This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Perry T, editor. Therapeutics Letter. Vancouver (BC): Therapeutics Initiative; 1994-.
Therapeutics Letter 39 adds new evidence to information previously published about NSAIDs in Therapeutics Letters 4 and Letter 17. It also responds to our commitment in Therapeutics Letter 31 to report on celecoxib when published trials became available. Conclusions: Patients on rofecoxib had less complicated and symptomatic ulcers but more myocardial infarctions than patients on naproxen. COX-2 selective NSAIDs were associated with the same incidence of serious adverse events as non-selective NSAIDs. Celecoxib and meloxicam caused fewer withdrawals due to adverse events than non-selective NSAIDs. Head-to-head RCTs comparing NSAIDS with dosing regimens to optimize efficacy and safety would be useful.
Keywords:
Anti-Inflammatory Agents, Non-Steroidal; Celecoxib; Meloxicam; Naproxen; Rofecoxib
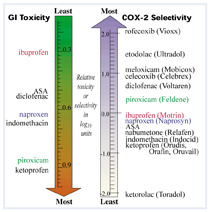
Figure
Does COX-2 selectivity explain GI toxicity?
What is the baseline risk of peptic ulcer complications?
The risk of hospitalization due to peptic ulcer complication is about 0.2% per year in non-users of NSAIDs (Saskatchewan data from 1982–1986).1 The most common presentation is gastrointestinal (GI) hemorrhage. Risk is higher in men than women and increases with age.1
What is the risk of peptic ulcer complications in patients taking NSAIDs?
Using data based on community dispensed NSAIDs, baseline incidence is increased 4-fold in patients currently taking NSAIDs.1,2 The risk varies widely with different NSAIDs and is illustrated in the left arrow of the Figure.3 Risk also varies with dose, 2.5 fold for low doses and 8.5 fold for high doses.3 Ibuprofen has the lowest risk, 2.0 fold, estimated absolute risk increase (ARI) 0.2%, number needed to harm (NNH) 500 per year.2
What can be done to reduce the risk of NSAID GI toxicity?
- prescribe NSAIDs only to patients who do not respond to acetaminophen
- select the NSAID with the lowest GI toxicity
- prescribe the lowest possible dose for the shortest duration of time2
Why might selective COX-2 inhibitors cause less peptic ulcer complications?
Potential for reduced GI toxicity with selective COX-2 inhibitors is based on the hypothesis that inhibition of COX-1 by effects on GI mucosa and platelets is the main cause of GI ulcers and bleeding. Testing by a single laboratory using human assay systems shows that rofecoxib is the most selective of available NSAIDs (>50-fold potency for COX-2 over COX-1).4 The relative COX-2 selectivity of common NSAIDs are shown in the right hand arrow of the Figure. The order of COX-2 selectivity does not appear to explain the order of GI toxicity.
How is the GI toxicity of different NSAIDs best compared?
Comparing different NSAIDs should be done in head-to-head randomized controlled trials (RCTs) using minimum effective doses and measuring serious GI outcomes. The most critical outcome to the patient is peptic ulcer complication (hemorrhage, perforation or obstruction) leading to hospitalization. A less serious but important outcome is symptomatic peptic ulcer (possibly requiring outpatient investigation and 6–8 weeks of treatment). Because these 2 outcomes have substantially different implications to the patient, they should be reported separately.
Do the newer more selective COX-2 inhibitors cause less serious GI toxicity?
Four published head-to-head RCTs provide a partial answer to this question. Unfortunately, in all these trials a single dose of drug was administered to all patients and not in a manner to minimize toxicity i.e. titrating the dose to the minimum effective dose. The CLASS trial randomized 7968 patients to 3 groups, celecoxib 400 mg BID, ibuprofen 800 mg TID and diclofenac, 75 mg BID; patients (69% female, mean age 60) with osteoarthritis (72.6%) and rheumatoid arthritis were treated for an average duration of 4.2 months.5 In the VIGOR trial, rofecoxib 50 mg per day was compared to naproxen 500 mg BID in 8076 patients (80% female, mean age 58) with rheumatoid arthritis over a median treatment period of 9 months.6 The Table shows the major outcomes from these 2 trials. Only rofecoxib significantly reduced complicated ulcers. While subgroup analyses were reported in these trials, these should be used to design future trials, not to support conclusions or clinical decisions.
Two large short-term RCTs (28 days) compared meloxicam with other NSAIDs in osteoarthritis patients. MELISSA7 (9323 patients) compared meloxicam 7.5 mg daily with diclofenac SR 100 mg daily and SELECT8 (8656 patients) compared meloxicam 7.5 mg daily with piroxicam 20 mg daily.
Interpretation of these trials is difficult as the doses were not equi-effective; measures of efficacy in the meloxicam patients were significantly less than the comparator in both trials. Complicated and symptomatic ulcers were uncommon and not significantly different. Total withdrawals due to adverse events were lower, 6.0%, with meloxicam than diclofenac, 8.0%, or piroxicam, 7.2%.7,8
Are the newer selective COX-2 inhibitors safer overall?
The best measure of overall safety, serious adverse events, is a required outcome of all clinical trials. This category includes death, life-threatening events, events leading to or prolonging hospitalization, and cancers. Total serious adverse events were not lower with the new drugs: celecoxib (see Table); meloxicam, 1.2%, versus diclofenac, 1.1%7; and meloxicam, 0.6%, versus piroxicam, 0.7%8. This outcome was not reported in the VIGOR paper, however, total withdrawals due to adverse events were similar for rofecoxib and naproxen, and myocardial infarctions were significantly increased in the rofecoxib group (see Table).6 Withdrawals due to rash were significantly increased in the celecoxib group, 2.7% vs ibuprofen/diclofenac, 1.2%, ARR = 1.5%, NNH = 67.5 Withdrawals due to renal adverse events were similar for the new drugs and the comparators.
What important data is not provided in these RCT publications?
CLASS: death and serious adverse events by category and cause, separate complete data for ibuprofen and diclofenac, incidence of GI investigations in 3 treatment groups.5
VIGOR: serious adverse events and withdrawals due to adverse events by category and cause, incidence of GI investigations. 6
MELISSA and SELECT: death, serious adverse events and withdrawals due to adverse events by category and cause.7,8
What is the dose and cost of newer NSAIDs?
Celecoxib 100 to 200 mg BID, cost $1.37–2.71 daily. Meloxicam 7.5 to 15 mg daily, cost $0.83–0.99 daily. Rofecoxib 12.5 to 25 mg daily, cost $1.34 daily (for either dose). The costs of the other NSAIDs are included in Letters 4 and 17. These costs have been updated to 2000 prices on our website (www.ti.ubc.ca).
Conclusions
- Patients on rofecoxib had less complicated and symptomatic ulcers but more myocardial infarctions than patients on naproxen.
- COX-2 selective NSAIDs were associated with the same incidence of serious adverse events as non-selective NSAIDs.
- Celecoxib and meloxicam caused fewer withdrawals due to adverse events than non-selective NSAIDs.
- Head-to-head RCTs comparing NSAIDS with dosing regimens to optimize efficacy and safety would be useful.
References
- 1.
- García Rodriguez LA, Walker AM, Pérez Gutthann, S. Nonsteroidal antiinflammatory drugs and gastrointestinal hospitalizations in Saskatchewan: a cohort study. Epidemiology. 1992; 3:337–342. [PubMed: 1637896]
- 2.
- Langman MJS, Weil J, Wainwright P, et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994; 343:1075–78. [PubMed: 7909103]
- 3.
- Henry D, Lim LL-Y, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 1996; 312:1563–6. [PMC free article: PMC2351326] [PubMed: 8664664]
- 4.
- Warner TD, Giuliano F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc Natl Acad Sci USA. Pharmacology. 1999; 96:7563–7568. [PMC free article: PMC22126] [PubMed: 10377455]
- 5.
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib versus nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The CLASS study: a randomized controlled trial. JAMA. 2000; 284:1247–1255. [PubMed: 10979111]
- 6.
- Bombardier C, Laine L, Reicin A, et al, for the VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000; 343:1520–8. [PubMed: 11087881]
- 7.
- Hawkey C, Kahan A, Steinbrück K, et al. Gastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients. Br. J. Rheumatol. 1998; 37:937–945. [PubMed: 9783757]
- 8.
- Dequeker J, Hawkey C, Kahan A, et al. Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the safety and efficacy large-scale evaluation of COX-inhibiting therapies (SELECT) trial in osteoarthritis. Br. J. Rheumatol. 1998; 37:946–951. [PubMed: 9783758]
- This Letter contains an assessment and synthesis of published (and whenever possible peer-reviewed) publications up to December 2000. We attempt to maintain the accuracy of the information in the Therapeutics Letter by extensive literature searches and verification by both the authors and the editorial board. In addition this Therapeutics Letter was submitted for review to 120 experts and primary care physicians in order to correct any inaccuracies and to ensure that the information is concise and relevant to clinicians.The Therapeutics Initiative’s objectives are unbiased review and dissemination of therapeutic evidence. Our recommendations are intended to apply to most patients; exceptional patients require exceptional approaches. We are committed to evaluate the effectiveness of our educational activities using the Pharmacare/PharmaNet databases without identifying individual physicians, pharmacies or patients. The Therapeutics Initiative is funded by the BC Ministry of Health through a 5-year grant to the University of BC. The Therapeutics Initiative provides evidence based advice about drug therapy, and is not responsible for formulating or adjudicating provincial drug policies.
Tables
Table
| Outcome | Celecoxib % | Other* % | RR 95%CI | ARR % | NNT 4 mo | Rofecoxib % | Naproxen % | RR 95%CI | ARR ARI % | NNT NNH 9 mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Myocardial infarction | 0.3 | 0.3 | 0.9 0.4–2.1 | NS | NS | 0.4 | 0.1 | 4.0 1.3–12 | 0.3 | 333 |
| Complicated ulcers | 0.3 | 0.6 | 0.6 0.3–1.2 | NS | NS | 0.4 | 0.9 | 0.4 0.2–0.8 | 0.5 | 200 |
| Serious adverse events | 4.3 | 4.2 | 1.02 0.8–1.3 | NS | NS | NR | NR | |||
| Symptomatic ulcers | 0.5 | 0.7 | 0.65 0.4–1.2 | NS | NS | 1.0 | 2.1 | 0.5 0.3–0.7 | 1.1 | 91 |
| Withdrawals due to adverse events | 18.4 | 20.6 | 0.89 0.8–0.97 | 2.2 | 44 | 16.4 | 16.1 | 1.02 0.9–1.1 | NS | NS |
- *
Half ibuprofen and half diclofenac.
RR = Risk Reduction, CI = Confidence Interval, ARR = Absolute Risk Reduction, NNT = Number Needed to Treat to prevent one event, ARI = Absolute Risk Increase, NNH = Number Needed to Treat to cause one Harmful event, NS = Non-Significant, NR = Not Reported.