NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cappellini MD, Farmakis D, Porter J, et al., editors. 2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition. Nicosia (Cyprus): Thalassaemia International Federation; 2023.

2021 Guidelines: For the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 4th edition.
Show detailsIntroduction
Infections and their complications were previously the second commonest cause of death in transfusion-dependent thalassaemia (TDT), prior to the new millennium (Borgna-Pignatti et al., 2004). Infections are becoming the leading cause of death in western countries due, in part, to a significant reduction in the number of deaths from iron-induced cardiac disease (Modell et al., 2008). Infections have already been reported as the primary cause of mortality among E-beta thalassaemia patients in Thailand years ago (Wanachiwanawin, 2000).
The variability in the epidemiology of infections, differences in socio-economic level, preventative strategies and accessibility to healthcare in each country should have an impact on variability in rates of infection-related morbidity and mortality in TDT throughout the world. In TDT, allogeneic packed red cell transfusions (pRBC) carry significant burden, including direct exposure to risk of transfusion-transmitted infections (Vamvakas & Blajchman, 2009), indirect risks of transfusion-related immunomodulation (TRIM) (Blajchman, 2005) and iron overload (IOL) (Marx, 2002). Underlying pathophysiological mechanisms of disease such as ineffective erythropoiesis (IE), haemolysis and anaemia may also have deleterious effects on the immune system and contribute to susceptibility to infections (Wanachiwanawin et al., 1993). Furthermore, some other therapeutic interventions such as iron chelation therapy, splenectomy, central venous catheters, and stem cell transplantation may contribute to infectious complications with resultant to morbidity and mortality (Figure 1).
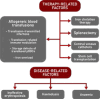
Figure 1
Factors contributing to infection risk in TDT.
Therapy-Related Risks of Infections in TDT and Preventive Measures
Allogeneic blood transfusion-related risks of infections
A. Transfusion-transmitted infections (TTIs)
The risk of transfusion-transmitted infections (TTI) in patients with transfusion-dependent thalassaemia does not differ from other multi-transfused patients. Hepatitis C virus (HCV), hepatitis B virus (HBV), human immunodeficiency virus (HIV) and syphilis are the most common infectious agents that may be transmitted via pRBC transfusions. There has also been a steadily increasing number of reported cases of transfusion-transmitted hepatitis E virus in blood donation recipients (Domanović et al., 2017). Transfusion transmitted malaria remains one of the most common TTI on a global scale (Kitchen & Chiodin, 2006). West-Nile virus (WNV) is also recognized as a transfusion-transmitted virus requiring preventive blood safety measures (Domanovic et al., 2019). Despite improved donor testing, long-term transfusion support has a substantial cumulative life-time residual risk of TTI (Kleinman & Stassinopoulos, 2015), due to low burden pathogens undetected by testing (e.g., Plasmodia, Babesia), emerging pathogens for which tests are not available (e.g., dengue, chikungunya), and unrecognised bacterial contamination (e.g., Yersinia entercolitica) (Stramer & Dodd, 2013; Damgaard et al., 2015). Further, the blood safety chain (donor selection, TTI testing, and haemovigilance, including post-transfusion surveillance) is suboptimal in low-income countries (Shyamala, 2014).
Fundamental principles for providing safe blood
- The deferral of high risk prospective donors is the first level of defence against TTIs.
- Strategies regarding donor recruitment through voluntary non-remunerated blood donation (VNRBD) should be implemented because such donors have been found to have lower risk for TTIs.
- The routine testing of donor blood for HBV, HCV, HIV and syphilis by validated technology should be implemented in Blood Banks (Bloch 2012).
Testing of donations for infectious agents is a key factor in ensuring that the risk of disease transmission is minimized and that blood components are suitable for their intended purpose. Current tests are based on the detection of relevant antigens and/or antibody and gene sequences.
The minimum mandatory serological blood donor screening tests are:
- antibody to HIV-1 and HIV-2 including outlying types e.g., HIV-1 type O,
- antibody to hepatitis C virus,
- hepatitis B surface antigen (HBsAg) assay
Treponema pallium haemagglutination assay (TPHA); Elisa for syphilis,
Additional Serological Screening Tests. These may be required by the national authorities for specific components or in particular epidemiological conditions, e.g.
- Anti-HTLV-l and HTLV-ll,
- Antibody to hepatitis B core antigen (anti-HBc)
- Testing for CMV antibodies for the transfusion of highly susceptible patients and in cases of candidates for HSCT.
- Chagas testing for Trypanosoma cruzi antibodies is employed in endemic areas and elsewhere for travellers returning from an endemic area.
Molecular testing
Nucleic Acid Screening or Nucleic Acid Amplification Techniques (NAT) for HCV-RNA, HIV-RNA and HBV-DNA in mini pools or single donations represents the state of the art in many countries. It was developed in order to remedy limitations in serological testing, e.g., the window period - the time lapse from the appearance of the virus in blood until the detectability of a given marker (antibody, antigen or nucleic acid) (Candotti & Allain, 2013). This method is technologically demanding and costly. Unfortunately, it has not yet gained worldwide application even where is most needed.
Silent /occult infections
Apart from seronegative donors during the infectious window period, the greatest threat to the safety of the blood supply using serological markers is posed by the occult period of HBV. This period is characterised by very low viral load and undetectable HBsAg at the tail end of chronic carriage or the occurrence of escape mutants interfering with HBsAg synthesis and the interference of other viruses in HBV replication (Paraskevis et al., 2013).
All patients with TDT should be protected by vaccination against HBV. Since the protection offered by vaccination is not absolute, patients should be tested annually for HBV markers as well as the other TTIs such as HCV and HIV. Booster dose of HBV vaccine is considered if anti-HBs titre decreases (Singh 2003) (A).
- The diversity of blood-borne infectious agents transmitted through transfusion of infected blood donated by apparently healthy and asymptomatic blood donors also includes human T-cell lymphotropic viruses (HTLV-1/2), cytomegalovirus (CMV), parvovirus B19, West Nile Virus (WNV), dengue virus, Babesia spp., Plasmodium spp., Trypanosoma cruzi and the prions that cause variant Creutzfeldt-Jakob disease (CJD) (Allain et al., 2009). The national authorities may require additional Serological Screening Tests for specific components or in particular epidemiological conditions, e.g. CMV antibodies for the transfusion of highly susceptible patients and in cases of candidates for HSCT or Chagas testing for Trypanosoma cruzi antibodies is employed in endemic areas and elsewhere for travelers returning from an endemic area.
- Haemovigilance is another crucial pillar for safeguarding blood safety through a system of epidemiological surveillance of adverse reactions and adverse events in donors and in recipients (European Commission Directive 2005/61/EC). Its ultimate goal is to prevent the recurrence of adverse events and reactions. To accomplish this task, haemovigilance must be a shared responsibility of the professionals in the field and the Competent Authorities for blood safety (Politis et al., 2016).
Preventative measures include
- Prestorage leucodepletion of pRBC units reduces the transmission of CMV, and may also be effective in reducing the risk of a number of additional transfusion-transmitted infections, including infections due to herpes viruses (e.g., Epstein–Barr virus [EBV] and human herpesvirus-8 [HHV-8]), retroviruses (e.g., HTLV-1 and HIV), bacteria (e.g., Yersinia enterocolitica), protozoa (e.g., Leishmania species and Trypanosoma cruzi) and infectious prions.
- It should be noted that leucodepletion does not provide 100% risk prevention from these infections, but it may provide an additional and justified measure of caution (Cervia, Wenz & Ortolano, 2007) (C).
Bacterial sepsis associated with transfusion of contaminated pRBC units is associated with high fever, rigors and hypotension, beginning during or shortly after the transfusion. The pRBC unit is presumably contaminated by transient donor bacteraemia due to a recent infection. Causative bacteria are most often Gram-negative bacilli – mainly Yersinia enterocolitica and Serratia marcescans (Lindholm, Annen & Ramsey, 2011).
The potential safeguard to mitigate the risk of TTI
Pathogen reduction treatment (PRT) of pRBC, which is referred to the inactivation of all viruses, bacteria, parasites and any replicating structures, could be a method to achieve the goal of almost absolute blood safety, albeit there are some limitations of these technologies. Some pathogens (Parvovirus B19, HAV, and HEV) show partially intrinsic resistance to the inactivation process constituting a limitation to the use of the PI-technology. (Kleinman & Stassinopoulos, 2015). Two methods are currently under development for supplying pathogen reduced pRBC: Photochemical inactivation of whole blood using Riboflavin (vitamin B2) and ultraviolet light energy (®Mirasol Pathogen Reduction System) has been investigated in a phase-III clinical trial for whole blood use (Allain, 2016) but not yet been evaluated for RBC use. Chemical inactivation of RBC using Amustaline-glutathione (®Intercept Blood System) has been evaluated in Phase-III clinical trials for supporting chronic transfusion program in patients with TDT (Aydinok, 2019). In the latter study, PRT of pRBC appeared to be well-tolerated and logistically feasible for chronic transfusion therapy without significant increase in pRBC utilization in TDT patients.
The universal utilization of an approved PRT pRBC could allow donor testing to be significantly revised and may ultimately be less complex and less expensive than continued assay development. However, the cost will be regarded as an important factor for implementing PRT technology, particularly in low-income countries.
Suspicion and approach to transfusion-related bacterial sepsis
- If bacterial contamination is suspected, the transfusion should be halted immediately.
- Intravenous infusion of a third generation cephalosporin (cefotaxime 2 g every 8h or ceftriaxone 2 g every 12 h) or carbapenem (meropenem or imipenem 2 g every 8 h) combined with vancomycin (1–1.5 g every 12 h).
- Gram stain and blood culture are obtained from both the blood bag and the recipient (A).
Preventative measures for bacterial sepsis
- Transfusion of pRBC units stored less than 2 weeks reduces the risk of transfusion-associated Yersinia septicaemia. It has been demonstrated that Yersinia grows in the contaminated RBC unit after a lag time of 2 weeks (C).
- Leucodepletion is able to eliminate or markedly reduce the growth of the bacterium in processed blood. However, it is not capable of providing100% protection from the risk of these infections. It may provide an additional and justified measure of caution (Kim et al., 1992) (C).
B. Transfusion-related immune modulation
TRIM may contribute to all immunological alterations observed in TDT patients and it is assumed that either allogeneic mononuclear cells in the pRBC unit, or the soluble substances that are released during storage, play a central role in pathogenesis of TRIM. Pre-storage leucodepletion of pRBC units has no protective effect on immune alterations observed in patients with thalassaemia (Sirchia et al., 1986).
C. Storage defects of transfused pRBCs
It is suggested that free haem compounds released from the lysis of transfused red cells can readily provide iron for bacteria and promote infection (Griffiths, 1999).This hypothesis could be augmented by evidence suggesting that low molecular mass iron complexes occur in pRBC units stored for more than 10 days (Marwah et al., 2002). In fact, marked increases in non-transferrin bound iron and a decrease in antioxidant capacity have been observed in pRBCs stored for more than 14 days (Ozment & Turi, 2009). A large comparative study is required to reveal whether prolonged storage of pRBC is associated with an increased risk of nosocomial infection.
- Transfusions with pRBC units that have been stored for less than 14 days may provide benefit to avoid deleterious effects of storage defects (C).
Table 1
Pathogens isolated from thalassaemic patients with infection:
D. Transfusional Iron overload
Iron overload is suggested to be a risk factor predisposing to infections, since all groups of protozoa, fungi, Gram-positive and negative bacteria require iron for survival and replication, with the only exception being pathogenic Borrelia burgdorferi which use manganese in place of iron. Some pathogens such as Yersinia enterocolitica, Klebsiella species, Escherichia coli, Streptococcus pneumoniae Pseudomonas aeruginosa, Listeria monocytogenes and Legionella pneumophila increase their virulence and pathogenicity in the presence of excess iron (Weinberg, 2000). A gram-negative bacterium, V. vulnificus can be transmitted by ingestion of uncooked warm seawater fish, crustaceans, and mollusks and can cause a lethal infection in 20-50% of iron-overloaded patients (Kuo et al., 2009). Although viruses do not require iron, studies have reported that iron increases the risk of viral infections (Weinberg, 2009) and impairs the clinical response to antiviral therapy in HCV infection (Pietrangelo, 2003). Further, iron overload is associated with faster HIV-1 disease progression and poor outcome in TDT patients (Gordeuk et al., 2001). Iron availability is linked to pathogenicity of Candida albicans and Aspergillum fumigates. Iron has subtle effects on cell-mediated immune effector pathways and systemic iron overload is associated with unfavourable outcomes in many types of infection (Nairz et al., 2010).
- Despite the lack of properly controlled studies, control of iron overload may have therapeutic benefit against infections (C).
Splenectomy
Splenectomy plays a significant role in susceptibility to infections in thalassaemia, since the spleen has a crucial function in immune defence as a phagocytic filter for blood-borne microorganisms, and also produces antibodies (Di Sabatino, Carsetti & Corazza, 2011).
Overwhelming post-splenectomy infection (OPSI) is defined as fulminating sepsis, meningitis or pneumonia triggered mainly by S. pneumoniae followed by H. influenzae type B and N. meningitidis. The risk of OPSI is more than 50 times higher than in the general population and is a permanent life-long condition (Hansen & Singer, 2001).
OPSI is a medical emergency. Following brief prodromal symptoms such as fever, shivering, myalgia, vomiting, diarrhoea and headache, septic shock develops in just a few hours, with anuria, hypotension, hypoglycaemia and, commonly, disseminated intravascular coagulation and massive adrenal gland haemorrhage (Waterhouse–Friderichsen syndrome), progressing to multiorgan failure and death (Brigden & Pattullo, 1999). The mortality rate is around 50 to 70% and most deaths occur within the first 24 hours; only prompt diagnosis and immediate treatment can reduce mortality (Holdsworth, Irving & Cuschieri, 1991).
Suspicion and approach to OPSI
- Physicians must be aware of the potential life-threatening infections in TDT patients who underwent splenectomy and patients should be educated to seek early care when fever develops.
- In patients at risk and with indicative symptoms, prompt initiation of empirical antibiotics is essential. Intravenous infusion of third generation cephalosporin (cefotaxime 2 g every 8 h or ceftriaxone 2 g every 12 h), combined with gentamicin (5–7 mg/kg every 24 h) or ciprofloxacin (400 mg every 12 h) or vancomycin (1–1.5 g every 12 h) (Brigden & Pattullo, 1999).
- While awaiting results of blood culture, bacteria can be visualised by Gram staining.
- A reverse transcriptase-polymerase chain reaction (RT-PCR) test for simultaneous identification of three main encapsulated bacteria (S. pneumonia, H. influenzae type B and N. meningitidis) is available (Di Sabatino, Carsetti & Corazza, 2011) (A).
The preventive strategy based on penicillin prophylaxis and vaccination is extremely important and has been discussed in Chapter 6 (The Spleen).
Iron chelation therapy
The control of systemic iron and withholding iron from invading microbes are important strategies of host defence. As a siderophore, some benefits of deferoxamine (DFO) have been demonstrated in particular infections; for example, DFO was able to promote recovery from coma in children with cerebral malaria (Gordeuk et al., 1992) and experimental studies indicate beneficial effects of DFO in infections with H. capsulatum and T. cruzi (Arantes et al., 2011). This is partly attributable to the immunomodulatory role of iron chelation via increased nitric oxide (NO) and decreased interleukin-4 (IL-4) production in DFO-treated patients. However, a certain amount of iron is important for the formation of oxygen radicals by the Fenton reaction and via the catalytic action of phagocyte oxidase (phox) while iron overload has immune-debilitating effects. In fact, treatment of Salmonella-infected mice with DFO impairs pathogen clearance due to reduced reactive oxygen species (ROS) generation (Collins, Kaufmann & Schaible, 2002). Furthermore, certain pathogens, including Y. enterocolitica, V. vulnificus and Mucorales, can utilise DFO as a siderophore for increasing their pathogenicity.
- As a measure, temporary discontinuation of DFO during a febrile illness until establishing whether the episode is caused by a pathogen that can use DFO as a siderophore or taken under control is strongly advised (B).
Nonsiderophoric iron chelators such as deferasirox are being studied for possible anti-infective properties. In an in vitro study, it has been observed that V. vulnificus was stimulated by DFO, whereas orally bioavailable iron chelators such as deferasirox (DFX) and deferiprone (DFP) had an inhibitory effect on the growth of V. vulnificus (Neupane & Kim, 2009). Further, DFX and DFP limit the growth of Chlamydia psittaci, C. trachomatis and L. pneumophila and may be suitable as add-on therapies in mucormycosis (Ibrahim et al., 2007; Paradkar et al., 2008). However, the latter could not be supported by a subsequent double-blind, placebo-controlled Phase II trial that aimed to define the safety and efficacy of short-term therapy with DFX for patients with acute mucormycosis (Spellberg et al., 2012).
- DFX or DFP can be continued during febrile episodes (C).
Disease-Related Risks of Infections in TDT and Preventive Measures
Ineffective erythropoiesis and haemolysis result in hyperplasia of monocyte/macrophages, which phagocytose defective erythroid precursors and erythrocytes. The increased phagocytic activity resulting from clearance of defective erythrocytes may reduce the capacity of the phagocytic system to defend against microorganisms (Wiener et al., 1996) and consequently overwhelms pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) (Ozinsky et al., 2000). In the clinical setting, severe anaemia, itself, has also been observed as a risk factor for bacterial infections in thalassaemia (Wanachiwanawin, 2000).
- Deleterious effects of anaemia, IE and haemolysis on the host defence mechanisms may be brought under control by the maintenance of pretransfusional haemoglobin levels between 90 and 95 g/l. This corrects anaemia while suppressing erythroid marrow (C).
Infectious Agents in Thalassaemia – Diagnosis and Treatment
Bacterial infections
Yersinia enterocolitic
Y. enterocolitica is of low pathogenicity and restricted to the gastrointestinal tract in an immune competent host. The availability of large amounts of iron in those with iron overload or undergoing DFO chelation increases the virulence of Y. enterocolitica. Fulminant Y. enterocolitica septicaemia has been reported as a common infectious risk in DFO-treated thalassaemic patients from western countries (Adamkiewicz et al., 1998), rather than eastern countries.
Clinical manifestations: Fever is the most common presenting feature, often associated with abdominal pain and enterocolitis. Pharyngitis-tonsillitis, acute respiratory distress syndrome and polyarthritis are other clinical manifestations of infection.
The mortality can reach 50% in septicaemia with complications including hepatic and splenic abscesses, osteomyelitis, intussusception, nephritis, meningitis and endocarditis.
Laboratory diagnosis: specific culture conditions (at 22°C for 48 hours) for blood and stool samples are necessary. The microbiology laboratory should be informed to enable correct culture conditions. Serological tests may display cross-reactivity. However, fourfold rises in IgG titres in samples obtained 15 days apart may be suggestive of recent infection.
Treatment: the basic and most important point is that a patient with thalassaemia manifesting the above symptomatology should be managed as follows:
- Stop DFO chelation*.
- Obtain suitable laboratory samples.
- Commence effective antibiotic treatment immediately.
Intravenous trimethoprim-sulfamethoxazole (400 mg sulfamethoxazole every 12 h) for 7 days (14 days in the case of septicaemia) plus gentamicin (5–7 mg/kg every 24 h) should be used for the treatment. Intramuscular ceftriaxone (2 g every 12 h) is an alternative in focal infections (e.g., enteritis, pharyngitis, tonsillitis). Ciproflaxacin (400 mg every 12 h) is also an effective antibiotic (A).
Klebsiella spp.
Klebsiella spp. has been reported as the major cause of severe bacterial infections in patients with thalassaemia from the Far East (Wanachiwanawin, 2000).
Clinical manifestations: infection presents with sinusitis, intracranial infections, septicaemia and pyogenic abscesses in liver, lung and kidney and parathyroid gland that are associated with high rates of morbidity and mortality.
Treatment
- Stop DFO chelation*.
- Obtain suitable laboratory samples.
- Commence effective antibiotic treatment immediately.
- Ceftazidime (2 g every 8 h) plus gentamicin (5–7 mg/kg every 24 h) should be used for treatment. Meropenem, imipenem and fluoroquinolones are alternative antibiotics for resistant species.
- Early surgical intervention should be considered (A).
Other bacterial infections
Thalassaemic patients appear to be at high risk of severe bacterial infections, particularly after splenectomy. The most common OPSIs are meningitis, pneumonia and sepsis caused by encapsulated bacteria (S. pneumonia, Haemophilus influenzae type B, Neisseria meningitidis). Other pathogens responsible for post-splenectomy infections include: E. coli, P. aeruginosa, group B streptococci, Enterococcus spp. and V. vulnificus (Cullingford et al., 1991).
Treatment
- Thalassaemic patients with fever and/or other signs of bacterial infection, particularly those who have undergone splenectomy, should be considered as having an emergency medical condition.
- Stop DFO chelation*.
- Obtain suitable laboratory samples.
- Commence effective antibiotic treatment immediately (A).
* Deferiprone does not have the virulence-enhancing effect observed with deferoxamine during experimental Y. enterocolitica infection in mice (Lesic, Foulon & Carniel, 2002). DFP and DFX chelation need not be interrupted on the suspicion of Y. enterocolitica infection (C).
Viral infections
Human parvovirus B19 (HPVB19)
Clinical manifestations: HPVB19 typically causes erythema infectiosum or fifth disease in children with the clinical course of a flu-like syndrome. HPVB19 DNA is present in the circulation for almost one week and disappears during the production of neutralizing antibodies (IgM for 6-8 weeks and IgG afterwards). This protective mechanism would not be present in immunocompromised subjects, leading to persistence of viral DNA.
HPVB19 particularly infects erythroid progenitors complicated by a transient red cell aplasia. Because of high erythroid turnover, patients with thalassaemia may develop severe anaemia with low reticulocyte counts during the course of HPV B19 infection (Ricerca, Di Girolamo & Rund, 2009). The patients require intensification of the transfusion regimen during acute infection. HPVB19 infection should be suspected in patients with increased blood consumption once other responsible factors (e.g. allo-immunization or hypersplenism) are excluded.
Although the main route of transmission is respiratory, transfusions of pRBC collected from persistently infected blood donors play a secondary role (Lefrère et al., 2005).
Human immunodeficiency virus (HIV)
HIV virus leads to CD4+ lymphocyte depletion that renders the individual at risk for many types of opportunistic infections. Due to continuous implementation and improvement of more sensitive serological methods and a nucleic acid amplification test (NAT), the residual risk of viral transmission decreased to less than 1:1.3 million for HIV in the European Union and the US (Velati et al., 2008; Allain, Thomas & Sauleda, 2002). No case has been recorded since implementation of NAT screening (August 2008-2015). However, in Africa, higher prevalence and less comprehensive testing still results in an estimated 10% to 15% of cases of HIV linked to unsafe blood transfusion (Safe Blood for Africa Foundation, 2008).
In a large multicentre study comprising 79 HIV-positive thalassaemia patients from different countries, the progression to overt acquired immune deficiency syndrome (AIDS) after seroconversion was 1.4% after 3 years and 9% after five years. There was no statistically significant relationship between disease progression and age, sex, acute infection or splenectomy (Costagliola et al., 1992). However, a significant inverse relationship between disease progression and the dose of DFO administered was reported; the rate of progression decreases as the mean daily DFO dose increases (Costagliola et al., 1994).
A 30-year multicentre study of about 3000 TDT patients (3-65 years old, 45% splenectomised) examined data on the survival of HIV infected patients along with national epidemiological data on donor blood. In 1982-2015, 43 patients tested positive for HIV infection (Politis et al., 2017). Forty-two who had received 819,000 units of RBCs (1:19,000) were infected by the end of 1987. TT-HIV infection risk was reduced significantly by 2005 with seroprevalence in only three patients transfused with seronegative blood donated from three different donors during the serologically silent window period. Therefore, the residual risk of TT-HIV in these patients in 1988-2015 was estimated at 1:290,666 serologically tested blood units. The corresponding figure in the blood donor population was 1:1,833,333 units. Of the 32 patients (74%) who died up to 2015, 26 (81%) progressed to AIDS at a mean age of 16.5±9.2 years. One deceased patient was co-infected with HBV and HCV. Hepatitis B and C were higher in HIV seropositive patients than in seronegative. Of the 13 survivors (mean age 43±7.1 years), two have a history of splenectomy and two are anti-HCV positive with PCR HCV RNA negative. Most patients have negative viral load and are free of HIV symptoms. A multivariate analysis demonstrates that serum ferritin levels are statistically associated with the duration of survival after diagnosis of HIV infection in this group. This 30-year study findings confirm Costagliola et al.’s earlier findings (1992, 1994) about the inverse relationship between the rate of disease progression and iron chelation therapy.
A large spectrum of therapeutic options is currently available for HIV-infected patients, which have also been used in patients with TDT. Since iron overload can ha ve an adverse effects on HIV-1 disease progression such as faster progression of HIV in patients with low doses of DFO and high serum ferritin (Gordeuk et al., 2001), optimal control of body iron burden with iron-chelation regimens is recommended in HIV-1-positive TDT patients. Although there is no evidence that splenectomy facilitates the progression of HIV infection, a splenectomy treatment strategy should be decided with caution in an HIV-1 positive patient.
Cytomegalovirus infection
Cytomegalovirus can be transmitted by fresh blood components containing leukocytes. It is estimated that approximately 2-12% of CMV-positive healthy donors can transmit the virus by blood donation to the recipients. Consequences of CMV infection are serious in immunocompromised patients such as thalassaemia patients who have had stem cell transplantation.
- The use of blood products from CMV-seronegative donors has been shown to be effective in preventing transmission. However, it does not completely eliminate the risk of transmission. Moreover, CMV seroprevalence reaches 50 to 100% in various geographical regions and the availability of CMV-seronegative products is limited.
- Pre-deposit leukodepletion of cellular blood products achieving a residual leukocyte count <5 x106 per unit allows the reduction of CMV transmission to a level at least equivalent to the transfusion of sero-negative blood components for those patients at major risk of severe CMV transfusion-associated disease (Bowden et al., 1995) (A).
West Nile virus
West Nile virus is a mosquito-borne flavivirus that primarily causes an asymptomatic or mild disease. However, in <1% it causes neurological disease, such as encephalitis, meningitis or - more rarely - acute flaccid paralysis. Elderly and immunocompromised persons are at higher risk of developing severe disease and having a fatal outcome. The risk of transmission through blood transfusion (TT-WNV) has been recognized and preventive blood safety measures have been implemented in the affected areas. The paucity of reported TT-WNV infections and the blood screening results in the USA and in Europe suggest that blood safety interventions are effective (Domanovic et al., 2019).
Investigation of WNV infection in 369 TDT patients in affected areas of a European country showed that 1.9% of the patients were positive for antibodies against the virus with mild clinical course. Transmission of TT-WNV before the implementation of blood screening with NAT-RNA was confirmed in two thalassaemia patients (Politis et al., 2011).
Hepatitis E
Hepatitis E is caused by infection with a non-enveloped, single-stranded RNA virus (HEV). Although underdiagnosed worldwide, it is responsible for 20 million infections yearly. Four major genotypes infect humans. Genotypes 1 and 2, endemic in many developing countries, are responsible for water-borne epidemics. Genotypes 3 and 4 are associated with zoonotic HEV infections causing sporadic infections in industrialised countries; they are transmitted to humans though consumption of uncooked infectious pork and game products, or by contact with infected animals. Transmissions through transfusion (TT-HEV) and transplantation have also been reported (Perez-Garcia et al., 2015; Schlosser et al., 2012).
HEV mainly causes acute self-limiting infections, but chronic infections may occur in immunocompromised patients and can lead to fulminant hepatitis and death. Locally-acquired HEV cases have been observed across Europe where genotype 3 infections have raised questions for Public Health and blood safety. HEV genotype 3 infection is commonly asymptomatic or mild and self-limiting without chronic sequelae. As acute phase viraemia persists for 6-8 weeks and because most cases are asymptomatic, it is possible for infected blood donors to donate blood while viraemic. A multicentre study in blood donors and thalassaemic patients in a European country, found an overall prevalence of 2.9% for HEV IgG antibodies (significantly greater in males and older donors, as well as in one particular city) and prevalence of 3.6% in thalassaemic patients (Zervou et al., 2015). The increasing incidence of TT-HEV has led several European countries to include blood screening in preventive strategies for this infection. The risk of TT-HEV in groups such as thalassaemia patients with heavy exposure to donor RBCs has raised new issues in their clinical management. Studies in donor blood in several European countries using NAT testing have shown a high frequency of viraemic donations of up to 1:726 (Hogema, 2016). However, the number of TT-HEV has until now been very low, probably due to under-reporting and under-recognition mainly because of asymptomatic infections in transfused patients.
Fungal infections
Mucor species
Mucormycosis or zygomycoses are opportunistic infections that may affect thalassaemics who have undergone stem cell transplantation. Iron is a key nutrient for fungi as well as bacteria. The notion that iron chelation may serve as an effective antifungal modality was proposed more than 30 years ago. However, administration of DFO resulted in exacerbation of mucormycosis. This was attributed to the fact that the DFO itself may act as a siderophore for the fungi. Observations that DFX chelation may be a useful adjunct to antifungal treatment (Ibrahim et al., 2007) led to a trial of DFX combined with liposomal amphotericin B (AmBisome) as short-term therapy for mucormycosis. The results were disappointing as patients treated with DFX had a higher mortality rate at 90 days, leading the authors to conclude that the data did not support a role for initial, adjunctive DFX therapy for mucormycosis.
Phytiosum insidiosi
Pythiosis is a very rare human infection caused by Phytiosum insidiosi, a fungus-like organism. Three forms of human pythiosis are recognised: 1) cutaneous form affecting the periorbital area, face and limbs as a granulomatous, ulcerating abscess-like cellulitis; 2) ophthalmic pythiosis affecting the eyes as corneal ulcers and keratitis; 3) systemic pythiosis affecting vascular tissue and resulting in arterial occlusions leading to gangrene and amputation (Vento, Cainelli & Cesario, 2006). Pythiosis has been reported in Thailand, Australia, Haiti, India, New Zealand and the US. The systemic form was common in patients with thalassaemia and associated with a high morbidity and mortality (most patients die within 6 months) (Prasertwitayakij et al., 2003).
Serological tests and PCR methods are being developed for diagnosis. Antifungal drugs are ineffective for providing disease control. Medical treatment alone is insufficient to salvage patients with systemic infections.
Two vaccines for pythiosis have been prepared. One vaccine has been prepared that has been prepared from soluble concentrated P. insidiosum antigen and is administered intradermally in the first, and subcutaneously in the following three injections and at 2 weekly intervals in patients with life threatening systemic infections. The vaccine was curative in a substantial number of cases (Wanachiwanawin et al., 2004).
Parasitic infections
Malaria
With reference to transfusion–transmitted diseases, parasitic infections play a very important role. Malaria is believed to be the most important parasitic disease currently facing humans (Angachaisuksiri et al., 2014, World Health Organization. Fact sheet: 2016). Malaria is a life-threatening disease caused by parasites that are transmitted to humans through the bites of infected female Anopheles mosquitoes (P. falciparum, P. vivax, P. malariae, P. ovale). The WHO estimates that 3.2 billion people live in areas at risk of malaria transmission in 106 countries and territories. In 2015, out of 91 countries and areas with ongoing malaria transmission, Africa was home to 90% of malaria cases and 92% of malaria deaths (mostly among children) (WHO, 2017). Transfusion transmitted malaria (TTM) is also reported sporadically in non-endemic areas, where the risk of transmission arises from travellers to endemic areas and permanent residents with origin in endemic areas. However, autochthonous transmissions are possible due to the presence of competent vectors.
An outbreak of locally acquired Plasmodium vivax malaria in Greece in 2009 which peaked in 2011 raised the question of how to define spatial boundaries of an affected area and when to trigger specific blood safety measures targeted to affected areas with ongoing local transmission. In this context, the ECDC advices the following expert opinion that could help the EU national blood safety authorities in developing a preventive strategy during malaria outbreaks (Domanovic et al., 2016)
- Suspension of blood sessions in the affected areas and in surrounding locations up to a radius of 6 km. The distance criterion is based in the dispersal range of the mosquito vector.
- Temporary deferral for 6 months from blood donation of asymptomatic persons residing or working in the above areas and visitors from the beginning of an outbreak up to 4 months after the end of the season of mosquito activity.
- Blood screening for malarial antibodies or for malarial DNA might be considered instead of temporary deferral that might jeopardize the blood supply. Pathogen reduction could be considered if a suitable methodology is available (Henschler et al., 2011, El Chaar et al., 2013)
There is evidence that carriers of certain haemoglobinopathies have a reduced risk of severe and fatal falciparum malaria. However, the same is not true for the homozygous state including thalassaemia major and intermedia (Vento, Cainelli & Cesario, 2006). The evolving patterns of drug resistance in malaria parasites and changes in recommendations for malaria prevention should be taken into account by physicians who advise chemoprophylaxis to patients before and during periods of travel into endemic areas (Chen & Keystone, 2005).
Box
Summary and Recommendations.
References
- Adamkiewicz T.V., Berkovitch M., Krishnan C., Polsinelli C., et al. Infection due to Yersinia enterocolitica in a series of patients with beta-thalassemia: incidence and predisposing factors. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. [Online] 1998;27(6):1362–1366. Available from: doi: [PubMed: 9868642] [CrossRef]
- Allain J.-P., Owusu-Ofori A.K., Assennato S.M., Marschner S., Goodrich R.P., Owusu-Ofori S. Effect of Plasmodium inactivation in whole blood on the incidence of blood transfusion-transmitted malaria in endemic regions: the African Investigation of the Mirasol System (AIMS) randomised controlled trial. Lancet. 2016;387:1753–61. [PubMed: 27116282] [CrossRef]
- Allain J.-P., Stramer S.L., Carneiro-Proietti A.B.F., Martins M.L., et al. Transfusion-transmitted infectious diseases. Biologicals: Journal of the International Association of Biological Standardization. [Online] 2009;37(2):71–77. Available from: doi: [PubMed: 19231236] [CrossRef]
- Allain J.-P., Thomas I., Sauleda S. Nucleic acid testing for emerging viral infections. Transfusion Medicine (Oxford, England). [Online] 2002;12(4):275–283. Available from: doi: [PubMed: 12220257] [CrossRef]
- Arantes J.M., Francisco A.F., de Abreu Vieira P.M., Silva M., et al. Trypanosoma cruzi: desferrioxamine decreases mortality and parasitemia in infected mice through a trypanostatic effect. Experimental Parasitology. [Online] 2011;128(4):401–408. Available from: doi: [PubMed: 21620835] [CrossRef]
- Aydinok Y., Piga A., Origa R., Mufti N., et al. Amustaline-glutathione pathogen-reduced red blood cell concentrates for transfusion-dependent thalassaemia. British Journal of Haematology. [Online] 2019;186(4):625–636. Available from: doi: [PMC free article: PMC6771954] [PubMed: 31148155] [CrossRef]
- Blajchman M.A. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology (Amsterdam, Netherlands). [Online] 2005;10 Suppl 1:208–214. Available from: doi: [PubMed: 16188675] [CrossRef]
- Borgna-Pignatti C., Rugolotto S., De Stefano P., Zhao H., et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–1193. [PubMed: 15477202]
- Bowden R.A., Slichter S.J., Sayers M., Weisdorf D., et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood. 1995;86(9):3598–3603. [PubMed: 7579469]
- Brigden M.L., Pattullo A.L. Prevention and management of overwhelming postsplenectomy infection--an update. Critical Care Medicine. [Online] 1999;27(4):836–842. Available from: doi: [PubMed: 10321679] [CrossRef]
- Candotti D., Allain J.P. Molecular virology in transfusion medicine laboratory. Blood Transfusion. 2013;11(2):203–16. [PMC free article: PMC3626471] [PubMed: 23356973] [CrossRef]
- Cervia J.S., Wenz B., Ortolano G.A. Leukocyte reduction’s role in the attenuation of infection risks among transfusion recipients. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. [Online] 2007;45(8):1008–1013. Available from: doi: [PubMed: 17879916] [CrossRef]
- Chen L.H., Keystone J.S. New strategies for the prevention of malaria in travelers. Infectious Disease Clinics of North America. [Online] 2005;19(1):185–210. Available from: doi: [PubMed: 15701554] [CrossRef]
- Collins H.L., Kaufmann S.H.E., Schaible U.E. Iron chelation via deferoxamine exacerbates experimental salmonellosis via inhibition of the nicotinamide adenine dinucleotide phosphate oxidase-dependent respiratory burst. Journal of Immunology (Baltimore, Md.: 1950). [Online] 2002;168(7):3458–3463. Available from: doi: [PubMed: 11907105] [CrossRef]
- Costagliola D.G., Girot R., Rebulla P., Lefrère J.J. Incidence of AIDS in HIV-1 infected thalassaemia patients. European and Mediterranean W.H.O. Working Group on Haemoglobinopathies and Cooleycare. British Journal of Haematology. [Online] 1992;81(1):109–112. Available from: doi: [PubMed: 1520608] [CrossRef]
- Costagliola D.G., de Montalembert M., Lefrère J.J., Briand C., et al. Dose of desferrioxamine and evolution of HIV-1 infection in thalassaemic patients. British Journal of Haematology. [Online] 1994;87(4):849–852. Available from: doi: [PubMed: 7986727] [CrossRef]
- Cullingford G.L., Watkins D.N., Watts A.D.J., Mallon D.F. Severe late postsplenectomy infection. BJS (British Journal of Surgery). [Online] 1991;78(6):716–721. Available from: doi: https://doi
.org/10.1002/bjs.1800780626. [PubMed: 2070242] - Damgaard C., Magnussen K., Enevold C., Nilsson M., et al. Viable bacteria associated with red blood cells and plasma in freshly drawn blood donations. PloS One. [Online] 2015;10(3):e0120826. Available from: doi: [PMC free article: PMC4353618] [PubMed: 25751254] [CrossRef]
- Di Sabatino A., Carsetti R., Corazza G.R. Post-splenectomy and hyposplenic states. Lancet (London, England). [Online] 2011;378(9785):86–97. Available from: doi: [PubMed: 21474172] [CrossRef]
- Domanović D., Gossner C.M., Lieshout-Krikke R., Mayr W., Baroti-Toth K., Dobrota A.M., Escoval M.A., Henseler O., Jungbauer C., Liumbruno G., Oyonarte S., Politis C., Sandid I., Vidović M.S., Young J.J., Ushiro-Lumb I., Nowotny N. West Nile and Usutu Virus Infections and Challenges to Blood Safety in the European Union. Emerg Infect Dis. [Online] 2019;25(6):1050–1057. [PMC free article: PMC6537739] [PubMed: 31107223]
- Domanovic D., Kitchen A., Politis C., Panagiotopoulos T., Bluemel J., Van Bortel W., Overbosch D., Lieshout-Krikke R., Fabra C., Facco G., Zeller H. Targeting of blood safety measures to affected areas with ongoing local transmission of malaria. Transfusion Medicine. 2016;26(3):161–165. [PubMed: 27238883] [CrossRef]
- Domanović D., Tedder R., Blümel J., Zaaijer H., et al. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Eurosurveillance. [Online] 2017;22(16) Available from: doi: [Accessed: 22 March 2021] [PMC free article: PMC5404480] [PubMed: 28449730] [CrossRef]
- El Chaar M., Atwal S., Freimanis G.L., Dinko B., Sutherland C.J., Allain J.-P. Inactivation of Plasmodium falciparum in whole blood by riboflavin plus irradiation. Transfusion. 2013;53(12):3174–3183. [PubMed: 23656538] [CrossRef]
- European Commission. Commission Directive 2005/61/EC. 2005. Available at: https://www
.ema.europa .eu/en/documents/regulatory-procedural-guideline /commission-directive-2005 /61/ec-30-september-2005-implementing-directive-2002 /98/ec-european-parliament-council-regards-traceability-requirements-notification-serious-adverse_en.pdf. - Gordeuk V., Mukiibi J., Hasstedt S.J., Samowitz W., et al. Iron overload in Africa. Interaction between a gene and dietary iron content. The New England Journal of Medicine. [Online] 1992;326(2):95–100. Available from: doi: [PubMed: 1727237] [CrossRef]
- Gordeuk V.R., Delanghe J.R., Langlois M.R., Boelaert J.R. Iron status and the outcome of HIV infection: an overview. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology. [Online] 2001;20(3):111–115. Available from: doi: [PubMed: 11166657] [CrossRef]
- Griffiths E. The iron-uptake systems of pathogenic bacteria, fungi and protozoa. Iron and infetion. 1999:87–212.
- Hansen K., Singer D.B. Asplenic-hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Pediatric and Developmental Pathology: The Official Journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. [Online] 2001;4(2):105–121. Available from: doi: [PubMed: 11178626] [CrossRef]
- Hogema B.M., Molier M., Sjerps M., de Waal M., van Swieten P., van de Laar T., Molenaar de Backer M., Zaaijer H.L. Incidence and duration of hepatitis E virus infection in Dutch blood donors. Transfusion. 2016;56(3):722–8. [PubMed: 26559806] [CrossRef]
- Holdsworth R.J., Irving A.D., Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. The British Journal of Surgery. [Online] 1991;78(9):1031–1038. Available from: doi: [PubMed: 1933181] [CrossRef]
- Ibrahim A.S., Gebermariam T., Fu Y., Lin L., et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. The Journal of Clinical Investigation. [Online] 2007;117(9):2649–2657. Available from: doi: [PMC free article: PMC1957543] [PubMed: 17786247] [CrossRef]
- Kim D.M., Brecher M.E., Bland L.A., Estes T.J., et al. Prestorage removal of Yersinia enterocolitica from red cells with white cell-reduction filters. Transfusion. [Online] 1992;32(7):658–662. Available from: doi: [PubMed: 1381532] [CrossRef]
- Kleinman S., Stassinopoulos A. Risks associated with red blood cell transfusions: potential benefits from application of pathogen inactivation. Transfusion. [Online] 2015;55(12):2983–3000. Available from: doi: [PMC free article: PMC7169855] [PubMed: 26303806] [CrossRef]
- Kuo C.-H., Dai Z.-K., Wu J.-R., Hsieh T.-J., et al. Septic arthritis as the initial manifestation of fatal Vibrio vulnificus septicemia in a patient with thalassemia and iron overload. Pediatric Blood & Cancer. [Online] 2009;53(6):1156–1158. Available from: doi: [PubMed: 19591224] [CrossRef]
- Lefrère J.-J., Servant-Delmas A., Candotti D., Mariotti M., et al. Persistent B19 infection in immunocompetent individuals: implications for transfusion safety. Blood. [Online] 2005;106(8):2890–2895. Available from: doi: [PubMed: 15976179] [CrossRef]
- Lesic B., Foulon J., Carniel E. Comparison of the effects of deferiprone versus deferoxamine on growth and virulence of Yersinia enterocolitica. Antimicrobial Agents and Chemotherapy. [Online] 2002;46(6):1741–1745. Available from: doi: [PMC free article: PMC127255] [PubMed: 12019084] [CrossRef]
- Lindholm P.F., Annen K., Ramsey G. Approaches to minimize infection risk in blood banking and transfusion practice. Infectious Disorders Drug Targets. [Online] 2011;11(1):45–56. Available from: doi: [PubMed: 21303341] [CrossRef]
- Marwah S.S., Blann A., Harrison P., Lumley M.A., et al. Increased non-transferrin bound iron in plasma-depleted SAG-M red blood cell units. Vox Sanguinis. [Online] 2002;82(3):122–126. Available from: doi: [PubMed: 11952985] [CrossRef]
- Marx J.J.M. Iron and infection: competition between host and microbes for a precious element. Best Practice & Research. Clinical Haematology. 2002;15(2):411–426. [PubMed: 12401315]
- Modell B., Khan M., Darlison M., Westwood M.A., et al. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance. [Online] 2008;10(1):42. Available from: doi: [PMC free article: PMC2563008] [PubMed: 18817553] [CrossRef]
- Nairz M., Schroll A., Sonnweber T., Weiss G. The struggle for iron - a metal at the host-pathogen interface. Cellular Microbiology. [Online] 2010;12(12):1691–1702. Available from: doi: [PubMed: 20964797] [CrossRef]
- Neupane G.P., Kim D.-M. Comparison of the effects of deferasirox, deferiprone, and deferoxamine on the growth and virulence of Vibrio vulnificus. Transfusion. [Online] 2009;49(8):1762–1769. Available from: doi: [PubMed: 19413741] [CrossRef]
- Ozinsky A., Underhill D.M., Fontenot J.D., Hajjar A.M., et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13766–13771. [PMC free article: PMC17650] [PubMed: 11095740]
- Ozment C.P., Turi J.L. Iron overload following red blood cell transfusion and its impact on disease severity. Biochimica Et Biophysica Acta. [Online] 2009;1790(7):694–701. Available from: doi: [PubMed: 18992790] [CrossRef]
- Paradkar P.N., De Domenico I., Durchfort N., Zohn I., et al. Iron depletion limits intra-cellular bacterial growth in macrophages. Blood. [Online] 2008;112(3):866–874. Available from: doi: [PMC free article: PMC2481528] [PubMed: 18369153] [CrossRef]
- Paraskevis D., Magiorkinis G., Magiorkinis E., Ho S.Y.W., Belshaw R., Allain J.-P., Hatzakis A. Hepatology. 2013;57(3):908–916. [PubMed: 22987324] [CrossRef]
- Perez-Gracia M.T., Garcia M., Suay B., Mateos-Lindemann M.L. Current knowledge on hepatitis E. Journal of Clinical & Translational Hepatology. 2015;3(2):117–26. [PMC free article: PMC4548356] [PubMed: 26355220] [CrossRef]
- Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. [Online] 2003;124(5):1509–1523. Available from: doi: [PubMed: 12730889] [CrossRef]
- Politis C., Ladis V., Karagiorga M., Fragodimitri C., Kattamis A., Chalkia P., Vlahaki E., Kouraklis A., Vgontza N., Maili X., Voskaridou E., Diamantidis M., Athanasiou K., Richardson C. What happened to the HIV infected patients with thalassaemia syndromes in Greece? A 30 year multicenter study. Vox Sanguinis. 2017;112 Suppl 1:280. [CrossRef]
- Politis C., Pappa A., Hassapopoulou H., Pantelidou D., Perifanis V., Teli A, et al. Transfusion transmitted West Nile virus (WNV) infection in thalassaemic patients in Greece. Vox Sanguinis. 2011;101 Suppl 1:236. [CrossRef]
- Politis C., Wiersum J.C., Richardson C., Robillard P., Jorgensen J., Renaudier P., Faber J.-C., Wood E.M. The International Haemovigilance Network database for the surveillance of adverse reactions and events in donors and recipients of blood components: technical issues and results. Vox Sanguinis. 2016;111(4):409–417. [PubMed: 27658188] [CrossRef]
- Zervou E.Z., Politis C.P., Hassapopoulou E.H., Vini M.V., Parara M.P., Kavallierou L.K., Fountouli K., Zaxarioudaki A., Hatzitaki M., Martinis G., Katopi D. Prevalence of hepatitis E virus (HEV) infection in blood donors and multi-transfused patients in Greece. Vox Sanguinis. 2015;109:242–3.
- Prasertwitayakij N., Louthrenoo W., Kasitanon N., Thamprasert K., et al. Human pythiosis, a rare cause of arteritis: case report and literature review. Seminars in Arthritis and Rheumatism. [Online] 2003;33(3):204–214. Available from: doi: [PubMed: 14671729] [CrossRef]
- Ricerca B.M., Di Girolamo A., Rund D. Infections in Thalassemia and Hemoglobinopathies: Focus on Therapy-Related Complications. Mediterranean Journal of Hematology and Infectious Diseases. [Online] 2009;1(1) Available from: doi: [Accessed: 14 February 2021] [PMC free article: PMC3033166] [PubMed: 21415996] [CrossRef]
- Safe Blood for Africa FoundationTM. Home. [Online]. 2008. 2008. Available from: https://www
.safeblood4africa.org/ [Accessed: 14 February 2021] - Schlosser B., Stein A., Neuhaus R., Pahl S., Ramez B., Krüger D.H., Berg T., Hofmann J. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. Journal of Hepatology. 2012;56(2):500–2. [PubMed: 21798217] [CrossRef]
- Shyamala V. Transfusion transmitted infections in thalassaemics: need for reappraisal of blood screening strategy in India. Transfusion Medicine (Oxford, England). [Online] 2014;24(2):79–88. Available from: doi: [PubMed: 24605952] [CrossRef]
- Sirchia G., Rebulla P., Mascaretti L., Greppi N., et al. The clinical importance of leukocyte depletion in regular erythrocyte transfusions. Vox Sanguinis. [Online] 1986;51 Suppl 1:2–8. Available from: doi: [PubMed: 3017002] [CrossRef]
- Spellberg B., Ibrahim A.S., Chin-Hong P.V., Kontoyiannis D.P., et al. The Deferasirox–AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. Journal of Antimicrobial Chemotherapy. [Online] 2012;67(3):715–722. Available from: doi: [PMC free article: PMC3383100] [PubMed: 21937481] [CrossRef]
- Stramer S.L., Dodd R.Y. Transfusion-transmitted emerging infectious diseases: 30 years of challenges and progress. Transfusion. [Online] 2013;53(10 Pt 2):2375–2383. Available from: doi: [PMC free article: PMC7169683] [PubMed: 23926897] [CrossRef]
- Vamvakas E.C., Blajchman M.A. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. [Online] 2009;113(15):3406–3417. Available from: doi: [PubMed: 19188662] [CrossRef]
- Velati C., Romanò L., Fomiatti L., Baruffi L., et al. Impact of nucleic acid testing for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus on the safety of blood supply in Italy: a 6-year survey. Transfusion. [Online] 2008;48(10):2205–2213. Available from: doi: [PubMed: 18631163] [CrossRef]
- Vento S., Cainelli F., Cesario F. Infections and thalassaemia. The Lancet. Infectious Diseases. [Online] 2006;6(4):226–233. Available from: doi: [PubMed: 16554247] [CrossRef]
- Wanachiwanawin W. Infections in E-beta thalassemia. Journal of Pediatric Hematology/Oncology. [Online] 2000;22(6):581–587. Available from: doi: [PubMed: 11132234] [CrossRef]
- Wanachiwanawin W., Mendoza L., Visuthisakchai S., Mutsikapan P., et al. Efficacy of immunotherapy using antigens of Pythium insidiosum in the treatment of vascular pythiosis in humans. Vaccine. [Online] 2004;22(27–28):3613–3621. Available from: doi: [PubMed: 15315840] [CrossRef]
- Wanachiwanawin W., Siripanyaphinyo U., Fucharoen S., Wasi P., et al. Activation of monocytes for the immune clearance of red cells in beta zero-thalassaemia/HbE. British Journal of Haematology. [Online] 1993;85(4):773–777. Available from: doi: [PubMed: 7918042] [CrossRef]
- Weinberg E.D. Iron availability and infection. Biochimica Et Biophysica Acta. [Online] 2009;1790(7):600–605. Available from: doi: [PubMed: 18675317] [CrossRef]
- Weinberg E.D. Microbial pathogens with impaired ability to acquire host iron. Biometals: An International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine. [Online] 2000;13(1):85–89. Available from: doi: [PubMed: 10831229] [CrossRef]
- Wiener E., Wanachiwanawin W., Chinprasertsuk S., Siripanyaphinyo U., et al. Increased serum levels of macrophage colony-stimulating factor (M-CSF) in alpha- and beta-thalassaemia syndromes: correlation with anaemia and monocyte activation. European Journal of Haematology. [Online] 1996;57(5):364–369. Available from: doi: [PubMed: 9003477] [CrossRef]
- World Health Organization. World Malaria Report. Geneva: World Health Organization; 2016.
- World Health Organization. World Malaria Report. Geneva: World Health Organization; 2017.
- Infectious disease - 2021 GuidelinesInfectious disease - 2021 Guidelines
Your browsing activity is empty.
Activity recording is turned off.
See more...