This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
The negative health impact of stroke can be drastically reduced by effective treatment options early poststroke. Because patients receive only a 4-day hospital length of stay, most care is provided after hospital discharge. However, evidence is not available to guide stroke survivors, caregivers, and health care professionals to make an informed decision at hospital discharge about postacute service referral.
Objectives:
The study sought to (1) describe stroke survivors' postacute service use and identify the person- and systems-level factors associated with the use of rehabilitation services following hospital discharge; (2) among stroke survivors discharged to short-term inpatient rehabilitation, examine the comparative effectiveness of high-intensity (in inpatient rehabilitation facilities [IRFs]) vs low-intensity (in skilled nursing facilities [SNFs]) care on 3- and 12-month outcomes; and (3) among stroke survivors discharged home from the hospital, examine early follow-up with community-based providers.
Methods:
For this retrospective observational study of patients aged 65 years and older, we linked national registry, longitudinal patient follow-up, and administrative claims data to identify predictors of health service use and to assess outcomes following hospitalization for an ischemic stroke. Among patients discharged from the hospital to continue care in short-term inpatient postacute settings, we conducted a comparative effectiveness evaluation of IRF compared with SNF using instrumental variables and inverse probability weighted estimation propensity score–based methods. Among patients discharged directly home from the hospital, we examined the association of early follow-up and rehospitalization, death, and home time (primary outcome) using multivariable analysis.
Results:
More than half of 162 432 stroke patients treated at 1192 hospitals experienced 3 or more care transitions after hospital discharge, and the number of days alive and living in the community (home time) varied by many factors. Nearly half of all stroke patients (43.7%) are discharged directly home from the hospital only 4 days after their stroke and 47.2% transition to another inpatient postacute facility. The likelihood of receiving short-term inpatient postacute care at IRFs vs SNFs varied nearly 3 times across hospitals (adjusted median odds ratio for receipt of IRF care vs SNF = 2.87; 95% CI, 2.68-3.11), even among patients with the same set of covariates, suggesting substantial variation even after accounting for all observed characteristics. Comparing outcomes for patients who received IRF or SNF care identified a higher likelihood of better outcomes for patients who received IRF care, particularly within 90 days of hospital discharge. IRF patients had better 12-month home time (primary outcome) and survival and were less likely to be rehospitalized or institutionalized in a nursing home. Care for patients discharged home from the hospital was fragmented and, in some cases, delayed. Only 13.68% received home health services after hospital discharge and 6.41% received outpatient rehabilitation. Stroke survivors shared with us that the latter was dependent on referral from a community-based provider. However, among patients discharged home, the average number of days to first medical follow-up was 27 days. Physician follow-up poststroke was more common with internal medicine or primary care (50.3% and 70.1%) and cardiology (53.3%) than neurology (42.4%). The median number of days to first visit with neurology was 35 days (interquartile range [IQR] = 18-87 days), whereas first visit with internal medicine was 18 days (IQR, 7-93 days) and primary care was 22 days (IQR, 7-159 days) from hospital discharge. The hospital-level rate of follow-up within 7 days of hospital discharge was 39%.
Conclusions:
The research conducted in this funded project strengthened the evidence base for the receipt of short-term inpatient rehabilitation and early follow-up with physicians after hospital discharge and revealed important variations in care for future research.
Background
Every 40 seconds, someone in the United States experiences a stroke. An overwhelming 800 000 strokes every year is giving way to a stroke epidemic from the aging baby boom generation that will substantially increase the number of stroke events to 2 million per year by 2050.1 Using the World Health Organization's measure of burden from disability, stroke is a leading cause of disability-adjusted life years lost (the sum of years of potential life lost owing to premature death and years of productive life lost owing to disability).2 Up to 90% of stroke survivors are living with limitations to their daily function.1 The number of potential medical complications poststroke is extensive, and 50% are related to mobility impairments.3 Although stroke is experienced as an acute event, stroke survivors live with long-term consequences and manage their resulting limitations and health status as a chronic condition. The negative impact of stroke on the health of individuals and the population can be drastically reduced by effective treatment options early poststroke.
A hospitalization for an acute ischemic stroke is, on average, 4 days in length. At that time stroke survivors, their caregivers, and health care professionals face a critical decision of what services to choose for after hospital discharge that would best meet needs and lead to better outcomes. Although clinical guidelines recommend intense inpatient rehabilitation poststroke, there is a paucity of supporting evidence.4 The benefits and risks of inpatient and community-based options for postacute care are not clear.
The significant knowledge gaps about the appropriate use and effectiveness of postacute stroke care largely result from the fragmentation of health services. This fragmentation contributes to the difficulty in measuring longitudinal outcomes regardless of the care received.4,5 Despite federal policies aimed to improve continuity and specifically address the quality of postacute care,6,7 at this time the United States is limited in the ability to examine the comparative effectiveness of postacute services or assess patient-centered outcomes for an episode of care beyond administratively measuring rehospitalizations and mortality. Consequently, outcomes research to date for patients who received poststroke inpatient rehabilitation, rehabilitation in a skilled nursing facility (SNF), home health (HH), outpatient rehabilitation (OPR), or neurology follow-up includes small samples of patients,8,9 has inadequate comparators,8,10,11 or is limited to covariates available in administrative claims data12,13 or setting-specific outcomes, such as those measured by the inpatient rehabilitation facility (IRF) patient assessment instrument, minimum data set for SNFs, and outcome and assessment information set) for home health.14-17 To overcome these limitations, we integrated clinical data and patient-reported outcomes with administrative claims data for a national cohort of patients to create statistically appropriate comparisons of care for stroke patients and to assess outcomes meaningful to patients.
This project's purpose was to determine the best options for postacute care. Our approach was to first create a robust data set that included patient clinical characteristics and hospital-based stroke care from a national acute stroke care registry, longitudinal patient follow-up, and administrative claims, to describe stroke patients' use of inpatient postacute rehabilitation options for care in IRFs and SNFs, community-based rehabilitation options including HH and OPR, and physician follow-up. As half of all stroke patients aged 65 and older are discharged from the hospital to inpatient postacute care,18 our primary aim was to compare 3- and 12-month outcomes for IRF vs SNF care. Among patients who were discharged home, we sought to determine the benefits of early follow-up with a physician. Analyses are limited to patients aged 65 and older; however, nearly 75% of all strokes occur in this age group.1 Deciding on where to receive care after an acute ischemic stroke is occurring now without key evidence for stroke patients, caregivers, and clinicians. This new evidence-based knowledge about the comparative benefits and harms of the different postacute options will be essential to guide individual decisions and improve future practice, policy, and patient-centered outcomes.
Participation of Patients and Other Stakeholders
This project was led by a team of investigators, an advisory group of stakeholders, and additional input from stroke survivors and caregivers. The team of investigators included an interdisciplinary team that met every second week for the 3-year funded period. The advisory group of 7 stakeholders were employees of federal agencies and professional organizations, health professionals, and patients identified as experts and leaders from publicly available reports and peer-reviewed literature.
Team members were invited to participate and accepted the invitation before the application for funding. The stakeholder advisory group met with the interdisciplinary investigator team 3 times a year—some participants in person and some on the phone. The principal investigator and patient stakeholder attended local stroke support groups to obtain additional input, guidance on interpretation of findings, and perspectives on significance and opportunities for additional research. Stakeholder input was a core operating principle for this project. As such, we made revisions to the study aims after the cohort of patients and their health care utilization were described.
Because this retrospective study used existing data, stakeholders provided input for defining the study population, the covariates to examine, and the descriptive analyses to examine; presenting the findings; interpreting the results; and disseminating findings as abstracts, oral presentations, and manuscripts. All manuscripts that included government employees included additional internal review according to that agency's processes for authorship. All proposals for analysis, and subsequently all abstracts and manuscripts, were reviewed by the American Heart Association's Get With the Guidelines review group to ensure all documents met their organization's policies on data use. As a result, dissemination of findings in some cases were reviewed by the study team, advisory group of stakeholders, government, and American Heart Association review committees before submitting for peer review with a journal. We felt this increased the rigor and quality of our research and supported transparency of the research process. Revisions to the study protocol that resulted from stakeholder engagement are described below in the Study Design section.
Methods
Study Design
Using a retrospective observational comparative effectiveness study design, we analyzed data from the nation's largest clinical registry for acute stroke—the American Heart Association Get With the Guidelines (GWTG)-Stroke program—linked with longitudinal Medicare claims of service use, and standardized measures of patient-reported 12-month outcomes from the Adherence eValuation After Ischemic Stroke–Longitudinal (AVAIL) study.19-21 We will refer to this integrated data as GWTG/Medicare/AVAIL. Using advanced analytic techniques, including the instrumental variable approach and the inverse probability weighted estimation propensity score–based method, we addressed the critical decision of what services to choose following an acute hospitalization. A scientific and stakeholder advisory team guided the concept, design and methodology, and interpretation of findings. The study was approved by the IRB and is registered on clinicaltrials.gov as protocol number Pro000044034. The specific aims as originally proposed, followed by the revised aim and the stakeholder rationale for the revisions, are below.
- Aim 1. Identify person- and systems-level factors associated with stroke survivors' use of rehabilitation services following hospital discharge.
- Aim 1–revised. Describe the patterns of postacute service use and identify person- and systems-level factors associated with stroke patients' use of postacute care following hospital discharge.
Rationale for revision: Stakeholders expressed interest in care transitions and wanted to explain the complexity of postacute stroke care.
- Aim 2 (no changes). Among stroke survivors discharged to short-term inpatient rehabilitation, examine the comparative effectiveness of high-intensity (in an IRF) vs low-intensity (in an SNF) care on 3-and 12-month outcomes.
- Aim 3. Among stroke survivors discharged from hospital to home, examine the comparative effectiveness of OPR, HH, and no rehabilitation on 3- and 12-month outcomes.
- Aim 3–revised. Among stroke survivors discharged from hospital to home, examine the association between early provider follow-up and 30- and 90-day outcomes.
Rationale for revision: Analysis of the patient population discharged home and health care policies that guide eligibility for HH and OPR indicated that the HH and OPR were not appropriate comparators. Also, the time lag from when this aim was proposed (2012) to when analysis began (2016) created an even larger gap from when these services were provided to patients, and some advisory group members felt the HH patients today were different than those 8 years prior. Addressing early follow-up was agreed to be a timely question, particularly with the more recent focus on bundled care and comprehensive stroke centers.
Data Sources
This study used a comprehensive data set created by linking and integrating data from several sources. The primary data sources and the data each provided are described below.
GWTG-Stroke
Our primary data source was the GWTG-Stroke registry, a nationwide stroke registry and quality improvement program sponsored and first launched by the American Heart Association in 2003. Still active, the registry as of 2016 had more than 1800 hospitals participating from across the United States, with data entered for more than 3 million patients. Trained hospital personnel are instructed to use a password-restricted web-based tool to collect patient-level data on acute stroke care provided in their hospital. The eligibility of each admission is confirmed through chart review by each hospital's personnel. Our team audited a random sample of records and confirmed that GWTG-Stroke is a reliable and accurate data source for clinical research.22 From this registry, we examined patient demographics, medical history, symptoms, complications, diagnostic testing, in-hospital treatment, adherence to evidence-based standards of care, and discharge status.23
GWTG-Stroke/AVAIL
The AVAIL study was a prospective national cohort study implemented in collaboration with the GWTG-Stroke program from 2006-2008. AVAIL was designed to supplement data in GWTG-Stroke. Patients treated at a GWTG-Stroke participating hospital that had also agreed to participate in AVAIL had all GWTG-Stroke data collected as part of usual care and before hospital discharge were recruited to participate in AVAIL.21 At enrollment, consented patients provided additional sociodemographic information not available in GWTG-Stroke, and contact information for themselves, 2 alternate contacts, and their primary physician. Trained research personnel at the Duke Clinical Research Institute (DCRI) conducted telephone interviews using the same standard scripts to collect poststroke patient-reported outcomes at 3- and 12-months postdischarge.
We used standardized measures reliable and valid for stroke for quality of life (EuroQol-5 dimensions [EQ5D]), function (modified Rankin Scale [mRS]), and depression (Patient Health Questionaire-8).21,24 The patient enrollee was the targeted interview respondent. When it was impossible to speak to the patient or when information provided was deemed unreliable by the interviewer, proxies (often family) were interviewed (20% at 12 months). Of patients, 92% completed the study. During active enrollment and follow-up (2006-2009), Outcome Sciences Inc (Cambridge, Massachusetts) managed GWTG and transferred data quarterly to the data coordinating center at DCRI for additional data validations. DCRI led AVAIL and continues to manage use of that study's data.
Medicare Claims Data
Medicare is the primary health insurer for approximately 97% of the US population aged 65 years and older. We used the denominator file and inpatient (hospital and IRF claims), SNF, HH, Outpatient, Carrier, and Beneficiary Summary analytic files. To reflect a full 12-month posthospitalization period for our patient sample recruited for GWTG-Stroke/AVAIL from 2006-2008, we included Medicare data for 2006-2009.
Additional Sources of Data
Stakeholder input advised the inclusion of 2 additional sources of data to supplement specific research questions. In partnership with the American Heart Association, we obtained hospital descriptors from the American Hospital Association. We used neighborhood characteristics obtained from publicly available American Community Survey data as additional sociodemographic characteristics for select studies.
GWTG-Stroke/Medicare Linked Data Set
From 2003-2012, 2 million records from 1800 hospitals were submitted to the GWTG-Stroke registry. Of those records, roughly 40% were ischemic stroke patients aged 65 years or older. These represent the group of patients eligible for matching to Medicare data. Because managed care plans account for 15% to 30% of Medicare-aged beneficiaries depending on region, the eligible population for matching to Medicare fee-for-services benefits ranges from 70% to 85% per year. Therefore, this study included only Medicare fee-for-service patients. We matched patients in GWTG-Stroke and the Medicare denominator and inpatient files using indirect identifiers of age, gender, admission, and discharge date.25 Prior analysis demonstrated that the GWTG-Stroke/Medicare matched cohort is representative of the overall registry and the national fee-for-service Medicare ischemic stroke population.21 Studies to date that have used these data have made significant advances to stroke outcomes research26; however, the effectiveness of rehabilitation or benefits with early follow-up have not been explored.
Study Population and Setting
The study population includes adults who experienced an acute ischemic stroke, were treated in a hospital participating in GWTG-Stroke from 2006-2008 and were Medicare beneficiaries with clinical registry records previously matched by our team to Medicare fee-for-service Part A (inpatient) data. Our starting population defined using GWTG/Medicare data included 162 432 acute ischemic stroke patients aged 65 years or older who received acute stroke care from 1192 US hospitals. After our study population was established, we defined the patient population specific to each aim and research question (Figures 1a-c). All analyses were either at the patient level, without identification of the site or location of care, or at the hospital level, without hospital identifiers. Although dates of service are known for each patient, the ability to reidentify patients in this study is limited.
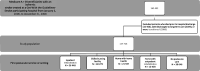
Figure 1a
Patient Flow Diagram for Aim 1.
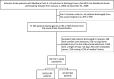
Figure 1b
Patient Flow Diagram for Aim 2, IRF Compared With SNF Care.

Figure 1c
Patient Flow Diagram for Aim 3, Early Follow-up After Acute Hospital Care.
Additional inclusion criteria applied for the different studies include the following: (1) community dwelling before stroke, (2) continuously enrolled in Medicare fee-for-service and for at least 3 months before stroke, (3) discharged alive from the acute hospital, and (4) had uninterrupted Medicare fee-for-service for at least 3 months after hospital discharge (or until death).
Interventions (Postacute Service) and Comparators
Administrative Medicare claims data indicate 2 dozen options for stroke survivors, caregivers, and clinicians to consider as possible hospital discharge destinations.27 However, except for stroke patients who are returning to live in a nursing home or are referred for hospice, the first decision is “Do I need/will I benefit from short-term rehabilitation in another inpatient setting or am I well enough to go home?” Examining the outcomes from comparing these 2 options is not possible because patients are not equally eligible for these pathways. Patients who are considered for home discharge have different needs and levels of stroke severity than those who continue with care in short-term inpatient postacute settings. There was only a 25% overlap for the interquartile range (IQR) of stroke severity scores for stroke patients discharged to IRFs and SNFs compared with those discharged home.
We used aim 1 to examine the different postacute service options, identified using Medicare claims that the service was provided or that the patient had an overnight stay in a particular setting. These included IRF, SNF, HH, and OPR. We also identified patients with hospital-to-hospital transfers using the inpatient files. Using the recommended algorithm provided by the Research Data Assistance Center (ResDAC) for use with Medicare claims, patients who at some point posthospital discharge transitioned to long-term care (LTC) in a nursing home were identified at hospital discharge. Face validity of the low frequency of nursing home patients was low among our stakeholders. As a result, we enhanced the ResDAC algorithm for identifying LTC residents to additionally include patients with a claim for a physician visit provided in a long-term nursing home facility. In the AVAIL cohort of patients (subset of the GWTG/Medicare cohort), transition to a nursing home was reported as place of residence in the 3- or 12-month follow-up phone call.
In aim 2, we compared the 2 most commonly used inpatient short-term options, IRF and SNF. We conducted the comparison similar to an intention-to-treat analysis and considered patients to have received care for 1 of these 2 options if they were referred at hospital discharge (documented as discharge destination) and had a Medicare claim for an admission to the IRF (in the inpatient file) or SNF (in the SNF file) within 1 day of hospital discharge.
In aim 3, we examined provider follow-up for patients discharged home. We defined provider follow-up as the first visit with a provider after the index admission's discharge where place of service was not an inpatient facility or the emergency department. We identified these using Current Procedural Terminology Evaluation and Monitoring codes 992XX-994XX. Provider types of interest included nurse practitioners; physician's assistants; and physician specialties of neurology, neurosurgery, cardiology, cardiovascular, internal medicine, endocrinology, primary care, general practice, family medicine, geriatrics, vascular, pulmonary, and physical medicine and rehabilitation. The comparison was early follow-up (any claim within 7 days of hospital discharge) compared with follow-up 8 days or later. The date of the visit was determined as the first visit after hospital discharge identified in either the outpatient facility claims revenue center part B file (revctrdt = visit date) or carrier claims part B (expnsdt1 = visit date).
Outcomes
For aim 1 (to describe the patterns of postacute service use and identify person- and systems-level factors associated with stroke patients' posthospital health care utilization), we examined discharge disposition and postacute service options as the outcome of interest as described above (hospital transfer, IRF, SNF, HH, OPR, and LTC).
For aim 2 (to assess the comparative effectiveness of high-intensity [in an IRF] vs low-intensity [in an SNF] postacute care for patients in the GWTG/Medicare sample), we measured all-cause mortality, a composite of all-cause mortality and rehospitalization, and days alive and out of inpatient care (ie, home time). Follow-up for endpoints began at discharge from the index hospitalization on the same day as assignment to an SNF or IRF care treatment setting, which we compared with respect to outcomes occurring within 14, 90, and 365 days of follow-up, with the exception of home time, which was not evaluated at 14 days. We selected the 14-day follow-up period because 14 days represents the median time spent in SNF care postdischarge, and therefore reflects the inpatient rehabilitation setting for most patients. The definition of rehospitalization included all acute care admissions (to either a short-term general hospital or a critical access hospital). We calculated home time, for example, using 90-day endpoint, as the total days minus those spent in inpatient care within 90 days of follow-up as INPTDAYS90. This included any readmission, IRF stay, or SNF stay, identified through Medicare Part A claims. This definition does not include long-term custodial care in an SNF or IRF, which is not covered by Medicare; it includes only admissions for which skilled nursing care was anticipated (covered by Medicare Part A), and these are generally short-term inpatient stays. We counted admissions to long-term care hospitals, which provide extended skilled care/rehabilitation, although this is <1% of inpatient claims in our population. If a patient survived past 90 days, 90-day home time = 90 − INPTDAYS90. If a patient died within 90 days, 90-day home time = (DEATH DATE − DISCHARGE DATE) − INPTDAYS90. Home time for patients who ceased Medicare fee-for-service eligibility not due to death was set to missing and subsequently excluded from the analysis (1.2% of patients at 90 days and 4.1% of patients within 365 days). A very small portion of patients (0.06%-0.08%) had home time <0; these were also set to missing and excluded.
Patient-reported outcomes examined for aim 2 were available in the GWTG/Medicare/AVAIL data set and obtained by telephone at 3 and 12 months after the patient's discharge from their acute hospitalization. Trained research personnel used standard scripts to conduct the telephone interview that included standardized scripts for questionnaires to ascertain disability, institutionalization in a nursing home, and quality of life. The patient enrolled was the targeted respondent, but when it was not possible to speak to the patient, when the information provided by the patient was deemed unreliable by the interviewer, or when the patient could not verbally respond, proxy interviews were conducted to assess both disability and institutionalization but not quality of life. We used the modified Rankin Scale as a global measure of disability that classifies function in 7 distinct stages.28 Patients or their proxies were asked to respond yes or no for whether assistance was needed for each mRS level (0 = no assistance needed; 5 = required constant care; 6 = death) and the corresponding score was recorded. We defined the outcome dependence as an mRS score of 3 to 6 (assistance needed with instrumental or basic activities of daily living, constant care was required, or death) and compared it with the favorable outcome of mRS = 0 to 2. We determined institutionalization by patient or proxy report of place of residence at each follow-up. We defined the composite outcome for institutionalization or death from data on the patient's residence that was reported as nursing home, hospice, or death at 3 or 12 months. The EuroQol group's EQ5D provided an assessment of quality of life (QOL) for patients able to respond to 5 questions.29 The EQ-5D is scored and examined as a continuous variable with a maximum value of 1 (greatest QOL). We assigned patients who died a score of 0.
For aim 3 on early provider follow-up among patients discharged home, we examined outcomes of rehospitalization and mortality within 30 and 90 days and home time within 90 days of hospital discharge. We defined these as previously described.
Covariates
We selected covariates by review of the literature and discussion with stakeholders and further narrowed them for each study aim. The full list is provided; the data sources are in parentheses.
Demographics (GWTG)
Age, sex, race.
Socioeconomic Status (American Community Survey)
Percentage college graduate or higher and median household income (log) corresponding to the patient Zip code. These data come from linking patient zip codes to the American Community Survey data 2007-2011, merged by Zip code tabulation areas code (approximately zip code) to patient zip code. We obtained patient Zip code from the index claim, if present, and otherwise from the denominator file for the index year.
Prior Service Use (Centers for Medicare & Medicaid Services Claims in the 6 Months Before the Index Admission)
Number of hospitalizations before the stroke admission, evidence of an SNF, evidence of an IRF, and HH care in 6 months before index.
Comorbidity (Combined GWTG Medical History and Centers for Medicare & Medicaid Services Claims Data for Each)
Stroke, ischemic heart disease, diabetes mellitus, peripheral vascular disease, congestive heart failure, and disability. Disability combined sources for patients with documentation as nonambulatory before the index stroke admission collected for GWTG, or Medicare claims for disability, hemiplegia, or paraplegia in the 6 months before the index stroke admission.
Comorbidity (GWTG Medical History)
Atrial fibrillation (AF), prosthetic heart valve, carotid stenosis, hypertension, dyslipidemia, smoking history.
Comorbidity (Centers for Medicare & Medicaid Services Claims in the 6 Months Before the Index Admission)
Any malignancy, chronic obstructive pulmonary disease, dementia, liver disease (mild, moderate, or severe), peptic ulcer disease, pneumonia renal disease, rheumatic disease, anemia, depression, extremity fracture, frailty, weight loss/malnutrition, osteoarthritis, osteoporosis, rheumatoid arthritis, substance abuse.
Hospital Care (GWTG)
Emergency medical services arrival, admitted to a stroke unit, deep vein thrombosis (DVT) prophylaxis initiated by the end of hospital day 2, tissue plasminogen activator (tPA) provided in index hospital.
In-Hospital Complications (GWTG)
Any serious complications (including intracerebral hemorrhage within 36 hours of tPA, life-threatening serious systemic hemorrhage within 36 hours, and other serious complications).
In-Hospital Complications (Centers for Medicare & Medicaid Services Claims for Index Hospitalization)
Cardiac/respiratory arrest, help breathing (tracheostomy, mechanical ventilator, etc), catheter use, delirium, dialysis, help feeding (gastrostomy, feeding tubes, etc), palliative care not hospice, pulmonary embolism, pneumonia, acute renal failure, septicemia/sepsis/septic shock, decubitus ulcer, urinary tract infection, venous thrombosis, length of stay during the acute index hospitalization, intensive care unit (ICU) during index hospitalization, length of ICU stay (days) during index hospitalization.
In-Hospital Status (GWTG)
Able to ambulate independently during the index or at discharge and stroke severity. We measured stroke severity by GWTG-participating hospital using the National Institutes of Health Stroke Scale (NIHSS), but data were missing for 55.5% of patients. The missingness was thought to be informative, involving numerous factors, some of which were not measured in the data; therefore, the assumptions for common missing data methods, such as multiple imputation, were not thought to be met. As a result, we implemented and compared 2 alternatives: (1) ignoring NIHSS and allowing it to be an unmeasured confounder; and (2) complete case analysis. We used the complete case as sensitivity analysis for aim 2. We used instrumental variable (IV) analyses to address unmeasured confounding, thereby increasing the validity of option No. 1 above.
Medications Prescribed at Hospital Discharge (GWTG)
Antihypertensive, lipid-lowering, antithrombotics, and smoking cessation.
Hospital Stroke Care Quality (GWTG)
Hospital composite of quality of care measure included documentation of tPA provided within 3 hours among arrival within 2 hours, early antithrombotics, DVT prophylaxis, antithrombotics at discharge, anticoagulants for AF, smoking cessation counseling, lipid-lowering drugs among low-density lipoprotein cholesterol (LDL) <100 or not documented.
Hospital Characteristics (American Hospital Association and American Heart Association)
Number of beds, teaching status, rural vs urban, region, certified primary stroke center hospital.
Analyses
Aim 1. Describe the Patterns of Postacute Service Use and Identify Person- and Systems-Level Factors Associated With Stroke Patients' Use of Postacute Care Following Hospital Discharge
We used mean, median, and percentages to describe the distribution of continuous and categorical variables. We compared baseline characteristics between patients discharged to the different postacute service options. In addition to analysis of the first postacute service used, overall and by stroke severity, we examined the trajectory of services used in the 90 days after hospital discharge to determine the total number of care transitions.
Aim 2. Among Stroke Survivors Discharged to Short-Term Inpatient Rehabilitation, Examine the Comparative Effectiveness of High-Intensity (in an IRF) vs Low-Intensity (in an SNF) Care on 3- and 12-Month Outcomes
We examined 3 outcomes (home time, readmissions, and mortality) using 2 approaches (inverse probability of treatment selection weighting [IPW] and instrumental variables), and using 2 instrumental variables for patients in the GWTG-Medicare study population. In the smaller GWTG/Medicare/AVAIL cohort of patients with patient-reported outcomes, we conducted unadjusted and adjusted analyses using IPW to examine outcomes of disability, institutionalization in a nursing home, and quality of life for patients who received IRF vs SNF care. The analytic strategy for aim 2 is described in more detail below. We conducted instrumental variable analyses in STATA version 13, and all other analyses in SAS V 9.4. We defined statistical significance as α = .05.
Among the patients discharged to an IRF or SNF in the GWTG/Medicare patient sample, we used the Kaplan-Meier method to estimate unadjusted event rates at specific time points, and we quantified differences between IRF and SNF by hazard ratios (HRs) and 95% from a Cox model. We modeled home time as count data using generalized estimating equations, with a log link, and a robust empirical variance to obtain rate ratios (RaRs) and 95% CI. We then adjusted all study comparisons for covariates thought to be related to both rehab selection and outcomes. We calculated a propensity score for the probability of receiving IRF rehab (rather than SNF) given the covariates. We assessed overlap in the covariate distribution and propensity scores between study groups and excluded patients with propensity scores less than 0.10 or greater than 0.90 (n = 4708; 7%). We reexamined the IPW assumptions (correct fit of the propensity model, positivity, assessment of balance, and unmeasured confounders) after these patients were excluded and then refit the models for treatment on outcomes using IPW.
IPW, like other propensity methods, relies on the assumption of no unmeasured confounders. This means that the results of IPW would be unbiased only if the covariate list contained all factors related to the treatment selection of IRF or SNF settings and outcomes. That cannot be guaranteed, particularly because we did not have stroke severity documented for all patients and information was not available for functional status at hospital discharge and availability of family support. To address the potential for unmeasured confounding, we conducted an instrumental variable analysis. An ideal instrument is something that influences the treatment choice arbitrarily (but is otherwise unrelated to outcome), after conditioning on the measured covariates. We evaluated and utilized 2 variables as instruments: (1) hospital practice patterns (instrumental variable referred to as %IRF) = the hospital-specific percentage of patients discharged to an IRF as opposed to an SNF, calculated over the whole study period; (2) differential distance (instrumental variable referred to as the same) = the distance to the nearest IRF minus the distance to the nearest SNF, where distance is calculated from the patient zip code centroid to the facility zip code centroid. In short, %IRF has a strong association with treatment selection (F = 14 658), although the relationship with differential distance is weaker (and F = 538). The observed patient characteristics are well balanced across quintiles of each instrumental variable (%IRF and differential distance), with the exception that hospitals with higher %IRF have modestly lower-risk patients. This in itself is not a problem because we used all of the measured covariates for adjustment.
However, IV analysis will be unbiased only if %IRF and differential distance are not related to additional, unmeasured confounders. Although differential distance achieved very good balance on measured patient characteristics and reasonable balance on hospital characteristics and, thus, may provide unbiased results, this instrument would be expected to have less precision and wider CIs than %IRF because the association of differential distance with treatment selection was not as strong. Where %IRF is strongly related to treatment selection, it is a more powerful instrumental variable and potentially more robust, as it is likely to have good precision and be representative of a very large portion of the total population.
To facilitate the use of classic instrumental variable methods, we converted time-to-event outcomes to binary indicators at the specific time points of interest. This was feasible because of the low levels of loss to follow-up. We estimated the relationship between IRF vs SNF rehab and these binary outcomes by fitting a simultaneous 2-equation bivariate probit model in STATA. The first equation estimated the probability of a patient receiving IRF treatment, as a function of the instrumental variable (%IRF or differential distance) and the measured covariates. The second equation estimated the relationship between IRF (vs SNF) and outcome, adjusting for other patient and hospital factors.
Estimating 2 equations jointly using a bivariate probit approach provides a consistent estimate of the treatment effect, given all of the instrumental variable assumptions. The parameters of the bivariate probit model are not easily interpreted and were, consequently, converted to adjusted relative risk using the margins command in Stata; this can be interpreted as the ratio of estimated event probabilities, if the “marginal” population were to receive IRF vs SNF. We estimated the variance of this parameter using a robust empirical variance that allowed for the clustering of patients within hospitals.
We estimated the relationship between IRF vs SNF settings and home time using IV Poisson modeling in Stata. Rather than the joint maximum likelihood method used in bivariate probit modeling, we implemented Poisson modeling using the generalized method of moments approach, which has been modified to incorporate instrumental variables into the sample moment conditions to be minimized. We assumed a multiplicative error term (ie, the magnitude of the error was constant in proportional terms) when estimating these models. While the coefficients that result from bivariate probit models are challenging to interpret, the Poisson model uses a log link function, so the exponentiation of the coefficients results in incidence rate ratios that are straightforward to interpret. As with the bivariate probit models, we used robust empirical variance estimators, allowing for the clustering of patients within hospitals. We selected Poisson rather than negative binomial regression models, owing to the greater maturity of Poisson vs negative binomial methods; the coefficient estimates from Poisson models are robust to overdispersion, and we corrected the variance estimate for any violation of the equality of mean and standard deviation assumption underlying Poisson modeling by virtue of robust standard error employment.
Among patients in the smaller AVAIL cohort with patient-reported outcomes, we performed a multivariable regression to examine the association between IRF vs SNF as the independent variables and 3 dependent variables. We used logistic regression for the binary outcomes of dependence and institutionalization; we used regression analysis for QOL because it is a continuous outcome. We excluded patients who missed the outcome (<2%) from regression analyses. We conducted unadjusted and adjusted analysis using IPW to adjust for patient- and hospital-level confounders. We used generalized estimating equations (GEEs) to account for within-hospital clustering and provide robust variance estimates for the regression model. The hospitals in this sample were primarily from urban areas, certified as primary stroke centers, and had access to both IRFs and SNFs.
In this AVAIL cohort, we estimated the propensity, or probability of receiving IRF treatment, for each patient, through a logistic regression model with IRF treatment (yes, no) as the dependent variable and all of the patient and hospital characteristics as the independent variables. We used simple imputation to address missing data for any of the covariates (<7% of any variable) where we used the median value for continuous variables and imputed the dominant or more frequent response or entered it for missing categorical variables. We used this approach to single imputation for missing data for sex, race, socioeconomic status, medications before admission, ability to ambulate independently during index admission or before hospital discharge, and discharge medications with less than 3% missing; medical history collected for the registry was 5% missing. We imputed missing for a treatment/intervention to “no treatment or intervention provided.” We assessed continuous variables for nonlinearity in the propensity model. We modeled the variables with nonsignificant, nonlinear relationships with IRF as a linear variable and modeled the variables with significant, nonlinear relationships with IRF with restricted cubic splines and 4 knot points. To assess the positivity assumption (that patients had a nonzero probability of receiving either treatment), we evaluated the distribution of propensity scores by IRF and SNF. Propensity scores near 0 or 1 were common, and we trimmed the population to exclude patients with propensity scores less than 0.10 or greater than 0.90, which reduced the sample size to 467 patients for 3-month outcomes (43% of eligible sample) and 473 at 12 months (42% of eligible sample).
Aim 3. GWTG/Medicare Population. Among Stroke Survivors Discharged From Hospital to Home, Examine the Association Between Early Provider Follow-up and 30- and 90-Day Outcomes
To accomplish this aim, we examined data for 50 170 acute ischemic stroke patients discharged home to the community from the index stroke hospitalization and measured home time, readmissions, and mortality for patients who received early provider follow-up. Stakeholders identified the following providers with interest in the first appointment after hospital discharge: neurologists, neurosurgeons, physiatrists (physical medicine and rehabilitation), cardiologists, endocrinologists, pulmonologists, geriatricians, internists, nurse practitioners and physician assistants, and general primary care. We examined time to first visit (mean and median) and categorical descriptive analysis of a visit within 7, 14, 21, 28, 30, 60, and 90 days overall and by provider type. We examined hospital-level variation in rate of follow-up and time to follow-up for any provider visit and by provider type across 675 hospitals that each had at least 25 patients discharged to the community. We divided hospitals into 4 groups, with an equal number of hospitals in each group based on the hospital-level rate of early 7-day follow-up. We compared patient and hospital characteristics across the 4 quartiles. We used multivariable analysis with generalized estimating equations to account for within- hospital clustering to examine 30-day and 90-day mortality and unplanned all-cause readmission, and 90-day home time.
Results
Aim 1. Patterns of Postacute Service Use
Among patients who had an acute ischemic stroke from 2006-2008, were treated at a GWTG participating hospital, and had Medicare FFS coverage, 47.2% were discharged directly from the hospital to short inpatient postacute care (IRF 22.4% and SNF 24.8%) and 43.7% were discharged home to the community (13.5% with HH, 6.4% with OPR, 23.8% without any postacute services in 90 days). Only 6.4% died before hospital discharge, 1.3% transferred to another acute hospital, and 1.3% were discharged directly to an LTC facility (Figure 2).

Figure 2
First Postacute Service After Index Stroke Hospitalization (N = 162 432).
Findings for the analysis of care transitions from the hospital by stroke severity were as expected among patients with an NIHSS score. More than two-thirds of patients discharged to the community had a mild stroke (NIHSS ≤5): 67.4% of patients discharged without any other services within 90 days of hospital discharge; 69.9% of patients discharged to HH; 76.3% of patients discharged to OPR. Patients discharged to an IRF or SNF had more severe strokes (NIHSS ≥6): 55.0% of IRF patients and 62.4% of SNF patients (Figure 2). Receiving more than 1 postacute service was common and 87.0% of patients experienced 2 or more care transitions within 90 days of their index stroke admission. The median number of transitions was 3.
Aim 1. Person-Level Factors Associated With Postacute Care Use
Patient factors associated with discharge to different sites of care are presented in Table 2. The median age was youngest for patients discharged to receive OPR (76 years; IQR, 70-82 years). Patients discharged without any postacute care had a median age of 78 years (IQR, 71-84 years), IRF patients were 79 years (IQR, 73-84 years), HH were 80 years (IQR, 74-85 years), and SNF patients were oldest (median age, 83 years; IQR, 78-88 years). Among patients discharged to short-term inpatient care, 56.4% of IRF and 66.6% of SNF patients were women. Among patients discharged home, 48.5% of OPR, 61.2% of HH, and 52.4% of those without services were women. IRF and SNF patients were similar by race. The percentage of HH patients who were Black or Hispanic was higher than for OPR.
Table 2
Patient Characteristics by First Postacute Service.
We measured the frequency of many comorbid conditions in this population. Approximately a third of patients had experienced a prior stroke or transient ischemic attack, ischemic heart disease, diabetes, and dyslipidemia (similar across groups). Three in 4 patients had hypertension. It was more common for patients with prior disability to be discharged to either an IRF or SNF than to the community. Dementia was documented for 13 116 patients and 47.4% of those were discharged to an SNF. This was similar for patients with recent weight loss or malnutrition: SNF was the first postacute service for 48.1% of malnourished patients.
Most patients were brought to the hospital by ambulance and approximately half of all stroke patients were cared for in a stroke unit. tPA use was less than 10% in this patient population: Only 3.3% of HH patients and 7.6% of IRF patients received IV or intra-arterial treatment (highest). In-hospital complications overall were generally low (0%-3%), with slightly higher rates (4%-10%) for complications from tPA, delirium, feeding assistance, pneumonia, acute renal failure, and urinary tract infection. Two-thirds of patients discharged to the community could ambulate independently before hospital discharge vs only 18.9% of IRF patients and 15.5% of SNF patients.
Aim 1. Hospital Characteristics by Postacute Service Use
The quality of acute stroke care measured using the composite measure was similar across patient groups (Table 3). DVT prophylaxis and use of lipid-lowering drugs were lowest among patients discharged to an SNF. A higher percentage of HH patients than the other service options were from hospitals in the South and the lowest from the Midwest. More patients discharged to an IRF were treated in academic teaching hospitals (63.2%) and certified primary stroke centers (54.6%).
Table 3
Hospital Characteristics by First Postacute Service.
Aim 2. Comparative Effectiveness of IRF vs SNF Care on 3- and 12-Month Death, Rehospitalization, and Home Time
GWTG/Medicare population
Among 69 212 acute ischemic stroke patients included in the study population and discharged to an IRF or SNF, 50.3% were discharged from the hospital to an IRF and 49.7% to an SNF. Patients who received SNF care had higher rates of premorbid dementia, hospitalizations, and SNF use in the 6 months before the index stroke admission; they also had documentation of delirium, urinary tract infections, and decubitus ulcers during the acute hospitalization. It was more common for IRF patients to have had prestroke physical disability, received tPA, and received acute care in a stroke unit. Differences in stroke severity (NIHSS median = 7 vs 6), the composite measure of acute stroke care quality (0.89 vs 0.90), and hospital length of stay (median = 5 days for both) were not clinically meaningful for SNF vs IRF patients. We conducted sensitivity analysis using all of the same covariates and approaches to modeling for patients with an NIHSS score; the findings were similar to our full study population; thus, only the full population findings are reported below.
Mortality
More patients who were discharged from the hospital to an SNF, rather than to an IRF, died by 14 days (6.4% vs 1.1%), 90 days (21.1% vs 7.2%), and 365 days (38.6% vs 17.9%; Table 4). After adjusting for patient- and hospital-level factors, IRF care was associated with a 35% lower risk of death within 365 days (HR, 0.65; 95% CI, 0.62-0.68). The reduction in 1-year risk of death associated with IRF vs SNF care determined using the %IRF as the instrumental variable was 8% (RR, 0.92; 95% CI, 0.86-0.98) with a similar estimate but wider confidence interval in analyses using differential distance as the instrumental variable (RR, 0.89; 95% CI, 0.75-1.05).
Table 4
Effectiveness of Care in Inpatient Rehabilitation Compared With SNFs on Mortality, Rehospitalization, and Home Time.
Rehospitalization or Death
All-cause rehospitalizations or death were higher for SNF patients than IRF patients at all time points: at 14 days (15.4% vs 8.4%); at 90 days (42.2% vs 29.6%); and at 365 days (68.1% vs 54.2%; Table 4). IRF care was associated with a 15% lower rate of rehospitalization/death within 1 year after IPW adjustment (HR, 0.85; 95% CI, 0.82-0.87). Analyses that used %IRF as the instrumental variable found IRF care to have a 6% reduction in 1-year risk of rehospitalization or death (RR, 0.94; 95% CI, 0.91-0.98); but when we used differential distance as the instrumental variable, the confidence interval was wider and the difference between groups was no longer significant.
Home Time
The number of days alive and out of inpatient care were higher for IRF patients than SNF patients at both 90 (51.8 ± 31.2 vs 32.5 ± 30.7) and 365 days (271.2 ± 112.5 vs 195.5 ± 138.5). After adjusting for measured confounders using IPW, IRF care was found to be associated with increased home time up to 365 days (RaR, 1.20; 95% CI, 1.19-1.22). IRF care continued to be associated with increased home time within 1-year compared with SNF care using instrumental variable analyses (Table 4).
Adjustment variables included demographics (age, female sex, Caucasian race), social economic status (percentage college graduate or higher and median household income), comorbidities documented from registry medical history and Centers for Medicare & Medicaid Services (CMS) comorbidities 6 months before index: stroke, ischemic heart disease, diabetes mellitus, peripheral vascular disease, congestive heart failure, atrial fibrillation, prosthetic heart valve, carotid stenosis, hypertension, dyslipidemia, smoking history, any malignancy, chronic obstructive pulmonary disease, dementia, liver disease (mild, moderate, or severe), peptic ulcer disease, renal disease, rheumatic disease, anemia, depression, extremity fracture, frailty, weight loss/malnutrition, osteoarthritis, osteoporosis, rheumatoid arthritis, substance abuse, surgery, index CMS claims: cardiac/respiratory arrest, help breathing (tracheostomy, mechanical ventilator, etc), catheter use, delirium, dialysis, help feeding (gastrostomy, feeding tubes, etc), palliative care not hospice, pulmonary embolism, acute renal failure, septicemia/sepsis/septic shock, decubitus ulcer, urinary tract infection, venous thrombosis, length of hospital stay and length of intensive care unit stay during index hospitalization, combined 6 months prior and index CMS claims: pneumonia, number of hospitalizations before the stroke admission, evidence of SNF, evidence of IRF, home health agency care, disability, admission information: emergency medical services arrival, stroke unit, in-hospital treatments and complications: deep vein thrombosis prophylaxis initiated by the end of hospital day 2, tPA in this hospital, any serious complications (including intracerebral hemorrhage within 36 hours, life-threatening serious systemic hemorrhage within 36 hours, and other serious complications), able to ambulate independently during the index or at discharge, registry discharge medications: antihypertensive, lipid-lowering, antithrombotics, smoking cessation, and hospital characteristics: number of beds, teaching status, rural vs urban, certified primary stroke center, hospitals' composite of quality of care measures (components include: tPA within 3 hours among arrival within 2 hours, early antithrombotics, DVT prophylaxis, antithrombotics at discharge, anticoagulants for atrial fibrillation, smoking cessation, lipid-lowering drugs among low-density lipoprotein cholesterol <100 or not documented).
Aim 2. Comparative Effectiveness of IRF vs SNF Care on 3- and 12-Month Function, Quality of Life, and Institutionalization in LTC
GWTG/AVAIL/Medicare Population
Of the 854 patients in this sample, 28 were missing outcome data at 3 months, resulting in a final sample of 826 ischemic stroke patients with 82% discharged to an IRF and 18% to an SNF. The analytic trimmed sample with a 10% to 90% probability of being discharged to an IRF included 467 patients with 72% discharged to an IRF and 28% to an SNF. It was more common for patients discharged to an IRF compared with an SNF patients to have received acute hospital care in the Northeast (25% vs 21%) or Midwest (34% vs 22%) and at hospitals with better composite scores for acute stroke care quality (0.90 vs 0.88).
Three-Month Outcomes
At 3 months after hospital discharge, 60% of patients discharged to an IRF and 67% of patients discharged to an SNF were dependent or dead (Table 5). Neither unadjusted nor adjusted odds of dependence at 3 months were significantly different between groups (adjusted odds ratio [OR] 0.90; CI, 0.53-1.54; P = .711). By 3 months, 14% of patients first discharged to an IRF were institutionalized in a nursing home or dead compared with 21% of SNF patients (P = .052). IRF patients were 49% less likely to be institutionalized after adjusting for measured confounders (OR, 0.51; CI, 0.30-0.88; P = .016). Patients first discharged to an IRF also reported to have a higher quality of life 3 months after hospital discharge. There was a statistically significant unadjusted and adjusted 9-point difference between means (CI, 0.03-0.16; P = .007).
Table 5
Effectiveness of Care in Inpatient Rehabilitation Compared With SNFs on Dependence, Institutionalization, and Quality of Life.
Twelve-Month Outcomes
Dependence 12 months after hospital discharge was not different for patients initially discharged to an IRF or SNF (Table 5). Institutionalization or death by 12 months was 19% for IRF patients compared with 32% for SNF patients. After adjusting for measured confounders, IRF patients were 46% less likely to be institutionalized in a nursing home or dead by 12 months (OR, 0.54; CI, 0.33-0.88; P = .013). Quality of life at 12 months descriptively was higher for IRF than SNF patients but without a significant difference in means between groups after adjusting for confounders.
Aim 3. Early Provider Follow-up After Discharge to the Community
Care for patients discharged home was fragmented and, in some cases, delayed. Only 13.5% received HH after hospital discharge and 6.4% received OPR. Stroke survivors shared with us that the latter was dependent on referral from a community-based provider. Analysis of provider follow-up among 50 170 patients discharged to the community after the index stroke hospitalization found that 48 338 (96.4%) had at least 1 follow-up within 90 days of hospital discharge. The mean number of days from discharge to follow-up was 27 days and the median was 10 days (IQR, 5-20). Early follow-up within 7 days of hospital discharge occurred for 19 573 (39%); 31 557 (62.9%) had a follow-up visit within 14 days; 40 686 (81.1%) within 30 days; and 44 547 (88.8%) within 60 days. Only 42.4% had a neurology visit within 90 days of hospital discharge.
Physician follow-up poststroke was more common with internal medicine or primary care (50.3% and 70.1%) and cardiology (53.3%) than neurology (42.4%). Within 7 days of hospital discharge, it was most common to see a general care provider (12.9% saw primary care, 1.6% nurse practitioner, 18.6% internist, 1.0% geriatrician), with only 7.9% seeing a nonneurology specialist first (0.5% physiatrist, 6.2% cardiologist, 0.4% endocrinologist, and 0.8% pulmonologist) compared with 3.3% having a visit with a neurologist or neurosurgeon within 7 days of hospital discharge (Figure 3). We observed the same trend for follow-up visits within 14, 21, and 28 days.

Figure 3
Rate of Follow-up in the First Month by Provider Type.
The hospital mean and median rate of early follow-up within 7 days was 39% (SD 9.7) with an IQR of 32.4% to 45.2% (Figure 4). Analysis of the association of hospital-level rates of early 7-day follow-up by quartiles and 30-day and 90-day mortality and unplanned all-cause rehospitalizations and 90-day home time did not identify significant differences by quartiles for all of these outcomes (Table 6). Thirty-day mortality ranged from 1.63% to 2.04% and 90-day mortality ranged from 4.15% to 4.81% across quartiles of hospital-level rates of early follow-up. After adjusting for patient- and systems-level covariates, 30-day mortality was significantly different only for patients treated at hospitals with medium-low rates of early provider follow-up compared with the lowest quartile (OR, 0.79; 95% CI, 0.63-0.98). Adjusted rates of 90-day mortality for patients treated at hospitals in the highest quartile of rates for early follow-up compared with lowest quartile of rates showed a 17% lower odds of death within 90 days of hospital discharge (OR, 0.83; 95% CI, 0.71-0.97), medium-high compared with lowest had a 12% reduced odds of death (OR, 0.88; 95% CI, 0.75-1.02), and medium-low compared with lowest had a 14% reduced odds of death (OR, 0.86; 95% CI, 0.75-0.99). Unplanned rehospitalizations for any cause within 30 and 90 days was 11% and 20%, respectively. This did not vary across quartiles and was not significantly different after adjusting for covariates. Similarly, patients discharged home spent 84 of 90 days at home in the community (out of any inpatient care) and this did not vary across quartiles and was not significantly different after adjusting for covariates (Table 6).

Figure 4
Hospital-Level Variation in Early Follow-up.
Table 6
Thirty-Day and 90-Day Mortality and Unplanned All-Cause Rehospitalization, and 90-Day Home Time by Quartile of Hospital-Level Rates of Early 7-Day Follow-up Care in the Community for Patients Discharged Home From the Hospital.
Adjustment variables included demographics (age, female sex, Caucasian race), social economic status (percentage college graduate or higher and median household income), comorbidities documented from registry medical history and CMS comorbidities 6 months before index: stroke, ischemic heart disease, diabetes mellitus, peripheral vascular disease, congestive heart failure, atrial fibrillation, prosthetic heart valve, carotid stenosis, hypertension, dyslipidemia, smoking history, any malignancy, chronic obstructive pulmonary disease, dementia, liver disease (mild, moderate, or severe), peptic ulcer disease, renal disease, rheumatic disease, anemia, depression, extremity fracture, frailty, weight loss/malnutrition, osteoarthritis, osteoporosis, rheumatoid arthritis, substance abuse, surgery, index CMS claims: cardiac/respiratory arrest, help breathing (tracheostomy, mechanical ventilator, etc), catheter use, delirium, dialysis, help feeding (gastrostomy, feeding tubes, etc), palliative care not hospice, pulmonary embolism, acute renal failure, septicemia/sepsis/septic shock, decubitus ulcer, urinary tract infection, venous thrombosis, length of hospital stay and length of intensive care unit stay during index hospitalization, combined 6 months prior and index CMS claims: pneumonia, number of hospitalizations before the stroke admission, evidence of SNF, evidence of IRF, home health agency care, disability, admission information: emergency medical services arrival, stroke unit, in-hospital treatments and complications: deep vein thrombosis prophylaxis initiated by the end of hospital day 2, tPA in this hospital, any serious complications (including intracerebral hemorrhage within 36 hours, life-threatening serious systemic hemorrhage within 36 hours, and other serious complications), able to ambulate independently during the index or at discharge, registry discharge medications: antihypertensive, lipid-lowering, antithrombotics, smoking cessation, and hospital characteristics: number of beds, teaching status, rural vs urban, certified primary stroke center, hospitals' composite of quality of care measures (components include: tPA within 3 hours among arrival within 2 hours, early antithrombotics, DVT prophylaxis, antithrombotics at discharge, anticoagulants for atrial fibrillation, smoking cessation, lipid-lowering drugs among low-density lipoprotein cholesterol <100 or not documented).
Discussion
Context for Study Results
In this largest-ever study integrating different data sources to examine the postacute care of patients with acute ischemic stroke, we described the complexity of service use and delays with provider follow-up and found that, relative to SNF care, IRF care after an acute stroke hospitalization was associated with better outcomes. Our findings for mortality and home time within 90 days of hospital discharge were robust across a variety of analytic strategies and sensitivity analyses, and across nearly all patient subgroups examined. We documented the complexity of poststroke recovery; for example, the benefits of IRF care had attenuated by 1 year after hospital discharge, and 28% of the entire Adolescent Idiopathic Scoliosis cohort discharged to an IRF or SNF had died.
Postacute stroke care in inpatient settings is a significant contributor to medical spending in the United States.30 Efficacy of the most common options, IRF and SNF care, is difficult to compare with randomized trials, and none have been conducted to date. This leads to the reliance on observational evidence in which confounding can be a significant threat to making valid comparisons. Previous observational studies that compared outcomes for stroke patients who received IRF or SNF care after hospital discharge found patients who received IRF care had better outcomes. However, these studies had limited and small samples,8,9,31 or they included only administrative claims data.10,12,32,33 Our study of IRF and SNF care is a departure from previous research in 2 important ways. First, we studied a large cohort of stroke survivors from a diverse group of hospitals from all regions of the country. Second, we moved beyond administrative data sets to combine several sources of data that more fully describe patient- and systems-level factors. Our multistrategy approach to statistical adjustment for confounding and our ability to adjust for key clinical characteristics reduces the potential for bias that may have been present in previous studies.11,16,17
Selection biases may confound observational comparative effectiveness studies. Thus, we adjusted for baseline differences in demographics and clinical and hospital characteristics using inverse propensity weighting analysis. In the analysis of IRF vs SNF in the GWTG/Medicare population, we also adjusted for potential unmeasured confounders, such as patient and family preference and social support, using 2 separate instrumental variables derived from provider and patient preferences (%IRF and differential distance, respectively). A good instrumental variable is one that meets a set of assumptions, some evaluated by examining the data and others evaluated conceptually. Two of these key assumptions are that the instrumental variable is strongly associated with the treatment, and that it does not have a direct effect on the outcome except through its influence on treatment selection. The 2 instruments had unique advantages regarding the IV assumptions. Wide variation occurred in the percentage of patients discharged to an IRF. This variation yielded a powerful instrument with %IRF, and its effect estimate is likely to have good precision and to be representative of a very large portion of the total population. However, hospitals that discharge more patients to an IRF had healthier patients and were more likely to be large, academic, urban, and Joint Commission certified as primary stroke centers. Because the %IRF variable was moderately associated with measured patient and hospital characteristics, there is a possibility for unmeasured confounding that would bias the results that favor IRF. The differential distance IV was not as strong a predictor of selecting posthospital care, which compromises precision and results in wider confidence intervals.
However, the differential distance IV was minimally associated with patient and hospital characteristics, especially when compared with the %IRF IV. This suggests the differential distance IV can minimize unmeasured confounding (particularly factors related to patient selection) and may provide unbiased results. Importantly, the short-term findings that used the 2 IVs were mostly similar, which should increase confidence in the finding that outcomes were better with discharge to an IRF.
Additionally, the IV analysis looked at a slightly different question. Rather than comparing the average difference in outcomes of all those who received IRF vs SNF care, the IV approach applied to a marginal population for which factors such as practice patterns and distance play a role in decision making and for which clinicians are more likely to be at equipoise between the choices of SNF vs IRF. These considerations should cause us to wonder whether the result of the IV analysis applies to typical stroke survivors. Although we could not identify individual members of this population, the IV analysis for %IRF is most representative of decisionmakers who might be influenced by their hospital infrastructure and historical referral patterns, and who are not fully committed to a preference for IRF vs SNF. The wide variation in %IRF across hospitals suggests that hospitals do have a strong impact on treatment selection. So, in this case, the marginal population of stroke survivors may still be representative of their counterparts in the general population. The treatment effect estimated by the IV analysis on differential distance is representative of people who might be influenced by the proximity of an IRF to their place of residence. The people who are not represented in the IV analysis are those for whom the choice of IRF is obvious (ie, compelling) based on their patient characteristics and care needs; these patients would be less likely to consider matters of convenience (ie, distance).
Generalizability of the Findings
This study integrates data for Medicare fee-for-service beneficiaries and patients who received care at a GWTG-Stroke participating hospital. While Medicare fee-for-service beneficiaries represent 70% of all Medicare beneficiaries, this study's findings may not be able to be generalized to other patient populations. Also, the GWTG-Stroke Registry is a voluntary program for hospitals and, similarly, these results might not extrapolate to patients treated in non-GWTG-Stroke Registry hospitals or to those in other countries. In the previous section, we also described in detail the implications on generalizability when using instrumental variables. Under monotonicity, the IV treatment effect estimate is not necessarily generalizable to the full population. Instead, it is representative of the type of people whose treatment selection is influenced by the instrument, also called a marginal population or compliers. A stronger IV indicates that a larger portion of the population may be characterized as a complier (ie, responsive to the IV). Therefore, the %IRF analysis may be more representative of the general population because the wide variation in %IRF across hospitals indicated a very strong IV (given the other assumptions).
Implementation of Study Results
Not applicable for observational studies.
Subpopulation Considerations
Our stakeholder advisory group identified the most likely clinical effect modifiers for our study on comparing outcomes for patients who received IRF or SNF care, and they chose to use these to define subgroups within the overall study population. We used the %IRF IV as the instrument with more precision to examine the comparative effectiveness of IRF vs SNF in prespecified patient subgroups defined by aged 65 to 79 or 80+ years, mild stroke severity (NIHSS = 0-5) or moderate-severe stroke (NIHSS 6+) and predicted mortality risk greater than 25%. Findings generally favor IRF care for each outcome and subgroup, especially within 90 days. Clinical trial data do not exist, and falsification endpoints could not be identified to help validate the success of adjustment or findings for specific subpopulations.
Study Limitations
Our study should be interpreted in the context of the following limitations. This was a retrospective observational analysis. To reduce the risk of confounding, we adjusted for patient and hospital characteristics in each individual study. Similarly, we conducted sensitivity analyses to explore any bias introduced by not including stroke severity in the primary analyses and additional unaddressed factors that would have indicated a higher risk of poor outcomes after hospital discharge. Nevertheless, selection bias and residual measured and unmeasured confounding could influence some of these findings. Even the instrumental variable analysis has limitations. First, the validity of instrumental variable assumptions can be proved empirically, but only explored by looking for visible problems. For example, it is possible that unmeasured variables are related to the instrument itself; thus, confounding that we cannot identify would remain. In addition, the interpretation of IV analysis as a treatment effect among compliers has limited generalizability. Finally, with continuous instrumental variables, the technical definition of compliers is less clear; previous authors have noted that the result is an average across different populations of compliers (who would be responsive to different increments of the IV).
Future Research
Patients and clinicians have difficulty deciding on posthospital care. Our study design and analyses were guided by the ambiguity expressed by stroke patients and their caregivers and clinicians of how to decide about postacute care and which would provide the best option for recovery. Patients and caregivers are often overwhelmed during the acute hospitalization and may have no point of reference for this decision. In addition, stroke is a complex condition with a high risk for adverse events, such as rehospitalizations, that could potentially be prevented. In this study, we examined care outcomes among patients who transitioned from the hospital to short-term inpatient postacute care and among patients who transitioned home. The disparate results between IPW and IV methods for the comparison of IRF and SNF care motivate the need for a randomized clinical trial. Future research is needed to examine community-based follow-up among patients who received inpatient postacute care (did not return home directly from the acute hospitalization), as these patients had more severe strokes and more comorbid disease and may need more intense community-based management once returning home. A significant opportunity remains for effective coordination of care across an episode of illness, inclusive of all health care services, and for collaboration for improved health care quality. In doing so, multiple providers can share responsibility in promoting recovery and unite to best support the patient's and family's needs. Little is understood about the different care patterns stroke patients navigate after hospital discharge, and efforts are needed to improve care transitions and increase the number of successful days at home in the community.
It will also be important to identify future opportunities to study care patterns and outcomes among young stroke patients and patients with health insurance or insurance coverage beyond government programs such as Medicare and Medicaid. Outside of integrated health care networks such as the Veterans Administration or Kaiser, data consortiums will need to consider how to integrate data not only across similar settings but also across the continuum. While our study is limited to Medicare fee-for-service beneficiaries, it is currently the most comprehensive data for studying national care trends and outcomes of stroke.
Finally, a significant effort is needed to develop an efficient approach to measuring patient-reported outcomes after formal health care services have ended. Several episode-based payment models exist and if a patient is living at home at the end of a time-specified episode, such as 90 days after the acute hospital admission, the actual health status beyond “not hospitalized and still alive” is unknown. Our work demonstrated the utility of home time as a viable outcome.34 Although this captures the number of days out of inpatient care, it cannot provide insight into repeated admissions—as we found for 5% of our population with 8 to 17 care transitions after the index hospitalization. There was also little variation in home time among discharged patients. The outcome does not factor in use of home health, dependence on family, or independence in self-care. The importance of patient-reported outcomes for patients discharged from an episode of care cannot be understated, and the process to measure these outcomes as part of routine care requires further investigation.
Conclusions
In summary, this study represents a robust and comprehensive assessment of the use of postacute services for Medicare beneficiaries who experienced an acute ischemic stroke and the factors associated with the receipt of care and outcomes from that care. We found that IRF compared with SNF care after hospital discharge was associated with a significantly lower risk of death and greater home time across every analytic approach. The association with improved outcomes attenuated somewhat by 1 year, which may reflect the complexity of poststroke management and the opportunity for improved postacute coordination and health care quality. The outcomes associated with IRF vs SNF care, especially in the short term, and the delay in follow-up care with a community-based provider, highlights the need for critical appraisal at hospital discharge on the optimal care pattern and postacute services selected for stroke patients. Additional research is needed to examine outcomes following complex postacute care patterns with multiple care transitions. Although our observational study design has limitations not present in randomized controlled trials, our findings are generated from data gathered across the country and in real-world practice, with implications for postacute stroke care policy.
References
- 1.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e60. [PubMed: 26673558]
- 2.
- Hong KS, Saver JL, Kang DW, et al. Years of optimum health lost due to complications after acute ischemic stroke: disability-adjusted life-years analysis. Stroke. 2010;41(8):1758-1765. [PMC free article: PMC2937160] [PubMed: 20595674]
- 3.
- Tong X, Kuklina EV, Gillespie C, George MG. Medical complications among hospitalizations for ischemic stroke in the United States from 1998 to 2007. Stroke. 2010;41(5):980-986. [PubMed: 20203317]
- 4.
- Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. [PubMed: 27145936]
- 5.
- Buntin MB. Access to postacute rehabilitation. Arch Phys Med Rehabil. 2007;88:1488-1493. [PubMed: 17964894]
- 6.
- RTI International. Overview of the Medicare Payment Reform Initiative—Fall 2009. Accessed July 25, 2012. http://www.pacdemo.rti.org/UserFiles/File/3_PAC_PRD2_Overview_9.15.09_ND.pdf [link no longer works]
- 7.
- Centers for Medicare and Medicaid Services. IMPACT Act of 2014 and cross setting measures. Accessed October 17, 2015. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014-and-Cross-Setting-Measures.html [link no longer works]
- 8.
- Kramer A, Holthaus D, Goodrish G, Epstein A. A Study of Stroke Post-acute Care Costs and Outcomes: Final Report. US Department of Health and Human Services; 2006.
- 9.
- Kane RL, Lin WC, Blewett LA. Geographic variation in the use of post-acute care. Health Serv Res. 2002;37:667-682. [PMC free article: PMC1434656] [PubMed: 12132600]
- 10.
- Kind AJ, Smith MA, Pandhi N, Frytak JR, Finch MD. Bouncing-back: rehospitalization in patients with complicated transitions in the first thirty days after hospital discharge for acute stroke. Home Health Care Serv Q. 2007;26(4):37-55. [PMC free article: PMC2205988] [PubMed: 18032199]
- 11.
- Deutsch A, Granger CV, Heinemann AW, et al. Poststroke rehabilitation: outcomes and reimbursement of inpatient rehabilitation facilities and subacute rehabilitation programs. Stroke. 2006;37:1477-1482. [PubMed: 16627797]
- 12.
- Buntin MB, Garten AD, Paddock S, Saliba D, Totten M, Escarce JJ. How much is postacute care use affected by its availability? Health Serv Res. 2005;40:413-434. [PMC free article: PMC1361149] [PubMed: 15762900]
- 13.
- Skolarus LE, Meurer WJ, Burke JF, Prvu Bettger J, Lisabeth LD. Effect of insurance status on post-acute care among working age stroke survivors. Neurology. 2012;78:1590-1595. [PMC free article: PMC3348849] [PubMed: 22551730]
- 14.
- Horner RD, Hoenig H, Sloane R, Rubenstein LV, Kahn KL. Racial differences in the utilization of inpatient rehabilitation services among elderly stroke patients. Stroke. 1997;28:19-25. [PubMed: 8996482]
- 15.
- Ottenbacher KJ, Campbell J, Kuo YF, Deutsch A, Ostir GV, Granger CV. Racial and ethnic differences in postacute rehabilitation outcomes after stroke in the United States. Stroke. 2008;39:1514-1519. [PMC free article: PMC2642622] [PubMed: 18340094]
- 16.
- Hoenig H, Sloane R, Horner RD, Zolkewitz M, Reker D. Differences in rehabilitation services and outcomes among stroke patients cared for in veterans hospitals. Health Serv Res. 2001;35(6):1293-1318. [PMC free article: PMC1089191] [PubMed: 11221820]
- 17.
- Horn SD, DeJong G, Smout RJ, Gassaway J, James R, Conroy B. Stroke rehabilitation patients, practice, and outcomes: is earlier and more aggressive therapy better? Arch Phys Med Rehabil. 2005;86(12 Suppl 2):S101-S114. [PubMed: 16373145]
- 18.
- Gage B, Morley M, Spain P, Ingber M. Examining Post Acute Care Relationships in an Integrated Hospital System. US Department of Health and Human Services; 2009.
- 19.
- Fonarow G, Reeves M, Smith E, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2010;3(3):291-302. [PubMed: 20177051]
- 20.
- Reeves M, Fonarow G, Smith EE, et al. Representativeness of the Get With the Guidelines-Stroke registry: comparison of patient and hospital characteristics among Medicare beneficiaries hospitalized with ischemic stroke. Stroke. 2012;43(1):44-49. [PubMed: 21980197]
- 21.
- Bushnell C, Zimmer L, Schwamm L, et al. The Adherence eValuation After Ischemic Stroke Longitudinal (AVAIL) registry: design, rationale, and baseline patient characteristics. Am Heart J. 2009;157:428-435.e2. [PubMed: 19249411]
- 22.
- Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With the Guidelines-Stroke: results from a national data validation audit. Am Heart J. 2012;(163):392-398. [PubMed: 22424009]
- 23.
- Schwamm L, Fonarow G, Reeves M, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or TIA. Circulation. 2009;119(1):107-115. [PubMed: 19075103]
- 24.
- Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke. 2000;31:1429-1438. [PubMed: 10835468]
- 25.
- Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995-1000. [PMC free article: PMC2732025] [PubMed: 19464409]
- 26.
- American Heart Association. Get With the Guidelines scientific publications and program results. Accessed July 15, 2012. http://www
.heart.org /HEARTORG/HealthcareResearch /GetWithTheGuidelinesHFStroke /Get-With-The-Guidelines-Scientific-Publications-and-Program-Results _UCM_306758_Article.jsp - 27.
- Centers for Medicare & Medicaid Services, TrailBlazer Health Enterprises, LLC. Part A UB-04 discharge status codes. Published March 12, 2012. Accessed June 25, 2012. http://www.trailblazerhealth.com/Publications/Job Aid/ub92a_discharge.pdf [link no longer works]
- 28.
- New PW, Buchbinder R. Critical appraisal and review of the Rankin scale and its derivatives. Neuroepidemiology. 2006;26:4-15. [PubMed: 16272826]
- 29.
- The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199-208. [PubMed: 10109801]
- 30.
- Mechanic R. Post-acute care—the next frontier for controlling Medicare spending. N Engl J Med. 2014;370:692-694. [PubMed: 24552315]
- 31.
- Stineman MG. Comparative effectiveness of alternative levels of stroke in the Veterans Healthcare Administration. NIH Research Portfolio Online Reporting Tools. Accessed June 8, 2012. http:
//projectreporter .nih.gov/project_info_description .cfm?aid =8080478&icde =13285814&ddparam =&ddvalue =&ddsub =&cr=3&csb =default&cs=ASC - 32.
- Liu K, Wissoker D, Rimes C. Determinants and costs of Medicare post-acute care provided by SNFs and HHAs. Inquiry. 1998;35:49-61. [PubMed: 9597017]
- 33.
- Ozdemir F, Birtane M, Tabatabaei R, Kokino S, Ekuklu G. Comparing stroke rehabilitation outcomes between acute inpatient and nonintense home settings. Arch Phys Med Rehabil. 2001;82:1375-1379. [PubMed: 11588740]
- 34.
- Fonarow GC, Liang L, Thomas L, et al. Assessment of home-time after acute ischemic stroke in Medicare beneficiaries. Stroke. 2016;47(3):836-842. [PubMed: 26892279]
Acknowledgment
Research reported in this report was [partially] funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#130) Further information available at: https://www.pcori.org/research-results/2012/comparing-recovery-options-stroke-patients
Suggested citation:
Bettger JP, Thomas L, Li L. (2019). Comparing Recovery Options for Stroke Patients. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/3.2019.CER.130
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Comparison of Functional Status Improvements Among Patients With Stroke Receiving Postacute Care in Inpatient Rehabilitation vs Skilled Nursing Facilities.[JAMA Netw Open. 2019]Comparison of Functional Status Improvements Among Patients With Stroke Receiving Postacute Care in Inpatient Rehabilitation vs Skilled Nursing Facilities.Hong I, Goodwin JS, Reistetter TA, Kuo YF, Mallinson T, Karmarkar A, Lin YL, Ottenbacher KJ. JAMA Netw Open. 2019 Dec 2; 2(12):e1916646. Epub 2019 Dec 2.
- Skilled Nursing and Inpatient Rehabilitation Facility Use by Medicare Fee-for-Service Beneficiaries Discharged Home After a Stroke: Findings From the COMPASS Trial.[Arch Phys Med Rehabil. 2022]Skilled Nursing and Inpatient Rehabilitation Facility Use by Medicare Fee-for-Service Beneficiaries Discharged Home After a Stroke: Findings From the COMPASS Trial.Freburger JK, Pastva AM, Coleman SW, Peter KM, Kucharska-Newton AM, Johnson AM, Psioda MA, Duncan PW, Bushnell CD, Rosamond WD, et al. Arch Phys Med Rehabil. 2022 May; 103(5):882-890.e2. Epub 2021 Nov 3.
- Unexplained Variation for Hospitals' Use of Inpatient Rehabilitation and Skilled Nursing Facilities After an Acute Ischemic Stroke.[Stroke. 2017]Unexplained Variation for Hospitals' Use of Inpatient Rehabilitation and Skilled Nursing Facilities After an Acute Ischemic Stroke.Xian Y, Thomas L, Liang L, Federspiel JJ, Webb LE, Bushnell CD, Duncan PW, Schwamm LH, Stein J, Fonarow GC, et al. Stroke. 2017 Oct; 48(10):2836-2842. Epub 2017 Aug 22.
- Review Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses[ 2014]Review Evidence Brief: The Quality of Care Provided by Advanced Practice NursesMcCleery E, Christensen V, Peterson K, Humphrey L, Helfand M. 2014 Sep
- Review Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.[Health Technol Assess. 2001]Review Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.Marshall M, Crowther R, Almaraz-Serrano A, Creed F, Sledge W, Kluiter H, Roberts C, Hill E, Wiersma D, Bond GR, et al. Health Technol Assess. 2001; 5(21):1-75.
- Comparing Recovery Options for Stroke PatientsComparing Recovery Options for Stroke Patients
Your browsing activity is empty.
Activity recording is turned off.
See more...
