Licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported license. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Nishihara S, Angata K, Aoki-Kinoshita KF, et al., editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021-.
Introduction
2-Azido-sugar, 2-azido-galactopyranose in particular, has been frequently used in the synthesis of complex oligosaccharides containing aminosugars. Among various forms of glycosides, N-acetyl galactosamine (GalNAc) often present in a form of α-glycoside. Having an azido group at the 2-position is advantageous for the synthesis of α-glycoside owing to the anomeric effect.
Lemieux and Ratcliffe introduced a famous and still frequently used azidonitration in 1979 (1). The desired compounds with galacto-configuration can be obtained in 75% yield after careful column chromatography. The anomeric nitrate can then be converted into various leaving groups.
In 1990, a direct preparation method for 1-chloro-2-azido-sugar from tri-O-acetyl glycal was patented (2). Later in 2011, the method was simplified (3), which was practical and provided slightly better yields compared to azidonitration in our hands.
Protocol
In this chapter, a protocol to prepare 2-azido-3,4,6-tri-O-acetyl-2-deoxy-D-galactopyranosyl chloride (compound 2) from 3,4,6-tri-O-acetyl-D-galactal (compound 1), as reported by Sewald et al., is described (Figure 1).
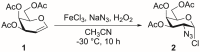
Figure 1:
Azidochrolination of acetyl galactal.
Materials
- 1.
3,4,6-Tri-O-acetyl-D-galactal (compound 1, prepared inhouse)
- 2.
CH3CN (Guaranteed Reagent stored over MS 3A)
- 3.
FeCl3·6H2O (Guaranteed Reagent)
- 4.
NaN3 (Guaranteed Reagent)
- 5.
30% H2O2 (Extra Pure Reagent)
- 6.
TLC silica gel 60 F254 25 glass plates for reaction monitoring with 5% H2SO4-EtOH.
- 7.
Ethyl acetate for extraction
- 8.
Saturated NaHCO3 for washing the organic phase
- 9.
Brine for washing the organic phase
- 10.
Na2SO4 for drying the organic phase
- 11.
Silica gel (Chromatorex PSQ 100B) for purification
- 12.
CDCl3 containing 1% (CH3)4Si (internal standard) for NMR measurement
Instruments
- 1.
Round bottom flask (reaction vessel)
- 2.
Magnetic stirring bar
- 3.
Rotary evaporator for removing organic solvents
- 4.
Circulating cooling bath
- 5.
Separating funnel
- 6.
Erlenmeyer flask
- 7.
Chromatography tube for silica gel chromatography (for purification).
- 8.
Fraction collector (for chromatography)
- 9.
Diaphragm pump (for filtration under reduced pressure)
- 10.
Vacuum pump (for drying up)
- 11.
AVANCE 500 spectrometer (Bruker Biospin Inc.) for obtaining 1H NMR (500 MHz).
Methods
- 1.
Dissolve compound 1 (109.7 mg, 0.403 mmol) in CH3CN (2 mL).
- 2.
Add FeCl3·6H2O (174 mL, 0.644 mmol), NaN3 (57.6 mg, 0.886 mmol), and 30% H2O2 (90.4 μL, 0.886 mmol) to a solution written above at −30°C.
- 3.
Stir the resulting mixture at the temperature for 10 h. (Note 1)
- 4.
Confirm the reaction completion by TLC (toluene-ethyl acetate = 4:1).
- 5.
Dilute the reaction mixture with 50 mL of ethyl acetate, and washed with water, saturated sodium bicarbonate, and brine.
- 6.
Dry organic solution over Na2SO4, and evaporate the solvents in vacuo to give crude compound 2 (132.2 mg, Figure 2).
- 7.
Perform column chromatography of the mixture to yield pure compound 2 (74%).

Figure 2:
1H NMR spectrum of a crude compound 2.
Note
- 1.
The homogeneous reaction prevents stirring problems in heterogeneous reaction.
References
- 1.
- Lemieux RU, Ratcliffe RM. The azidonitration of tri-O-acetyl-D-galactal. Can J Chem. 1979 May;57(10):1244–51. [CrossRef]
- 2.
- Naicker S, Noujaim A, Inventors; Oncothyreon Canada Inc., assignee. Azidochlorination and diazidization of glycals. United States patent US 4,935,503A, 1990 Jun 19.
- 3.
- Plattner C, Höfener M, Sewald N. One-pot azidochlorination of glycals. Org Lett. 2011 Feb 18;13(4):545–7. [PubMed: 21244046] [CrossRef]
Footnotes
The authors declare no competing or financial interests.
- PubMedLinks to PubMed
- Syntheses of T(N) building blocks Nalpha-(9-fluorenylmethoxycarbonyl)-O-(3,4,6-tri-O-acetyl-2-azido-2-deoxy-alpha-D-galactopyranosyl)-L-serine/L-threonine pentafluorophenyl esters: comparison of protocols and elucidation of side reactions.[Carbohydr Res. 2005]Syntheses of T(N) building blocks Nalpha-(9-fluorenylmethoxycarbonyl)-O-(3,4,6-tri-O-acetyl-2-azido-2-deoxy-alpha-D-galactopyranosyl)-L-serine/L-threonine pentafluorophenyl esters: comparison of protocols and elucidation of side reactions.Liu M, Young VG Jr, Lohani S, Live D, Barany G. Carbohydr Res. 2005 May 23; 340(7):1273-85.
- Comparative conformational studies of 3,4,6-tri-O-acetyl-1,5-anhydro-2-deoxyhex-1-enitols at the DFT level.[Carbohydr Res. 2018]Comparative conformational studies of 3,4,6-tri-O-acetyl-1,5-anhydro-2-deoxyhex-1-enitols at the DFT level.Nowacki A, Liberek B. Carbohydr Res. 2018 Jun 15; 462:13-27. Epub 2018 Mar 28.
- The use of tri-O-acetyl-D-glucal and -D-galactal in the synthesis of 3-acetamido-2,3-dideoxyhexopyranoses and -hexopyranosides.[Carbohydr Res. 2002]The use of tri-O-acetyl-D-glucal and -D-galactal in the synthesis of 3-acetamido-2,3-dideoxyhexopyranoses and -hexopyranosides.Liberek B, Dabrowska A, Frankowski R, Matuszewska M, Smiatacz Z. Carbohydr Res. 2002 Nov 5; 337(20):1803-10.
- Solution and solid-state structure of N-acetamido-3,4,6-tri-O-acetyl-2-azido-2-deoxy-alpha-D-galactopyranosylamine.[Carbohydr Res. 2004]Solution and solid-state structure of N-acetamido-3,4,6-tri-O-acetyl-2-azido-2-deoxy-alpha-D-galactopyranosylamine.Srinivas O, Muktha B, Radhika S, Guru Row TN, Jayaraman N. Carbohydr Res. 2004 Jun 1; 339(8):1447-51.
- Review 4-Thiofuranoid Glycal: Versatile Glycosyl Donor for the Selective Synthesis of β-anomer of 4'-thionucleoside and its Biological Activities.[Curr Med Chem. 2022]Review 4-Thiofuranoid Glycal: Versatile Glycosyl Donor for the Selective Synthesis of β-anomer of 4'-thionucleoside and its Biological Activities.Haraguchi K, Kumamoto H, Tanaka H. Curr Med Chem. 2022; 29(21):3684-3731.
- Azidochrolination reaction of tri-O-acetyl-D-galactal - Glycoscience Protocols (...Azidochrolination reaction of tri-O-acetyl-D-galactal - Glycoscience Protocols (GlycoPODv2)
Your browsing activity is empty.
Activity recording is turned off.
See more...
