NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Toxicological Profile for Nitrophenols. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2023 Apr.
4.1. CHEMICAL IDENTITY
Nitrophenols (also referred to as mononitrophenols) exist in three isomeric forms: 2-nitrophenol (or ortho- or o-), 3-nitrophenol (or meta- or m-), and 4-nitrophenol (or para- or p-). All three of these nitrophenols are manmade. Table 4-1 lists common synonyms, trade names, and other pertinent identification information for 2-nitrophenol, Table 4-2 lists this information for 3-nitrophenol, and Table 4-3 lists this information for 4-nitrophenol.
4.2. PHYSICAL AND CHEMICAL PROPERTIES
2-Nitrophenol is a light yellow, aromatic solid; 3- and 4-nitrophenol are colorless to pale yellow solids. The nitrophenols are expected to be highly soluble in water. They also have low vapor pressures, and therefore, low potential for long range atmospheric transport (Harrison et al. 2005). Tables 4-4, 4-5, and 4-6. List important physical and chemical properties of 2-, 3-, and 4-nitrophenol, respectively.
Tables
Table 4-1Chemical Identity of 2-Nitrophenol
| Characteristic | Information |
|---|---|
| Chemical Name | 2-Nitrophenol |
| Synonym(s) and Registered trade name(s) | O-Hydroxynitrobenzene; 2-Hydroxynitrobenzene; o-Nitrophenol; Phenol, o-nitro-; Phenol, 2-nitro |
| Chemical formula | C6H5NO3 |
| Chemical structure |
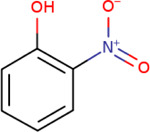
|
| CAS registry number | 88-75-5 |
CAS = Chemical Abstracts Service
Source: NLM 2022a
Table 4-2Chemical Identity of 3-Nitrophenol
| Characteristic | Information |
|---|---|
| Chemical Name | 3-Nitrophenol |
| Synonym(s) and Registered trade name(s) | M-Hydroxynitrobenzene; 3-Hydroxynitrobenzene; m-Nitrophenol; Phenol, m-nitro-; Phenol, 3-nitro-; |
| Chemical formula | C6H5NO3 |
| Chemical structure |
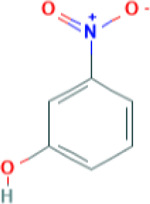
|
| CAS registry number | 554-84-7 |
CAS = Chemical Abstracts Service
Source: NLM 2022b
Table 4-3Chemical Identity of 4-Nitrophenol
| Characteristic | Information |
|---|---|
| Chemical Name | 4-Nitrophenol |
| Synonym(s) and Registered trade name(s) | P-Hydroxynitrobenzene; 4-Hydroxynitrobenzene; Niphen; P-Nitrophenol; Paranitrophenol; Phenol, p-nitro-; Phenol, 4-nitro-; PNP |
| Chemical formula | C6H5NO3 |
| Chemical structure |
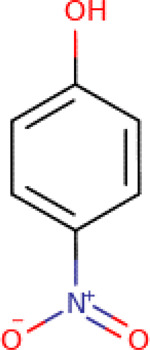
|
| CAS registry number | 100-02-7 |
CAS = Chemical Abstracts Service
Source: NLM 2022c
Table 4-4Physical and Chemical Properties of 2-Nitrophenol
| Property | Information | Reference |
|---|---|---|
| Molecular weight | 139.109 | NLM 2022a |
| Color | Light yellow | O’Neil 2006 |
| Physical state | Crystalline solid | NLM 2022a |
| Melting point | 44–45°C | O’Neil 2006 |
| Boiling point | 216°C | Haynes et al. 2015 |
| Density: | ||
| 1.29 g/cm3 at 40°C | Haynes et al. 2015 | |
| Odor | Aromatic | O’Neil 2006 |
| Odor threshold: | ||
| Water | 10 mg/L | Verschueren 1983 |
| Air | 8×10−11 moles/m3 | Fazzalari 1978 |
| Taste threshold | 0.001 mg/L | Verschueren 1983 |
| Solubility: | ||
| Water | 2,100 mg/L at 20°C | Verschueren 1983 |
| 2,500 mg/L at 25°C | Yalkowsky et al. 2010 | |
| 10,800 mg/L at 100°C | Verschueren 1983 | |
| Organic solvent(s) | Very soluble in ethanol, ether, acetone, benzene, pyridine, chlorine; freely soluble in carbon sulfide, alkali hydroxides | Budavari 1996; Haynes et al. 2015 |
| Partition coefficients: | ||
| Log Kow | 1.79 | Hansch et al. 1995 |
| Log Koc | 1.76–2.04 | Gawlik et al. 1998; Tülp et al. 2009 |
| Vapor pressure | ||
| At 20°C | 0.113 mm Hg at 25°C | NLM 2022a |
| Henry’s law constant | 1.3×10−5 at 20°C | Tremp et al. 1993 |
| 1.63×10−5 at 25°C | Harrison et al. 2002 | |
| Disassociation constant (pKa) | 7.23 | NLM 2022a |
| Autoignition temperature | 550°C | NLM 2022a |
| Flashpoint | 108°C | NLM 2022a |
| Flammability limits | No data | NLM 2022a |
| Conversion factors | ||
| ppm (v/v) to mg/m3 in air at 20°C | 1 ppm=5.783 mg/m3 | NLM 2022a |
| mg/m3to ppm (v/v) in air at 20°C | 1 mg/m3=0.173 ppm | |
| Explosive limits | No data | NLM 2022a |
Table 4-5Physical and Chemical Properties of 3-Nitrophenol
| Property | Information | Reference |
|---|---|---|
| Molecular weight | 139.11 g/mol | NLM 2022b |
| Color | Colorless to pale yellow | NLM 2022b |
| Physical state | Crystalline solid | NLM 2022b |
| Melting point | 96.8°C | NLM 2022b |
| Boiling point | 194°C | NLM 2022b |
| Density: | ||
| At 20°C/4°C | 1.485 | O’Neil 2006 |
| Odor | Aromatic to sweetish | Sittig 1981 |
| Odor threshold: | ||
| Water | 0.6 m/L | Verschueren 1983 |
| Air | 3.0 mg/m3 | |
| Taste threshold | No data | |
| Solubility: | ||
| Water Organic solvent(s) | 13,550 mg/L at 25°C | Yalkowsky et al. 2010 |
| 133,000 mg/L at 90°C | Verschueren 1983 | |
| Organic solvent(s) | Very soluble in acetone, ether, ethanol, benzene | Haynes et al. 2015 |
| Partition coefficients: | ||
| Log Kow | 2.00 | Hansch et al. 1995 |
| Log Koc | 1.68 | Borisover and Graber 1997 |
| Vapor pressure | ||
| 5.85×10−5 mm Hg at 25°C | Bannan et al. 2017 | |
| Henry’s law constant | 2.00×10−9 atm-m3/mole at 25°C | NLM 2022b |
| Dissociation constant | 8.36 | NLM 2022b |
| Autoignition temperature | 400°C | NLM 2022b |
| Flashpoint | >100°C | NLM 2022b |
| Flammability limits | No data | NLM 2022b |
| Conversion factors | ||
| ppm (v/v) to mg/m3 in air at 20°C | 1 mg/m3=0.18 ppm | NLM 2022b |
| mg/m3 to ppm (v/v) in air at 20°C | 1 ppm=5.69 mg/m3 | |
| Explosive limits | No data | NLM 2022b |
Table 4-6Physical and Chemical Properties of 4-Nitrophenol
| Property | Information | Reference |
|---|---|---|
| Molecular weight | 139.11 | O’Neil 2006 |
| Color | Colorless to slightly yellow | O’Neil 2006 |
| Physical state | Solid | NLM 2022c |
| Melting point | 113–114°C | O’Neil 2006 |
| Boiling point | 279°C | Lewis 2007 |
| Density: At 20°C | 1.479 g/cm3 | Lewis 2007 |
| Odor | Odorless | O’Neil 2006 |
| Odor threshold: | ||
| Water | 2.5 mg/L | Verschueren 1983 |
| Air | 2.3 mg/m3 | |
| Taste threshold | 43.4 mg/L | NLM 2022c |
| Solubility: | ||
| Water | 10,000 mg/L at 15°C | Verschueren 1983 |
| 15,600 mg/L at 25°C | Yalkowsky et al. 2010 | |
| Organic solvent(s) | Very soluble in ethanol, ether, and acetone; freely soluble in alcohol, chloroform; soluble in solution of fixed alkali hydroxides and carbonates | Haynes et al. 2015; O’Neil 2006 |
| Partition coefficients: | ||
| Log Kow | 1.91 | Hansch et al. 1995 |
| Log Koc | 2.37 | Schüürmann et al. 2006 |
| Vapor pressure | 1.2×10−5 mm Hg at 25°C | Bannan et al. 2017 |
| Henry’s law constant | 1.28×10−8 atm-m3/mol at 20°C | Tremp et al. 1993 |
| Dissociation constant | 7.15 | NLM 2022c |
| Autoignition temperature | 490°C | NLM 2022c |
| Flashpoint | 169°C | NLM 2022c |
| Flammability limits | No data | NLM 2022c |
| Conversion factors | ||
| ppm (v/v) to mg/m3 in air at 20°C | 1 mg/m3=0.173 ppm mg/m3 | NLM 2022c |
| mg/m3 to ppm (v/v) in air at 20°C | 1 ppm=5.783 mg/m3 | |
| Explosive limits | No data | NLM 2022c |