This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Show detailsIntroduction
As the utilization of the robotic platform gains momentum in managing various urologic pathologies, its application to bladder surgery continues to evolve. Traditionally, open radical cystectomy with bilateral pelvic node dissection and urinary diversion has been the standard of care for managing muscle-invasive and select high-risk nonmuscle-invasive bladder cancer.[1][2] However, robotic radical cystectomy has emerged as a minimally invasive surgical technique for managing these conditions and reducing surgical morbidity for advanced localized bladder cancer without compromising oncologic outcomes.
Since the first documented case series on robotic cystectomy in 2003, the utilization of this approach has been gradually gaining popularity.[3][4] Despite its technical challenges, long learning curve, and longer operating times, robotic radical cystectomy has been associated with several benefits compared to open radical cystectomy. These include less intraoperative blood loss, reduced need for transfusions, fewer major postoperative complications, a lower rate of positive surgical margins, 40% more lymph nodes recovered on average, and earlier hospital discharge.[5][6][7][8][9][10][11][12] In addition, robotic surgery also offers a reduced risk of wound-related complications and thromboembolic events.[8][13]
Quality of life scores are generally similar between the robotic and open surgical approaches, but when the robotic intracorporeal urinary diversion was added, quality of life scores improved.[7][8] Comparative studies have demonstrated that oncological outcomes are comparable between open and robotic surgical approaches to radical cystectomy over 10 years with regard to recurrence-free, progression-free, and overall survival.[14][15][16][17][18] Given its potential value, long learning curve, high technical complexity, and limitations, the role of minimally invasive major robotic bladder surgery continues to evolve and is the subject of ongoing investigation and study.
Anatomy and Physiology
The primary histological type of bladder cancer is urothelial carcinoma, accounting for approximately 90% of all bladder tumors. Less common types include schistosoma-related squamous cell carcinoma, which makes up about 5% of bladder tumors and is more prevalent in the Middle East and Africa, and adenocarcinoma, which accounts for about 2% of bladder tumors and may develop from the urachus.[19]
Robotic radical cystectomy in males involves the removal of the bladder, distal ureters, prostate, and vas deferens. In females, the procedure classically involves anterior pelvic exenteration, removing the bladder, urethra, uterus, cervix, and anterior vaginal wall, although pelvic organ-sparing cystectomy may be performed in selected patients. When performing bilateral pelvic lymphadenectomy, surgeons should remove, at a minimum, the external and internal iliac nodes as well as the obturator lymph nodes (standard lymphadenectomy).
Indications
Indications for robotic radical cystectomy are similar to those for open cystectomy. These include the absence of metastatic urothelial lesions and the patient's willingness and ability to undergo a major surgical intervention.
Indications for cystectomy surgery include muscle-invasive bladder cancer or non-muscle–invasive cancer with at least 1 of the following criteria:[20][21][22][23][24][25]
- High-risk patients with persistent high-grade T1 disease on repeat transurethral resection or recurrent disease.
- High-risk patients with persistent or recurrent carcinoma in situ within 1 year after 2 cycles of Bacillus Calmette-Guerin (BCG) induction therapy or while on maintenance BCG treatment.
- High-risk patients with persistent or recurrent T1 tumors associated with lymphovascular invasion or variant bladder cancer histology, as well as low glomerular filtration rate (GFR) and larger tumor size (>3 cm) who are not responding to BCG.
- T1 disease associated with carcinoma in situ.
Special considerations arise with the robotic approach, particularly regarding patient positioning, tolerance of pneumoperitoneum, and abdominal access. The combination of the steep Trendelenburg position and pneumoperitoneum used during this procedure can lead to cardiopulmonary consequences, such as decreased lung compliance, reduced functional residual capacity, increased ventilation/perfusion mismatch, and heightened risk of hypercarbia/metabolic acidosis. Previous intrabdominal or pelvic surgery, as well as radiation therapy, may complicate robotic access to the abdomen.[26] Therefore, it is crucial to thoroughly evaluate and discuss these critical preoperative considerations on an individual basis with the entire care team before proceeding with robotic radical cystectomy.
Patients with indications for a radical cystectomy but are unwilling or medically unfit to undergo surgery are best addressed with trimodal therapy. This approach typically involves maximal transurethral bladder tumor resection followed by chemotherapy and external beam radiation treatment.[27][28][29]
Contraindications
Robotic radical cystectomy is a major operative procedure that can carry significant perioperative morbidity and mortality risks.[30] Therefore, patients with localized disease being considered for this surgery must undergo a comprehensive preoperative medical evaluation of their functional status. This evaluation aims to identify any significant or unexpected medical comorbidities, such as bleeding diathesis, poor pulmonary function, or cardiac issues, which could potentially prevent them from undergoing surgery or lead to preventable major complications.
Specific contraindications for robotic radical cystectomy surgery include disease that extends into the pelvis or surrounding structures with bladder fixation, as well as an uncorrected bleeding diathesis.[31]
Morbid obesity, prior abdominal intestinal or vascular surgery, previous radiation therapy to the abdomen, locally advanced disease, and advanced age of older patients may pose technical challenges for robotic cystectomy surgery. However, they are not absolute contraindications, as good outcomes can still be achieved with careful surgical planning and management.[32][33][34][35]
Various contraindications may exist for specific types of urinary diversion, including:
- Patients with short bowel syndrome, inflammatory small bowel disease, and a history of extensive radiation to the ileum should not undergo an ileal conduit diversion.
- Continent cutaneous diversions may have relative contraindications, such as poor renal function and severe hepatic dysfunction, due to the higher risk of metabolic complications associated with these techniques.
- Mental impairment, disability, or extreme frailty are other potential contraindications for continent cutaneous diversions, as they require the ability to self-catheterize postoperatively.[36]
Equipment
The techniques and equipment used can vary. The described technique for robotic radical cystectomy involves the use of 6 ports, as outlined in the "Technique or Treatment" section below.
Standard robotic instruments utilized in this procedure include the permanent cautery hook on the right arm, Maryland bipolar forceps, SynchroSeal, or Vessel Sealer on the left arm, the Tip-Up Fenestrated grasper, and the 60-mm robotic stapler on the fourth arm. Large needle drivers are utilized for suturing during the urinary diversion.
The assistant's primary instruments include a laparoscopic grasper, a long suction-irrigator, and laparoscopic scissors.
Personnel
Performing and caring for a patient undergoing robotic cystectomy of the bladder requires a multidisciplinary team of specialized personnel. This includes a complete surgical team comprising the urologic surgeon, anesthesiologist, circulating nurse, scrub technician, bedside assistant, and other operating room personnel essential for performing this operation.
Postoperatively, the post-anesthesia recovery nurses, intensive care unit nurses, floor nurses, stoma nurses, and case managers play vital roles in aftercare. A dedicated team comprising physical therapists, dietitians, and oncologists also contributes to the patient's rehabilitation and long-term care, ensuring a comprehensive approach to recovery and overall health management.
Preparation
Preparation for this procedure includes administering a high carbohydrate diet to the patient 2 to 3 days before surgery, along with participation in a preoperative education class. Clear liquids are started the day before surgery, followed by nothing-by-mouth starting at midnight. Routine use of laxatives or mechanical bowel preparation before surgery is avoided, as they have not been shown to reduce rates of anastomotic leaks or wound infections.[37]
In the preoperative holding area, the patient receives a single dose of broad-spectrum antimicrobial prophylaxis, deep venous thrombosis prophylaxis, and a μ-opioid receptor antagonist (alvimopan 12 mg) to promote early return of bowel function postoperatively.[38] Alvimopan can be continued postoperatively at 12 mg BID.[39][40]
A stoma therapist will mark the site if the patient is expected to receive an ileal conduit. This practice is often beneficial, even if another type of urinary diversion is planned, due to potential unforeseen developments during surgery.
Technique or Treatment
Radical cystectomy surgery for urothelial cancer should be preceded by neoadjuvant cisplatin-based chemotherapy.[23][41][42] Robotic radical cystectomy, especially when combined with intracorporeal urinary diversion, presents significant challenges and complexities. Yet, it has become the predominant surgical approach for invasive bladder cancer in tertiary care centers.[8] Notably, it is estimated that over 130 procedures are required for surgeons to achieve a stable level of maximum proficiency with minimal 90-day postoperative complication rates.[43] Patients requiring this advanced type of robotic surgery are recommended to be referred to high-volume centers of excellence (performing more than 50 such cases per year) to optimize outcomes and minimize complications.[44][45]
Treatment Procedures for Robotic Radical Cystectomy
Patient positioning and equipment: The patient is initially placed in a supine position on an anti-skid foam pad to prevent sliding and pressure injuries. Female patients requiring vaginal access are positioned in a low lithotomy position. The patient's arms are wrapped in foam padding and tucked at their sides, ensuring padding for the hands and elbows. A chest and leg straps may also be utilized to secure the patient further. A warming blanket and intermittent compression hose are applied for comfort and circulation support.
Once appropriately positioned, it is crucial to test the patient in steep Trendelenburg to confirm stability and minimize movement. A Foley catheter is then inserted and allowed to drain under gravity. Best practice typically involves temporarily reducing the steep Trendelenburg position after about 4 hours to mitigate the risk of positional injuries.
The robot is positioned on the patient's left side, with the primary assistant and surgical technician seated on the right side. While different positioning setups are acceptable, it is preferable for the assistant to sit on the side where bowel work is conducted, opposite the robotic stapler. Alternatively, the robot may be placed and secured between the patient's legs.[46][47][48][49]
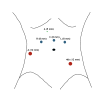
Port placement and robotic equipment: A Veress needle is inserted through a periumbilical puncture to insufflate the peritoneum for port placement. Notably, 6 ports are utilized, positioned similarly to a robotic radical prostatectomy but more cephalad.[50] This strategic port placement is crucial for executing the urinary diversion and extended lymph node dissection (see Image. Port Placement for Robotic Radical Cystectomy).
- The robotic arm and camera ports are positioned approximately 5 to 7 cm cephalad from the umbilicus to facilitate bowel work on the ileum. This also permits a cephalad dissection of lymph nodes if necessary.
- An 8-mm supraumbilical camera port is initially placed, and a 30° camera is utilized. Subsequently, the patient is then placed in a steep Trendelenburg position.
- Under direct vision, a 12-mm assistant port is placed 3 fingerbreadths cranial to the right iliac crest in a location anterior to the cecum.
- An 8-mm right robotic instrument port is positioned 1 handbreadth (10-12 cm) lateral to the camera port.
- A 5-mm assistant port is then placed between the camera port and the right robotic instrument port.
- Another 8-mm robotic instrument port is positioned on the left side of the abdomen to replicate the placement of the right robotic instrument port.
- A 12-mm robotic port is placed in the left lower quadrant, approximately 2 fingerbreadths (4 cm) off of the left anterior superior iliac spine.
This positioning is designed to enhance the maneuverability of the fourth robotic arm, facilitating easier placement of the stapler during the creation of the urinary diversion.
Alternative approaches include adding a 15-mm port to facilitate easier manipulation of the GIA stapler by the assistant.[46] This additional port also facilitates the placement of an intracorporeal specimen bag.[46] Another option involves adding a 12-mm suprapubic port after placing the bladder in the specimen bag.[51] This modification enhances access for side-to-side bowel anastomoses and helps prevent torsion.[51]
The pneumoperitoneum is generally set at 10 mm Hg throughout the procedure unless there is limited visualization necessitating adjustment.
Bowel mobilization and ureteral dissection: The procedure begins by releasing the white line of Toldt adjacent to the ascending and descending colon to maximize exposure of the deep pelvis. The sigmoid peritoneal attachments are then released, facilitating the retraction of the large bowel from the pelvis. This mobilization is crucial for exposing the pelvis and accessing and mobilizing the small bowel to create the urinary diversion. Once the retroperitoneum is exposed, the right ureteral dissection is performed (see Image. Right Ureteral Identification, Dissection, and Transection). The right ureter is identified at approximately the level of the right iliac artery bifurcation. The ureter is then circumferentially dissected distally toward the ureterovesical junction.
If an intracorporeal urinary diversion is planned, the ureter should not be dissected deeply close to the bladder, as much of the distal ureter will be discarded. Thus, it is important to utilize an atraumatic technique during ureteral dissection and to preserve a healthy margin of surrounding fat and periureteral vasculature.
Once the distal portion of the right ureter is dissected, the ureter is transected sharply. A section from the distal right ureter is sent for frozen section analysis to rule out malignancy. The distal end of the proximal ureter should be clipped to prevent urine from dripping into the peritoneum and to induce proximal hydronephrosis and mild ureteral distension. This distension will be helpful when creating the ureteral-intestinal anastomoses for urinary diversion (see Image. Clipping and Transection of Urethra).
The left ureteral dissection is performed similarly but must be continued distally to account for its passage under the sigmoid colon. A sample from the distal edge of the left ureter is sent for frozen section analysis to ensure a negative surgical margin.
Posterior dissection: The posterior dissection is performed before entry into the space of Retzius, providing anterior traction for exposure of the posterior plane and dissection of the vascular pedicles.
The bladder is retracted proximally and anteriorly using the fourth robotic arm. A transverse incision is made through the peritoneum at the rectovesical cul-de-sac. The seminal vesicles and vas deferens are identified bilaterally and dissected.
The dissection plane is carried posteriorly between the seminal vesicles and the rectum until Denonvilliers fascia is reached and incised. This allows the posterior dissection to be carried bluntly distally toward the prostatic apex, taking care to avoid injury to the rectum, which lies immediately inferior. To aid exposure, the fourth arm and/or an assistant are used to help retract the seminal vesicles cephalad.
Control of the vascular pedicles: An incision is made in the parietal peritoneum just lateral to the medial umbilical ligament bilaterally to separate the bladder from the pelvic side wall and pelvic vasculature. The vas deferens are clipped and divided.
Blunt dissection is continued caudally toward the endopelvic fascia, exposing the superior vesical pedicle. The superior vesical artery is ligated bilaterally at the origin of the hypogastric artery using clips, a SynchroSeal, or a Vessel Sealer. The exposed endopelvic fascia is then opened to define the lateral plane of the prostatic pedicles (see Image. Release of Endopelvic Fascia).
Dissection between the pelvic side wall and the bladder can be facilitated by medial retraction of the bladder using the fourth robotic arm. This maneuver ensures clearance from the obturator nerve and external iliac vessels and enhances the exposure of the bladder's lateral pedicles.
- Non-nerve–sparing procedures: In non-nerve-sparing procedures, anterior-cranial traction is applied by the assistant or robotic arm. The lateral pedicles of the bladder can then be managed, ligated, and divided using 60-mm laparoscopic vascular staplers, clips, or similar devices (see Image. Control of Bladder Pedicle).
- Nerve-sparing procedures: The neurovascular bundles may be spared similarly to nerve-sparing techniques used during radical prostatectomy in appropriately selected patients. In a nerve-sparing approach, the neurovascular bundles located on the posterior-lateral aspects of the prostate can be released along the surface of the prostate or vagina.
The dissection begins at the seminal vesicles and proceeds toward the prostate. The neurovascular bundles are meticulously separated from the bladder and the posterolateral prostate by gently peeling them off in a superolateral direction. In such cases, it is typically preferable to clip the inferior vesical and prostate pedicles rather than dividing them sharply.
Anterior dissection: Anterior dissection involves retracting the urachus cranially with the fourth robotic arm to facilitate transection of the urachus and medial umbilical ligaments. This maneuver exposes the space of Retzius, allowing the bladder to descend from the anterior abdominal wall. The avascular plane between the anterior abdominal wall and bladder is then carefully extended toward the pubic bone, with blunt dissection in the soft areolar tissue anterior to the prostate and bladder.
The levator ani muscles can be dissected after the endopelvic fascia has been incised. The lateral pelvic fascia is then incised above the level of the neurovascular bundles, allowing them to fall posterolaterally, which is crucial for nerve-sparing procedures.
The fourth robotic arm and assistant provide cephalad traction on the bladder to expose the dorsal venous complex (DVC). The puboprostatic ligaments and DVC are identified and then transected using monopolar cautery or cold scissors. Optionally, the transected DVC complex may be oversewn with a running suture ligature to minimize bleeding.
The prostatic apex and urethra are circumferentially dissected. Once the urethral stump is isolated, the Foley catheter is removed, and a clip is placed proximally on the urethra to prevent spillage. Alternatively, the apical prostate can be oversewn. The urethra is transected proximal to the verumontanum, and the freed specimen is then fully released and placed into an intracorporeal specimen bag. A specimen of the urethral margin from the apical prostatic urethra is sent to pathology for a frozen section.
A urethrectomy should be performed if high-grade malignant tissue is identified by a frozen or permanent section of the surgical margin of the apical prostatic urethra after its surgical removal.[23][52] In women, a urethrectomy should be considered if they are not planning to undergo orthotopic neobladder reconstruction, aiming to reduce the risk of recurrence or inadvertent positive surgical margins.[23]
Bilateral pelvic lymph node dissections (standard and extended): Bilateral pelvic lymph node dissection involves a standard bilateral lymphadenectomy, which includes the removal of the external iliac, obturator, and internal iliac (hypogastric) nodes.[53][54][55] More than 12 nodes should be resected for adequate staging.[23] A larger number of identifiable lymph nodes is also considered a positive indicator of the quality of the surgery.[22][56]
The anatomic boundaries of the standard dissection template typically include the genitofemoral nerves laterally, the internal iliac artery medially, the node of Cloquet inferiorly, and the common iliac artery superiorly. Careful attention is crucial during lymphadenectomy to prevent injury to critical nerves and vasculature.
Optionally, the lymphadenectomy can be expanded to encompass the common iliac and presacral lymph nodes (extended lymph node dissection) using a "split and roll" technique. However, an extended lymph node dissection adds considerable time to an already lengthy procedure, heightens the risk of complications and inadvertent injury to surrounding structures, and increases the likelihood of lymphocele formation. Importantly, large-scale clinical trials have not shown significant survival benefits compared to standard lymph node dissection.[57]
A few important tips for pelvic lymph node dissections include:
- All major lymphatic channels should be clipped to avoid chylous ascites and the development of lymphoceles.
- Nodes should not be cut to avoid the possibility of tumor spillage.
- All lymph nodes collected should be placed in separate packets based on the anatomical region of their dissection.
Pelvic lymph node dissections usually commence at the node of Cloquet on the right side and proceed superiorly up to the aortic bifurcation. Proximal freeing of the right ureter may be necessary to avoid inadvertent injury during this process.
The external iliac vessels can be gently reflected to facilitate the removal of the entire Triangle of Marcille lymph node packet at the origin of the obturator nerve, approached laterally. Lateral retraction of the sigmoid colon provides access to the presacral lymph nodes, enabling their dissection and retrieval.
On the left side, a small opening can be made posterior to the sigmoid colon, which will later facilitate the movement of the left ureter to the right side during the creation of the urinary diversion. The sigmoid is then retracted medially, allowing for a left-sided lymph node dissection performed in a manner similar to the right side.
- Earlier studies suggested that more extensive lymph node dissections, including up to the aortic bifurcation, might lead to lower overall and urothelial cancer-specific mortality rates. However, these findings have been questioned due to methodological issues, variations in surgical techniques, and inconsistent results across studies.[58][59][60][61][62] More recent studies indicate minimal or no significant benefit from extended lymph node dissections in terms of overall survival.[63][64][65][66]
- A recent systematic review and meta-analysis indicated that a moderately extended lymph node dissection may offer optimal benefits by improving recurrence-free survival without the additional time or morbidity associated with superextended dissections (above the aortic bifurcation).[67] However, the review found that overall survival rates were comparable to those achieved with the more limited standard dissection template.[67]
- Many surgeons opt for the extended lymph node dissection template based on studies suggesting improvements in recurrence-free and cancer-specific survival; however, this approach remains optional.[67][68][69] Evidence does not support the use of the superextended dissection, as it does not provide any oncological or survival advantage; hence, it is not recommended.[67][70][71]
- The current guidelines from the American Urological Association (AUA) and European Urological Association (EUA) recommend only a standard bilateral lymph node dissection as the minimal requirement. This recommendation is based on insufficient evidence to justify the routine use of the extended template at this time.[57][72][73]
Female radical cystectomy and anterior exenteration: In female patients, radical cystectomy typically involves a total anterior pelvic exenteration, which includes removal of the bladder, urethra, anterior vagina, uterus, and cervix. After completing ureteral dissection and controlling the vessels, the next step is to proceed with the dissection of the uterus and vagina.
A vaginal vault manipulator, such as a sponge stick, is inserted into the vagina to facilitate anterior traction and expose the posterior vaginal fornix, which is then incised. Subsequently, the infundibulopelvic ligaments and uterine vessels are transected and ligated using GIA staplers. The pouch of Douglas is also incised during this step.
Dissection of the bladder and transection of the pedicles, urachus, and DVC are performed as previously described, allowing the bladder to fall posteriorly. The dissection plane of the posterior vaginal canal is then extended anteriorly toward the urethra. The presence of the Foley catheter facilitates anatomical identification. The urethra is transected as described previously. This facilitates the removal of the specimen, which includes a small strip of the anterior vaginal wall along with the bladder, adnexa, and uterus.
Using a sponge stick, the uterus is directed posteriorly, while the fourth robotic arm provides anterior traction to the bladder. Posterior dissection of the bladder proceeds toward the vaginal canal. Laterally to the cervix, the ureter is identified, running posteriorly to the cardinal ligament, which houses the uterine artery. The ureter is dissected free, preserving the uterine pedicle. A urethrectomy can now be performed if an orthotopic urinary diversion is not planned.
An ongoing discussion regarding the potential role of organ-sparing and sexual function-sparing cystectomy exists in select patients, which emphasizes the preservation of reproductive organs, including the vagina, uterus, fallopian tubes, and ovaries. To optimize postoperative sexual and urinary function while maintaining oncologic control, organ-sparing radical cystectomy may be considered in female patients who are young, sexually active with early, unifocal, organ-confined disease, and in whom an orthotopic neobladder may be planned.[74][75] Women with a family history of breast or ovarian cancer should undergo bilateral salpingo-oophorectomy.
Patients with evidence of malignancy at the bladder neck and posterior bladder are generally considered poor candidates for vaginal sparing cystectomy due to concern for positive margins. In most cases, the entire specimen can be extracted through the vaginal canal, thereby forgoing a large extraction site incision on the abdomen. A sponge is used to pack the vaginal canal to preserve the pneumoperitoneum until the vaginal canal is closed using a 2-0 suture.
Urinary diversion: Urinary diversion options, based on preoperative patient characteristics, may now include intracorporeal techniques. These techniques commonly involve intracorporeal ileal conduit, orthotopic neobladder, or continent cutaneous diversion.[8][76][77]
Robotic radical cystectomy with bilateral lymph node dissection and intracorporeal urinary diversion has become the preferred approach for bladder cancer patients in high-volume tertiary care centers.[8][43][77] The technique of robotic radical cystectomy and orthotopic intracorporeal ileal neobladder is well described by Abreu, Simone, Truong, and others.[74][78][79][80][81][82][83][84]
The AUA maintains a series of instructional videos on robotic radical cystectomy and intracorporeal urinary diversions, accessible to members via their website. Additional educational resources on robotic radical cystectomy and intracorporeal urinary diversion can be found on YouTube, featuring videos by experts such as Steven Chang from Brigham and Women's Hospital, Raj Pruthi MD from the University of California, San Francisco (UCSF), Mark Tyson from the Mayo Clinic, Joan Palau, Richard Gaston from the European Congress on Robotics, among others.
Complications
Robotic radical cystectomy is a complex procedure involving both the genitourinary and gastrointestinal tracts, which may lead to frequent morbidity and high complication rates. The most common complications include gastrointestinal (up to 20%), infectious (up to 17%), and genitourinary complications (up to 10%), such as urinary tract infections, wound infections, ileus formation, and anemia requiring transfusion.[85] Despite an overall high 30-day complication rate (up to 48%), most complications are low-grade. Furthermore, the complication rate is similar to the corresponding open surgical technique.[85][86][87] Efforts have been focused on perioperative care to reduce the risk of complications following surgery.
Multimodal, interdisciplinary Enhanced Recovery After Surgery (ERAS) protocols have been established to shorten hospital stays, accelerate recovery of bowel function, and decrease overall morbidity following robotic cystectomy.[88][89][90][90][91][92][93] These protocols typically emphasize early ambulation, goal-directed fluid management, multimodal postoperative analgesia, and early enteral feeding.[94][95][88]
Clinical Significance
The surgical management of muscle-invasive bladder cancer has seen increasing adoption of robotic cystectomy. This approach offers comparable oncologic outcomes and quality of life scores to the open approach, albeit with longer operating times and higher costs. However, robotic cystectomy also demonstrates advantages such as reduced operative blood loss, lower transfusion rates, decreased incidence of positive margins, fewer major complications, increased lymph node yields, and shorter length of hospital stays.[46] As robotic training advances and expands, robotic radical cystectomy is expected to become more commonplace in the urological and surgical armamentarium.
Enhancing Healthcare Team Outcomes
Providing patient-centered care for individuals undergoing robotic cystectomy requires a collaborative approach among healthcare professionals. Bladder cancer can be a life-altering diagnosis for patients, which requires high-quality, integrated, multidisciplinary care throughout the perioperative period.
Before undergoing robotic cystectomy, patients will need comprehensive multidisciplinary education and counseling. This includes information about the benefits and risks of surgery and anesthesia, preoperative nutrition, future self-care for a stoma (if applicable), and expectations for recovery.
During the intraoperative phase, each member of the surgical team must fulfill their designated roles to ensure an efficient and safe procedure. Postoperatively, efforts are directed at reducing the risk of complications and optimizing the restoration of normal physiological functions. This involves a coordinated effort involving the entire interprofessional healthcare team, including, but not limited to, bedside nurses, physical therapists, occupational therapists, stoma nurses, dieticians, pharmacists, and physicians. Ultimately, each member of the healthcare team plays a crucial role in ensuring a safe and enhanced recovery for patients undergoing robotic cystectomy.
Nursing, Allied Health, and Interprofessional Team Interventions
Postoperative interprofessional care is essential for a successful outcome after robotic radical cystectomy. Regarding nursing care, key focuses in the immediate postoperative period include postoperative analgesia, early ambulation, and an emphasis on the return of bowel function.[96][97]
Healthcare professionals should regularly assess patients' pain levels and treat them using a multimodal approach. Early ambulation should be encouraged to reduce the risk of cardiovascular and pulmonary complications. Frequent assessments of gastrointestinal function should be made to determine the appropriate timing for enteral feeding. Interdisciplinary ERAS protocols should be implemented. Postoperatively, patients often have many externalized tubes (such as drains, ureteral stents, urostomy, and catheters), which require strict monitoring to assess fluid balance and ensure optimal recovery.
Stoma nurses play a pivotal role in the comprehensive aftercare of patients following conduit diversion. They may offer personalized guidance on stoma care techniques, including proper cleaning, appliance fitting, and troubleshooting potential problems.[96] Before discharge from the hospital, the interprofessional healthcare team should educate patients and their family members on dietary adjustments, physical activities, and lifestyle modifications essential for optimal recovery.
Figure
Port Placement for Robotic Radical Cystectomy Contributed by M Lee, MD

Figure
Right Ureteral Identification, Dissection, and Transection Contributed by M Lee, MD

Figure
Control of Bladder Pedicle Contributed by M Lee, MD
Figure
Release of Endopelvic Fascia Contributed by M Lee, MD

Figure
Clipping and Transection of Urethra Contributed by M Lee, MD
References
- 1.
- Aminoltejari K, Black PC. Radical cystectomy: a review of techniques, developments and controversies. Transl Androl Urol. 2020 Dec;9(6):3073-3081. [PMC free article: PMC7807330] [PubMed: 33457280]
- 2.
- Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, Adams JL, Saigal CS., Urologic Diseases in America Project. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010 Jun 02;102(11):802-11. [PMC free article: PMC3245689] [PubMed: 20400716]
- 3.
- Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, Shaaban A, Abol-Enein H, Ghoneim MA. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003 Aug;92(3):232-6. [PubMed: 12887473]
- 4.
- Pak JS, Lee JJ, Bilal K, Finkelstein M, Palese MA. Utilization Trends and Short-term Outcomes of Robotic Versus Open Radical Cystectomy for Bladder Cancer. Urology. 2017 May;103:117-123. [PubMed: 28189553]
- 5.
- Sathianathen NJ, Kalapara A, Frydenberg M, Lawrentschuk N, Weight CJ, Parekh D, Konety BR. Robotic Assisted Radical Cystectomy vs Open Radical Cystectomy: Systematic Review and Meta-Analysis. J Urol. 2019 Apr;201(4):715-720. [PubMed: 30321551]
- 6.
- Kowalewski KF, Wieland VLS, Kriegmair MC, Uysal D, Sicker T, Stolzenburg JU, Michel MS, Haney CM. Robotic-assisted Versus Laparoscopic Versus Open Radical Cystectomy-A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. Eur Urol Focus. 2023 May;9(3):480-490. [PubMed: 36529645]
- 7.
- Rai BP, Bondad J, Vasdev N, Adshead J, Lane T, Ahmed K, Khan MS, Dasgupta P, Guru K, Chlosta PL, Aboumarzouk OM. Robotic versus open radical cystectomy for bladder cancer in adults. Cochrane Database Syst Rev. 2019 Apr 24;4(4):CD011903. [PMC free article: PMC6479207] [PubMed: 31016718]
- 8.
- Han JH, Ku JH. Robot-assisted radical cystectomy: Where we are in 2023. Investig Clin Urol. 2023 Mar;64(2):107-117. [PMC free article: PMC9995950] [PubMed: 36882169]
- 9.
- Feng D, Liu S, Tang Y, Yang Y, Wei W, Han P. Comparison of perioperative and oncologic outcomes between robot-assisted and laparoscopic radical cystectomy for bladder cancer: a systematic review and updated meta-analysis. Int Urol Nephrol. 2020 Jul;52(7):1243-1254. [PubMed: 32078116]
- 10.
- Mortezavi A, Crippa A, Kotopouli MI, Akre O, Wiklund P, Hosseini A. Association of Open vs Robot-Assisted Radical Cystectomy With Mortality and Perioperative Outcomes Among Patients With Bladder Cancer in Sweden. JAMA Netw Open. 2022 Apr 01;5(4):e228959. [PMC free article: PMC9051984] [PubMed: 35482309]
- 11.
- Zhou N, Tian F, Feng Y, Zhao K, Chen L, Fan R, Lu W, Gu C. Perioperative outcomes of intracorporeal robot-assisted radical cystectomy versus open radical cystectomy: A systematic review and meta-analysis of comparative studies. Int J Surg. 2021 Oct;94:106137. [PubMed: 34600124]
- 12.
- Murthy PB, Lone Z, Munoz Lopez C, Ericson JZK, Thomas L, Caveney M, Gerber D, Khanna A, Abouassaly R, Haber GP, Lee BH. Comparison of Oncologic Outcomes Following Open and Robotic-assisted Radical Cystectomy with both Extracorporeal and Intracorporeal Urinary Diversion. Urology. 2021 Aug;154:184-190. [PubMed: 33891929]
- 13.
- Catto JWF, Khetrapal P, Ricciardi F, Ambler G, Williams NR, Al-Hammouri T, Khan MS, Thurairaja R, Nair R, Feber A, Dixon S, Nathan S, Briggs T, Sridhar A, Ahmad I, Bhatt J, Charlesworth P, Blick C, Cumberbatch MG, Hussain SA, Kotwal S, Koupparis A, McGrath J, Noon AP, Rowe E, Vasdev N, Hanchanale V, Hagan D, Brew-Graves C, Kelly JD., iROC Study Team. Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial. JAMA. 2022 Jun 07;327(21):2092-2103. [PMC free article: PMC9109000] [PubMed: 35569079]
- 14.
- Venkatramani V, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, Weizer AZ, Konety BR, Tollefson M, Krupski TL, Smith ND, Shabsigh A, Barocas DA, Quek ML, Dash A, Kibel AS, Pruthi RS, Montgomery JS, Weight CJ, Sharp DS, Chang SS, Cookson MS, Gupta GN, Gorbonos A, Uchio EM, Skinner E, Soodana-Prakash N, Becerra MF, Swain S, Kendrick K, Smith JA, Thompson IM, Parekh DJ. Predictors of Recurrence, and Progression-Free and Overall Survival following Open versus Robotic Radical Cystectomy: Analysis from the RAZOR Trial with a 3-Year Followup. J Urol. 2020 Mar;203(3):522-529. [PMC free article: PMC7487279] [PubMed: 31549935]
- 15.
- Hussein AA, Elsayed AS, Aldhaam NA, Jing Z, Osei J, Kaouk J, Redorta JP, Menon M, Peabody J, Dasgupta P, Khan MS, Mottrie A, Stöckle M, Hemal A, Richstone L, Hosseini A, Wiklund P, Schanne F, Kim E, Ho Rha K, Guru KA. Ten-Year Oncologic Outcomes Following Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol. 2019 Nov;202(5):927-935. [PubMed: 31188729]
- 16.
- Ip KL, Javier-DesLoges JF, Leung C, Nie J, Khajir G, Nawaf CB, Syed J, Rosoff JS, Martin TV, Hesse DG. Comparison of long-term outcomes in a 10-year experience of robotic cystectomy vs. open cystectomy. J Robot Surg. 2021 Oct;15(5):773-780. [PubMed: 33226567]
- 17.
- Elsayed AS, Gibson S, Jing Z, Wijburg C, Wagner AA, Mottrie A, Dasgupta P, Peabody J, Hussein AA, Guru KA. Rates and Patterns of Recurrences and Survival Outcomes after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol. 2021 Feb;205(2):407-413. [PubMed: 32945729]
- 18.
- Zennami K, Sumitomo M, Takahara K, Nukaya T, Takenaka M, Fukaya K, Ichino M, Fukami N, Sasaki H, Kusaka M, Shiroki R. Intra-corporeal robot-assisted versus open radical cystectomy: a propensity score-matched analysis comparing perioperative and long-term survival outcomes and recurrence patterns. Int J Clin Oncol. 2021 Aug;26(8):1514-1523. [PubMed: 34009486]
- 19.
- Mori K, Abufaraj M, Mostafaei H, Quhal F, Karakiewicz PI, Briganti A, Kimura S, Egawa S, Shariat SF. A Systematic Review and Meta-Analysis of Variant Histology in Urothelial Carcinoma of the Bladder Treated with Radical Cystectomy. J Urol. 2020 Dec;204(6):1129-1140. [PubMed: 32716694]
- 20.
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD, Skinner EC, Smith ND, McKiernan JM. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016 Oct;196(4):1021-9. [PubMed: 27317986]
- 21.
- Power NE, Izawa J. Comparison of Guidelines on Non-Muscle Invasive Bladder Cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer. 2016 Jan 07;2(1):27-36. [PMC free article: PMC4927900] [PubMed: 27376122]
- 22.
- Holzbeierlein JM, Bixler BR, Buckley DI, Chang SS, Holmes R, James AC, Kirkby E, McKiernan JM, Schuckman AK. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J Urol. 2024 Apr;211(4):533-538. [PubMed: 38265030]
- 23.
- Holzbeierlein J, Bixler BR, Buckley DI, Chang SS, Holmes RS, James AC, Kirkby E, McKiernan JM, Schuckman A. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/SUO Guideline (2017; Amended 2020, 2024). J Urol. 2024 Jul;212(1):3-10. [PubMed: 38661067]
- 24.
- McFadden J, Tachibana I, Adra N, Collins K, Cary C, Koch M, Kaimakliotis H, Masterson TA, Rice KR. Impact of variant histology on upstaging and survival in patients with nonmuscle invasive bladder cancer undergoing radical cystectomy. Urol Oncol. 2024 Mar;42(3):69.e11-69.e16. [PubMed: 38267301]
- 25.
- Iida K, Miyake M, Murakami K, Komiyama M, Okajima E, Sazuka T, Nishiyama N, Yasumoto H, Kimura T, Ito A, Shiga K, Yamagishi A, Kikuchi H, Sugimoto M, Taoka R, Kobayashi T, Kojima T, Kitamura H, Nishiyama H, Fujimoto K. Bacillus Calmette-Guérin-unresponsive non-muscle invasive bladder cancer outcomes in patients without radical cystectomy. Int J Clin Oncol. 2021 Nov;26(11):2104-2112. [PubMed: 34313904]
- 26.
- Hsu RL, Kaye AD, Urman RD. Anesthetic Challenges in Robotic-assisted Urologic Surgery. Rev Urol. 2013;15(4):178-84. [PMC free article: PMC3922322] [PubMed: 24659914]
- 27.
- Sell A, Jakobsen A, Nerstrøm B, Sørensen BL, Steven K, Barlebo H. Treatment of advanced bladder cancer category T2 T3 and T4a. A randomized multicenter study of preoperative irradiation and cystectomy versus radical irradiation and early salvage cystectomy for residual tumor. DAVECA protocol 8201. Danish Vesical Cancer Group. Scand J Urol Nephrol Suppl. 1991;138:193-201. [PubMed: 1785004]
- 28.
- Zlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G, Drumm M, Mari A, Thio E, Fleshner NE, Kulkarni GS, Jewett MAS, Bristow RG, Catton C, Berlin A, Sridhar SS, Schuckman A, Feldman AS, Wszolek M, Dahl DM, Lee RJ, Saylor PJ, Michaelson MD, Miyamoto DT, Zietman A, Shipley W, Chung P, Daneshmand S, Efstathiou JA. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023 Jun;24(6):669-681. [PubMed: 37187202]
- 29.
- Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, Kaufman DS, Heney NM, Zietman AL. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014 Dec 01;32(34):3801-9. [PMC free article: PMC4239302] [PubMed: 25366678]
- 30.
- Lowrance WT, Rumohr JA, Chang SS, Clark PE, Smith JA, Cookson MS. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008 Apr;179(4):1313-8; discussion 1318. [PubMed: 18289578]
- 31.
- Abreu AL, Chopra S, Azhar RA, Berger AK, Miranda G, Cai J, Gill IS, Aron M, Desai MM. Robotic radical cystectomy and intracorporeal urinary diversion: The USC technique. Indian J Urol. 2014 Jul;30(3):300-6. [PMC free article: PMC4120218] [PubMed: 25097317]
- 32.
- Al-Daghmin A, Aboumohamed A, Din R, Khan A, Raza SJ, Sztorc J, Mehedint D, Sharif M, Shi Y, Wilding G, Guru KA. Readmission after robot-assisted radical cystectomy: outcomes and predictors at 90-day follow-up. Urology. 2014 Feb;83(2):350-6. [PMC free article: PMC4431627] [PubMed: 24468509]
- 33.
- Al-Daghmin A, Kauffman EC, Shi Y, Badani K, Balbay MD, Canda E, Dasgupta P, Ghavamian R, Grubb R, Hemal A, Kaouk J, Kibel AS, Maatman T, Menon M, Mottrie A, Nepple K, Pattaras JG, Peabody JO, Poulakis V, Pruthi R, Palou Redorta J, Rha KH, Richstone L, Schanne F, Scherr DS, Siemer S, Stöckle M, Wallen EM, Weizer A, Wiklund P, Wilson T, Wilding G, Woods M, Guru KA. Efficacy of robot-assisted radical cystectomy (RARC) in advanced bladder cancer: results from the International Radical Cystectomy Consortium (IRCC). BJU Int. 2014 Jul;114(1):98-103. [PMC free article: PMC4196689] [PubMed: 24219170]
- 34.
- Lee DJ, Rothberg MB, McKiernan JM, Benson MC, Badani KK. Robot-assisted radical cystoprostatectomy in complex surgical patients: single institution report. Can J Urol. 2009 Jun;16(3):4664-9. [PubMed: 19497175]
- 35.
- Phillips EA, Uberoi V, Tuerk IA. Robot-assisted radical cystectomy in octogenarians. J Endourol. 2014 Feb;28(2):219-23. [PubMed: 24074288]
- 36.
- Lee DJ, Tyson MD, Chang SS. Conduit Urinary Diversion. Urol Clin North Am. 2018 Feb;45(1):25-36. [PubMed: 29169448]
- 37.
- Hashad MM, Atta M, Elabbady A, Elfiky S, Khattab A, Kotb A. Safety of no bowel preparation before ileal urinary diversion. BJU Int. 2012 Dec;110(11 Pt C):E1109-13. [PubMed: 23167296]
- 38.
- Kauf TL, Svatek RS, Amiel G, Beard TL, Chang SS, Fergany A, Karnes RJ, Koch M, O'Hara J, Lee CT, Sexton WJ, Slaton JW, Steinberg GD, Wilson SS, Techner L, Martin C, Moreno J, Kamat AM. Alvimopan, a peripherally acting μ-opioid receptor antagonist, is associated with reduced costs after radical cystectomy: economic analysis of a phase 4 randomized, controlled trial. J Urol. 2014 Jun;191(6):1721-7. [PubMed: 24342144]
- 39.
- Reichert M, Willis F, Post S, Schneider M, Vilz T, Willis M, Hecker A. Pharmacologic prevention and therapy of postoperative paralytic ileus after gastrointestinal cancer surgery: systematic review and meta-analysis. Int J Surg. 2024 Jul 01;110(7):4329-4341. [PMC free article: PMC11254286] [PubMed: 38526522]
- 40.
- Hanna P, Regmi S, Kalapara A, Mulpuri KS, Zabell J, Albersheim J, Wahr J, Randle D, Kaizer A, Patten L, Konety B, Weight C. Alvimopan as part of the Enhanced Recovery After Surgery protocol following radical cystectomy is associated with decreased hospital stay. Int J Urol. 2021 Jun;28(6):696-701. [PubMed: 33769634]
- 41.
- International Collaboration of Trialists. Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group). European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group. Australian Bladder Cancer Study Group. National Cancer Institute of Canada Clinical Trials Group. Finnbladder. Norwegian Bladder Cancer Study Group. Club Urologico Espanol de Tratamiento Oncologico Group. Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011 Jun 01;29(16):2171-7. [PMC free article: PMC3107740] [PubMed: 21502557]
- 42.
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003 Aug 28;349(9):859-66. [PubMed: 12944571]
- 43.
- Wijburg CJ, Hannink G, Michels CTJ, Weijerman PC, Issa R, Tay A, Decaestecker K, Wiklund P, Hosseini A, Sridhar A, Kelly J, d'Hondt F, Mottrie A, Klaver S, Edeling S, Dell'Oglio P, Montorsi F, Rovers MM, Witjes JA. Learning Curve Analysis for Intracorporeal Robot-assisted Radical Cystectomy: Results from the EAU Robotic Urology Section Scientific Working Group. Eur Urol Open Sci. 2022 May;39:55-61. [PMC free article: PMC9068730] [PubMed: 35528784]
- 44.
- Arora S, Keeley J, Patel A, Eleswarapu SV, Bronkema C, Alanee S, Menon M. Defining a "High Volume" Radical Cystectomy Hospital: Where Do We Draw the Line? Eur Urol Focus. 2020 Sep 15;6(5):975-981. [PubMed: 30772360]
- 45.
- Pyrgidis N, Volz Y, Ebner B, Kazmierczak PM, Enzinger B, Hermans J, Buchner A, Stief C, Schulz GB. The effect of hospital caseload on perioperative mortality, morbidity and costs in bladder cancer patients undergoing radical cystectomy: results of the German nationwide inpatient data. World J Urol. 2024 Jan 10;42(1):19. [PMC free article: PMC10781819] [PubMed: 38197902]
- 46.
- Martin AS, Corcoran AT. Contemporary techniques and outcomes of robotic assisted radical cystectomy with intracorporeal urinary diversion. Transl Androl Urol. 2021 May;10(5):2216-2232. [PMC free article: PMC8185677] [PubMed: 34159105]
- 47.
- Chan KG, Guru K, Wiklund P, Catto J, Yuh B, Novara G, Murphy DG, Al-Tartir T, Collins JW, Zhumkhawala A, Wilson TG., Pasadena Consensus Panel. Robot-assisted radical cystectomy and urinary diversion: technical recommendations from the Pasadena Consensus Panel. Eur Urol. 2015 Mar;67(3):423-31. [PubMed: 25595099]
- 48.
- Collins JW, Tyritzis S, Nyberg T, Schumacher M, Laurin O, Khazaeli D, Adding C, Jonsson MN, Hosseini A, Wiklund NP. Robot-assisted radical cystectomy: description of an evolved approach to radical cystectomy. Eur Urol. 2013 Oct;64(4):654-63. [PubMed: 23769588]
- 49.
- Hussein AA, Ahmed YE, Kozlowski JD, May PR, Nyquist J, Sexton S, Curtin L, Peabody JO, Abol-Enein H, Guru KA. Robot-assisted approach to 'W'-configuration urinary diversion: a step-by-step technique. BJU Int. 2017 Jul;120(1):152-157. [PubMed: 28220593]
- 50.
- Abreu AL, Chopra S, Berger AK, Leslie S, Desai MM, Gill IS, Aron M. Management of large median and lateral intravesical lobes during robot-assisted radical prostatectomy. J Endourol. 2013 Nov;27(11):1389-92. [PubMed: 23859125]
- 51.
- Azzouni FS, Din R, Rehman S, Khan A, Shi Y, Stegemann A, Sharif M, Wilding GE, Guru KA. The first 100 consecutive, robot-assisted, intracorporeal ileal conduits: evolution of technique and 90-day outcomes. Eur Urol. 2013 Apr;63(4):637-43. [PubMed: 23265384]
- 52.
- Laukhtina E, Rajwa P, Mori K, Moschini M, D'Andrea D, Abufaraj M, Soria F, Mari A, Krajewski W, Albisinni S, Teoh JY, Quhal F, Sari Motlagh R, Mostafaei H, Katayama S, Grossmann NC, Enikeev D, Zimmermann K, Fajkovic H, Glybochko P, Shariat SF, Pradere B., European Association of Urology Young Academic Urologists Urothelial Carcinoma Working Group (EAU YAU). Accuracy of Frozen Section Analysis of Urethral and Ureteral Margins During Radical Cystectomy for Bladder Cancer: A Systematic Review and Diagnostic Meta-Analysis. Eur Urol Focus. 2022 May;8(3):752-760. [PubMed: 34127436]
- 53.
- Packiam VT, Tsivian M, Boorjian SA. The evolving role of lymphadenectomy for bladder cancer: why, when, and how. Transl Androl Urol. 2020 Dec;9(6):3082-3093. [PMC free article: PMC7807370] [PubMed: 33457281]
- 54.
- Herr H, Lee C, Chang S, Lerner S., Bladder Cancer Collaborative Group. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J Urol. 2004 May;171(5):1823-8; discussion 1827-8. [PubMed: 15076285]
- 55.
- Abol-Enein H, Tilki D, Mosbah A, El-Baz M, Shokeir A, Nabeeh A, Ghoneim MA. Does the extent of lymphadenectomy in radical cystectomy for bladder cancer influence disease-free survival? A prospective single-center study. Eur Urol. 2011 Sep;60(3):572-7. [PubMed: 21684070]
- 56.
- Bochner BH, Cho D, Herr HW, Donat M, Kattan MW, Dalbagni G. Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. J Urol. 2004 Oct;172(4 Pt 1):1286-90. [PubMed: 15371825]
- 57.
- Gschwend JE, Heck MM, Lehmann J, Rübben H, Albers P, Wolff JM, Frohneberg D, de Geeter P, Heidenreich A, Kälble T, Stöckle M, Schnöller T, Stenzl A, Müller M, Truss M, Roth S, Liehr UB, Leißner J, Bregenzer T, Retz M. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol. 2019 Apr;75(4):604-611. [PubMed: 30337060]
- 58.
- Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002 Mar;167(3):1295-8. [PubMed: 11832716]
- 59.
- Shirotake S, Kikuchi E, Matsumoto K, Yazawa S, Kosaka T, Miyajima A, Nakagawa K, Oya M. Role of pelvic lymph node dissection in lymph node-negative patients with invasive bladder cancer. Jpn J Clin Oncol. 2010 Mar;40(3):247-51. [PubMed: 19887521]
- 60.
- Brunocilla E, Pernetti R, Schiavina R, Borghesi M, Vagnoni V, Rocca GC, Borgatti F, Concetti S, Martorana G. The number of nodes removed as well as the template of the dissection is independently correlated to cancer-specific survival after radical cystectomy for muscle-invasive bladder cancer. Int Urol Nephrol. 2013 Jun;45(3):711-9. [PubMed: 23666588]
- 61.
- Zehnder P, Studer UE, Skinner EC, Dorin RP, Cai J, Roth B, Miranda G, Birkhäuser F, Stein J, Burkhard FC, Daneshmand S, Thalmann GN, Gill IS, Skinner DG. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol. 2011 Oct;186(4):1261-8. [PubMed: 21849183]
- 62.
- Simone G, Papalia R, Ferriero M, Guaglianone S, Castelli E, Collura D, Muto G, Gallucci M. Stage-specific impact of extended versus standard pelvic lymph node dissection in radical cystectomy. Int J Urol. 2013 Apr;20(4):390-7. [PubMed: 22970939]
- 63.
- Kosiba M, Stolzenbach LF, Collà Ruvolo C, Nocera L, Mansour M, Tian Z, Roos FC, Becker A, Kluth LA, Tilki D, Shariat SF, Saad F, Chun FKH, Karakiewicz PI. Contemporary Trends and Efficacy of Pelvic Lymph Node Dissection at Radical Cystectomy for Urothelial and Variant Histology Carcinoma of the Urinary Bladder. Clin Genitourin Cancer. 2022 Apr;20(2):195.e1-195.e8. [PubMed: 34906434]
- 64.
- von Deimling M, Furrer M, Mertens LS, Mari A, van Ginkel N, Bacchiani M, Maas M, Pichler R, Li R, Moschini M, Bianchi A, Vetterlein MW, Lonati C, Crocetto F, Taylor J, Tully KH, Afferi L, Soria F, Del Giudice F, Longoni M, Laukhtina E, Antonelli A, Rink M, Fisch M, Lotan Y, Spiess PE, Black PC, Kiss B, Pradere B, Shariat SF., CLIPOLY Study Group Collaborators. Impact of the extent of lymph node dissection on survival outcomes in clinically lymph node-positive bladder cancer. BJU Int. 2024 Mar;133(3):341-350. [PubMed: 37904652]
- 65.
- Crocerossa F, Autorino R, Carbonara U, Cantiello F, Damiano R, Mir MC. Extent of lymph node dissection and impact on survival in radical cystectomy for advanced bladder cancer. Curr Opin Urol. 2022 Nov 01;32(6):607-613. [PubMed: 36101521]
- 66.
- Heck MM, Gschwend JE. Extended Lymph Node Dissection for Bladder Cancer: Do Clinical Trials Rule Out a Benefit? Eur Urol Focus. 2020 Jul 15;6(4):617-619. [PubMed: 31439506]
- 67.
- Qi W, Zhong M, Jiang N, Zhou Y, Lv G, Li R, Shi B, Chen S. Which lymph node dissection template is optimal for radical cystectomy? A systematic review and Bayesian network meta-analysis. Front Oncol. 2022;12:986150. [PMC free article: PMC9732561] [PubMed: 36505883]
- 68.
- Perera M, McGrath S, Sengupta S, Crozier J, Bolton D, Lawrentschuk N. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nat Rev Urol. 2018 Nov;15(11):686-692. [PubMed: 30104615]
- 69.
- Bruins HM, Veskimae E, Hernandez V, Imamura M, Neuberger MM, Dahm P, Stewart F, Lam TB, N'Dow J, van der Heijden AG, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Sherif A, Witjes JA. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. Eur Urol. 2014 Dec;66(6):1065-77. [PubMed: 25074764]
- 70.
- Muilwijk T, Akand M, Gevaert T, Joniau S. No survival difference between super extended and standard lymph node dissection at radical cystectomy: what can we learn from the first prospective randomized phase III trial? Transl Androl Urol. 2019 Mar;8(Suppl 1):S112-S115. [PMC free article: PMC6511709] [PubMed: 31143684]
- 71.
- Møller MK, Høyer S, Jensen JB. Extended versus superextended lymph-node dissection in radical cystectomy: subgroup analysis of possible recurrence-free survival benefit. Scand J Urol. 2016 Jun;50(3):175-80. [PubMed: 27049808]
- 72.
- Soria F, Gontero P. Re: Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol. 2019 Sep;76(3):408-409. [PubMed: 30987842]
- 73.
- Alfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, Fietkau R, Gakis G, Lorch A, Martini A, Mertens LS, Meijer RP, Milowsky MI, Neuzillet Y, Panebianco V, Redlef J, Rink M, Rouanne M, Thalmann GN, Sæbjørnsen S, Veskimäe E, van der Heijden AG. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur Urol. 2024 Jan;85(1):17-31. [PubMed: 37858453]
- 74.
- Truong H, Maxon V, Goh AC. Robotic Female Radical Cystectomy. J Endourol. 2021 Sep;35(S2):S106-S115. [PMC free article: PMC8558089] [PubMed: 34499552]
- 75.
- Niver BE, Daneshmand S, Satkunasivam R. Female reproductive organ-sparing radical cystectomy: contemporary indications, techniques and outcomes. Curr Opin Urol. 2015 Mar;25(2):105-10. [PubMed: 25581538]
- 76.
- Chopra S, Abreu AL, Gill IS. Robotic urinary diversion: the range of options. Curr Opin Urol. 2016 Jan;26(1):107-13. [PubMed: 26574875]
- 77.
- Thress TM, Cookson MS, Patel S. Robotic Cystectomy with Intracorporeal Urinary Diversion: Review of Current Techniques and Outcomes. Urol Clin North Am. 2018 Feb;45(1):67-77. [PubMed: 29169452]
- 78.
- Simone G, Papalia R, Misuraca L, Tuderti G, Minisola F, Ferriero M, Vallati G, Guaglianone S, Gallucci M. Robotic Intracorporeal Padua Ileal Bladder: Surgical Technique, Perioperative, Oncologic and Functional Outcomes. Eur Urol. 2018 Jun;73(6):934-940. [PubMed: 27780643]
- 79.
- Hussein AA, Li Q, Guru KA. Robot-assisted radical cystectomy: surgical technique, perioperative and oncologic outcomes. Curr Opin Urol. 2022 Jan 01;32(1):116-122. [PubMed: 34798640]
- 80.
- Elsayed AS, Aldhaam NA, Nitsche L, Siam A, Jing Z, Hussein AA, Shigemura K, Fujisawa M, Guru KA. Robot-assisted radical cystectomy: Review of surgical technique, and perioperative, oncological and functional outcomes. Int J Urol. 2020 Mar;27(3):194-205. [PubMed: 31981379]
- 81.
- Wiklund NP, Poulakis V. Robotic neobladder. BJU Int. 2011 May;107(9):1514-37. [PubMed: 21518236]
- 82.
- Chen AB, Polotti CF, Zhang M, Yip W, Desai M. Robotic Intracorporeal Ileal Conduit Urinary Diversion Technique. J Endourol. 2021 Sep;35(S2):S116-S121. [PubMed: 34499542]
- 83.
- Murthy PB, Campbell RA, Lee BH. Intracorporeal Urinary Diversion in Robotic Radical Cystectomy. Urol Clin North Am. 2021 Feb;48(1):51-70. [PubMed: 33218594]
- 84.
- Tan WS, Lamb BW, Sridhar A, Briggs TP, Kelly JD. A comprehensive guide to perioperative management and operative technique for robotic cystectomy with intracorporeal urinary diversion. Urologia. 2017 Apr 28;84(2):71-78. [PubMed: 28256704]
- 85.
- Katsimperis S, Tzelves L, Tandogdu Z, Ta A, Geraghty R, Bellos T, Manolitsis I, Pyrgidis N, Schulz GB, Sridhar A, Shaw G, Kelly J, Skolarikos A. Complications After Radical Cystectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials with a Meta-regression Analysis. Eur Urol Focus. 2023 Nov;9(6):920-929. [PubMed: 37246124]
- 86.
- Johar RS, Hayn MH, Stegemann AP, Ahmed K, Agarwal P, Balbay MD, Hemal A, Kibel AS, Muhletaler F, Nepple K, Pattaras JG, Peabody JO, Palou Redorta J, Rha KH, Richstone L, Saar M, Schanne F, Scherr DS, Siemer S, Stökle M, Weizer A, Wiklund P, Wilson T, Woods M, Yuh B, Guru KA. Complications after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2013 Jul;64(1):52-7. [PubMed: 23380164]
- 87.
- Novara G, Catto JW, Wilson T, Annerstedt M, Chan K, Murphy DG, Motttrie A, Peabody JO, Skinner EC, Wiklund PN, Guru KA, Yuh B. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol. 2015 Mar;67(3):376-401. [PubMed: 25560798]
- 88.
- Ivan SJ, Roebuck EH, Sinks AL, Robinson MM, Clark PE, Gaston KE, Matulay JT, Riggs SB. It's complicated: The relationship of non-narcotic medications and postoperative opioid use in radical cystectomy patients. Urol Oncol. 2024 Oct;42(10):332.e1-332.e9. [PubMed: 38735799]
- 89.
- Çetin B, Çilesiz NC, Ozkan A, Onuk Ö, Kır G, Balci MBC, Özdemir E. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Length of Hospital Stay in Radical Cystectomy: A Prospective Randomized Controlled Study. Cureus. 2024 Mar;16(3):e55460. [PMC free article: PMC10988186] [PubMed: 38571847]
- 90.
- Zennami K, Kusaka M, Tomozawa S, Toda F, Ito K, Kawai A, Nakamura W, Muto Y, Saruta M, Motonaga T, Takahara K, Sumitomo M, Shiroki R. Impact of an enhanced recovery protocol in frail patients after intracorporeal urinary diversion. BJU Int. 2024 Mar 19; [PubMed: 38500447]
- 91.
- Grilo N, Crettenand F, Bohner P, Rodrigues Dias SC, Cerantola Y, Lucca I. Impact of Enhanced Recovery after Surgery® Protocol Compliance on Length of Stay, Bowel Recovery and Complications after Radical Cystectomy. Diagnostics (Basel). 2024 Jan 25;14(3) [PMC free article: PMC10855147] [PubMed: 38337779]
- 92.
- Yanada BA, Dias BH, Corcoran NM, Zargar H, Bishop C, Wallace S, Hayes D, Huang JG. Implementation of the enhanced recovery after surgery protocol for radical cystectomy patients: A single centre experience. Investig Clin Urol. 2024 Jan;65(1):32-39. [PMC free article: PMC10789537] [PubMed: 38197749]
- 93.
- Lee G, Patel HV, Srivastava A, Ghodoussipour S. Updates on enhanced recovery after surgery for radical cystectomy. Ther Adv Urol. 2022 Jan-Dec;14:17562872221109022. [PMC free article: PMC9280843] [PubMed: 35844831]
- 94.
- Williams SB, Cumberbatch MGK, Kamat AM, Jubber I, Kerr PS, McGrath JS, Djaladat H, Collins JW, Packiam VT, Steinberg GD, Lee E, Kassouf W, Black PC, Cerantola Y, Catto JWF, Daneshmand S. Reporting Radical Cystectomy Outcomes Following Implementation of Enhanced Recovery After Surgery Protocols: A Systematic Review and Individual Patient Data Meta-analysis. Eur Urol. 2020 Nov;78(5):719-730. [PubMed: 32624275]
- 95.
- Collins JW, Patel H, Adding C, Annerstedt M, Dasgupta P, Khan SM, Artibani W, Gaston R, Piechaud T, Catto JW, Koupparis A, Rowe E, Perry M, Issa R, McGrath J, Kelly J, Schumacher M, Wijburg C, Canda AE, Balbay MD, Decaestecker K, Schwentner C, Stenzl A, Edeling S, Pokupić S, Stockle M, Siemer S, Sanchez-Salas R, Cathelineau X, Weston R, Johnson M, D'Hondt F, Mottrie A, Hosseini A, Wiklund PN. Enhanced Recovery After Robot-assisted Radical Cystectomy: EAU Robotic Urology Section Scientific Working Group Consensus View. Eur Urol. 2016 Oct;70(4):649-660. [PubMed: 27234997]
- 96.
- Jensen BT, Retinger NL, Lauridsen SV. From fast-track to enhanced recovery after surgery in radical cystectomy pathways: A nursing perspective. Asia Pac J Oncol Nurs. 2022 Jul;9(7):100048. [PMC free article: PMC9136268] [PubMed: 35647225]
- 97.
- Overstreet DL, Sims TW. Care of the patient undergoing radical cystectomy with a robotic approach. Urol Nurs. 2006 Apr;26(2):117-22. [PubMed: 16703919]
Disclosure: Matthew Lee declares no relevant financial relationships with ineligible companies.
Disclosure: Daniel Eun declares no relevant financial relationships with ineligible companies.
- Robotic versus open radical cystectomy for bladder cancer in adults.[Cochrane Database Syst Rev. 2019]Robotic versus open radical cystectomy for bladder cancer in adults.Rai BP, Bondad J, Vasdev N, Adshead J, Lane T, Ahmed K, Khan MS, Dasgupta P, Guru K, Chlosta PL, et al. Cochrane Database Syst Rev. 2019 Apr 24; 4(4):CD011903. Epub 2019 Apr 24.
- Large Single Institution Comparison of Perioperative Outcomes and Complications of Open Radical Cystectomy, Intracorporeal Robot-Assisted Radical Cystectomy and Robotic Extracorporeal Approach.[J Urol. 2020]Large Single Institution Comparison of Perioperative Outcomes and Complications of Open Radical Cystectomy, Intracorporeal Robot-Assisted Radical Cystectomy and Robotic Extracorporeal Approach.Zhang JH, Ericson KJ, Thomas LJ, Knorr J, Khanna A, Crane A, Mittal R, Zampini A, Fascelli M, Murthy PB, et al. J Urol. 2020 Mar; 203(3):512-521. Epub 2019 Oct 3.
- Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial.[Eur Urol. 2015]Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial.Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, Coleman JA, Mathew S, Vickers A, Schnorr GC, et al. Eur Urol. 2015 Jun; 67(6):1042-1050. Epub 2014 Dec 8.
- Review The role of laparoscopic and robotic cystectomy in the management of muscle-invasive bladder cancer with special emphasis on cancer control and complications.[Eur Urol. 2011]Review The role of laparoscopic and robotic cystectomy in the management of muscle-invasive bladder cancer with special emphasis on cancer control and complications.Challacombe BJ, Bochner BH, Dasgupta P, Gill I, Guru K, Herr H, Mottrie A, Pruthi R, Redorta JP, Wiklund P. Eur Urol. 2011 Oct; 60(4):767-75. Epub 2011 May 17.
- Review Utility of robot-assisted radical cystectomy with intracorporeal urinary diversion for muscle-invasive bladder cancer.[Int J Urol. 2019]Review Utility of robot-assisted radical cystectomy with intracorporeal urinary diversion for muscle-invasive bladder cancer.Koie T, Ohyama C, Makiyama K, Shimazui T, Miyagawa T, Mizutani K, Tsuchiya T, Kato T, Nakane K. Int J Urol. 2019 Mar; 26(3):334-340. Epub 2019 Jan 28.
- Robotic Radical Cystectomy of the Bladder - StatPearlsRobotic Radical Cystectomy of the Bladder - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...