NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The flavoenzyme methylenetetrahydrofolate reductase (MTHFR) catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which serves as a methyl group donor in the conversion of homocysteine to methionine. In rats, experimental riboflavin deficiency is associated with low MTHFR activity and reduced levels of 5-methyltetrahydrofolate. In humans, reduced enzyme activity caused by the commonly occurring 677C→T substitution of the MTHFR gene is associated with elevated plasma homocysteine. The mutant enzyme has lower affinity for its flavin cofactor than the wild-type enzyme, and recent studies show that plasma homocysteine is inversely related to riboflavin in subjects with the T-allele. This indicates that the metabolic effect of the 677C→T polymorphism is related to riboflavin status, which may have implications for future studies on the relationship between this polymorphism and various clinical and biochemical endpoints.
Introduction
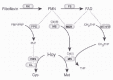
Riboflavin is a water-soluble vitamin, which serves as the precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD).1 FMN is formed by the phosphorylation of riboflavin, and FAD is formed in a subsequent ATP-dependent reaction as most of the FMN is adenylated.1 FMN and FAD are cofactors for more than 150 reduction-oxidation enzymes, some of which are involved in the metabolism of folate, vitamin B6 and cobalamin 1,2 (Fig. 1).
The majority of flavoenzymes, including methylenetetrahydrofolate reductase (MTHFR), are FAD-dependent.1,3 Mammalian MTHFR is a cytosolic homodimer, and each subunit contains a catalytic N-terminal domain as well as a regulatory C-terminal domain,4 which binds the allosteric inhibitor S-adenosylmethionine (AdoMet).3,4 The enzyme uses NADPH as a cofactor in addition to FAD, and catalyzes the transformation of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (5-methylTHF). The reaction is irreversible in vivo and is the only source of 5-methylTHF, which serves as the methyl donor for the cobalamin-dependent conversion of homocysteine to methionine.5 In most tissues, this provides the sole pathway for homocysteine remethylation. Concentrations of homocysteine in tissues and blood also depend upon its degradation through vitamin B6-dependent transsulfuration in the liver and kidneys.5
Thermolabile MTHFR associated with lower catalytic activity was reported approximately 15 years ago.6 Later, this thermolability was shown to be caused by a 677C→T transition in the MTHFR gene, resulting in an alanine to valine substitution in the enzyme.7 MTHFR in lymphocytes from subjects with the TT genotype has approximately 30% of the catalytic activity of the wild-type, while the CT genotype has 65% of the catalytic activity.7 The frequency of the T-allele is about 0.3 - 0.4 in several populations of Asian and European descent, but is much lower in sub-Saharan Africa and in some other areas.8
Guenther et al studied the biochemical properties of MTHFR in an E. coli model.9 They found that the enzyme variant homologous to the human Ala222Val substitution had lower binding affinity for FAD than the wild-type, and that folate stabilized the enzyme and increased FAD affinity.9 Purified human MTHFR was later shown to have similar biochemical properties.4 Folate and AdoMet stabilized the enzyme, particularly the mutant, and protected against flavin loss. The stabilizing effect of FAD was studied with respect to loss of enzyme activity after heating and after enzyme dilution, and in both cases the flavin cofactor was found to have a protective effect.4
Animal Studies
In 1968, Narisawa et al investigated the relationship between riboflavin status and MTHFR activity, and found lower enzyme activity in livers of riboflavin-deficient rats than in controls.10 Bates and Fuller confirmed these findings by demonstrating a dose-dependent relationship between riboflavin status and MTHFR activity.11 They also reported that MTHFR was particularly sensitive to riboflavin deficiency, and that enzyme activity decreased relatively more than intracellular concentrations of FAD when riboflavin was scarce. Low MTHFR activity in riboflavin deficiency is associated with altered distribution of liver folates, and 5-methylTHF is reduced relative to other folate forms.10-12 Redistribution of folates may explain why the riboflavin-deficient rats compared to controls,10,12 and possibly why homocysteine is elevated in the skin of riboflavin-deficient rats.13
MTHFR activity has also been investigated in hypothyroid rats.14-16 Hypothyroidism is associated with low intracellular FMN and FAD, which has been explained by low activity of riboflavin kinase, the enzyme catalysing the conversion of riboflavin to FMN 17,18 (Fig. 1). As with alimentary riboflavin deficiency, MTHFR activity is low,15 and intracellular levels of 5-methylTHF are reduced relative to other folates.14-16 Histidine oxidation is increased,14-16 both at high and low intakes of methionine.14,15 Hypothyroidism is also associated with higher levels of AdoMet, which may inhibit MTHFR activity.16
Human Studies
Assessment of Riboflavin Status
Glutathione reductase is a FAD-dependent enzyme, which is sensitive to riboflavin deficiency. 19 This probably explains why the erythrocyte glutathione reductase activation coefficient (EGRAC), which is the ratio between in vitro enzyme activity determined with and without the addition of FAD, is useful for the assessment of riboflavin status.20,21 High levels of EGRAC indicate low FAD saturation of the apoenzyme and biochemical riboflavin deficiency. The method has been used as an indicator of riboflavin status in several clinical studies.21
In some human studies, concentrations of plasma riboflavin have been used for the assessment of riboflavin status.22,23 This parameter appears to be a more sensitive indicator of riboflavin status than plasma concentrations of flavin cofactors.24 In severe riboflavin deficiency, plasma concentrations of FAD are probably lowered as well.24,25
Blood Levels of tHcy and Folate in MTHFR Deficiency
The role of MTHFR in homocysteine remethylation is illustrated by the finding that both mild MTHFR deficiency, which is observed in subjects with the 677C→T transition, and severe MTHFR deficiency are associated with elevated concentrations of plasma tHcy.7,8 High plasma tHcy in subjects with the TT genotype is usually observed only under conditions of impaired folate status,26 which probably reflects the role of folate as a substrate and as a genotype-dependent regulator of enzyme stability. Moreover, the TT genotype is frequently associated with low levels of plasma folate.27 5-methylTHF is the predominant folate species in plasma,27 and low folate is probably related to impaired synthesis of 5-methylTHF. Published data on erythrocyte folate may appear contradictory, and increased27 or decreased28 concentrations have been reported in subjects with the TT genotype compared to the CT and CC genotypes. Such apparent inconsistencies may be method dependent and reflect the ability of different assays to detect various cellular folate species.29 This idea is supported by the finding of formylated tetrahydrofolates in erythrocytes from subjects with the TT genotype, whereas CC cells contain only 5-methylTHF.30
Riboflavin Intake and Plasma tHcy
The relationship between dietary riboflavin and plasma concentrations of tHcy has been studied in a few cross-sectional studies.31-33 In individuals from the Framingham Offspring cohort (n = 1960), plasma tHcy was 1.0 µmol/l higher in the lowest compared to the highest quintile of riboflavin intake in a multivariate model adjusted for intakes of folate, cobalamin, vitamin B6 and other possible determinants of tHcy.31 Samples were collected prior to the implementation of mandatory folic acid fortification in 1998, and individuals who were regular users of B-vitamin supplements were excluded from the analysis.31 A strong inverse relationship between riboflavin intake and plasma tHcy was reported in another American study, but the data were not adjusted for folate or other B-vitamins.32 Dietary intakes of folate, riboflavin, vitamin B6 and cobalamin were inversely related to plasma tHcy in 2435 men and women from a Dutch population-based cohort,33 but only folate remained associated with tHcy in a multivariate model, adjusted for B-vitamins and other determinants of plasma tHcy. Individuals who used B-vitamin supplements were excluded from the study. None of the above studies investigated the possible effect of MTHFR 677C→T genotype on the riboflavin-tHcy relationship.31,32
Riboflavin and the MTHFR 677C→T Polymorphism As Determinants of tHcy
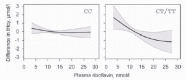
An inverse relationship between plasma concentrations of riboflavin and tHcy was reported by Hustad et al in a study of 423 Norwegian blood donors22 (Fig. 2). Plasma tHcy was 1.4 µmol/l higher in the lowest compared to the highest riboflavin quartile in a multiple regression model adjusted for folate and other determinants of tHcy. The riboflavin-tHcy relationship was modified by the MTHFR 677C→T polymorphism and was essentially confined to subjects with the T allele (Fig. 2). The riboflavin-tHcy relationship was not significantly modified by levels of serum folate.
Jacques et al studied 450 subjects from the Framingham Offspring cohort, selected according to the MTHFR 677C→T polymorphism and equally distributed between the CC, CT and TT genotypes.23 They found an inverse association between plasma concentrations of riboflavin and plasma tHcy, but only in subjects with the TT genotype and plasma folate below the median (12.5 nmol/l). In this group, tHcy was 2.9 µmol/l higher in the lowest compared to the highest riboflavin tertile after adjustment for sex, age and folate. There was no relationship between concentrations of flavin cofactors and tHcy.23
Thus, both the American and the Norwegian studies22,23 demonstrated an association between riboflavin and tHcy, which was dependent on the MTHFR 677C→T polymorphism. In the Framingham Offspring cohort, the riboflavin-tHcy relationship was weaker, however. In addition, plasma tHcy was less strongly associated with the MTHFR genotype, and concentrations of serum folate were independent of genotype.22,23 Thus, the polymorphism apparently had less metabolic effects in the American than in the Norwegian study.
This disparity might be explained by different intakes of vitamins or nutrients, which modulate MTHFR activity. Riboflavin intake was probably higher in the Americans, because grain products have been riboflavin fortified in the USA since the 1940s23,34 and levels of fortification have been rising over the years.34 Although samples were collected prior to the implementation of mandatory folic acid fortification in the USA, many breakfast cereals were fortified at the time of the study,23,34 and folate status may have been better in the Americans. The idea that vitamin intake differs between the two populations is further supported by the finding of an overall correlation between concentrations of riboflavin and folate in the American study (Spearman correlation coefficient = 0.31, P <0.001),23 but not in the Norwegian study.22 Such a correlation might be related to common dietary sources for these vitamins.
In a recent study by McNulty et al of 286 healthy individuals from Northern Ireland, EGRAC was used to determine riboflavin status.35 The authors demonstrated a significant inverse association between riboflavin status and plasma concentrations of tHcy. As in previous studies, this relationship was modified by MTHFR genotype, and in the lowest tertile of riboflavin status, mean tHcy was 18.09 µmol/l in the TT group as compared to 10.15 µmol/l in the CT and 8.32 µmol/l in the CC groups. When riboflavin status was higher, tHcy was independent of genotype. The genotype-tHcy relationship was also dependent on folate, and was only observed in subjects with concentrations of erythrocyte folate below the median.35 The authors did not assess the combined effects of riboflavin, folate and MTHFR genotype on tHcy in a multivariate model, but they found no correlation between riboflavin and erythrocyte folate,35 which makes serious confounding from folate unlikely.
The relationship between plasma tHcy and riboflavin status has also been investigated in end-stage renal disease.36 In a study of 54 nonvitamin supplemented patients with a mean age of 54 years who were maintained on peritoneal dialysis, mean plasma tHcy was 33.0 µmol/l. Ten patients had EGRAC equal to or greater than 1.52, indicating riboflavin deficiency.36 The authors found a positive association between EGRAC and tHcy, which is consistent with high tHcy when riboflavin status is low. Riboflavin was significantly related to plasma tHcy in multivariate models, which included folate and other B-vitamins. The riboflavin-tHcy relationship was not studied in relation to the MTHFR 677C→T polymorphism, because of the relatively low number of patients.36
Riboflavin Intervention Studies
The homocysteine lowering effect of 15 days of riboflavin (10 mg/d; n = 10) or vitamin B6 (20 mg/d; n = 10) supplementation was investigated in a small study of riboflavin and vitamin B6-deficient Indian women.37 Mean tHcy was 12.1 and 14.7 µmol/l in the riboflavin and B6 groups, respectively, and tHcy decreased only in the B6 group.37 There was no placebo group, and no data on MTHFR 677C→T genotype.
Another riboflavin intervention study was published by McKinley et al.38 In the first phase of this study, 46 subjects with suboptimal riboflavin status (EGRAC ≥1.20) received low-dose riboflavin (1.6 mg/d; n = 23) or placebo (n = 23) for 12 weeks. In the second phase of the study, participants originally on placebo received 400 µg/d of folic acid for 6 weeks followed by a combination of folic acid and riboflavin for 12 weeks. Folic acid supplementation lowered tHcy, but no effect of riboflavin was observed in either phase, and apparently riboflavin status was not a determinant of plasma tHcy in this study. A possible reason is that only five subjects had the TT genotype. In addition, riboflavin status was only modestly impaired, and no subject had EGRAC higher than 1.40.38
Implications
MTHFR may be sensitive to riboflavin status,11 particularly in subjects with the 677C→T substitution of the MTHFR gene. In subjects with the TT genotype, higher riboflavin intake could be necessary for the formation of adequate amounts of 5-methyl-THF involved in homocysteine remethylation. Although the TT genotype comprises only around 10% of the population in many countries,8 it is more prevalent in subjects with hyperhomocysteinemia. In a Norwegian population-based study of men and women aged 40 — 67 years, 73% of individuals with plasma tHcy equal to or higher than 40 µmol/l had the TT genotype, compared to 10% of the controls.39 In men from Northern Ireland, the TT genotype occurred in 48, 35, and 23% of the top 5, 10, and 20% of individuals ranked by plasma tHcy levels.40 This indicates that the MTHFR 677C→T polymorphism may be important for the development of moderate hyperhomocysteinemia, and an effect of the enzyme variant associated with the TT genotype might be partly attributed to riboflavin.
Much of the interest in the MTHFR 677C→T polymorphism stems from its association with moderate hyperhomocysteinemia , which is a risk factor for occlusive arterial disease, venous thrombosis, neural tube defects and pregnancy complications.5,8 It is still not clear whether elevated tHcy is the cause of these conditions or if it is mainly a surrogate marker. In several studies, the MTHFR 677C→T polymorphism itself has been shown to modify the risk of certain diseases,8 including cardiovascular disease,41,42 neural tube defects27 and colon cancer,43,44 while other studies show no such relationship.8 Inconsistent reports on the MTHFR 677C→T polymorphism and disease risk could be explained through effect modification by nutritional factors. This has been demonstrated for folate,42,44 whereas the role of riboflavin has received less attention.45
Further research is warranted to investigate the importance of riboflavin as a regulator of MTHFR activity, particularly with respect to its interaction with folate and its relationship to various clinical endpoints.
References
- 1.
- Rivlin RS, Pinto JT. Riboflavin (Vitamin B2). In: Rucker RB, Suttie JW, McCormick DB, Machlin LJ, eds. Handbook of Vitamins. 3rd ed. New York: Marcel Dekker, Inc 2001:255-273.
- 2.
- Sauberlich HE. Interactions of thiamin, riboflavin, and other B-vitamins. Ann N Y Acad Sci. 1980;355:80–97. [PubMed: 7015958]
- 3.
- Kutzbach C, Stokstad EL. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1971;250:459–477. [PubMed: 4399897]
- 4.
- Yamada K, Chen ZT, Rozen R. et al. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Nat Acad Sci USA. 2001;98:14853–14858. [PMC free article: PMC64948] [PubMed: 11742092]
- 5.
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. [PubMed: 10448523]
- 6.
- Kang SS, Zhou J, Wong PW. et al. Intermediate homocysteinemia: A thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988;43:414–421. [PMC free article: PMC1715503] [PubMed: 3177384]
- 7.
- Frosst P, Blom HJ, Milos R. et al. A candidate genetic risk factor for vascular disease: A common mutation at the methylenetetrahydrofolate reductase. Nature Genetics. 1995;10:111–113. [PubMed: 7647779]
- 8.
- Ueland PM, Hustad S, Schneede J. et al. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. 2001;22:195–201. [PubMed: 11282420]
- 9.
- Guenther BD, Sheppard CA, Tran P. et al. The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nature Struct Biol. 1999;6:359–365. [PubMed: 10201405]
- 10.
- Narisawa K, Tamura T, Tanno K. et al. Tetrahydrofolate-dependent enzyme activities of the rat liver in riboflavin deficiency. Tohoku J Exp Med. 1968;94:417–430. [PubMed: 4970398]
- 11.
- Bates CJ, Fuller NJ. The effect of riboflavin deficiency on methylenetetrahydrofolate reductase (NADPH) (EC 1.5.1.20) and folate metabolism in the rat. Br J Nutr. 1986;55:455–464. [PubMed: 3676170]
- 12.
- Honda Y. Folate derivatives in the liver of riboflavin-deficient rats. Tohuko J Exp Med. 1968;95:79–86. [PubMed: 4972728]
- 13.
- Lakshmi R, Lakshmi AV, Bamji MS. Mechanism of impaired skin collagen maturity in riboflavin or pyridoxine deficiency. J Biosci. 1990;15:289–295.
- 14.
- Chan MM, Stokstad EL. Metabolic responses of folic acid and related compounds to thyroxine in rats. Biochim Biophys Acta. 1980;632:244–253. [PubMed: 7417525]
- 15.
- Stokstad EL, Chan MM, Watson JE. et al. Nutritional interactions of vitamin B12, folic acid, and thyroxine. Ann N Y Acad Sci. 1980;355:119–129. [PubMed: 6972186]
- 16.
- Keating JN, Kusano G, Stokstad EL. Effect of thiouracil in modifying folate function in severe vitamin B12 deficiency. Arch Biochem Biophys. 1988;267:119–124. [PubMed: 3143307]
- 17.
- Rivlin RS, Langdon RG. Effects of thyroxine upon biosynthesis of flavin mononucleotide and flavin adenine dinucleotide. Endocrinology. 1969;84:584–588. [PubMed: 4304263]
- 18.
- Lee SS, McCormick DB. Thyroid hormone regulation of flavocoenzyme biosynthesis. Arch Biochem Biophys. 1985;237:197–201. [PubMed: 2982328]
- 19.
- Ross NS, Hansen TP. Riboflavin deficiency is associated with selective preservation of critical flavoenzyme-dependent metabolic pathways. Biofactors. 1992;3:185–190. [PubMed: 1599612]
- 20.
- Glatzle D, K_r WF, Christeller S. et al. Method for the detection of a biochemical riboflavin deficiency. Stimulation of NADPH2-dependent glutathione reductase from human erythrocytes by FAD in vitro. Investigations on the vitamin B2 status in healthly people and geriatric patients. Int Z Vitaminforsch. 1970;40:166–183. [PubMed: 4393763]
- 21.
- Bates C. Riboflavin. Int J Vitam Nutr Res. 1993;63:274–277. [PubMed: 8157434]
- 22.
- Hustad S, Ueland PM, Vollset SE. et al. Riboflavin as a determinant of plasma total homocysteine: Effect modification by the methylenetetrahydrofolate reductase C677T polymorphism. Clin Chem. 2000;46:1065–1071. [PubMed: 10926884]
- 23.
- Jacques PF, Kalmbach R, Bagley PJ. et al. The relationship between riboflavin and plasma total homocysteine in the Framingham offspring cohort is influenced by folate status and the C677T transition in the methylenetetrahydrofolate reductase gene. J Nutr. 2002;132:283–288. [PubMed: 11823591]
- 24.
- Hustad S, McKinley MC, McNulty H. et al. Riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in human plasma and erythrocytes at baseline and after low-dose riboflavin supplementation. Clin Chem. 2002;48:1571–1577. [PubMed: 12194936]
- 25.
- Burch HB, Bessey OA, Lowry OH. Fluorometric measurements of riboflavin and its natural derivatives in small quantities of blood serum and cells. J Biol Chem. 1948;175:457–470. [PubMed: 18873321]
- 26.
- Jacques PF, Bostom AG, Williams RR. et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. [PubMed: 8616944]
- 27.
- van der PutNM, Steegers-Theunissen RP. et al. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet. 1995;346:1070–1071. [PubMed: 7564788]
- 28.
- Molloy AM, Daly S, Mills JL. et al. Thermolabile variant of 5,10-methylenetetrahydrofolate reductase associated with low red-cell folates: Implications for folate intake recommendations. Lancet. 1997;349:1591–1593. [PubMed: 9174561]
- 29.
- Molloy AM, Mills JL, Kirke PN. et al. Whole-blood folate values in subjects with different methylenetetrahydrofolate reductase genotypes: Differences between the radioassay and microbiological assays. Clin Chem. 1998;44:186–188. [PubMed: 9550581]
- 30.
- Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA. 1998;95:13217–13220. [PMC free article: PMC23763] [PubMed: 9789068]
- 31.
- Jacques PF, Bostom AG, Wilson PW. et al. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001;73:613–621. [PubMed: 11237940]
- 32.
- Shimakawa T, Nieto FJ, Malinow MR. et al. Vitamin intake: A possible determinant of plasma homocyst(e)ine among middle-aged adults. Ann Epidemiol. 1997;7:285–293. [PubMed: 9177112]
- 33.
- de BreeA, Verschuren WMM, Blom HJ. et al. Association between B vitamin intake and plasma homocysteine concentration in the general Dutch population aged 20-65 y. Amer J Clin Nutr. 2001;73:1027–1033. [PubMed: 11382655]
- 34.
- Backstrand JR. The history and future of food fortification in the United States: A public health perspective. Nutr Rev. 2002;60:15–26. [PubMed: 11842999]
- 35.
- McNulty H, McKinley MC, Wilson B. et al. Impaired functioning of thermolabile methylenetetrahydrofolate reductase is dependent on riboflavin status: Implications for riboflavin requirements. Am J Clin Nutr. 2002;76:436–441. [PubMed: 12145019]
- 36.
- Skoupy S, F_ger M, Veitl M. et al. Riboflavin is a determinant of total homocysteine plasma concentrations in end-stage renal disease patients. J Amer Soc Nephrol. 2002;13:1331–1337. [PubMed: 11961021]
- 37.
- Lakshmi AV, Ramalakshmi BA. Effect of pyridoxine or riboflavin supplementation on plasma homocysteine levels in women with oral lesions. Natl Med J India. 1998;11:171–172. [PubMed: 9808973]
- 38.
- McKinley MC, McNulty H, McPartlin J. et al. Effect of riboflavin supplementation on plasma homocysteine in elderly people with low riboflavin status. Eur J Clin Nutr. 2002;56:850–856. [PubMed: 12209373]
- 39.
- Guttormsen AB, Ueland PM, Nesthus I. et al. Determinants and vitamin responsiveness of intermediate hyperhomocysteinemia (40 µmol/liter)-the Hordaland homocysteine study. J Clin Invest. 1996;98:2174–2183. [PMC free article: PMC507663] [PubMed: 8903338]
- 40.
- Harmon DL, Woodside JV, Yarnell JWG. et al. The common thermolabile variant of methylene tetrahydrofolate reductase is a major determinant of mild hyperhomocysteinemia. Q J Med. 1996;89:571–577. [PubMed: 8935478]
- 41.
- Brattströ L, Wilcken DE, Örvik J. et al. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: The result of a meta-analysis. Circulation. 1998;98:2520–2526. [PubMed: 9843457]
- 42.
- Klerk M, Verhoef P, Clarke R. et al. MTHFR 677C -→ T polymorphism and risk of coronary heart disease-A meta-analysis. JAMA. 2002;288:2023–2031. [PubMed: 12387655]
- 43.
- Chen J, Giovannucci E, Kelsey K. et al. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996;56:4862–4864. [PubMed: 8895734]
- 44.
- Ma J, Stampfer MJ, Giovannucci E. et al. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997;57:1098–1102. [PubMed: 9067278]
- 45.
- Verhoef P, Rimm EB, Hunter DJ. et al. A common mutation in the methylenetetrahydrofolate reductase gene and risk of coronary heart disease: Results among U.S. men. J Am Coll Cardiol. 1998;32:353–359. [PubMed: 9708460]
- Riboflavin and Methylenetetrahydrofolate Reductase - Madame Curie Bioscience Dat...Riboflavin and Methylenetetrahydrofolate Reductase - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...