Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- Evidence from 1 randomized controlled trial suggests that subcutaneous azacitidine prolongs disease-free survival among patients with acute myeloid leukemia who have achieved complete remission and who are ineligible for stem cell transplant.
- The oral and subcutaneous formulations are different pharmacokinetically. The studied regimens are also very different in the dose and treatment duration per cycle. The approved oral regimen is 300 mg once daily for 14 days per 28-day cycle, whereas the studied subcutaneous dose is 50 mg/m2 per day for 5 days per 28-day cycle.
- Given that there is no direct head-to-head comparison between the oral and subcutaneous formulations of azacitidine, there is insufficient evidence to extrapolate conclusions about the clinical effectiveness of oral formulation to the subcutaneous formulation of azacitidine as a maintenance therapy for acute myeloid leukemia.
- For the subcutaneous administration of azacitidine, there are potentially additional health care costs associated with handling hazardous medications and the need for trained personnel for administration. More research is needed to inform decision-making in the context of routes of administration.
Background
Acute myeloid leukemia (AML) is the most common acute leukemia among adults.1,2 It is an aggressive and heterogeneous hematologic malignancy that is characterized by abnormal production and differentiation of clonal myeloid stem cells in the bone marrow, peripheral blood, and other tissues.2,3 The proliferation of abnormal leukemic cells interferes with normal blood cell production, which, in turn, causes weakness, infection, and bleeding, among other symptoms.1,2 The most recent Canadian estimates suggest that 1,090 cases of AML were diagnosed in 2016 and 1,184 people living in Canada died as a result of AML in 2017.4 The median age of diagnosis is approximately 68 years and incidence increases with age, with approximately one-third of AML cases diagnosed among those aged 75 and older.1-3
AML is typically classified into 1 of 6 types according to morphology, immunophenotype, and clinical presentation, as defined by WHO.5 These classifications include: (i) AML with certain genetic abnormalities; (ii) AML with myelodysplasia-related changes, (iii) therapy-related myeloid neoplasms; (iv) AML, not otherwise specified; (v) myeloid sarcoma; and (vi) myeloid proliferations of Down syndrome.5 Classifying AML into an appropriate subtype has several advantages, such as helping to determine which therapy would be most suitable, providing prognostic information, and helping clarify the underlying molecular pathogenesis of the disease to advance therapeutic development.5,6 Poorer prognosis is associated with increased age, poor performance status, cytogenetic and/or molecular genetic findings in tumour cells, therapy-related AML, and AML associated with a prior hematological malignancy.2,7
Another classification system of AML is the French-American-British (FAB). In the 1970s, a group of leukemia experts from France, America, and Britain divided AML into M0 through M7subtypes, based on the type of cell the leukemia develops from and the maturity of the cells.
FAB subtype names:
M0 = undifferentiated acute myeloblastic leukemia
M1 = acute myeloblastic leukemia with minimal maturation
M2 = acute myeloblastic leukemic with maturation
M3 = acute promyelocytic leukemia (APL)
M4 = acute myelomonocytic leukemia
M4 eos = acute myelomonocytic leukemia with eosinophilia
M5 = acute monocytic leukemia
M6 = acute erythroid leukemia
M7 = acute megakaryoblastic leukemia
Overall, treatment options for AML are diverse and depend on multiple aspects. Patients with AML who can tolerate aggressive treatment first undergo induction therapy with the goal of achieving complete remission (CR).1,3 Success of induction treatment with respect to CR and cure varies with age; however, even if CR is achieved, nearly all patients will eventually relapse if treatment is discontinued even with residual disease of AML.2,3 Those with a demonstrated response to induction therapy should have subsequent consolidation therapy to attain lasting remission.2,3 Consolidation therapy options generally include more intensive chemotherapy and/or hematopoietic stem cell transplant (HSCT).2
For those who cannot tolerate aggressive treatment (i.e., elderly patients > 65 years, those with poor-risk cytogenetics), lower intensity therapies may be more appropriate to slow progression of the disease, prolong survival, and reduce symptoms; however, these are unlikely to lead to long-term benefits.1-3 These lower intensity therapies include immune-modulating therapies, targeted therapies, and/or hypomethylating agents.8 Of particular interest is the hypomethylating agent azacitidine, which has demonstrated therapeutic benefit as an alternative to supportive care or low-dose chemotherapy among those previously described patients who are not eligible for intensive therapy.1,3
More recently, an oral formulation of azacitidine has been studied as a maintenance therapy among patients with AML who have achieved CR or complete remission with incomplete blood count recovery (CRi), and who are ineligible for HSCT.9 In this context, azacitidine is taken orally for the first 14 days of a 28-day cycle until disease progression or unacceptable toxicity.9 Against placebo, oral azacitidine has demonstrated longer overall survival (OS) (24.7 months and 14.8 months, respectively; P < 0.001) and relapse-free survival (10.2 months and 4.0 months, respectively, P < 0.001).9
Policy Issue
In January 2021, oral azacitidine (Onureg) was approved for market in Canada and in January 2022 it was reviewed for reimbursement by CADTH.10 The Health Canada–approved indication for oral azacitidine is as maintenance therapy in adult patients with AML who achieved CR or CRi following induction therapy with or without consolidation treatment, and who are not eligible for HSCT.11
The CADTH pan-Canadian Oncology Drug Review Expert Review Committee recommended that oral azacitidine be reimbursed for the indication approved by Health Canada with conditions.12 Despite this recommendation, the cost associated with oral azacitidine10 may be prohibitive for payers in the context of a generic version of an injectable formulation. This report sought to assess the evidence in support of the injectable formulation of azacitidine (Vidaza), which is already available in some jurisdictions and costs less than the oral formulation, as maintenance therapy for patients with AML.
Purpose of This Report
The purpose of this report was to systematically review the literature related to dosing and/or clinical effectiveness of IV or subcutaneous formulations of azacitidine as maintenance therapy for patients diagnosed with AML who have achieved CR or CRi following induction therapy with or without consolidation therapy and who are ineligible for HSCT.
Research Question(s)
- What is the pharmacokinetic or pharmacodynamics profile of IV or subcutaneous azacitidine compared to oral azacitidine among patients with AML or related indications (e.g., hematological malignancies)?
- What is the clinical effectiveness of IV or subcutaneous azacitidine as maintenance therapy among patients with AML who have achieved CR or CRi following induction therapy with or without consolidation treatment and who are ineligible for HSCT?
Methods
Literature Search Methods
The literature search for clinical studies was performed by an information specialist using a peer-reviewed search strategy according to the PRESS Peer Review of Electronic Search Strategies checklist.13 The complete search strategy is presented in Appendix 1.
Published literature was identified by searching the following bibliographic databases: MEDLINE All (1946—) and Embase (1974—) via Ovid. All Ovid searches were run simultaneously as a multi-file search. Duplicates were removed using Ovid deduplication for multi-file searches, followed by manual deduplication in Endnote. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s Medical Subject Headings, and keywords. The main search concepts were azacitidine, subcutaneous or IV administration, and acute myeloid leukemia. Clinical trials registries were searched: the US National Institutes of Health’s ClinicalTrials.gov, WHO’s International Clinical Trials Registry Platform search portal, Health Canada’s Clinical Trials Database, the EU Clinical Trials Register, and the Canadian Cancer Trials search.
No filters were applied to limit the retrieval by study type. Retrieval was not limited by publication date but was limited to the English or French language. Where possible, retrieval was limited to the human population. Conference abstracts were excluded from the search results.
The bibliographic databases search was completed on June 6, 2022; the clinical trial registries search was completed on June 9, 2022.
Grey literature (literature that is not commercially published) was identified by searching sources listed in relevant sections of the CADTH Grey Matters resource.14 Included in this search were the websites of key regulatory agencies (US FDA and European Medicines Agency) and Canadian drug formularies. Google was used to search for additional internet-based materials. See Appendix 1 for more information on the grey literature search strategy.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, or were duplicate publications.
Critical Appraisal of Individual Studies
The included publications (3 studies) that directly meet the selection criteria and population for question 2 (outlined in Table 1) were critically appraised by 1 reviewer using the Downs and Black checklist15 for randomized and non-randomized studies. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively. The summary is outlined in Appendix 2.
Studies evaluating the pharmacokinetics and pharmacodynamics were excluded from appraisal with the Downs and Black checklist,15 as their overall goals and methodologies do not align with the standards required for clinical studies. Studies evaluating the use of oral azacitidine in related malignancies were excluded as they were only included to provide additional context for safety and dose tolerability.
Summary of Evidence
Quantity of Research Available
A total of 300 citations were identified in the literature search. Following screening of titles and abstracts, 279 citations were excluded and 21 potentially relevant reports from the electronic search were retrieved for full-text review. One potentially relevant publication was retrieved from the grey literature search for full-text review. Of these potentially relevant articles, 11 publications were excluded for various reasons, and 11 publications met the inclusion criteria and were included in this report (Figure 1). These comprised 3 randomized controlled trial (RCT)16-18 and 8 non-randomized studies (NRSs).19-26
Among the 11 publications, 3 -21 evaluated the pharmacokinetics and pharmacodynamics of azacitidine, 3,25,26 evaluated the clinical effectiveness of azacitidine meeting the specific population criteria (adult patients with AML who have achieved CR or CRi following induction therapy with or without consolidation treatment and who are ineligible for HSCT), and 5 evaluating the clinical effectiveness of azacitidine in AML or related hematological malignancies.18,22-24,27
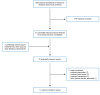
Figure 1
Selection of Included Studies.
Summary of Study Characteristics
Study Design
Studies Evaluating the Pharmacokinetics and Pharmacodynamics of Azacitidine
Three studies have been identified that evaluated the pharmacokinetics and pharmacodynamics of azacitidine. One pilot pharmacokinetic study was done to evaluate the oral formulation of azacitidine at 60 mg and 80 mg to determine if that drug in those dosage forms can be absorbed and provide detectable plasma levels.19 One study investigated the pharmacokinetics and pharmacodynamics of subcutaneous azacitidine compared to oral azacitidine in a phase I dose escalation trial focused on oral azacitidine.21 Azacitidine has also been studied in different hematological indications with different dosing regimens to evaluate the pharmacokinetics indicators.20,23,24
Studies Evaluating the Clinical Effectiveness of Azacitidine as a Maintenance Therapy in AML
Three primary studies17,25,26 investigated the clinical effectiveness of subcutaneous azacitidine as a maintenance therapy in patients with AML. Among these 3 primary studies, the most recent was a 2-arm, phase III RCT that was conducted across multiple sites.17 The other 2 primary studies evaluating the clinical effectiveness of subcutaneous azacitidine as a maintenance therapy were single-arm, phase II, open-label studies.25,26
Studies Evaluating the Clinical Effectiveness of Azacitidine in Related Hematological Malignancies
Given that there have been limited studies evaluated the role of azacitidine in this specific patient population, 5 additional studies that evaluated azacitidine in related indications (e.g., myelodysplastic syndromes or other hematological malignancies) have also been reviewed to potentially identify relevant dosing of azacitidine that may be applicable in the population of interest. The first study was a retrospective review that evaluated 74 patients with low-risk myelodysplastic syndromes who received azacitidine on a national named patient program.28 The second study was a phase II, multicentre, randomized, open-label trial conducted to evaluate 3 alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes.24 The third study was a multicentre, open-label study conducted to evaluate efficacy and safety of extended dosing schedule of azacitidine in patients with lower-risk myelodysplastic syndromes.23 The fourth study was a phase III, multicentre randomized trial conducted to evaluate the clinical effectiveness of azacitidine as compared with conventional care regimens in elderly patients with low bone marrow blast count AML.27 Finally, a phase III randomized study was conducted that compared subcutaneous azacitidine treatment with supportive care in patients with myelodysplastic syndrome.18
Country of Origin
All 3 pharmacokinetic studies were conducted in the US.19-21 Among the 3 studies evaluating azacitidine in patients with AML in remission, 1 was conducted in the US,26 1 in the Netherlands,17 and 1 in Sweden.25 The remaining 5 single-centre or multicentre studies evaluating azacitidine in related hematological malignancies were conducted in the US, France, Italy, Sweden, and Australia.16,18,23,28,29
Patient Population
The 3 studies evaluating the pharmacokinetics and pharmacodynamics of azacitidine all included patients with AML or other related indications. Specifically, they included adult male or female participants with myelodysplastic syndrome (MDS), AML, or malignant solid tumours who were 18 years or older and had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 to 2.19-21
The 3 studies evaluating azacitidine in patients with AML in remission included broader eligible patient populations. In the study by Huls et al. (2019),17 adults at least 60 years of age with a cytopathologically confirmed diagnosis of AML and at least 20% blast infiltrate of the bone marrow were eligible, along with patients with the initial subtype of MDS (i.e., refractory anemia with excess blasts in transformation [RAEB-t]) and lower than 5% blasts after 2 cycles of induction chemotherapy. Similarly, the studies by Garcia-Manero et al. (2011)21 and Grövdal et al. (2010)25 enrolled adult patients with intermediate-2 or high-risk MDS, chronic myelomonocytic leukemia with more than 10% blasts, and AML. The study by Griffin et al. (2015)26 enrolled adult patients aged 60 years and older with a diagnosis of AML by WHO criteria in their first CR or CRi.
The remaining 5 studies included patients with AML or related hematological malignancies. Fenaux et al. (2010) have included patients with 20% or higher bone marrow or peripheral blasts based on centra bone marrow review (e.g., with FAB-defined RAEB-t and WHO-defined AML).16 Additional eligibility criteria include aged 18 years or older, ECOG PS of 0 to 2, and an estimated life expectancy of 3 months or longer. For the study evaluating the extended dosing schedule of azacitidine, the eligible patients included individuals 18 years or older with an ECOG PS of 0 to 2 and International Prognostic Scoring System–defined lower-risk MDS (low or intermediate) as diagnosed by the treating physician.23 Lyons et al. (2009) enrolled male and female patients with MDS who were 18 years or older with a diagnosis of FAB criteria-defined refractory anemia (RA), RA with ringed sideroblasts, RA with excess blasts, RAEB-T, or chronic myelomonocytic leukemia (CMML).24 This study specifically excluded patients with secondary MDS, a history of AML, or other malignant disease.24 Musto et al. (2010) have included patients with lower-risk myelodysplastic syndromes.28 Silverman et al. (2002) have included patients who have fulfilled FAB-classified criteria for MDS.18
Interventions and Comparators
Studies Evaluating the Pharmacokinetics and Pharmacodynamics of Azacitidine
In the pilot pharmacokinetic study by Garcia-Manero et al. (2008), patients were administered a 60 mg dose of oral azacitidine initially.19 If the dose was well tolerated, dose escalation would occur with an 80 mg dose and continue to escalate until the dose administration was deemed intolerable, the appropriate concentrations of drug were found or dose escalation reached the 200 mg level, which is approximately equivalent to a maximum approved daily dose of subcutaneous 100 mg/m2.
In the phase I study of oral azacitidine, patients were administered with 75 mg/m2 subcutaneously on the first 7 days of the first cycle, followed by oral azacitidine daily ranging from 120 mg to 600 mg for the first 7 days on each additional 28-day cycle.21 For the oral formulation, the starting dose was 120 mg and doses were escalated in 60 mg increments up to a dose of 360 mg, followed by 120 mg increments until the maximum tolerated dose was achieved; this was also delivered on a 7 out of 28-day schedule.21 The pharmacokinetic parameters were compared between the subcutaneous and the oral formulations.
In the pharmacokinetic and pharmacodynamic study by Laille et al. (2015) that evaluated the extended dosing of azacitidine, patients were sequentially assigned to 1 of the 4 following extended dosing regimens:20
- 300 mg once daily for 14 days every 28 days
- 300 mg once daily for 21 days every 28 days
- 200 mg twice daily for 14 days every 28 days
- 200 mg twice daily for 21 days every 28 days.
Studies Evaluating the Clinical Effectiveness of Azacitidine as a Maintenance Therapy in AML
Azacitidine was administered subcutaneously in each of the 3 included primary studies that evaluated azacitidine as a maintenance therapy; however, the dosing and schedule differed in each study. In the study by Huls et al. (2019),17 subcutaneous azacitidine was administered at a dose of 50 mg/m2 for 5 days every 4 weeks for 12 cycles. Griffin et al. (2015)26 randomly assigned participants to 1 of 3 subcutaneous azacitidine dosing schedules for 12 cycles: 50 mg/m2 daily for 5 days; 50 mg/m2 daily for 7 days; or 50 mg/m2 daily for 10 days. The majority of participants were treated on the 5-day schedule.26 In the Grövdal et al. (2010)25 study, participants were started on subcutaneous azacitidine at 75 mg/m2 for the first 5 days every 4 weeks, but this was reduced to 60 mg/m2 after the first 5 participants were enrolled. The number of treatment cycles was not reported.25 In terms of comparators, the Huls et al. (2019)17 study was the only 2-arm study included and the authors used no other treatment as their comparator.
Studies Evaluating the Clinical Effectiveness of Azacitidine in Related Hematological Malignancies
In the study by Fenaux et al. (2010), patients received azacitidine 75mg/m2 per day subcutaneously for 7 days every 28 days for at least 6 cycles as compared to conventional care regimens, which included best supportive care only, low-dose cytarabine, or intensive chemotherapy.30
In Garcia-Manero et al. (2016) patients were sequentially assigned to receive azacitidine 300 mg orally once daily for the first 14 or 21 days of each 28-day cycle. After 6 cycles, patients whose disease did not respond could discontinue, remain on study, or cross over to receive azacitidine 75 mg/m2 per day subcutaneously for 7 days.
Musto et al. (2010) have patients evaluated on the following dosing regimens: azacitidine 75 mg/m2 per day subcutaneously for 5 days, 7 days, or 10 days every month or compared to a fixed dose of 100 mg daily for a median of 7 cycles.28
In the study by Lyons et al. (2009), the following 3 dosing regimens were compared:24
- AZA 5-2-2: azacitidine 75mg/m2 per day subcutaneously for 5 days, followed by 2 days of no treatment, then 75mg/m2 per day for 2 days (for a total cumulative dose of 525mg/m2 per cycle)
- AZA 5-2-5: azacitidine 50mg/m2 per day subcutaneously for 5 days, followed by 2 days of no treatment, then 50mg/m2 per day for 2 days (for a total cumulative dose of 500mg/m2 per cycle)
- AZA 5: azacitidine 75mg/m2 per day subcutaneously for 5 days (for a total cumulative dose of 375mg/m2 per cycle).
Finally, in the RCT by Silverman et al. (2002), patients were either assigned to azacitidine 75mg/m2 per day subcutaneously for 7 days every 28 days or supportive care.18
Outcomes
Studies Evaluating the Pharmacokinetics and Pharmacodynamics of Azacitidine
To evaluate the pharmacokinetic parameters of azacitidine, the outcomes of interest include time of maximum observed plasma concentration (Tmax),19-21 maximum observed plasma concentration (Cmax),19-21 area under the plasma concentration-time curve from zero to infinity (AUC(0-∞)),19-21 half-life (T1/2),20,21 relative oral bioavailability (F),20,21 and apparent volume of distribution (Vd/F).20,21
In addition to evaluating the pharmacokinetics of azacitidine, DNA methylation levels were measured.20,21 Maximum tolerated dose and safety parameters (e.g., dose-limiting toxicity), as well as clinical activity such as overall response rate (e.g., CR, hematologic improvement or red blood cell or platelet transfusion independence) were measured in the phase I study by Garcia-Manero et al. (2011).21
Studies Evaluating the Clinical Effectiveness of Azacitidine as a Maintenance Therapy in AML
To assess the clinical effectiveness of parenteral formulations of azacitidine as a maintenance therapy for patients with AML who have achieved CR or CRi with or without consolidation therapy and who are ineligible for HSCT, the main outcomes of interest were OS,17,25,26 disease-free survival (DFS),17,26 probability of relapse and death,17 number and duration of hospitalizations,17 transfusion requirements,17 impact of pretreatment parameters on prognosis,25 and safety.17,25,26
Studies Evaluating the Clinical Effectiveness of Azacitidine in Related Hematological Malignancies
The main outcomes evaluated in these studies were OS;16 overall response rate, including CR or partial response;18,23,28 hematological improvements;18,23,28 total days in hospital;27 transfusion requirements;18,23,24 and safety specifically for grade 3 or 4 hematological adverse events.16,23,24,28
Additional characteristics of the include primary studies are available in Table 2.
Table 2
Characteristics of Included Studies.
Summary of Findings
Studies Evaluating Pharmacokinetics and Pharmacodynamics of Azacitidine
In the pilot pharmacokinetic study, 4 patients were treated with single doses of oral azacitidine, ranging from 120 mg to 160 mg with detectable azacitidine concentration.19 However, in the log scale comparison of plasma concentration versus time for a single dose of oral azacitidine in 4 subjects, the mean and individual concentration verse time curves were all below the curve as compared to the single-dose subcutaneous dose administration. The reported oral drug exposure as measured by AUC(0-∞) ranges from 22.6 to 112.6 ng times h/mL; however, the historical subcutaneous azacitidine median AUC(0-∞) is 777 ng times h/mL.19 These results all suggest that the oral formulation is different from the subcutaneous formulation of azacitidine, providing overall lower drug exposure.
In the phase I study of oral azacitidine, the azacitidine plasma concentration versus time curve was also constructed to compare the subcutaneous formulation (75 mg/m2 on day 1) to oral formulation (480 mg on day 1). In this study, the subcutaneous formulation was able to achieve much higher peak concentration over time as compared to the oral formulation. The authors also determined that the mean relative oral bioavailability ranged from 6.3% to 20%. In this study, pharmacodynamic analysis was performed by evaluating the DNA methylation levels. This was done by determining DNA hypomethylating activity of azacitidine when administered subcutaneously or orally. Both oral and subcutaneous azacitidine were able to decrease DNA methylation in blood, with maximum effect at day 15 of each cycle.21
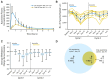
Figure 2
Overview of Pharmacokinetic and Pharmacodynamic Data of Oral and Subcutaneous Azacitidine.

Figure 3
Detailed Pharmacokinetic Parameters After Single Subcutaneous and Oral Azacitidine Administration.
In the pharmacokinetics and pharmacodynamics study by Laille et al. (2015),20 the azacitidine plasma concentrations over time were measured for the 4 dosing regimens (300 mg once daily or 200 mg twice daily for 14 or 21 days) as well as the changes in global DNA methylation score. The authors concluded the both the 300 mg once-daily schedules and the 200 mg twice-daily 21-day schedule significantly (P < 0.05) reduced global DNA methylation in whole blood at all measured time points (days 15, 22, and 28 of the treatment cycle), with sustained hypomethylation at cycle end compared with baseline. The authors also concluded that azacitidine exposure and reduced DNA methylation were significantly correlated. This study illustrates that extending the oral dosing of azacitidine can increase the duration of azacitidine exposure.
Studies Evaluating the Clinical Effectiveness of Azacitidine as a Maintenance Therapy in AML
To assess the clinical effectiveness of parenteral formulations of azacitidine as a maintenance therapy for patients with AML who have achieved CR or CRi with or without consolidation therapy and who are ineligible for HSCT, the main outcomes of interest were OS,17,25,26 DFS,17,26 probability of relapse and death,17 number and duration of hospitalizations,17 transfusion requirements,17 impact of pretreatment parameters on prognosis,25 and safety.17,25,26
In the study by Huls et al. (2019),17 the DFS was significantly better for the azacitidine treatment group (log-rank test; P = 0.04), as well as after adjustment for poor-risk cytogenetic abnormalities at diagnosis and platelet count randomization as surrogate for CR versus CRi; Cox regression; hazard ratio = 0.62; 95% confidence interval, 0.41 to 0.95; P = 0.026). The 12-month DFS was estimated at 64% for the azacitidine group and 42% for the control group. The OS did not differ between treatment groups, with or without censoring for allogeneic hematopoietic cell transplant. The authors have concluded that azacitidine maintenance after CR or CRi after intensive chemotherapy is feasible and significantly improves DFS.
In the study by Griffin et al. (2015),26 the primary objective was to determine the 1-year DFS with the secondary objectives were to determine safety and tolerability. From the time of first CR, the estimated 1-year DFS was 50% and the median OS was 20.4 months. Thrombocytopenia and neutropenia were most common grade 3 or 4 toxicities. “Although the numbers were quite small, patients who discontinued therapy early due to toxicity (n=7) appeared to have inferior DFS (HR 5.07; 95% CI, 1.03 to 24.92) and OS (HR 12.02; 95% CI, 1.30 to 103.01) compared to patients who completed 1 year of azacitidine maintenance (n=5)” (p.798). 26
In the prospective phase II study by Grövdal et al. (2010),25 25 the median OS was 20 months. Hypermethylation of CDH1, specifically epithelial cadherin, was significantly associated with low CR rate, early relapse, and short OS (P = 0.003). It was reported that 5-azacitidine treatment at a dose of 60 mg/m2 was well tolerated. Grade III thrombocytopenia occurred in 9.5% of the treatment courses. Grade IV neutropenia occurred in 30.5% of patients.
Studies Evaluating the Clinical Effectiveness of Azacitidine in Related Hematological Malignancies
While only 3 studies have been identified that meet the studied population criteria of patients with AML in remission, it is useful to also identify studies evaluating the use of azacitidine in a related population or hematological malignancies, particularly in patients with low-risk MDS). There are some overall similar characteristics between AML and MDS with some patients with MDS have the potential to progress to AML.7,31
In the study16 evaluating the OS of azacitidine when compared with conventional care regimens in elderly patients with low bone marrow blast count AML, the primary outcome was OS. At a median follow-up of 20.1 months, median OS for patients treated with azacitidine was 24.5 months compared with 16.0 months for patients treated with conventional care regimens (CCR) (hazard ratio = 0.47; 95% confidence interval, 0.28 to 0.79; P = 0.005), and the 2-year OS rates were 50% and 16%, respectively (P = 0.001). Two-year OS rates were higher with azacitidine versus CCR in patients considered unfit for intensive chemotherapy (P = 0.0003).
Garcia-Manero et al. (2016)23 evaluated the efficacy and safety of extended dosing schedules of azacitidine in patients with lower-risk MDS. The primary outcomes include overall response (complete or partial remission, red blood cell or platelet transfusion independence, or hematologic improvement) rates. Overall response was achieved by 36% of patients taking the 14-day dosing and 41% taking the 21-day dosing of azacitidine. Red blood cell transfusion independence rates were similar between both dosing schedules (31% and 38%, respectively). The authors have concluded that extended dosing schedules of oral azacitidine may provide effective long-term treatment for patients with lower-risk MDS.23
In the retrospective study evaluating patients with low risk or intermediate 1-risk MDS,28 the overall response rate was 45.9%, including CR (10.8%), partial responses (9.5%), hematologic improvements (20.3%), and bone marrow complete responses (5.4%) in 64 patients who completed 4 or more cycles of azacitidine subcutaneous treatment for 5 days every month at 75mg/m2 daily. The median duration of response was 6 months (range, 1 to 3 months). After a median follow-up of 15 months, 71% of patients remained alive. A survival benefit was observed in responders versus nonresponders (94% versus 54% of patients projected to be alive at 2.5 years, respectively; P < 0.014). The most common grade 3 or 4 adverse events were myelosuppression (21.6%) and infection (6.8%).
Lyons et al. (2009) evaluated the hematologic response to 3 alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes.24 Efficacy was measured based on hematologic improvement and transfusion independence rates, as defined by international working group (IWG) 2000 criteria and determined by computer-generated assessment. Hematologic improvement was achieved by 44% (22 of 50), 45% (23 of 51), and 56% (28 of 50) of AZA 5-2-2, AZA 5-2-5, and AZA 5 arms, respectively. Proportions of patients who were red blood cell transfusion-dependent and achieved transfusion independence were 50% (12 of 24), 55% (12 of 22), and 64% (16 of 25) and of patients who were FAB lower-risk transfusion-dependent were 53% (9 of 17), 50% (6 of 12), and 61% (11 of 18), respectively. In the AZA 5-2-2, AZA 5-2-5, and AZA 5 groups, 84%, 77%, and 58% respectively, experienced 1 or more grade 3 to 4 adverse event.
In the RCT by Silverman et al. (2002)18 evaluating the use of azacitidine in patients with the MDS, responses were measured as the primary outcome. Complete Response was defined as normal bone marrow or less than 5% blasts in the bone marrow. Partial response was defined as 50% or less of initial bone marrow blasts and the criteria for improvement are satisfied by either monolineage or bilineage response (defined by 50% or more restitution of the initial deficit from normal in 1 or 2 peripheral blood cell counts) or 50% or more decrease in transfusion requirement from baseline. In total, 60% of patients achieved responses from the azacitidine arm, with 7% achieving complete response, 16% achieving partial response, and 37% with improved response (P < 0.001). Median time to leukemic transformation or death was 21 months for the azacitidine group versus 13 months for the supportive care group (P = 0.007). Transformation to acute myelogenous leukemia occurred as the first event in 15% of patients in the azacitidine group and in 38% receiving supportive care (P = 0.001). After accounting for the confounding effect of early cross over to the azacitidine group, a landmark analysis after 6 months showed median survival of an additional 18 months for the azacitidine group and 11 months for the supportive care group (P = 0.03). Quality of life assessment found significant major advantages in physical function, symptoms, and psychological state for patients initially randomized to azacitidine.
In summary, one can infer that the oral formulation of azacitidine achieves much less overall drug exposure as compared to the subcutaneous formulation based on the pharmacokinetic and pharmacodynamic studies.19-21 This is demonstrated by achieving a lower Cmax and lower AUC when the oral formulation was compared to the subcutaneous formulation. However, the pharmacodynamic evaluation suggests that there is still sustained hypomethylating activity with the oral formulation, especially if given at extended dosing duration.20
In the 1 randomized controlled study evaluating azacitidine as a maintenance therapy in older patients with AML,17 there were clinical benefits in improving DFS with azacitidine 50mg/m2 per day subcutaneously for 5 days every 4 weeks for a maximum of 12 cycles (64% versus 42% at 12 months; log-rank test; P = 0.04). Two additional NRSs, which dosed with azacitidine 50 mg/m2 to 60 mg/m2 per day subcutaneously for 5 to 10 days, have provided further experience with these dosing regimens as a maintenance dose following CR.25,26
Other studies16,18,23,24,28 also evaluated subcutaneous azacitidine in related settings (e.g., MDS) with the most common regimen being 75mg/m2 per day subcutaneously for 7 days every 28 days. While their results may not be applicable in the setting of AML in remission, they do provide additional experience that higher dosing is likely safe and tolerated with appropriate hematological monitoring in place.
Without a direct head-to-head comparative trial between the oral formulation and subcutaneous formulation of azacitidine, clinical benefits cannot be inferred9 across formulations.
Table 3 presents the main studies’ findings by outcome.
Table 3
Summary of Findings of Main Primary Clinical Studies.
Summary of Critical Appraisal
Small Sample Size and Heterogenous Patient Population
In general, there is some limited evidence supporting the use of parenteral azacitidine (mainly administered subcutaneously) as a maintenance therapy for AML with experience based on a variety of dosing regimens. Among all studies identified, the overall sample size is small, and even smaller if identifying only those with a diagnosis of AML. All reported studies have a very heterogenous patient population that includes individuals with AML, MDS, or other hematological malignancies.
Inconsistent Diagnostic Criteria Among Studies
Further, some of the older studies may have followed the diagnostic criteria for AML that would no longer be current. The most current diagnostic criteria include the FAB classification of AML, or the WHO classification of AML, which was updated in 201632 to incorporate factors that can affect prognosis.32 Furthermore, patients who would previously be considered to have AML (e.g., as in the case of AML, not otherwise specified with previous defined case with 50% or more bone marrow erythroid precursors and 20% or more myeloblasts among noneyrthyroid cells) may now be classified as having MDS (usually with excess blast).5 It has also been suggested that a fixed blast percentage may not be optimal to distinguish AML from MDS.33 Possible reasons stemming from biologic data include33 that AML-associated abnormalities may present as MDS, that there is genetic overlap between high-grade MDS and secondary AML, and that patients with MDS can progress to AML. Given the diagnostic challenge in differentiating between AML and MDS (for some patients), it is possible that patients belonging to different subgroups of AML may have different response rates to azacitidine.
Other Considerations
There may be additional reasons for why a difference in efficacy results was observed between the oral and subcutaneous formulations of azacitidine. Wei et al. (2022) were able to demonstrate improved OS via the randomized trial9 for the oral regimen, while Huls et al. (2019) were only able to show improved DFS in the randomized trial17 for the subcutaneous regimen.
Different Formulations and Treatment Duration
Results from pharmacokinetic studies have consistently confirmed that oral and subcutaneous regimens are not interchangeable because that the overall drug exposure for oral formulation is lower than the subcutaneous formulation.20 But azacitidine appears to have sustained pharmacodynamic activities, especially with an extended dosing duration.20 This may have led to the use of a longer duration of the oral regimen (300 mg by mouth daily for 14 days every 28 days). With the subcutaneous regimen evaluated by Huls et al. (2019), patients received azacitidine 50mg/m2 per day for 5 days every 28 days. There is a difference of 9 days in the treatment duration per cycle, which may play a factor in differential efficacy observed in their respective clinical trials, though no direct or indirect comparison was performed.
Age and Disease Severity Difference in Patient Population
It is also worth noting that Wei et al. (2022)9 have enrolled patients from a slightly younger age group (inclusion age criteria ≥ 55, median age 68 [range, 55 to 85]), whereas Huls et al. (2019) have recruited older patients (inclusion age criterion ≥ 60, median age 69 [range, 60 to 81]). In the study by Wei et al. (2022),9 more than 90% of patients had an ECOG PS of 0 to 1, suggesting their diseases were milder overall. On the other hand, Huls et al. (2019)17 enrolled patients with a WHO performance score of 0 and 1, with unequal distribution of patients between the placebo group and the azacitidine group. This difference in age and disease severity may impact health status, prognosis, and overall response to the maintenance therapy.
Table 4
RCT Evidence for Oral and Subcutaneous Regimen of Azacitidine.
Cytogenetic Risk at Diagnosis
Another point worth highlighting is that while both studies have included process to evaluate the cytogenetic risk at diagnosis, this information is only reported by Wei et al. (2022)9 and not readily reported by Huls et al. (2019).17 Given that cytogenic risk is associated with prognosis, this information could have helped to contextualize the differences of their results.
All in all, there are few considerations worth noting that may explain the difference in results as observed in the oral versus subcutaneous regimen of azacitidine as used in patients with AML in remission. Without a head-to-head trial comparing the clinical outcomes between oral azacitidine and subcutaneous azacitidine, one cannot make any conclusion related to whether subcutaneous azacitidine can offer similar outcomes as oral azacitidine. While there is some limited evidence supported by 1 randomized trial17 and 2 non-randomized trials,25,26 there are limitations with the methodologies and differences in patient populations that prevent any meaningful extrapolation.
Please refer to Appendix 2 for a critical appraisal of the 3 primary clinical studies using the Downs and Blacks checklist.
Conclusions and Implications for Decision- or Policy-Making
This review identified 3 primary studies17,25,26 that investigated the clinical effectiveness of subcutaneous azacitidine as a maintenance therapy among patients with AML who have achieved CR or CRi and are ineligible for HSCT.
Based on the available evidence, there was a weak signal from a single RCT17 that subcutaneous azacitidine may be effective as a maintenance therapy compared to no further treatment based on DFS; however, this finding did not persist in terms of OS. Results from the NRSs25,26 demonstrated favourable outcomes in terms of DFS and OS, and a tolerable safety profile for subcutaneous azacitidine as a maintenance therapy. However, given the open-label, single arm nature of these phase II studies, it is difficult to draw robust conclusions based on these data.
While there may be a weak signal from the evidence to use subcutaneous azacitidine as a maintenance treatment for patients with AML in remission, there are additional considerations that may impact implementation. With an oral formulation, patients can self-administer at home, with less risk for hematological adverse events. This can translate into improved quality of life and potential savings in health care costs related to the administration of the medication. If azacitidine is to be given subcutaneously, the patient may need to receive this treatment at an ambulatory cancer clinic, which may necessitate additional monitoring for hematological adverse events. There are also additional costs associated with handling hazardous medications during preparation, administration, and final disposal. The subcutaneous formulation of azacitidine can also be administered at home but this would require additional resources for proper handling and administration of the medications with trained personnel.
Overall, there is limited high-quality evidence available from which to draw conclusions and inform decision-making, and more research is needed to address these questions.
Abbreviations
- AML
acute myeloid leukemia
- CR
complete remission
- CRi
complete remission with incomplete blood count recovery
- DFS
disease-free survival
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- FAB
French-American-British
- HSCT
hematopoietic stem cell transplant
- MDS
myelodysplastic syndrome
- NRS
non-randomized study
- OS
overall survival
- RA
refractory anemia
- RAEB-t
refractory anemia with excess blasts in transformation
- RCT
randomized controlled trial
References
- 1.
- Kolitz J. Overview of acute myeloid leukemia in adults. In: Larson RA, Rosmarin AG, eds. UpToDate. Waltham (MA): UpToDate; 2022: https://www
.uptodate .com/contents/overview-of-acute-myeloid-leukemia-in-adults. Accessed 2022 Jun 26. - 2.
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. [PMC free article: PMC5030376] [PubMed: 27367478]
- 3.
- Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026. [PubMed: 34615640]
- 4.
- Acute myeloid leukemia statistics. Toronto (ON): Canadian Cancer Society; 2022: https://cancer
.ca/en /cancer-information/cancer-types /acute-myeloid-leukemia-aml /statistics. Accessed 2022 Sep 20. - 5.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [PubMed: 27069254]
- 6.
- Schiffer CA, Gurbuxani S. Clinical manifestations, pathologic features, and diagnosis of acute myeloid leukemia. In: Larson RA, Rosmarin AG, eds. UpToDate. Waltham (MA): UpToDate; 2022: https://www
.uptodate .com/contents/clinical-manifestations-pathologic-features-and-diagnosis-of-acute-myeloid-leukemia. Accessed 2022 Jun 26. - 7.
- Schiffer CA. Prognosis of acute myeloid leukemia. In: Larson RA, Rosmarin AG, eds. UpToDate. Waltham (MA): UpToDate; 2022: https://www
.uptodate .com/contents/prognosis-of-acute-myeloid-leukemia. Accessed 2022 Jun 26. - 8.
- Bewersdorf JP, Tallman MS, Stahl M. Maintenance therapies in acute myeloid leukemia: the renaissance of an old therapeutic concept. Curr Opin Oncol. 2021;33(6):658-669. [PubMed: 34341323]
- 9.
- Wei AH, Döhner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. [PubMed: 33369355]
- 10.
- Drug Reimbursement Review clinical guidance report: azacitidine (Onureg) for acute myeloid leukemia. Ottawa (ON): CADTH; 2021: https://www
.cadth.ca/azacitidine. Accessed 2022 Jun 26. - 11.
- Onureg: azacitidine tablets. Tablets, 200 mg, 300 mg, azacitidine, oral [product monograph]. Saint-Laurent (QC): Celgene; 2021: https://www
.bms.com/assets /bms/ca/documents /productmonograph/ONUREG_EN_PM.pdf. Accessed 2022 Jun 26. - 12.
- pan-Canadian Oncology Drug Review Committee (pERC) final recommendation: azacitidine (Onureg - Celgene Inc.). Ottawa (ON): CADTH; 2022: https://www
.cadth.ca /sites/default/files /DRR/2021/PC0245%20Onureg %20-%20CADTH%20Final%20Rec.pdf. Accessed 2022 Jun 26. - 13.
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. [PubMed: 27005575]
- 14.
- Grey matters: a practical tool for searching health-related grey literature. Ottawa (ON): CADTH; 2019: https://www
.cadth.ca/grey-matters. Accessed 2022 Aug 16. - 15.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 16.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562-569. [PubMed: 20026804]
- 17.
- Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. [PubMed: 30630862]
- 18.
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429-2440. [PubMed: 12011120]
- 19.
- Garcia-Manero G, Stoltz ML, Ward MR, Kantarjian H, Sharma S. A pilot pharmacokinetic study of oral azacitidine. Leukemia. 2008;22(9):1680-1684. [PubMed: 18548103]
- 20.
- Laille E, Shi T, Garcia-Manero G, et al. Pharmacokinetics and pharmacodynamics with extended dosing of CC-486 in patients with hematologic malignancies. PLoS One. 2015;10(8):e0135520. [PMC free article: PMC4546409] [PubMed: 26296092]
- 21.
- Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29(18):2521-2527. [PMC free article: PMC3675699] [PubMed: 21576646]
- 22.
- Musto P, Maurillo L, Spagnoli A, et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes: a retrospective study of 74 patients enrolled in an Italian named patient program. Cancer. 2010;116(6):1485-1494. [PubMed: 20151429]
- 23.
- Garcia-Manero G, Gore SD, Kambhampati S, et al. Efficacy and safety of extended dosing schedules of CC-486 (oral azacitidine) in patients with lower-risk myelodysplastic syndromes. Leukemia. 2016;30(4):889-896. [PMC free article: PMC4832070] [PubMed: 26442612]
- 24.
- Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850-1856. [PubMed: 19255328]
- 25.
- Grovdal M, Karimi M, Khan R, et al. Maintenance treatment with azacytidine for patients with high-risk myelodysplastic syndromes (MDS) or acute myeloid leukaemia following MDS in complete remission after induction chemotherapy. Br J Haematol. 2010;150(3):293-302. [PubMed: 20497178]
- 26.
- Griffin PT, Komrokji RS, De Castro CM, et al. A multicenter, phase II study of maintenance azacitidine in older patients with acute myeloid leukemia in complete remission after induction chemotherapy. Am J Hematol. 2015;90(9):796-799. [PubMed: 26089240]
- 27.
- Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562-569. [PubMed: 20026804]
- 28.
- Musto P, Maurillo L, Spagnoli A, et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes: a retrospective study of 74 patients enrolled in an Italian named patient program. Cancer. 2010;116(6):1485-1494. [PubMed: 20151429]
- 29.
- Atallah E, Kantarjian H, Garcia-Manero G. The role of decitabine in the treatment of myelodysplastic syndromes. Expert Opin Pharmacother. 2007;8(1):65-73. [PubMed: 17163808]
- 30.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232. [PMC free article: PMC4086808] [PubMed: 19230772]
- 31.
- Schiffer M, Zhao J, Johnson A, Lee J, Bewersdorf JP, Zeidan AM. The development and clinical use of oral hypomethylating agents in acute myeloid leukemia and myelodysplastic syndromes: dawn of the total oral therapy era. Expert Rev Anticancer Ther. 2021;21(9):989-1002. [PubMed: 33853476]
- 32.
- American Cancer Society. Acute myeloid leukemia early detection, diagnosis, and types. 2018; https://www
.cancer.org /cancer/acute-myeloid-leukemia /detection-diagnosis-staging.html. Accessed 2022 Aug 4. - 33.
- Estey E, Hasserjian RP, Dohner H. Distinguishing AML from MDS: a fixed blast percentage may no longer be optimal. Blood. 2022;139(3):323-332. [PMC free article: PMC8832464] [PubMed: 34111285]
Appendix 1. Literature Search Strategy
Note that this appendix has not been copy-edited.
Clinical Literature Search
Overview
Interface: Ovid
Databases:
- MEDLINE All (1946-present)
- Embase (1974-present)
Note: Subject headings and search fields have been customized for each database. Duplicates between databases were removed in Ovid.
Date of search: June 6, 2022.
Alerts: None.
Search filters applied: No filters were applied to limit the retrieval by study type.
Limits:
- Language limit: English- and French-language
- Humans
- No publication date limits were applied.
- Conference abstracts were excluded.
Table 5
Syntax Guide.
Multi-Database Strategy – Medline and Embase, via Ovid
- exp Administration, Intravenous/
- exp Injections, Subcutaneous/
- exp Infusions, Subcutaneous/
- (intravenous* or injection? or injected or IV or bolus or infusion?).ti,ab,kf.
- (subcutaneous* or sub-cutaneous* or sub-cu or sub-Q or subQ or subcut).ti,ab,kf.
- or/1-5
- Azacitidine/
- (azacitidin* or azacytidin* or Vidaza* or Ladakamycin* or Mylosar* or Azadine or Celazadine or 5-AZCR or 5AZCR or “BRN 0620461” or BRN0620461 or CCRIS 60 or CCRIS60 or EINECS 206-280-2 or EINECS 2062802 or EINECS2062802 or HSDB 6879 or HSDB6879 or NCI C01569 or NCIC01569 or NSC 102816 or NSC102816 or CC-486 or CC486 or U 18496 or U18496 or WR 183027 or WR183027 or M801H13NRU).ti,ab,ot,kf,hw,nm,rn.
- 7 or 8
- exp Leukemia, Myeloid, acute/
- (acute adj5 (granulocytic or myeloblastic or myelocytic or myelogenous or myeloid or nonlymphoblastic or non-lymphoblastic or nonlymphocytic or non-lymphcytic or megakaryocytic or monocytic or myelomonocytic or basophilic or eosinophilic or erythroblastic or megakaryoblastic or promyelocytic) adj5 leuk?emia*).ti,ab,kf.
- (erythroleuk?emia* or mast cell leuk?emia* or promyelocytic leuk?emia*).ti,ab,kf.
- (AML or ANLL).ti,ab,kf.
- or/10-13
- 9 and 14
- 6 and 15
- use medall
- exp intravenous drug administration/
- subcutaneous drug administration/
- (intravenous* or injection? or injected or IV or bolus or infusion?).ti,ab,kf,dq.
- (subcutaneous* or sub-cutaneous* or sub-cu or sub-Q or subQ or subcut).ti,ab,kf,dq.
- or/18-21
- *Azacitidine/
- (azacitidin* or azacytidin* or Vidaza* or Ladakamycin* or Mylosar* or Azadine or Celazadine or 5-AZCR or 5AZCR or “BRN 0620461” or BRN0620461 or CCRIS 60 or CCRIS60 or EINECS 206-280-2 or EINECS 2062802 or EINECS2062802 or HSDB 6879 or HSDB6879 or NCI C01569 or NCIC01569 or NSC 102816 or NSC102816 or CC-486 or CC486 or U 18496 or U18496 or WR 183027 or WR183027).ti,ab,kf,dq.
- 23 or 24
- exp Acute myeloid leukemia/
- (acute adj5 (granulocytic or myeloblastic or myelocytic or myelogenous or myeloid or nonlymphoblastic or non-lymphoblastic or nonlymphocytic or non-lymphcytic or megakaryocytic or monocytic or myelomonocytic or basophilic or eosinophilic or erythroblastic or megakaryoblastic or promyelocytic) adj5 leuk?emia*).ti,ab,kf,dq.
- (erythroleuk?emia* or mast cell leuk?emia* or promyelocytic leuk?emia*).ti,ab,kf,dq.
- (AML or ANLL).ti,ab,kf,dq.
- or/26-29
- 25 and 30
- 22 and 31
- use oemezd
- not (conference abstract or conference review).pt.
- 17 or 34
- remove duplicates from 35
- exp animals/
- exp animal experimentation/ or exp animal experiment/
- exp models animal/
- nonhuman/
- exp vertebrate/ or exp vertebrates/
- or/37-41
- exp humans/
- exp human experimentation/ or exp human experiment/
- or/43-44
- 42 not 45
- 36 not 46
- limit 47 to (english or french)
Clinical Trial Registries
ClinicalTrials.gov
Produced by the US National Library of Medicine. Targeted search used to capture registered clinical trials.
Search – (azacitidine or azacitidine or Vidaza) AND (maintenance) AND (IV OR intravenously OR injection OR injected OR IV OR bolus OR infusion OR subcutaneous OR subcutaneously OR sub-cutaneous) | Condition: leukemia OR AML
WHO ICTRP
International Clinical Trials Registry Platform, produced by the WHO. Targeted search used to capture registered clinical trials.
Search – (leukemia OR AML) AND (azacitidine or azacitidine or Vidaza) AND (maintenance) AND (IV OR intravenously OR injection OR injected OR IV OR bolus OR infusion OR subcutaneous OR subcutaneously OR sub-cutaneous)
Health Canada’s Clinical Trials Database
Produced by Health Canada. Targeted search used to capture registered clinical trials.
Search – leukemia and azacitidine
EU Clinical Trials Register
European Union Clinical Trials Register, produced by the European Union. Targeted search used to capture registered clinical trials.
Search - (leukemia OR AML) AND (azacitidine or azacitidine or Vidaza) AND (maintenance) AND (IV OR intravenously OR injection OR injected OR IV OR bolus OR infusion OR subcutaneous OR subcutaneously OR sub-cutaneous)
Canadian Cancer Trials
Created by the Canadian Partnership Against Cancer and partners.
Search – Leukemia adult, keyword azacitidine
Grey Literature
Search dates: June 2 to 9, 2022.
Keywords: azacitidine; acute myeloid leukemia; subcutaneous or IV administration; and synonyms.
Limits: Publication years: 1996-present
Relevant websites from the following sections of the CADTH grey literature checklist, the Grey Matters resource, were searched:
- Canadian Drug Formularies, including Canadian oncology formularies
- Clinical Trial Registries
- Health Technology Assessment (HTA) Agencies – major only, including NICE
- Regulatory Approvals – major only, including Health Canada, FDA, EMA
- Specific databases: Cochrane Library, Trip Database, CMA Infobase
- Internet Search
Appendix 2. Critical Appraisal Using the Downs and Black Checklist
Note that this appendix has not been copy-edited.
Among the 3 studies evaluating azacitidine in adult patients with AML who have achieved complete remission or complete remission with incomplete blood count recovery following induction therapy with or without consolidation treatment and who are ineligible for HSCT, the assessment of the methodological quality of their studies was done using Downs and Black Checklist.15
Randomized Controlled Trial
A single RCT was included in this report.17 In general, this RCT demonstrated some strengths and a few important limitations, with clear descriptions of appropriate methods and clear reporting of findings.17 This RCT also demonstrated internal validity with random assignment to treatment groups, adequate reporting of sufficient statistical testing methods, and no evidence of data dredging or post-hoc analyses.17 The authors did not report if patients and outcome assessors were blinded to the intervention or is randomization was concealed until recruitment was complete, but based on the comparator of observation only, it is almost certain that patients and providers were aware of which treatment arm they were part of, which is a limitation that could negatively impact the internal validity of the findings.17 In addition, no information was provided about the methods used to facilitate the randomization of participants, so there is uncertainty about whether the randomization process was robust and/or free from any biases.17
A power calculation and planned sample size were reported by the study authors; however, the RCT was terminated early before the planned sample size was reached.17 As a consequence, the statistical efficiency of the RCT may be compromised and any statistically significant findings should be interpreted with caution.17
In terms of external validity, insufficient information was reported by the authors to fully understand whether the study participants and/or settings would be representative of the entire population or health care settings in which the intervention of interest would be administered.17
It was also noted that the RCT was funded by a for-profit, private industry pharmaceutical manufacturer, which manufactures subcutaneous azacitidine under the brand name Vidaza®.17 This may or may not introduce risk of bias, as the authors do report that the study was designed by a working group from the Dutch-Belgian Hematology-Oncology Cooperative Group and that data were managed centrally within the same group.17 It may be presumed that external oversight would represent objective oversight of the study, thus be considered a strength; however, it is unclear if some members of the oversight group have received grant monies, honoraria, etc. from the same manufacturer.17
Non-Randomized Studies
The included NRSs demonstrated both strengths and weaknesses. There was clear reporting in each study of the aim, study objectives, patient characteristics, interventions, potential confounders, estimates of random variability and adverse events.25,26 Nonetheless, some details were either not reported or not clearly reported including actual P values and whether different lengths of follow-up were adequately considered in the analyses.25,26 For 1 of the NRS, the findings were generated from planned analyses,21,25 but the study by Griffin et al. (2015)26 discusses comparing DFS and OS to fixed historical values in the Methods section and it was unclear from the reporting of the findings what the results of this comparison were. In addition, Griffin et al. (2015)26 report on subgroup analyses that were not described as part of the Methods, so it is unclear if these analyses were completed post hoc. Patients were also not randomized, which could lead to an imbalance of unmeasured confounding factors in each of the 2 NRSs.25,26 These limitations pose a threat to the internal validity of each study and should be considered in the interpretation of study results.
It was unclear from the reporting across each study whether the recruited participants would be representative of the entire population or if the study settings would be representative of those in Canada; thus, limiting the confidence that can be placed on the external validity of the findings.25,26 Sample sizes were also relatively small across each of the NRS,25,26 which could be a limiting factor in determining a true estimation of the effects of the intervention under study.
Similar to the RCT described above,17 the 2 NRSs25,26 were funded by the same for-profit, private industry pharmaceutical manufacturer, which manufactures subcutaneous azacitidine under the brand name Vidaza®. It is unclear if this introduces any risk of bias with respect to study results.
Additional details regarding the strengths and limitations of included publications are provided in Table 6.
Table 6
Strengths and Limitations of Clinical Studies Using the Downs and Black Checklist.
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
- Subcutaneous Injection of Azacitidine for the Treatment of Acute Myeloid Leukemi...Subcutaneous Injection of Azacitidine for the Treatment of Acute Myeloid Leukemia
Your browsing activity is empty.
Activity recording is turned off.
See more...
