Copyright © 2023 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- In acute care settings with low-risk of infection transmission, discontinuing contact precautions (i.e., gloves and gown) may result in similar rates of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and may lower the risk of hospital-acquired vancomycin-resistant enterococci (VRE), compared to scenarios in which such precautions were employed.
- Rates of late-onset infections in a neonatal intensive care unit were similar when standard infection control precautions were used compared with universal glove use.
- Two guidelines recommend that nonsterile gloves should be worn for nonsterile procedures when it is anticipated that there will be contact with blood, body fluids, non-intact skin, mucous membranes, lesions, or hazardous drugs and chemicals; for environmental cleaning; and when contact precautions for infection control are in effect.
Context and Policy Issues
The use of nonsterile gloves (NSGs) reduces the risk of contamination of hands of health care workers (HCWs) after patient contact1,2 and, presumably, the transmission of pathogens between patients. Canadian clinical practice guidelines recommend the use of gloves when contact with body fluids is anticipated and when contact precautions are in place for a patient who has tested positive for a pathogen requiring such precautions.3 Gloves should be changed between patient contacts and hand hygiene should be performed before putting on gloves and immediately after removing gloves.3
While glove use is necessary to prevent cross-contamination and transmission of pathogens in many acute care settings and situations, excess glove use contributes to increased medical waste and environmental contamination, which has been exacerbated by the COVID-19 pandemic.4-6 Using gloves when it is not indicated can occur in up to 50% of patient contacts and such use can lead to missed opportunities for hand hygiene, potentially resulting in cross-contamination.7-9 Inappropriate use of gloves combined with inadequate hand hygiene after use may result in the transmission of pathogens to the patient via gloved hands.9 Contaminated NSGs have been associated with a hospital-wide outbreak of Paenibacillus spp pseudobacteremia,10 suggesting that even unused gloves can become contaminated. Unnecessary glove use in this type of scenario may result in harm to patients that may not otherwise occur.
Occupational skin disease in HCWs is common and has been associated with occupational “wet work” including repetitive handwashing and the use of disposable gloves.11-13 The rate of skin disease related to personal protective equipment such as gloves has been reported as almost doubled in HCWs compared with non-HCWs.11
Recent calls to reduce NSG use cite overuse and consequent cost implications, occupational skin disease, medical waste, and environmental contamination as rationale for advocating appropriate glove use.14,15 While NSGs are indicated in many health care settings, it is important to determine the appropriate circumstances for the use and non-use of NSGs to develop effective education campaigns.
The objective of this report is to evaluate and summarize the evidence for the effectiveness of non-use of NSGs in inpatient settings compared with use of NSGs during activities with low risk of infection transmission. The report will describe the manner in which NSG use can impact patient outcomes, which may help to inform future initiatives aiming to reduce overuse of NSGs.
Research Questions
- What is the clinical effectiveness of non-use versus use of nonsterile gloves for individuals receiving inpatient care considered at low risk of infection transmission?
- What are the evidence-based guidelines regarding the use of nonsterile gloves for individuals receiving inpatient care considered at low risk of infection transmission?
Methods
Literature Search Methods
An information specialist conducted a literature search on key resources including MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane Database of Systematic Reviews, the International HTA Database, and the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search approach was customized to retrieve a limited set of results, balancing comprehensiveness with relevancy. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. Search concepts were developed based on the elements of the research questions and selection criteria. The main search concepts were nonsterile gloves and health care. CADTH-developed search filters were applied to limit retrieval to health technology assessments, systematic reviews (SRs), meta-analyses, indirect treatment comparisons, any types of clinical trials or observational studies, and guidelines. The search was completed on July 24, 2023, and limited to English-language documents published since January 1, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, if they were duplicate publications, or if they were published before 2018. SRs in which all relevant studies were captured in other more recent or more comprehensive SRs were excluded. Guidelines with unclear methodology were also excluded.
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the following tools as a guide: A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2)16 for systematic reviews, the Downs and Black checklist17 for randomized and nonrandomized studies, and the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument18 for guidelines. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 496 citations were identified in the literature search. Following screening of titles and abstracts, 455 citations were excluded and 41 potentially relevant reports from the electronic search were retrieved for full-text review. Six potentially relevant publications were retrieved from the grey literature search for full-text review. Of these potentially relevant articles, 43 publications were excluded for various reasons, and 4 publications met the inclusion criteria and were included in this report. These comprised 1 SR, 1 pilot randomized controlled trial (RCT), and 2 evidence-based guidelines. Figure 1 presents the PRISMA19 flow chart of the study selection. Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
Four relevant reports were identified comprising 1 SR,20 1 pilot RCT,21 and 2 evidence-based guidelines.22,23 A summary of study characteristics for each is included below. Additional details regarding the characteristics of included publications are provided in Appendix 2.
Study Design
The SR was published in 2021 and included 15 nonrandomized, quasi-experimental studies and 2 prospective, observational studies evaluating rates of methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococci (VRE) with the discontinuation of contact precautions (i.e., gloves and gown) for patients testing positive for MRSA and VRE in acute care settings.20 The SR included studies that were published no more than 10 years before and including August 2019.20
One single-centre pilot crossover RCT was published in 2023.21 This study, based in a neonatal intensive care unit (NICU), compared the rate of late-onset infections (LOIs) during 6 months of standard infection control precautions (i.e., hand hygiene, glove use only when in contact with bodily fluids) with the rate of LOIs during 6 months of universal gloving (NSGs before any contact with any patient). The centre was randomly assigned to the order of treatment with a 2-week washout period between treatment arms.
One guideline was published in 2021 jointly by the Healthcare Infection Society and Infection Prevention Society in the UK.22 An SR of controlled trials, cohort studies, interrupted time series studies, case control studies, diagnostic accuracy studies, and controlled before-after studies was conducted to inform the recommendations. The quality of the evidence was graded using SIGN Methodology, Cochrane Effective Practice and Organization of Care Risk of Bias, and the Joanna Briggs Institute Critical Appraisal Checklist for Quasi-Experimental Studies. The evidence was graded using the following ratings: high, moderate, low, and very low, based on the characteristics of the studies included in the evidence base. The wording of the recommendations reflected their rating. For example, a strong recommendation used the word “must” if failure to follow the recommendation may have serious consequences. A conditional recommendation used the word “consider” if the evidence did not support a strong recommendation but indicated that the intervention may be beneficial in some circumstances. Final recommendations were based on external consultation with relevant stakeholders.
One guideline was published in 2022 by the Antimicrobial Resistance and Healthcare Associated Infection (ARHAI) Scotland Infection Control team.23 An SR of all study types was conducted to inform the recommendations. The quality of the evidence was graded using SIGN Methodology. Evidence grades ranged from 1++ (highest quality) to 4 (lowest quality). The highest grade of recommendation was “Mandatory,” which reflected directives from government policy, regulations, or legislation. The lowest grade was “Category C,” which was based solely on expert opinion. Final recommendations were based on external consultation with relevant stakeholders.
Both guidelines included recommendations that were outside the scope of this review. Only recommendations for glove use were included in this report.
Country of Origin
The lead author of the SR was from the US.20 The primary studies included in the SR were conducted in the US (n = 15), Canada (n = 1), and France (n = 1). The pilot RCT was conducted in Canada.21 One of the evidence-based guidelines was developed for Scotland23 and the other for the UK as a whole.22
Patient Population
All studies, including those included in the SR, were conducted in acute care settings. The setting for most (11 of 17) of the studies in the SR was the entire hospital, while the other 6 studies were conducted in specific units of a hospital (i.e., leukemia, bone marrow transplant, and lymphoma service of a cancer institute; hospital intensive care units; trauma service; a skilled care unit of hospital).20 No other information with regard to patient characteristics was provided.
The pilot RCT was conducted in a NICU and included a total of 750 neonates.21 Baseline characteristics were similar between treatment groups. Among the entire cohort, 61% were male and 39% were female, gestational age at birth was approximately 34 weeks, and 0% to 76.9% had at least 1 risk factor for infection.
The target population of both evidence-based guidelines is hospital inpatients of all age groups, and the intended users are infection prevention and control teams as well as HCWs providing patient care in acute care settings.22,23
Interventions and Comparators
Interventions reported in the SR were the discontinuation of contact precautions (i.e., gown and gloves) for patients testing positive for MRSA or VRE.20 Fifteen studies discontinued contact precautions for patients testing positive for MRSA, 11 studies discontinued contact precautions for patients testing positive for VRE, and 9 studies discontinued contact precautions for both patients testing positive for MRSA and VRE.20 The time period before discontinuation of contact precautions was used as the comparator.20
The intervention assessed in the pilot RCT was universal use of NSGs before any contact with a patient, and the comparator was the use of standard precautions, which included glove use only in the context of contact with bodily fluids.21
Both guidelines assessed a wider variety of interventions than the scope of this review. Specific to this review, the guidelines addressed appropriate settings for glove use in general,23 appropriate settings for NSG use,23 and glove use for minimizing the transmission of MRSA.22
Outcomes
The outcome measures reported in the SR were rates of laboratory-confirmed hospital-associated MRSA infection (11 studies) and rates of laboratory-confirmed hospital-associated VRE infection (7 studies) before and after cessation of contact precautions.20 Random-effects and fixed-effects models were used to determine the pooled risk ratios of hospital-associated infection rates with contact precautions compared to rates without contact precautions.20
The outcomes reported in the pilot RCT were rates of LOIs in NICU patients.21 LOI episodes were categorized as follows: sterile-site LOI (i.e., culture-positive meningitis, bacteremia, urinary tract infection) and nonsterile-site LOI (culture-negative meningitis, single blood culture positive with coagulase-negative staphylococci, abdominal infection, pneumonia, clinically diagnosed cellulitis, and “culture-negative” sepsis). This study also assessed compliance with hand hygiene.
One guideline considered new laboratory-confirmed MRSA infections as the outcome in making recommendations for the use of contact precautions for patients with MRSA (i.e., gloves and gown).22 One guideline reported no primary evidence for glove use, and instead relied on expert opinion to make recommendations.23
Summary of Critical Appraisal
Systematic Review
The SR used a comprehensive literature search strategy and 2 reviewers to select studies, which increased the likelihood that all relevant studies were included; however, only studies of moderate quality were identified for inclusion.20 The meta-analysis included all studies and, while there was a discussion of bias, there was no formal assessment of the impact of bias on results.20 Excluded studies were not listed; however, the rational for exclusion was reported and each excluded study was cited in the reference list.20
Primary Clinical Study
The study objectives, variables of interest, and main outcomes were clearly described in the pilot RCT.21 The authors listed and compared the presence of potential confounding factors between treatment groups, which can provide assurance that the groups were similar before the study and that the difference between the groups is the intervention.21 Study personnel who conducted the retrospective chart review were blinded to the treatment arm to reduce the risk of observer bias.21 While this study was a randomized crossover trial, rather than individual patients being randomized, the single centre was randomly assigned in terms of the order of treatment arms. This means that different patients were included during each of the time periods the treatment arms were in effect, and risk of infection may have been different during each of the time periods. There was no sample size or power calculation, as this pilot study was meant to inform the development of a larger multicentre RCT.21
Evidence-Based Guidelines
The main limitation of both evidence-based guidelines was the lack of clinical data on which to base recommendations that were relevant to this review. While both guideline development groups used a systematic approach to gather and evaluate the evidence and to develop recommendations for the use of gloves in acute care settings, there was little evidence identified, and thus all of the relevant recommendations from 1 guideline23 and 1 of 2 recommendations from the other guideline22 were based on expert opinion.
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Summary of Findings
Appendix 4 presents the main study findings.
Clinical Effectiveness of Nonsterile Glove Use
Pooled data from the SR found a statistically significant difference in the rates of hospital-associated VRE infection, with evidence suggesting lower rates of hospital-associated VRE infection after cessation of contact precautions, compared with the time period during which contact precautions (i.e., gown and gloves) were used in hospitalized patients.20 The SR also found no evidence of a difference in the rate of hospital-associated MRSA infection before and after cessation of contact precautions.20
The pilot RCT found the rate of LOI in the NICU was similar with universal glove use for all patients compared to standard precautions.21 In addition, the odds of hand hygiene compliance before and after patient contact was significantly lower in the study arm using universal gloving compared to that using standard precautions alone.21
Guidelines Regarding the Use of NSGs
Recommendations for Glove Use in Acute Care Settings
One guideline made several recommendations regarding the use of gloves and standard infection control precautions in acute care settings, which were all based on expert opinion with no supporting evidence from primary studies.23
This guideline recommends that disposable gloves be worn to protect the health care worker and/or the patient if there is risk of contact with blood, body fluids, non-intact skin, mucous membranes, lesions and/or vesicles, hazardous drugs and chemicals, and that gloves should be worn during environmental cleaning and cleaning of used medical equipment.23
Gloves are not recommended for administering immunizations unless recommended by vaccine manufacturers or there is risk of contact with body fluids or non-intact skin.23
In cases where gloves are worn, it is recommended that NSGs are used for nonsterile procedures (e.g., patient examination), communal care equipment, environmental cleaning, and administering immunizations (where gloves are indicated).23
Recommendations for Glove Use Specific to MRSA in Acute Care Settings
One guideline recommends that standard infection prevention and control precautions be used in the care of all patients to minimize the risk of MRSA transmission (based on expert opinion).22
One guideline conditionally recommends that providers consider using contact precautions (i.e., gloves and gown) for direct contact with patients known to be colonized or infected with MRSA or their immediate environment.22 If contact precautions are used, it is recommended that gloves be changed between care procedures and that hand hygiene be performed after glove removal.22 This recommendation was based on limited primary clinical evidence from 2 RCTs and 7 quasi-experimental studies with inconsistent results.22
Limitations
This report is limited by the quantity and quality of evidence available on the use and non-use of NSGs for individuals receiving inpatient care considered at low risk of infection transmission. While the 2 guideline documents used a systematic method of gathering evidence on which to base their recommendations, very few relevant studies were identified.22,23 The only RCT included in this review was a pilot project conducted to determine the feasibility of designing a large, multicentre RCT.21 While the results showed no statistically significant differences in rates of infection between standard precautions and universal gloving, the study reported no sample size calculation, and it is unknown whether it was powered to detect a difference between treatment arms.21 Completion and publication of the findings from the full study (i.e., a large, multicentre, cluster crossover RCT), will further inform the safety and benefits of NSG use in the NICU.
The SR examining the effect of discontinuation of contact precautions (I.e., gloves and gown) for MRSA and VRE was well conducted and of high quality; however, only studies of moderate quality were identified for inclusion.20 Surveillance of these pathogens was variable among the studies, and 2 of the included studies discontinued active surveillance of VRE (i.e., screening) during the study period.20 Thus, it is impossible to know whether a reduction in the rate of VRE was due to the intervention or due to lack of measurement of the outcome.
No studies were identified that examined the impact of NSGs in direct comparison with non-use of NSGs on contamination events or transmission of infections. Little evidence was found for the impact of NSG use on hand hygiene practices. Furthermore, no comparative evidence was found for the impact of gloves outcomes such as occupational skin conditions.
There was considerable heterogeneity in the evidence evaluating NSG use. The SR included studies that evaluated the discontinuation of contact precautions, which include both gloves and gowns.20 Thus, conclusions cannot be made about glove use alone. It was not reported in the SR whether the gloves used were NSGs, and none of the included studies explicitly stated that the care activities were considered to have a low risk of infection transmission; rather, it was inferred that the care being provided was considered at low risk of infection transmission based on the use of “standard precautions” as the alternative intervention (i.e., hand hygiene before and after patient contact). The use of standard infection control precautions implies that the use of gloves would be based on risk of exposure to body fluids.23 Finally, the populations included in the studies are mixed, with the pilot RCT specific to neonates21 while the studies included in the SR were mainly whole-hospital settings.20 The 2 guideline documents were directed toward all inpatients in acute care settings regardless of age.22,23
Conclusions and Implications for Decision- or Policy-Making
The body of evidence comparing non-use of gloves versus use of gloves in acute care settings where there is low risk of infection transmissions is limited and of moderate quality. One SR assessed rates of nosocomial MRSA and VRE infections before and after discontinuation of contact precautions (i.e., gloves and gown) for such infections.20 One pilot RCT compared the rate of LOIs in neonates during a period when standard infection control precautions were used versus a time period when universal gloving of HCWs was employed.21 Finally, 2 evidence-based guidelines included recommendations for the use of gloves for infection control in acute care settings.22,23
Recommendations for the Use of Gloves in Acute Care Settings
In general, gloves should be worn to protect the HCW and patient when it is anticipated that there will be contact with blood, body fluids, non-intact skin, mucous membranes, lesions, or hazardous drugs and chemicals, and for environmental cleaning (1 guideline, based on expert opinion).23
It is recommended that NSGs be worn under the following circumstances (1 guideline, based on expert opinion):23
- during nonsterile procedures
- when using communal care equipment
- during environmental cleaning
- when administering immunizations, if indicated.
Specifically related to MRSA, it is conditionally recommended to consider using contact precautions (i.e., gloves and gown) for direct contact with patients testing positive for MRSA or their immediate environment.22 This recommendation was based on inconsistent evidence that did not support a strong recommendation, but the intervention may be beneficial in some circumstances.
Clinical Effectiveness of the Non-Use of NSGs
The included SR demonstrated a lower risk of VRE infection and no statistically significant difference in the risk of MRSA infection with discontinuation of contact precautions (i.e., gloves and gown) for patients in acute care settings.20 This evidence suggests that in situations with low-risk of infection transmission, non-use of gloves and gowns may have little to no difference in the rate of hospital-acquired MRSA infections, and may lower the rate of hospital-acquired VRE infections; however, it is important to consider the limitations of this evidence when interpreting these findings (e.g., evidence from 1 SR based on a limited number of moderate-quality nonrandomized studies).
In a neonatal population, no statistically significant differences were found in the rates of LOIs with standard infection control precautions (i.e., use of gloves with risk of contact with bodily fluids) compared with time periods in which gloves were used before any contact with the patient.21 This suggests that universal gloving may have little added benefit in the prevention of LOIs in the NICU compared with standard infection control precautions (e.g., hand hygiene).
Generalizability
The evidence and recommendations summarized in this review are generalizable to the Canadian health care context. One study included in the SR and the pilot RCT were conducted in Canada.20,21 In addition, the recommendations for the use of gloves in general23 and specific to MRSA22 are similar to those in the Canadian infection control guidelines published before 2018 (i.e., outside the time frame of this review).3
Considerations for Future Research
A substantial research gap exists for good-quality prospective RCTs that evaluate the effectiveness of NSGs in comparison with no gloves in terms of transmission of pathogens. Researchers should consider that the current findings of equivalence between NSGs and standard infection control precautions should be confirmed with well-conducted RCTs to better inform decisions regarding NSG use when providing inpatient care considered at low risk of infection transmission. Finally, the design of education campaigns or interventions to reduce misuse and overuse of gloves in acute care settings should be guided by an evidence-informed understanding of the drivers of glove-use behaviour.
Implications for Clinical Practice
In acute care settings, when providing care considered at low risk of infection transmission, standard precautions (e.g., hand hygiene,20 or glove use only when there is a risk of contact with bodily fluids21) may offer a similar level of protection against infection,20,21 or may have the potential to lower the risk of certain infections20 when compared to the use of contact precautions20 (i.e., gloves and gown) or the universal use NSGs.21 However, decision-makers should also consider the limitations of this evidence (e.g., based on a limited number of moderate-quality studies, the heterogeneity of the evidence).
While guidelines recommend wearing gloves to protect HCWs and patients from exposure to bodily fluids, the misuse and overuse of gloves can be a problem in health care settings.7-9 Excessive glove use when not medically necessary may add an unnecessary expense to the already high cost of health care, contribute to unnecessary medical waste, and contribute to adverse skin effects in wearers. Hand eczema or contact dermatitis in the previous year has been found to be common in HCWs, and higher levels of glove use are associated with harm to skin integrity.12,13 In addition, glove use can negatively impact compliance with hand hygiene, which has the potential to impact the transmission of pathogens.9 However, no evidence was identified that met the criteria for this review that directly compared the impact of non-use versus use of NSGs on health care staff safety (e.g., dermatitis).
Decision-makers may also wish to consider the environmental impacts of NSG use. Medical waste has become an increasing concern, as it contributes to environmental pollution. Physiochemical analysis showed weathered gloves released leachable substances, including microparticles, organic matter, and heavy metals contaminating drinking water and the food chain,4,5 potentially resulting in a number of health effects in humans and animals.5
Abbreviations
- HCW
health care worker
- LOI
late-onset infection
- MRSA
methicillin-resistant Staphylococcus aureus
- NICU
neonatal intensive care unit
- NSG
nonsterile glove
- RCT
randomized controlled trial
- SR
systematic review
- VRE
vancomycin-resistant enterococci
Contributors: Kendra Brett
References
- 1.
- Kpadeh-Rogers Z, Robinson GL, Alserehi H, et al. Effect of glove decontamination on bacterial contamination of healthcare personnel hands. Clin Infect Dis. 2019;69(Suppl 3):S224-S227. [PMC free article: PMC6761364] [PubMed: 31517972]
- 2.
- Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis. 2001;32(5):826-829. [PubMed: 11229854]
- 3.
- Public Health Agency of Canada. Routine practices and additional precautions for preventing the transmission of infection in healthcare settings. 2016; https://www
.canada.ca /en/public-health/services /publications /diseases-conditions /routine-practices-precautions-healthcare-associated-infections.html. Accessed 2023 Aug 17. - 4.
- Wang Z, An C, Lee K, et al. Physicochemical change and microparticle release from disposable gloves in the aqueous environment impacted by accelerated weathering. Sci Total Environ. 2022;832:154986. [PubMed: 35395312]
- 5.
- Jędruchniewicz K, Ok YS, Oleszczuk P. COVID-19 discarded disposable gloves as a source and a vector of pollutants in the environment. J Hazard Mater. 2021;417:125938. [PMC free article: PMC8076738] [PubMed: 34010776]
- 6.
- Andeobu L, Wibowo S, Grandhi S. Medical waste from COVID-19 pandemic: A systematic review of management and environmental impacts in Australia. Int J Environ Res Public Health. 2022;19(3):1381. [PMC free article: PMC8835138] [PubMed: 35162400]
- 7.
- Fuller C, Savage J, Besser S, et al. “The dirty hand in the latex glove”: A study of hand hygiene compliance when gloves are worn. Infect Control Hosp Epidemiol. 2011;32(12):1194-1199. [PubMed: 22080658]
- 8.
- Wilson J, Prieto J, Singleton J, O’Connor V, Lynam S, Loveday H. The misuse and overuse of non-sterile gloves: Application of an audit tool to define the problem. J Infect Prev. 2015;16(1):24-31. [PMC free article: PMC5074137] [PubMed: 28989395]
- 9.
- Loveday HP, Lynam S, Singleton J, Wilson J. Clinical glove use: Healthcare workers’ actions and perceptions. J Hosp Infect. 2014;86(2):110-116. [PubMed: 24412643]
- 10.
- Sorio GGL, Arns B, Kawski C, et al. Large hospital-wide outbreak of Paenibacillus spp pseudobacteremia associated with contaminated nonsterile gloves. Infect Control Hosp Epidemiol. 2023:1-4. [PubMed: 36987857]
- 11.
- Montero-Vilchez T, Cuenca-Barrales C, Martinez-Lopez A, Molina-Leyva A, Arias-Santiago S. Skin adverse events related to personal protective equipment: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2021;35(10):1994-2006. [PubMed: 34077565]
- 12.
- Hamnerius N, Svedman C, Bergendorff O, Bjork J, Bruze M, Ponten A. Wet work exposure and hand eczema among healthcare workers: A cross-sectional study. Br J Dermatol. 2018;178(2):452-461. [PubMed: 28722122]
- 13.
- Mekonnen TH, Yenealem DG, Tolosa BM. Self-report occupational-related contact dermatitis: Prevalence and risk factors among healthcare workers in Gondar town, Northwest Ethiopia, 2018-a cross-sectional study. Environ Health Prev Med. 2019;24(1):11. [PMC free article: PMC6376784] [PubMed: 30764759]
- 14.
- Barton A. Kicking the glove habit. Br J Nurs. 2023;32(2):S3. [PubMed: 36715526]
- 15.
- Gould D. Gloves: A COVID-19-driven habit nurses need to break. Nurs Manage. 2021;28(4):13-13.
- 16.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [PMC free article: PMC5833365] [PubMed: 28935701]
- 17.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 18.
- Agree Next Steps Consortium. The AGREE II Instrument. [Hamilton, ON]: AGREE Enterprise; 2017: https://www
.agreetrust .org/wp-content/uploads /2017/12/AGREE-II-Users-Manual-and-23-item-Instrument-2009-Update-2017.pdf. Accessed 2023 Aug 17. - 19.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 20.
- Kleyman R, Cupril-Nilson S, Robinson K, et al. Does the removal of contact precautions for MRSA and VRE infected patients change health care-associated infection rate?: A systematic review and meta-analysis. Am J Infect Control. 2021;49(6):784-791. [PubMed: 33276000]
- 21.
- Khan S, Tsang KK, Hu ZJ, et al. GloveCare: A pilot study in preparation for a cluster crossover randomized controlled trial of non-sterile glove-based care in preventing late-onset infection in the NICU. Pilot Feasibility Stud. 2023;9(1):50. [PMC free article: PMC10035220] [PubMed: 36959636]
- 22.
- Coia JE, Wilson JA, Bak A, et al. Joint Healthcare Infection Society (HIS) and Infection Prevention Society (IPS) guidelines for the prevention and control of methicillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect. 2021;118S:S1-S39. [PubMed: 34757174]
- 23.
- ARHAI Scotland. Standard infection control and transmission based precautions literature review: Personal protective equipment (PPE) gloves. 2022; https://www
.nipcm.scot .nhs.uk/documents/sicp-ppe-gloves/. Accessed 2023 Aug 17. - 24.
- ARHAI Scotland. National infection prevention and control manual: Development process. 2022; https://www
.nipcm.hps .scot.nhs.uk/resources /development-process/. Accessed 2023 Aug 17.
Appendix 1. Selection of Included Studies
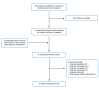
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Note that this appendix has not been copy-edited.
Table 2
Characteristics of the Included Systematic Review.
Table 3
Characteristics of Included Primary Clinical Study.
Table 4
Characteristics of Included Guidelines.
Table 5
SIGN 50 Levels of Evidence,.
Table 6
Grades of Recommendation,.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 7
Strengths and Limitations of the Systematic Review Using AMSTAR 216.
Table 8
Strengths and Limitations of the Clinical Study Using the Downs and Black Checklist.
Table 9
Strengths and Limitations of Guidelines Using AGREE II.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 10
Summary of Findings by Outcome — Clinical Effectiveness of NSG Use.
Table 11
Summary of Recommendations in Included Guidelines.
Appendix 5. References of Potential Interest
Note that this appendix has not been copy-edited.
- Smith A, Severn S. Reducing the environmental impact of clinical care. Can J Health Technol. 2023;3(4). https://www
.cadth.ca /sites/default/files /pdf/htis/2023/EH0112%20v.7_%20Final.pdf. [PubMed: 37903201] - Hand sanitizer on glove exteriors: Clinical effectiveness and guidelines. (CADTH Rapid response report: reference list). Ottawa (ON): CADTH; 2020: https://www
.cadth.ca /hand-sanitizer-glove-exteriors-clinical-effectiveness-and-guidelines. - Disposable gloves for use in healthcare settings: Clinical effectiveness, cost-effectiveness, and guidelines. (CADTH Rapid response report: summary of abstracts). Ottawa (ON): CADTH; 2019: https://www
.cadth.ca /disposable-gloves-use-healthcare-settings-clinical-effectiveness-cost-effectiveness-and-guidelines. - Hand hygiene and glove use in dental settings: Clinical effectiveness, cost-effectiveness, and guidelines. (CADTH Rapid response report: reference list). Ottawa (ON): CADTH; 2019: https://www
.cadth.ca /hand-hygiene-and-glove-use-dental-settings-clinical-effectiveness-cost-effectiveness-and-guidelines. - Disposable, non-sterile gloves for minor surgical procedures: A review of clinical evidence. (CADTH Rapid response report: summary with critical appraisal). Ottawa (ON): CADTH; 2017: https://www
.cadth.ca /disposable-non-sterile-gloves-minor-surgical-procedures-review-clinical-evidence. [PubMed: 29293297] - Health care practitioner hand hygiene practices and health care associated infections: A review of the qualitative patient perspectives and experiences literature. (CADTH Rapid response report: summary with critical appraisal). Ottawa (ON): CADTH; 2017: https://www
.cadth.ca /health-care-practitioner-hand-hygiene-practices-and-health-care-associated-infections-review. [PubMed: 29293301] - Sterile versus clean gloves during suctioning of orotracheal, nasotracheal, and nasopharyngeal sites: Comparative clinical effectiveness, cost-effectiveness, and guidelines. (CADTH Rapid response report: summary of abstracts). Ottawa (ON): CADTH; 2015: https://www
.cadth.ca /sterile-versus-clean-gloves-during-suctioning-orotracheal-nasotracheal-and-nasopharyngeal-sites. - Leonard A, Dunn H, Wilson N. 068 The ‘gloves are off’ – can we reduce inappropriate glove usage through an educational based intervention and risk assessment. Arch Dis Child. 2018;103:A28.
- European Centre for Disease Prevention and Control. Covid-19 guidance: Use of gloves in healthcare and non-healthcare settings in the context of the COVID 19 pandemic. 2020; https://www
.ecdc.europa .eu/en/publications-data /gloves-healthcare-and-non-healthcare-settings-covid-19. - Public Health Agency of Canada. Guideline on the prevention of transmission of bloodborne viruses from infected healthcare workers in healthcare settings. 2019; https://www
.canada.ca /content/dam/phac-aspc /documents/services /infectious-diseases /nosocomial-occupational-infections /prevention-transmission-bloodborne-viruses-healthcare-workers /guideline _accessible_aug-2-2019.pdf. [PMC free article: PMC7041653] [PubMed: 32167087] - NHS. Great Ormond Street Hospital – reducing single use plastics case study. 2020; https://www
.england.nhs .uk/greenernhs/whats-already-happening /great-ormond-street-hospital-reducing-single-use-plastics-case-study/ - Mahase E. Sixty seconds on . . . gloves off. BMJ. 2019;366:l4498. [PubMed: 31266766]
- Nursing Times. A programme to cut inappropriate use of non-sterile medical gloves. 2019; https://www
.nursingtimes .net/clinical-archive /infection-control /programme-cut-inappropriate-use-non-sterile-medical-gloves-20-08-2019/
Previous CADTH Reports
Nonrandomized Studies
Guidelines and Recommendations
Additional References
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for noncommercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to ac.HTDAC@stseuqeR
- Key Messages
- Context and Policy Issues
- Research Questions
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Nonsterile Glove UseNonsterile Glove Use
Your browsing activity is empty.
Activity recording is turned off.
See more...
