to 2 to 3 weeks for MA users. Users of ice are reputed to have the most intense and persistent psychoses (Sowder and Beschner, 1993).
Toxic stimulant psychosis can have typical and atypical presentations. Case reviews have established that approximately 80 percent of these psychotic clients experience paranoid delusions; 60 to 70 percent have hallucinations; 12 percent have tactile hallucinations (e.g., cocaine bugs crawling on the skin); olfactory hallucinations are present in fewer than 10 percent; and about 7 percent become disoriented. Hyperactivity and excitation are usually present (Tinklenberg, 1975; Ellinwood, 1975; Angrist, 1994). The client is generally oriented and has intact memory and an appropriate level of consciousness. Clients remember the psychotic episodes with remarkable clarity (Tinklenberg, 1975; Ellinwood, 1975). Thought disorder, if present, is usually mild and transient (CSAT, 1997).
A few clients are confused--usually delirious from high doses to which they have not developed tolerance; bizarre, usually autoerotic, sexual behavior is present in some, and others have destructive outbursts or make unmotivated assaults. One investigator found no differences in symptoms between relatively naive and chronic amphetamine users with psychotic reactions, but others claim that individuals who binge on high doses over a few days have more delusions and disorganized hallucinations and paranoid ideation than chronic users who have more systematic delusions. Intravenous drug administration caused no change in symptoms, but more rapid progression to psychosis (Angrist, 1994; CSAT, 1997).
The role of drug sensitization
Several issues pertaining to stimulant-induced psychosis remain unresolved. There is some disagreement about the role of drug sensitization (kindling) in precipitating more frequent toxic psychotic reactions at smaller-than-previously-required doses and sooner after drug use is reinitiated following a period of abstinence. There is also disagreement about the role of sensitization in deepening the depression experienced after withdrawal. The mechanisms for this "reverse tolerance" are not fully understood. Although animal experiments have shown that daily, intermittent dosing with stimulants results in sensitization, studies of amphetamine-induced psychosis in humans have yielded more ambiguous results (CSAT, 1997). However, a 1991 survey by Satel and colleagues of 50 cocaine-dependent clients consecutively admitted to a treatment program found that two-thirds (68 percent) had experienced paranoid psychosis while intoxicated and during the immediate postdrug crash (Satel et al., 1991).
The reported characteristics of this paranoia were consistent with a sensitization process. All of those with a paranoid reaction had, on average, years of binge use before paranoid symptoms gradually emerged. Anxiety gradually intensified during binges before frank paranoid delusions were experienced. Once paranoia emerged, every subsequent binge produced intensified reactions (despite use of anxiolytic street drugs by half of the group to ameliorate paranoid reactions), and the onset of these delusions after starting a run accelerated over time. Half of those who had experienced paranoid psychosis acknowledged engaging in bizarre behavior such as hiding or compulsively "checking up" on things; nearly two-fifths had secured weapons to protect themselves from imagined assailants. This paranoia persisted for an average of 12 hours, with near total resolution in 97 percent of cases before awakening after the postbinge crash (Gawin and Khalsa-Denison, 1996).
Similar results are reported by Brady and colleagues after a 1991 study of another 55 cocaine-dependent clients in treatment--53 percent had experienced cocaine psychosis, most with less drug and increasing frequency over time and with more rapid onset for the majority (Brady et al., 1991). It seems clear that sensitization to the psychosis-inducing effects of cocaine does occur, although the evidence for sensitization with amphetamines is somewhat less clear (Angrist, 1994).
Stress-induced psychosis
The role of stress or other "triggers" such as alcohol use or insomnia in precipitating the return of psychotic symptoms that were initially induced by stimulants is also controversial.
Some investigators have reported that stress can evoke the return of psychotic symptoms (delusions, hallucinations, paranoia, suicidal thoughts) without further amphetamine or MA use and after long periods of abstinence (NIDA, 1998a; Sowder and Beschner, 1993; Spotts and Spotts, 1980).
However, Angrist questions the accuracy of such reports of spontaneous or stress-induced psychosis following amphetamine psychosis because the reported cases were not carefully monitored with urine toxicologies to rule out continuing substance use or examined for the possibility of simultaneous development of another psychiatric disorder (Angrist, 1994).
Duration of toxic psychosis
The duration of toxic stimulant psychosis is another issue in some dispute. Typically, uncomplicated psychosis induced by stimulants resolves rapidly unless more of the drug is taken. However, several Japanese investigators (i.e., Tatesu, 1964; Nakatani, 1990; Iwanami et al., 1994 [as cited in Angrist, 1994]) have reported persisting psychoses in chronic stimulant users for up to 1 year after abstinence when amphetamine metabolites were no longer present.
Angrist argues that Western investigators do not see prolonged psychoses very frequently, and the persisting psychoses observed in Japan may actually be cases where stimulants precipitated latent schizophrenia (or bipolar disorder), or the disorder was present but undiagnosed before amphetamine use began (Angrist, 1994). He concludes that the potential for amphetamine to cause long-standing psychosis may be a complication for some individuals. This conclusion is, however, unproven because the premorbid state of clients in reported studies has not been known and because continued substance use has not been ruled out by urine toxicology monitoring.
Treatment of toxic psychosis
Treatment of the client who presents with toxic stimulant psychosis entails rapid, systematic visual assessment, continued observation and monitoring, and symptom management. All unnecessary stimulation should be reduced, but complete sensory deprivation should be avoided by providing quiet rooms with moderate lighting and sufficient space and insisting on subdued talking without any rapid or unexpected movements. The clinician should reassure the client that the condition is drug-induced and will subside (Tinklenberg, 1975). Restraints may be required initially to gain control of the client, but should be checked frequently to ensure that risk to extremities is minimized, that respiration is not compromised, and that heat loss is not inhibited. Agitation should be controlled promptly by sedation with parenteral benzodiazepines--usually diazepam.
Differential diagnosis of acute confusional states must be instituted immediately. Consideration should be given to the possibility of head injury, intracranial hemorrhage, or thyrotoxicosis. Information from significant others is helpful, and toxicology testing is also useful to confirm a diagnosis (Schrank, 1993).
Acute stimulant-induced psychosis should generally be managed in a hospital psychiatric department or similar facility. Minor psychotic episodes with low-grade symptoms that respond readily to neuroleptic medications may, on some occasions, be managed in a well-staffed, free-standing chemical dependency unit if sufficient personnel with training and experience in treating dual diagnosis are readily available. Urine testing is recommended to confirm a diagnosis of drug-induced psychosis because the syndrome can closely mimic other psychotic disorders such as schizophrenia, hypomania, depression, obsessive-compulsive reactions, or catatonia. However, a negative urine report does not necessarily mean that stimulants were not present (Ellinwood, 1975; Tinklenberg, 1975). The criteria for placement should reflect the persistence of the condition, the competence and training of personnel, and the drug taken. MA users who have accumulated high plasma levels from longer binges and larger doses of stimulants with longer half-lives are particularly prone to violence during psychosis--their paranoia makes them suspicious of attempts to medicate them, they are likely to become aggressive, and they don't comply with medication instructions after release from the hospital.
The criteria for continued hospitalization or inpatient care during psychosis are perceived risk or threats to self and others, as well as elevated vital signs, severe suicidal ideation, persistence of psychological or cognitive impairments beyond the usual time for spontaneous resolution, and severity of any medical problems such as serious heart disease, a history of infarcts, concomitant alcohol, barbiturate or opiate dependence, or diabetes and similar conditions that require careful monitoring (Tinklenberg, 1975). Release should not be considered until the medical crisis is resolved or until the patient has been stabilized psychologically for 24 hours and is able to self-calm without continuing use of neuroleptics. Buspirone hydrochloride (Buspar) is used experimentally in Hawaii to treat low-grade residual psychosis and is anecdotally effective when given in larger than usual doses (over 60 mg). It is relatively safe, although not advisable if the client has a history of benzodiazepine abuse.
Aggression and Violence
A major perceived problem associated with amphetamine/MA abuse is the potential for sudden and intense violence (Miczek and Tidey, 1989). MA has been associated with crime and combative behavior (Sowder and Beschner, 1993). Anecdotal reports by law enforcement officials, psychiatrists, and drug users themselves, as well as some surveys, link stimulants to aggression and unprovoked assaults. Users have committed murders and other violent acts while intoxicated with amphetamines. Nonetheless, the effects of stimulants on aggression and violence are complex and paradoxical. There are sharply differing opinions about the nature and extent of the problem (King and Ellinwood, 1997; Miczek and Tidey, 1989).
In a 1987 review of clinical observations and survey results, Miczek found differing representations of amphetamine effects (Miczek, 1987). Some surveys found that sizeable proportions of prison populations and juvenile delinquents commit crimes of violence while intoxicated by amphetamines, but other studies identified only rare cases and small percentages of juvenile delinquents and hostile persons who were amphetamine users. The reliability of these studies was unfortunately compromised by the lack of matched samples and reliance on verbal self-reports containing questionable information about dose and frequency of drug administration. It may be that intense violent acts are more prominent among chronic high-dose users, but no reports linked amphetamines to a high incidence of excessively violent behavior or other offensive social behavior (Miczek and Tidey, 1989).
Although well-controlled experimental studies of stimulant-associated aggression or violence in humans are scarce, the conclusions are similarly ambivalent. Early studies of cocaine or amphetamines did not focus on this aspect or note any increased aggression as a behavioral side effect. In fact, low doses (10 to 30 mg) of particular stimulants (i.e., Ritalin) have well-known and carefully studied beneficial effects on children 5 to 14 years old who are diagnosed with attention deficit/hyperactivity disorder (AD/HD) which manifests as aggressive, destructive, irritable, hyperactive behavior. Animal studies of mice, rats, squirrels, monkeys, and cats have examined aggression induced by isolation, pain, and brain stimulation in combination with amphetamines, with mixed results.
The most important determinants of aggression and defensive responses seem to be situation, species, prior experience with these behaviors, dosage, and chronicity of the stimulus. For example, a substantial increase in aggressive behavior can be evoked if amphetamines are administered to animals that are repeatedly confronting an intruder. Most importantly, there may be a biphasic dose effect on aggressive behavior in some animals: Aggression can be enhanced at low doses and also at higher doses, up to a point at which stereotypy and social withdrawal interfere (Miczek and Tidey, 1989; King and Ellinwood, 1997).
Other research more clearly confirms the effect of amphetamines on human aggression. A recent Japanese study ascertained that MA users scored higher on tests of verbal and physical aggressiveness and on impulsiveness than either alcoholic or normal control groups (Mukasa, 1990 [cited in Sowder and Beschner, 1993]). An earlier investigator found that participants in a task that enabled them to reward competitors with money or punish them with white noise increased their aggressiveness after 5 and 10 mg doses of amphetamines, whereas caffeine reduced the frequency of this aggressive behavior (Cherek et al., 1986 [cited in King and Ellinwood, 1997]).
Probably the most useful explanation of amphetamine effects on violence is offered by researchers who claim that stimulants do have a specific, but complex association with violent behavior. Chronic, moderate-to-high dose MA use, especially if the drug is injected or used by another rapid route of administration, often results in assaultive behavior and other forms of violence in the context of an interaction of behavioral and psychological effects (e.g., hyperactivity, agitation, emotional lability, and paranoid delusional thinking) combined with personality factors and social environment (King and Ellinwood, 1997). In other words, certain individuals who are regularly using high doses of amphetamine may be prone to intense violence, especially if experiencing paranoid delusions, but it is not known how frequently this occurs or what circumstances/personality characteristics promote this reaction (Miczek and Tidey, 1989).
Prevention of aggressive behavior
The Consensus Panel notes that the combination of low impulse control, paranoia, poor judgment, and grandiosity experienced by the chronic MA user, especially during a psychotic or prepsychotic episode, is a natural setup for violence. Similarly, the combination of a long-acting drug and a sustained high--because of MA's ready availability and low cost--results in a more severe/intense withdrawal reaction and accompanying susceptibility toward violence. The tweaker who is ready to crash after bingeing on ice does not need provocation to react aggressively, but confrontation increases the likelihood of a violent reaction.
Because drug-induced psychoses apparently increase the potential for violence in response to perceived persecution and paranoia, sound behavioral management techniques to prevent this negative and dangerous response are essential. The techniques listed in Figure 5-8 have been demonstrated to be useful and should be adopted by ER personnel as well as emergency medical technicians and police.
Co-Occurring Disorders Among People With Stimulant Use Disorders
Stimulant users have a surprising number of co- or preexisting disorders that can make differential diagnosis challenging or complicate treatment. Recently, investigators have become more interested in the implications of premorbid conditions as potential indicators of vulnerability to stimulant dependence. Majewska points out the need for more research to establish the epidemiological relationships between preexisting neurological deficits resulting from genetic, developmental, traumatic, or neurotoxic factors and vulnerability to drug addiction (Majewska, 1996). More specifically, preclinical studies and some surveys seem to indicate that neurological deficits associated with AD/HD, neuroanatomical abnormalities, lead poisoning, alcoholism, posttraumatic brain lesions, and posttraumatic stress disorder (PTSD) may be correlated with increased vulnerability to stimulant addiction. Another investigator (Bauer, 1996) lists another set of conditions or disorders that frequently co-occur with cocaine use disorders and notes that these correlates represent potential confounds to research regarding the sequelae of cocaine abuse and dependence as well as potential risk factors for developing those disorders. These include antisocial personality disorder, depression, other DSM Axis I disorders, polysubstance use, aggression, a family history of alcoholism or other substance use disorders, prescribed psychoactive medications, seizures, head injury, HIV/AIDS, and other major medical problems.
The following sections describe some of the most commonly identified premorbid and co-occurring disorders among stimulant users, with some comments on treatment precautions.
Polysubstance Use
Concomitant use of a variety of other licit and illicit psychoactive substances is a common correlate of stimulant use. These substances are frequently used to attenuate aversive symptoms experienced in the posteuphoric phase of use (Weis, 1997) or may be administered to prolong or counter particular effects of stimulant intoxication. Different combinations of substances are used to titrate mood states or effects (CSAT, 1997).
Cocaine users tend to prefer alcohol, marijuana, or opiates. There is generally less alcohol use but more marijuana use among MA users than cocaine users (CSAT, 1997). Cigarette smoking is almost ubiquitous among stimulant users, usually to relieve perceived stress. Speedballing--simultaneous use of opioids and cocaine or other stimulants--is still prevalent in many places because the combination is perceived to smooth the effects of each drug. Some clients who are taking prescribed neuroleptics for psychiatric problems take stimulants to counteract the sedating properties of these antipsychotic medications (Weis, 1997).
Various reports indicate that 62 to 90 percent of cocaine users concurrently drink alcohol to prolong the high and attenuate unpleasant agitation and sleeplessness that emerge at the end of a binge (Gold, 1997; Gold and Miller, 1997). However, the combination of cocaine and alcohol appears to be particularly dangerous. Researchers have established that cocaethylene, an ethyl ester of benzoylecgonine, is formed in the liver when these two substances are used together and that this metabolite is particularly toxic to the liver. A substance user who combines cocaine and alcohol may experience more intense pleasure from the experience than using either substance alone, but is also exposed to the combined toxicities of cocaine and the even more potent cocaethylene (Gold, 1997; Cornish and O'Brien, 1996). Mendelson and colleagues found that a combination of ingested alcohol and injected MA increased users' perception of intoxication as well as cardiac responses, with potential for more serious cardiovascular consequences (Mendelson et al., 1995). Yamamura and colleagues found the combination aggravated both somatic and mental disorders (Yamamura et al., 1992). Because cocaethylene has a longer half-life (2 hours) than cocaine (38 to 60 minutes), the cumulative and additive effects found in the combination increase the incidence of lethal heart attacks and stroke (18 times higher risk of sudden death than with cocaine alone).
Cocaethylene appears to prolong the duration of cocaine-related increases in blood pressure and, in turn, to increase the likelihood of small vessel intercerebral infarcts. In addition, cocaethylene increases the risk of panic and anxiety attacks that chronic cocaine users experience, especially those that persist for some time. There is some indication that cocaethylene produces greater irritability and more persistent withdrawal complaints (Gold and Miller, 1997). The role of cocaethylene in evoking violence and intensifying agitation is also being investigated (Schrank, 1993).
Concomitant use of benzodiazepines and cocaine to blunt dysphoric effects is also common. This combination may enhance respiratory depression and prolong altered mental states, but decrease risk of seizures--especially if diazepam is taken before cocaine is used (Schrank, 1993).
The popularity of marijuana among stimulant users is explained by its pharmacologic properties. Because marijuana induces vasodilation of nasal mucosa, it attenuates the vasoconstriction of cocaine so that absorption is increased. Smoking marijuana before snorting cocaine decreases the time to peak euphoric effects, decreases dysphoric effects, and increases peak cocaine levels apparently by increasing bioavailability (Gold, 1997).
Psychiatric Disorders
It is believed that most stimulant users have concurrent psychiatric disorders. A 1991 survey of nearly 300 treatment-seeking cocaine users found that more than 70 percent had a lifetime history of psychiatric disorders such as alcoholism, major depression, bipolar disorder, anhedonia, anxiety, phobias, antisocial personality, and childhood AD/HD (Rounsaville and Carroll, 1991). At least four other earlier studies found similar comorbidity of cocaine with most of these same psychiatric diagnoses in addition to PTSD (Majewska, 1996). As many as half of surveyed cocaine users in treatment have lifetime diagnoses of depression; 20 to 25 percent have cyclic mood disorders; and sizeable percentages of these clients report borderline or antisocial personality, PTSD, or residual AD/HD (Gold, 1997). These psychiatric disorders are more common among stimulant users than in the general population (Weis, 1997).
Identified anxiety, phobias, AD/HD, and antisocial personality disorder typically precede cocaine dependence, whereas alcoholism, depression, and paranoia generally follow stimulant use. Although the symptoms of stimulant-induced psychosis closely mimic those of schizophrenia, and heavy use of cocaine/amphetamines may precipitate latent schizophrenia, the two disorders are not closely correlated (Majewska, 1996). Panic attacks are another correlate of cocaine use. Risk for this problem may increase because of sensitization to cocaine (Gold, 1997).
Differentiating comorbid psychiatric disorders from stimulant-related disorders can be challenging. Acute or chronic stimulant intoxication can elicit symptoms of anxiety that are indistinguishable from phobias, obsessive compulsiveness, panic, and generalized anxiety. The parallels between symptoms of stimulant-induced psychosis and schizophrenia are well known. Withdrawal from stimulants can cause depression that is indistinguishable from major depression from other causes (Gold and Miller, 1997). It can take at least a month of abstinence from all stimulant use to differentiate stimulant-induced dysphoria, depression, paranoia, or anxiety from a true psychiatric disorder.
The prognosis for substance use disorders is worsened by the presence of other untreated psychiatric disorders (or substance use disorders). Clients with comorbid psychiatric and drug dependence disorders need to have both treated; the psychiatric problems usually improve with abstinence. Antidepressant and neuroleptic medications with low anticholinergic and sedative properties are preferred in order to avoid another addiction. Sedative-hypnotics and benzodiazepines must be used with caution in high-risk populations (Gold and Miller, 1997).
Medical Conditions
Any preexisting acute or chronic physical conditions are also likely to be complicated and exacerbated by the stress of stimulant intoxication and withdrawal. Particularly dangerous coexisting medical conditions include any history of seizures, coronary heart disease, cardiac or thyroid problems, hypertension, or respiratory and pulmonary disease. Hypertension, renal failure, and diabetes mellitus, which are risk factors for stroke, can be exacerbated if cocaine/crack is smoked (Cornish and O'Brien, 1996).
Clients who are already taking medications for other medical conditions may be at special risk if stimulants are mixed with, for example, antidepressants, medications for high blood pressure, or antipsychotics. The effects of such drug interactions may be difficult to predict.
Crack- or MA-using mothers may be identified during prenatal care or in the delivery room through pregnancy or delivery complications, positive urine toxicologies, or acknowledged histories of substance use. Newborns who were exposed to stimulants in utero may manifest neurobehavioral problems that are less obvious and dangerous than those seen in opiate- or alcohol-exposed counterparts. The symptoms of stimulant exposure in newborns are likely to be transient and not require direct intervention. However, the young babies are typically irritable, tremulous, lethargic, emotionally labile, and somnolent. They may have a pronounced startle reaction, CNS instability, and prolonged and inconsolable crying. A few have signs of vascular disruptions and, rarely, congenital malformations, particularly of the heart, gastrointestinal tract, or skeletal system. Risk of sudden infant death syndrome may be heightened slightly.
Management is primarily by close observation in a quiet nursery environment, gentle handling, careful attention to feeding habits, and promoting positive bonding with the mother. More information about assessing, diagnosing, and managing the stimulant-exposed neonate can be found in TIP 5, Improving Treatment for Drug-Exposed Infants (CSAT, 1993).
Traumatic Injury
Patients appearing in hospital emergency departments following mild to severe traumas may be stimulant users who have been involved in fights or accidents of various types. The incidence of broken hands after fighting seems to be particularly high among MA users. TIP 16, Alcohol and Other Drug Screening of Hospitalized Trauma Patients (CSAT, 1995b), provides relevant information regarding how to identify and manage trauma patients with acute or chronic substance use disorders, including stimulant abuse. Several widely used screening instruments that can help hospital personnel determine substance use status of conscious trauma patients are described, as are laboratory tests to determine substance use status of any individual with potential symptoms of substance use, abuse, or dependence.
Assessment and Diagnosis
Diagnosis can be based on established DSM-IV criteria for amphetamine or cocaine abuse/dependence and other listed composites (American Psychiatric Press, 1994). For treatment reimbursement, the diagnosis may also need to reflect criteria according to the International Classification of Diseases (American Medical Association, 1997). Arriving at a diagnosis is simplified by having information available from a relevant and accurate client history, a urine toxicology screen or similar laboratory tests, and clinical observations of physical signs and mental status.
History
An appropriate substance use history should include the substance(s) and medications used during the last 30 days; the specific substance(s) or combinations typically used with the usual dose, frequency, and route of administration; the duration of use; and the time and amount of last use, as well as when the symptoms or complaints developed and how they have progressed. If the client has been bingeing, a brief description of this and previous episodes is helpful. In addition, the history should include information about any previous seizures, delirium tremens, heart and pulmonary problems, paranoid reactions (with or without delusions and hallucinations), and other serious medical and psychological conditions and psychiatric diagnoses, as well as all current medications the client is taking. Although people with stimulant use disorders are not as likely as those with other types of substance use disorders (i.e., alcoholics) to have a genetic component or familial history, information about other substance abuse or psychiatric problems in the family can be enlightening.
For most patients presenting in an ER, the substance and medical history will, of necessity, be brief and focus on the potential causes for the observed symptoms and complaints and any potential medical or psychological problems that are likely to complicate management and the patient's response. Stabilize the patient medically before trying to take a history and assess potential danger to self or others; beware of exaggeration or dismissal by the patient of his symptoms and condition; and use significant others, whenever possible, to validate his history. In situations where the patient is delirious, psychotic, or unable to respond, information from accompanying friends or significant others about the antecedents of the problem is particularly important. Sometimes, the substance history must await symptomatic management.
The history may be supplemented by a variety of screening instruments constructed to ascertain substance use disorders, although these are not notably reliable if used with acutely psychotic or intoxicated individuals. A number of these screening instruments are described in detail in TIP 16, Alcohol and Other Drug Screening of Hospitalized Trauma Patients (CSAT, 1995b).
Urine Toxicology
A urine screen or toxicology test may be used to identify which substances the client has used recently. This testing is vital to confirm clinicians' personal assessments and observations. Some ERs have bedside or patient-side urine immunoassay testing kits (dipstick tests) that can be used for a quick turnaround without waiting on more formal assays. These can be validated by additional laboratory studies that require 6 to 8 hours or longer for processing in a hospital setting.
The results of either dipstick or Enzyme Multiplied Immunoassay Technique (EMIT) tests are appropriate to use for medical purposes, but cannot be used for criminal prosecution because no chain of custody is established. Alternative techniques for determining substance use are analyses of hair, blood, sweat, or tissue samples. In general, however, urine has become the standard method of determining substance use in an individual, and tests are readily available in the medical setting where other types of testing are not. Urine screens are less expensive than drawing blood samples for testing or other alternatives. Both qualitative and quantitative urine assays are usually needed to verify use and time/amount taken. Repeated assays may be used to track elimination of stimulants from the system if large amounts have been detected.
Because no standard set of substances is tested in a urine substance screen, medical personnel should make certain that assays for suspected substances are included. Also, no toxicology screen can determine with certainty that any particular substance--or any substances at all--was ingested. The detection limitations may be too broad or the specific substance may have been completely metabolized before a urine specimen was collected. A positive report will not necessarily indicate when the substance was last used: Metabolites for some substances are detectable for days or weeks after last use, but take some time after substance administration to be detectable in urine (CSAT, 1995b).
Stimulants can be detected in urine for approximately 24 to 48 hours following use and, maximally, for 3 days after a single dose and 7 to 12 days following repeated high doses (American Psychiatric Press, 1994). Cocaine is excreted more rapidly and is more difficult to detect in urine samples than MA. However, an EMIT test can detect benzoylecgonine, an inactive cocaine metabolite, in urine for up to 72 hours after last ingestion (Weis, 1997). Benzoylecgonine has been found in urine as late as 22 days after last cocaine intoxication in three asymptomatic clients with substantial histories of cocaine use (Goldfrank and Hoffman, 1993). Many prescription and over-the-counter drugs (e.g., diet aids, cold remedies) contain phenylpropanolamine or ephedrine that may yield positive EMIT or RIA tests for amphetamines. A procedure that does not have cross-reactivity to phenylpropanolamine or ephedrine will be needed to confirm that amphetamine was consumed (Hawks and Chiang, 1986).
Physical Signs and Mental Status
Data acquired from monitoring vital signs (temperature, blood pressure, pulse rate, respiration rate) can be used to document physical indicators. In addition, observations of physical manifestations listed for acute or chronic users and from the withdrawal stage can be documented. Similarly, a variety of instruments exists to determine mental status, although observational data regarding psychological and mental status may be adequate.
Differential Diagnosis
In the diagnostic process, other disorders and conditions with similar or identical presentations must be considered to rule out or include them. As already noted, many stimulant users have coexisting mental illnesses such as bipolar disorders, borderline personality, and so on. Similarly, the cause of a heart attack or seizure must be determined for optimal continuing care and medical management.
Before a differential diagnosis of a coexisting psychiatric disorder (dual diagnosis) can be made, the client must be abstinent for some period of time, at least 3 to 4 weeks. The syndrome and symptoms presented can be treated meanwhile, and a diagnosis of psychotic disorder, not otherwise specified (NOS), can be given. More information regarding the diagnostic process for clients with symptoms that indicate coexisting substance use and mood disorders can be found in TIP 9, Assessment and Treatment of Patients With Coexisting Mental Illness and Alcohol and Other Drug Abuse (CSAT, 1994a).
New forms of brain imaging techniques offer a promising approach for making a differential diagnosis if current research determines that these techniques are useful for distinguishing among drug-induced and other forms of psychosis.
Developing Linkages Between Treatment Programs and Medical Facilities
Because the ER may be the stimulant user's first point of contact with the medical system and potential treatment, attention needs to be given to establishing and supporting a continuum of care in which appropriate linkages among all necessary services and programs for substance users are represented. Although the burden of developing and encouraging these linkages among treatment components cannot fall to hospital staff alone, and it would be unrealistic to expect this, cooperation and enlightened self-interest are encouraged. If not hooked up to the treatment system, cocaine and MA users are likely to return repeatedly to the ER and other parts of the hospital for care of more and more serious health and mental health problems. Stimulant abuse/dependence, as all substance use disorders, is a life-long, relapsing condition that requires ongoing management and support.
Hence, treatment programs should take primary responsibility for developing linkages with hospitals, using several approaches. The most exemplary approach--and that most likely to succeed--is to have a substance abuse treatment counselor or trained nurse/social worker visit the hospital and other medical facilities regularly in order to identify, screen, encourage, and follow up clients who have stimulant-related and other substance use problems and need access to the ongoing treatment continuum. A face-to-face visit by an outreach specialist is particularly effective in supporting the crisis-precipitated motivation to enter treatment, especially if the potential client is hospitalized for some length of time. Because a crisis creates an intervention opportunity, clients may be unusually receptive to considering lifestyle alternatives and the need for longer term treatment.
It also may be realistic for hospital staff to hand out a list of available treatment facilities for stimulant use and/or other substance use disorders that is developed and provided by the substance use disorder treatment staff. However, it is not very likely that clients in crisis will follow up the suggested referral, especially if they are in the early stages of crashing (and terribly sleepy) or paranoid.
Some educational literature might also be helpful--particularly regarding withdrawal symptoms, drug-induced psychoses, and medical complications--if the client or a significant other is willing to read it. Because it is imperative for doctors and other medical staff to know about the addiction process in order to understand clients they see everyday, cross-training in the field of substance use disorder treatment is vital for learning about and actively supporting the development and use of linkages and referral mechanisms. It is believed that at least one-fourth of those treated in hospitals has some type of substance use-related problem.
Motivation for change is often difficult to determine in the substance user. Health problems may, however, be the motivation to move the individual from contemplation to action (Prochaska et al., 1992). Health care personnel working with a patient hospitalized for an acute drug episode may capitalize on the fact that the situation was so acute from drug use that he had to be hospitalized.
Hospitals often deal with a population known as "frequent flyers," that is, persons with frequent, revolving admissions to hospital ERs or inpatient hospital beds because of medical or psychiatric complications resulting from their substance use. The financial burdens can be severe for the patient and, in the case of those lacking insurance, the hospital's costs of care may be unrecoverable. A collaborative arrangement between the hospital and a local treatment facility can allow for door-to-door drug treatment.
Obtaining Consent for Treatment
In obtaining the client's consent for treatment, gathering information from others about his history of substance use, making referrals for continuing care, or seeking reimbursement from insurance carriers, hospital staff must be familiar with the provisions of special Federal and State laws and regulations for protection of clients' confidentiality as set forth in 42 U.S.C. §290dd-2 (1992) and C.F.R. Part 2. Intoxicated or psychotic clients may have diminished capacity for providing informed consent to treatment. If consent is obtained, even temporarily, from a relative, this may be considered a "disclosure of identifying information" and subject to Federal guidelines. In referring a client from a hospital to another treatment program and making an appointment, staff are also making a disclosure and will ordinarily need a written consent form from the client containing specified information (see Figure 5-9).
Special exceptions, however, apply to information needed in a medical emergency that can be provided to medical personnel who need health- or treatment-related facts about a client in order to treat his life-threatening condition. However, the treatment program that is, for example, providing this information to a hospital before transferring the client for emergency care, must document specific data in his record regarding the nature of the emergency, what information was released, the name of the person making the disclosure, and the date and time. Additional information about consent, confidentiality, and other types of communications governed by Federal regulations is presented in TIP 19, Detoxification From Alcohol and Other Drugs, (CSAT, 1995d).
Publication Details
Copyright
Publisher
Substance Abuse and Mental Health Services Administration (US), Rockville (MD)
NLM Citation
Center for Substance Abuse Treatment. Treatment for Stimulant Use Disorders. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 1999. (Treatment Improvement Protocol (TIP) Series, No. 33.) Chapter 5—Medical Aspects of Stimulant Use Disorders.
