Abbreviations
- AMSTAR 2
A MeaSurement Tool to Assess systematic Reviews 2
- EQ-5D
EuroQol 5-Dimensions questionnaire
- HTA
health technology assessment
- ICER
incremental cost-effectiveness ratios
- NYHA
New York Heart Association
- PARTNER
Placement of AoRTic TraNscathetER Valve
- QALY
quality-adjusted life-years
- SAVR
surgical aortic valve replacement
- TAVI
transcatheter aortic valve implantation
Key Messages
The cost-effectiveness of transcatheter aortic valve implantation (TAVI) compared to surgical aortic valve replacement was examined in patients with severe symptomatic aortic stenosis at high, intermediate, and low surgical risk, and the findings were mixed. When compared to surgical aortic valve replacement, some studies suggest that TAVI is cost-effective (less costly and/or more effective) and some studies suggest that TAVI is not cost-effective.
Factors such as the type of TAVI system used, the cost of treatment-associated expenses (such as post-operative follow-up costs and hospitalization costs), and the characteristics of patients selected for treatment likely impact the cost-effectiveness of TAVI for patients with severe symptomatic aortic stenosis.
Context and Policy Issues
Aortic stenosis is a narrowing of the aortic valve that interferes with the flow of blood from the left ventricle of the heart to the aorta. This progressive valvular disorder affects approximately 2% of the population aged 65 years or older1 and 5% of those older than 75 years of age.2 People who are affected by aortic stenosis typically remain asymptomatic for many years and the condition may not measurably impact their health; however, patients who become symptomatic generally have a poor prognosis.3,4 Symptoms of aortic stenosis — such as decreased capacity for exercise, heart murmur, chest pain or tightness, heart palpitations, shortness of breath, and feeling faint or dizzy with physical activity — tend to gradually develop as the condition worsens.5 Serious complications of aortic stenosis include heart failure, stroke, blood clots, endocarditis, and sudden death.3,6
Treatment options for severe aortic stenosis include medical therapy (e.g., statin therapy, non-statin lipid-lowering therapy, antihypertensive therapy, and therapies that target or prevent aortic valve calcification) or various surgical procedures, such as aortic valvuloplasty, openheart surgical aortic valve replacement (SAVR), or TAVI (also known as transcatheter aortic replacement).7 Aortic valvuloplasty may be offered to some patients as a bridge therapy or to provide temporary palliation and symptomatic relief;8 however, aortic valve replacement using SAVR or TAVI is considered the definitive treatment for severe symptomatic aortic stenosis.7,9 TAVI is a minimally invasive procedure, where a prosthetic valve that functionally replaces the damaged aortic valve is implanted through a catheter inserted through the blood vessels. The replacement valve is typically delivered via the femoral artery in the groin,10 but other access routes such as the subclavian artery, the common carotid artery, the femoral vein, or a route that enters directly into the ascending aorta may be considered as alternatives in some patients.11–13
CADTH has previously reviewed evidence regarding the clinical effectiveness of TAVI for the treatment of patients with severe aortic stenosis14–16 or with degenerated mitral or tricuspid valve bioprostheses.17 Additionally, 2 rapid qualitative reviews18,19 conducted by CADTH have examined how people with aortic stenosis experience TAVI. While these previous reviews have summarized some of the literature on TAVI, the cost-effectiveness of this procedure is unclear. The objective of this report is to evaluate the cost-effectiveness of TAVI to support decisions involving the use of this therapy for the treatment of patients with severe symptomatic aortic stenosis.
Research Question
What is the cost-effectiveness of transcatheter aortic valve implantation or replacement in low-risk, intermediate-risk, and high-risk patients with severe symptomatic aortic stenosis undergoing this procedure?
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including MEDLINE, the Cochrane Database of Systematic Reviews, the international HTA database, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were transcatheter aortic valve implementation and aortic valve stenosis. CADTH-developed search filters were applied to limit retrieval to health technology assessments (HTAs), systematic reviews, meta-analyses, or network meta-analyses; and economic studies. Where possible, retrieval was limited to the human population. The search was also limited to English-language documents published between January 1, 2016 and June 2, 2021.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published before 2018. Systematic reviews in which all relevant studies were captured in other more recent or more comprehensive systematic reviews were excluded. Economic evaluations retrieved by the search were excluded if they were captured in 1 or more included systematic reviews.
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the following tools as a guide: A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2)20 for systematic reviews and the Drummond checklist21 for economic evaluations. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Quantity of Research Available
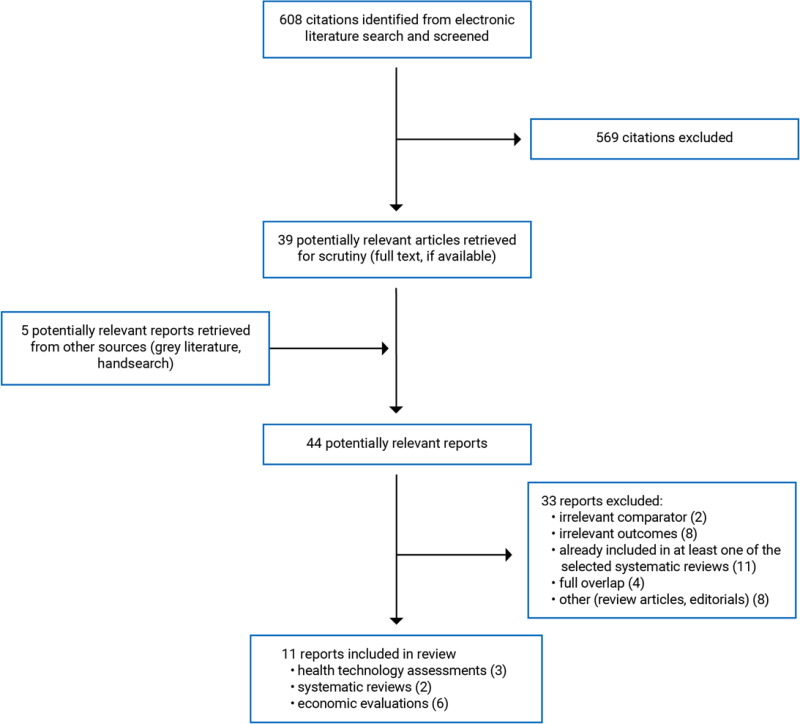
A total of 608 citations were identified in the literature search. Following the screening of titles and abstracts, 569 citations were excluded and 39 potentially relevant reports from the electronic search were retrieved for full-text review. Five potentially relevant publications were retrieved from the grey literature search for full-text review. Of these 44 potentially relevant articles, 33 publications were excluded for various reasons and 11 publications met the inclusion criteria and were included in this report. These comprised 3 HTAs (each included a systematic review22–24; 2 included primary economic evaluations22,24), 2 systematic reviews,25,26 and 6 economic evaluations.27–32
Appendix 1 presents the PRISMA33 flow chart of the study selection. Additional references of potential interest are provided in Appendix 6.
Summary of Study Characteristics
Three relevant HTAs (which included 3 systematic reviews22–24 and 2 de novo economic evaluations22,24), 2 systematic reviews,25,26 and 6 economic evaluations27–32 were included in this review. The components of the identified HTAs that are relevant to this report (i.e., 3 systematic reviews and 2 economic evaluations) will be described individually, as much of the other information contained in these HTAs is beyond the scope of the current report. Detailed study characteristics are available in Appendix 2, , and .
One included systematic review26 had objectives and inclusion criteria that were wider in scope than the present review. Specifically, Gialama et al. (2018)26 examined the cost-effectiveness of any interventions for the treatment of valvular heart disease. Only the characteristics and results of the subset of relevant studies will be described in this report.
Study Design
The systematic review by Health Technology Wales (2020)22 included a systematic review of cost-utility analyses published up to September 8, 2020. Two previously completed HTAs were used as an initial source of published evidence, which were supplemented by additional literature searches. There were 10 eligible studies included in the systematic review,22 all of which were relevant to the current report. The systematic review of cost-effectiveness literature conducted by Ontario Health (2020)23 designated cost-benefit analyses, coste-effectiveness analyses, and cost-utility analyses as relevant study designs. Eligible studies were identified from a 2016 Ontario Health HTA that evaluated TAVI for patients with severe aortic stenosis and through economic literature searches to identify studies published from January 1, 2015 to July 12, 2019. One relevant cost-utility analysis was included in the review.23 The authors of the Health Information and Quality Authority SR24 searched for economic evaluations (e.g., cost-utility analyses or cost-effectiveness analyses) published between January 1, 2013 and June 28, 2019. Six cost-utility analyses and 1 cost-effectiveness analysis were included in their review24 (all were relevant to the current report). The systematic review by Azraai et al. (2020)25 included cost-effectiveness studies published between January 2010 and November 2019. Eight economic evaluations were included in the review.25 The authors of the Gialama et al. (2018)26 systematic review included economic evaluations published up to June 2017. A total of 27 articles were included in the systematic review26 (11 were relevant to the current report). In total, the 5 systematic reviews22–26 included 24 unique economic evaluations relevant to the current report. The relevant primary study overlap between these systematic reviews is summarized in Appendix 5, Table 12.
In addition to those identified in the systematic reviews, 8 relevant economic evaluations22,24,27–32 were identified. All 8 economic evaluations22,24,27–32 were conducted as cost-utility analyses and incorporated Markov models that included between 231 and 924,28,29 health states. The time horizons were 8 years,32 15 years,24,27–29 and lifetime.22,30,31
The analysis by Himmels et al. (2021)27 employed a 3-state Markov model with 1-month cycle lengths from the Norwegian health care perspective, using a 15-year time horizon. The 3 health states were: alive and well, post major complications, and dead. Model inputs were derived from the Placement of AoRTic TraNscathetER Valve (PARTNER) 3 and PARTNER 2 trials, from Norwegian activity-based payment system, and from various sources of literature.
The model used in the study by Lorenzoni et al. (2021)28 was constructed using a 15-year time horizon from the perspective of the Italian national health system. The model was structured as a Markov model with a 1-month cycle length, comprising 9 different health states and categorized using New York Heart Association (NYHA) functional classifications: NYHA I, NYHA I with a history of stroke, NYHA II, NYHA II with a history of stroke, NYHA III, NYHA III with a history of stroke, NYHA IV, NYHA IV with a history of stroke, and death. Effectiveness inputs, transition probabilities, and utility values were extrapolated from the PARTNER trials and other key clinical studies. Cost inputs were derived from Italian national tariffs and various sources of published literature.
Pinar et al. (2021)29 conducted their analysis using a Markov model with a 1-month cycle length, comprising 9 different health states: NYHA I, NYHA I with a history of stroke, NYHA II, NYHA II with a history of stroke, NYHA III, NYHA III with a history of stroke, NYHA IV, NYHA IV with a history of stroke, and death. The analysis used a 15-year horizon from the perspective of the Spanish national health system. Transition probabilities and health state utilities were derived from key clinical studies, including the PARTNER trials. Costs were estimated using published literature, information provided by the accounts service at a Spanish hospital, and expert opinion.
The cost-utility analysis by Zhou et al. (2021)30 was conducted from the perspective of the Australian health care system, using a lifetime horizon. Cost-effectiveness was estimated using a decision-analytic Markov model with 30-day cycles. The model included 4 health states: procedure, alive and well, alive with previous stroke, and dead. Key clinical data inputs, including utility values, were drawn from the PARTNER trials and the Evolut Low-Risk trial. Additional clinical inputs were derived from Australian life tables and other sources of clinical literature. Cost inputs were retrieved from the Australian Medicare Benefits Schedule and published costs associated with the provision of TAVI and SAVR.
The economic evaluation by Health Technology Wales (2020)22 used a Markov model with a 1-month cycle length from the perspective of the UK NHS–National Health Service and personal social services with a lifetime horizon, comprising 3 main health states: alive with no complications, disabling stroke, and dead. Mortality and complication rates were obtained from the PARTNER 2 study, cost inputs were obtained from NHS reference costs 2018–2019 and clinical expert opinion, and utility values were derived from EuroQol 5-Dimensions questionnaire (EQ-5D) scores measured in the PARTNER 2 study.
The cost-utility analysis by Inoue et al. (2020)31 used a decision tree model for the initial 2 years of analysis that fed into a Markov model with 1-year cycles with 2 states: alive and dead. The model was from the perspective of public health care payers and used a lifetime horizon. Clinical inputs for were retrieved using a systematic review of the literature. Costs associated with treatments were calculated using a medical claims database. Utility values after TAVI were extrapolated from the PARTNER trials, whereas utility values from other health states were taken from a previously published economic evaluation.
Kuntjoro et al. (2020)32 used a 2-phase economic model that had a decision tree model for the initial 30 days, followed by a long-term Markov model with 1-year cycles. The analysis was conducted using an 8-year horizon from the perspective of the National University Health System in Singapore. Clinical parameters, such as post-operative mortality rates and risk for complications, were derived from the PARTNER studies and Singapore life tables. Cost inputs were retrieved from a national database and published literature. Health utility values were taken from the Singapore population norm for EQ-5D scores using local preference weights.
The Health Information and Quality Authority (2019)24 analysis used a 9-state Markov model that simulated patient outcomes in 1-month cycles. The health states included: alive and well, major complications, post major complications, re-hospitalization, and death. The major complications and post major complication each included 3 health states (i.e., acute kidney injury, disabling stroke, and myocardial infarction) to reflect the different risks of mortality associated with each complication. The analysis was conducted using a 15-year time horizon from the perspective of the publicly funded health and social care system in Ireland. Clinical inputs, including utility estimates, were derived from the PARTNER trials following a systematic review of the literature and from national life tables for Ireland from 2015. Cost estimates were retrieved from relevant Diagnostic Related Group codes in Ireland or from previously published HTAs.
Country of Origin
The included systematic reviews were conducted by groups in Australia,25 Canada,23 Greece,26 Ireland,24 and Wales.22
The economic evaluations were by authors in Australia,30 Japan,31 Ireland,24 Italy,28 Norway,27 Singapore,32 Spain,29 and Wales.22
Patient Population
The systematic review by Health Technology Wales (2020)22 was specific to adults with symptomatic aortic stenosis who were considered to be at intermediate surgical risk. The Ontario Health (2020)23 systematic review included studies of adults with severe aortic valve stenosis and low surgical risk. Two systematic reviews24,25 included studies of patients with aortic stenosis at low or intermediate risk of surgical complications. The systematic review by Gialama et al. (2018)26 included studies of people with valvular heart disease, regardless of their surgical risk; however, only primary studies of patients with aortic stenosis were considered relevant to the current report.
The study populations in all 8 included economic evaluations comprised patients with severe aortic stenosis. One study31 was specific to high-risk patients, 1 study22 was specific to intermediate-risk patients, 2 studies27,30 were specific to low-risk patients, and 4 studies24,28,29,32 considered patients at varying levels of surgical risk.
While the included studies22–32 referenced various methods to determine the surgical risk of patients, such as the Society of Thoracic Surgeons predicted risk of mortality calculator and the logistic European System for Cardiac Operative Risk Evaluation (versions I and II), standardized criteria for categorizing patients were not detailed and applied consistently throughout. This reflects the absence of an ideal risk model and emphasizes the importance of assessment by multidisciplinary heart teams.24
Interventions and Comparators
The 5 systematic reviews22–26 included economic evaluations that examined the coste-effectiveness of TAVI devices compared to SAVR. In all cases, there were no restrictions on the types of TAVI devices (e.g., self-expanding or balloon-expandable) or on the routes of access (e.g., transfemoral, subclavian, transapical) that were eligible for inclusion.
Of the 8 included economic evaluations, 3 studies reported the brand name of the TAVI system (i.e., SAPIEN, manufactured by Edwards Lifesciences): 1 study29 was specific to TAVI performed using the balloon-expandable SAPIEN 3 system, 1 study31 was specific to TAVI performed using the balloon-expandable SAPIEN XT system, 1 study32 was specific to balloon-expandable SAPIEN systems; and 5 studies22,24,27,28,30 were not specific to a particular type of TAVI. Consistent with the inclusion criteria for the current report, all 8 economic evaluations22,24,27–32 used SAVR as a comparator.
Outcomes
The included systematic reviews22–26 reported on various measures of costs and benefits, such as projected treatment-associated costs, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and probabilities of cost-effectiveness at specified willingness-to-pay thresholds. ICERs were generally reported as costs per QALY gained; however, some primary studies reported costs per life-year gained or costs per life saved.
Similarly, model outputs from the 8 economic evaluations22,24,27–32 included treatment costs (reported in local currencies), life-years gained, QALYs gained, ICERs (expressed as cost per QALY gained or cost per life-year gained), and incremental net monetary benefits. In some cases, the analyses also included cost-effectiveness acceptability curves that showed the probability of each treatment being cost-effective over a range of willingness-to-pay thresholds.
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of the included publications are provided in Appendix 3, and .
Systematic Reviews
The 5 systematic reviews22–26 were considered to be of variable methodological quality based on the assessments using AMSTAR 2.20 The authors of all included systematic reviews clearly defined their objectives and primary study eligibility criteria, conducted literature searches in multiple databases, provided a description of key search terms and restrictions (e.g., on language or date of publication), and included a flow chart that illustrated study selection. These methodological strengths increase the reproducibility of the systematic reviews. Additionally, the authors of all 5 systematic reviews justified their selection of study designs eligible for inclusion in the reviews and summarized the included studies in adequate detail. The quality of included primary studies was assessed using satisfactory techniques in 4 systematic reviews22–24,26 and the authors of 3 systematic reviews22,24,26 reported the sources of funding for the included primary studies.
As for methodological limitations, none of the included systematic reviews contained an explicit statement that the review methods were established before conducting the review (and did not reference a published protocol) or a list of studies excluded after full-text review. These limitations increase the risk for reporting bias and decrease the overall transparency of the review process. Additionally, sources of grey literature were not searched in 2 systematic reviews,22,25 increasing the risk for missing relevant, non-indexed studies. In 4 systematic reviews, the methods for article selection and data extraction were poorly documented22,25,26 or were conducted using a single reviewer,23 increasing the likelihood for inaccuracies in these processes. Similarly, it was unclear how many reviewers were involved in the quality assessment process in 3 systematic reviews22,23,26 and there was no quality assessment of included studies performed in the systematic review by Azraai et al.25 Finally, the authors of 3 systematic reviews22,23,25 did not state their potential conflicts of interest and the sources of funding for 2 reviews25,26 were unclear.
Economic Evaluations
In all 8 economic evaluations,22,24,27–32 the authors clearly stated their research questions, the economic importance of the research questions, described the interventions and comparators in detail, provided rationale for choosing alternative interventions, justified their selection of the form of economic evaluations, and explained model structures using figures. The selected time horizons, which were 8 years,32 15 years,24,27–29 and lifetime,22,30,31 were appropriate given the nature of severe aortic stenosis, TAVI, and SAVR. Additional methodological strengths common to all 8 economic evaluations22,24,27–32 included:
the perspectives of the analyses were clearly stated and justified
the sources of clinical, cost, and utility data were appropriately referenced
the approaches to sensitivity analyses and the choice of variables for sensitivity analyses were justified
outcome measures for the economic evaluation were clearly stated
currency and price data were recorded
incremental analyses were reported
the conclusions made by study authors followed the reported data and were accompanied by appropriate caveats.
As for methodological limitations, descriptions of the methods used for currency price adjustments for inflation were not included in 6 studies,22,24,27–29,32 the authors of 3 studies22,24,32 did not disclose their potential conflicts of interest, 3 studies28,29,31 were industry-funded (increasing the risk for sponsorship bias), and 2 studies28,29 provided limited information on the characteristics of patient populations from whom model inputs were obtained. While various discount rates ranging between 2% and 5% were applied to costs and benefits in each of the included studies, the authors of 5 studies27–29,31,32 did not provide a justification for their selected discount rates, making it unclear if the selected discount rates accurately represent the views and preferences of patients and payers. In some cases, advice from clinical experts or the extrapolation of results from key clinical trials to extend beyond the available followup periods was necessary to inform the economic models.22,24,27–32 While the parameters that were estimated from expert clinical advice and the techniques used for extrapolation appeared to be reasonable, these inputs add additional uncertainty to the economic models.
Summary of Findings
The overall findings of the included studies are highlighted here, categorized by the surgical risk level of patients included in the studies. A consistent definition to categorize patients by their levels of surgical risk was not applied across all studies. Instead, studies were categorized based on the description of the patient population by study authors. There was overlap in the primary studies included in the systematic reviews, as described in Appendix 5, Table 12. The data from primary studies described in multiple systematic reviews are only presented once. Detailed summaries of the main findings and authors’ conclusions are available in Appendix 4, and Table 11.
Cost-Effectiveness of TAVI
High Surgical Risk Populations
Evidence regarding the cost-effectiveness of TAVI for the treatment of severe symptomatic aortic stenosis in patients at high surgical risk was available from 7 economic evaluations summarized in 1 included systematic review26 and 3 additional economic evaluations.28,29,31 These findings are described by primary study and presented in .
Cost-Effectiveness Findings for TAVI Versus SAVR in Patients With Severe Aortic Stenosis at High Surgical Risk.
There was substantial variation in the cost-effectiveness results from studies in high-risk patients. The findings from 1 study suggested that TAVI was dominant versus SAVR (i.e., treatment with TAVI resulted in more QALYs and less costs),26 5 studies suggested TAVI was cost-effective versus SAVR at their specified willingness-to-pay thresholds,26,28,29,31 3 studies suggested that TAVI was not cost-effective or was dominated by SAVR (i.e., treatment with TAVI resulted in less QALYs and more costs),26 and 1 study suggested that the cost-effectiveness of TAVI versus SAVR depended on the type of TAVI procedure (i.e., transfemoral TAVI was cost-effective, transapical TAVI was not cost-effective).26
Intermediate Surgical Risk Populations
The cost-effectiveness of TAVI for the treatment of severe symptomatic aortic stenosis in patients at intermediate surgical risk was examined in 8 economic evaluations summarized in 4 included systematic reviews22,24–26 and 4 additional economic evaluations.22,24,28,29 Findings are described by primary study; summarizes the cost-effectiveness findings in intermediate-risk patients by primary study, including the country that the study was conducted in, ICERs, and the willingness-to-pay threshold that was referenced in the study.
Cost-Effectiveness Findings for TAVI Versus SAVR in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk.
Of the 12 economic evaluations that provided results specific to intermediate-risk patients, 4 studies suggested that TAVI was dominant compared to SAVR,22,24,25 5 studies (including 3 studies that were conducted from Canadian perspectives) suggested that TAVI was cost-effective versus SAVR at their specified willingness-to-pay thresholds,22,24,25,28,29 and 3 studies suggested that TAVI was not cost-effective.22,24–26 None of the identified studies indicated that TAVI was dominated by SAVR for the treatment of aortic stenosis in intermediate-risk patients.
Low Surgical Risk Populations
Evidence regarding the cost-effectiveness of TAVI for the treatment of severe symptomatic aortic stenosis in patients at low surgical risk was available from 4 economic evaluations summarized in 3 included systematic reviews23,25,26 and 2 additional economic evaluations.27,30 These findings are described by primary study and summarized in .
Cost-Effectiveness Findings for TAVI Versus SAVR in Patients With Severe Aortic Stenosis at Low Surgical Risk.
The authors of these economic evaluations concluded that TAVI was dominant (1 study),27 cost-effective at their specified willingness-to-pay thresholds (2 studies, including 1 conducted in Canada),23,25 cost-ineffective (1 study),26 or that the cost-effectiveness of TAVI depended on the type of TAVI procedure (i.e., self-expanding TAVI was dominant, balloon-expanding TAVI was cost-effective, transapical TAVI was not cost-effective; 1 study).30 The findings of 1 economic evaluation in low-risk patients were not summarized in detail in the systematic review that included it.25
Populations With Mixed or Unclear Risk of Surgical Complications
Evidence regarding the cost-effectiveness of TAVI for the treatment of severe symptomatic aortic stenosis in patient populations with mixed or unclear levels of surgical risk was available from 3 economic evaluations summarized in 2 included systematic reviews24,26 and 1 additional economic evaluation.32 These findings are described by primary study and presented in .
Cost-Effectiveness Findings for TAVI Versus SAVR in Patients With Aortic Stenosis at Mixed or Unclear Surgical Risk.
The findings from these economic evaluations were inconsistent. The authors of 1 study suggested that TAVI was cost-effective versus SAVR at their specified willingness-to-pay threshold.32 The findings from 2 studies, including 1 conducted in Canada, indicated that TAVI was not cost-effective or was dominated by SAVR.26 The fourth economic evaluation reported an incremental cost of €1,486,118 per life saved, but the authors’ specified willingness-to-pay threshold was not reported in the systematic review that included this study.24
Limitations
There were some concerns relating to the generalizability of the findings from the included economic evaluations to Canadian settings, as these analyses were conducted using effectiveness and cost inputs from Australia,30 Ireland,24 Italy,28 Japan,31 Norway,27 Singapore,32 Spain,29 and Wales.22 Any differences in the expected clinical effectiveness or costs associated with TAVI and SAVR between Canadian health care systems and the systems of these Asian, European, and Oceanian countries would affect the applicability of the cost-effectiveness findings.
The 8 economic evaluations22,24,27–32 used PARTNER trials as sources of clinical inputs for their analyses; however, it was not always clearly reported which of the PARTNER trials were used (e.g., PARTNER 1, PARTNER 2, or PARTNER 3) and the degree of overlap in clinical inputs from the PARTNER trials was unclear. Similarly, detailed descriptions of the clinical inputs used in the economic evaluations summarized in the included systematic reviews22–26 were often unavailable. As a result, it is unclear how variations in the clinical inputs extrapolated from the PARTNER trials have impacted the cost-effectiveness findings summarized in this report.
Several of the included economic evaluations incorporated data from studies on first- or early-generation TAVI devices (e.g., Edwards Lifesciences SAPIEN, Medtronic CoreValve) into their economic models. Data from these first-generation devices may not precisely reflect the clinical and cost-effectiveness of newer systems (e.g., Edwards Lifesciences SAPIEN 3 valve, Medtronic Evolut R).34
The included literature stratified the cost-effectiveness of TAVI devices across patient populations with various levels of surgical risk; however, it is unclear how other patient characteristics — such as age, gender, body mass index, ethnicity, or comorbidities — may impact the cost-effectiveness of TAVI.
There was heterogeneity in the way that surgical risk was determined across included studies, often involving clinical assessment by a cardiac team or using various risk algorithms, such as the Society of Thoracic Surgeons Predicted Risk of Mortality and the logistic European System for Cardiac Operative Risk Evaluation (versions I or II). It was unclear how applying different criteria to assess surgical risk may impact the cost-effectiveness findings summarized in this review.
While the volume of cost-effectiveness evidence summarized in this report is substantial (i.e., 32 unique economic evaluations), only 5 economic evaluations (summarized in the included systematic reviews22–26) were conducted using Canadian perspectives, none of which were conducted in populations with high surgical risk. The cost-effectiveness of TAVI is expected to be context-specific and to be influenced by many factors (e.g., local costs of the procedure and associated costs, patient characteristics). Therefore, the applicability of cost-effectiveness findings from studies conducted outside of Canada should be considered when interpreting the results summarized in this report.
Conclusions and Implications for Decision- or Policy-Making
This review comprised 5 systematic reviews22–26 (3 conducted as part of HTAs22–24) and 8 primary economic evaluations22,24,27–32 (2 conducted as part of HTAs22,24) regarding the cost-effectiveness of TAVI for the treatment of severe aortic stenosis.
The evidence summarized in this review provided inconsistent findings regarding the cost-effectiveness of TAVI compared to SAVR across the 3 categories of surgical risk. While the authors of many included studies concluded that TAVI was dominant22,24–27,30 (i.e., cost-saving and generated more QALYs) or that TAVI was cost-effective at commonly cited willingness-to-pay thresholds versus SAVR,22–26,28–32 the findings of other studies22,24,26 suggested that TAVI was not cost-effective for treating patients with severe symptomatic aortic stenosis compared to SAVR. Of the 10 economic evaluations22,24,26 that suggested TAVI was not cost-effective or was dominated by SAVR, 8 were published before 2014. On the other hand, 17 of the 18 studies22–32 published since 2018 suggested that TAVI was cost-effective or dominant versus SAVR. This observation may indicate that the cost-effectiveness of TAVI has improved over time.
The cost-effectiveness of TAVI is likely to be sensitive to the access route, the costs of the procedure and related expenses, and the characteristics of patients selected for treatment. Despite the mixed conclusions provided by authors of identified economic evaluations, 4 of the 5 studies22–26 conducted between 2018 and 2020 from Canadian perspectives indicated that TAVI was cost-effective compared to SAVR in patients at intermediate to low surgical risk. It is likely that the findings from these analyses have the highest generalizability to Canadian settings and may be most useful to Canadian decision-makers. The remaining Canadian study, which was on patients with unclear surgical risk (as summarized in the review by Gialama et al.26), suggested that TAVI was dominated by SAVR; however, this study was conducted in 2013, indicating that the results of more recent clinical trials on TAVI devices were unavailable for incorporation into the analysis and that the costs associated with providing TAVI may not accurately reflect the current landscape in Canada. It’s worth noting that, while these 5 economic evaluations22–26 were conducted from Canadian perspectives (and thus incorporate Canadian-specific cost data), the clinical inputs used in these models were not derived entirely from patients treated with TAVI or SAVR in Canada.
The limitations of the included literature22–32 (e.g., uncertainty in the economic models) should be considered when interpreting the findings of this report. Future economic evaluations conducted from Canadian perspectives may be useful to further inform clinical and policy decisions, especially those that integrate real-world data collected in Canada regarding the clinical effectiveness of TAVI and the costs associated with the provision of TAVI or those that include patients considered to be of high surgical risk.
References
- 1.
- 2.
- 3.
- 4.
Grimard
BH, Safford
RE, Burns
EL. Aortic Stenosis: Diagnosis and Treatment.
Am Fam Physician. 2016;93(5):371–378.
PubMed [
PubMed: 26926974]
- 5.
- 6.
- 7.
Marquis-Gravel
G, Redfors
B, Leon
MB, Généreux
P. Medical Treatment of Aortic Stenosis.
Circulation. 2016;134(22):1766–1784.
PubMed [
PubMed: 27895025]
- 8.
Baber
U, Kini
AS, Moreno
PR, Sharma
SK. Aortic Stenosis: Role of Balloon Aortic Valvuloplasty.
Cardiol Clin. 2013;31(3):327–336.
PubMed [
PubMed: 23931097]
- 9.
Szerlip
M, Arsalan
M, Mack
MC, et al
Usefulness of Balloon Aortic Valvuloplasty in the Management of Patients With Aortic Stenosis.
Am J Cardiol. 2017;120(8):1366–1372.
PubMed [
PubMed: 28865895]
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
Shea
BJ, Reeves
BC, Wells
G, et al
AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both.
Bmj. 2017;358:j4008.
PubMed [
PMC free article: PMC5833365] [
PubMed: 28935701]
- 21.
- 22.
- 23.
Ontario
H. Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Valve Stenosis at Low Surgical Risk: A Health Technology Assessment.
Ont Health Technol Assess Ser. 2020;20(14):1–148.
PubMed [
PMC free article: PMC7670297] [
PubMed: 33240455]
- 24.
Health Technology Assessment of transcatheter aortic valve implantation (TAVI) in patients with severe symptomatic aortic stenosis at low and intermediate risk of surgical complications. Dublin (IE): Health Information and Quality Authority; 2019:
https://www.hiqa.ie/sites/default/files/2019-12/TAVI_HTA.pdf Accessed 2021 Jun 28.
- 25.
Azraai
M, Gao
L, Ajani
AE. Cost-Effectiveness of Transcatheter Aortic Valve Intervention (TAVI) Compared to Surgical Aortic Valve Replacement (SAVR) in Low- to Intermediate-Surgical-Risk Patients.
Cardiovasc Revasc Med. 2020;21(9):1164–1168.
PubMed [
PubMed: 31980399]
- 26.
Gialama
F, Prezerakos
P, Apostolopoulos
V, Maniadakis
N. Systematic review of the cost-effectiveness of transcatheter interventions for valvular heart disease.
Eur Heart J Qual Care Clin Outcomes. 2018;4(2):81–90.
PubMed [
PubMed: 29325012]
- 27.
- 28.
Lorenzoni
V, Barbieri
G, Saia
F, et al
The cost-effectiveness of transcatheter aortic valve implantation: exploring the Italian National Health System perspective and different patient risk groups.
Eur J Health Econ. 2021;21:21.
PubMed [
PMC free article: PMC8558181] [
PubMed: 34019220]
- 29.
Pinar
E, Garcia de Lara
J, Hurtado
J, et al
Cost-effectiveness analysis of the SAPIEN 3 transcatheter aortic valve implant in patients with symptomatic severe aortic stenosis.
Rev Esp Cardiol (Engl). 2021;17:17.
PubMed [
PubMed: 34016548]
- 30.
Zhou
JY, Liew
D, Duffy
SJ, Walton
A, Htun
N, Stub
D. Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients With Severe Aortic Stenosis.
Heart Lung Circ. 2021;30(4):547–554.
PubMed [
PubMed: 33189571]
- 31.
Inoue
S, Nakao
K, Hanyu
M, et al
Cost-Effectiveness of Transcatheter Aortic Valve Implantation Using a Balloon-Expandable Valve in Japan: Experience From the Japanese Pilot Health Technology Assessment.
Value Health Reg Issues. 2020;21:82–90.
PubMed [
PubMed: 31670112]
- 32.
Kuntjoro
I, Tay
E, Hon
J, et al
Cost-Effectiveness of Transcatheter Aortic Valve Implantation in Intermediate and Low Risk Severe Aortic Stenosis Patients in Singapore.
Ann Acad Med Singapore. 2020;49(7):423–433.
PubMed [
PubMed: 33000105]
- 33.
Liberati
A, Altman
DG, Tetzlaff
J, et al
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34.
PubMed [
PubMed: 19631507]
- 34.
Pilgrim
T, Lee
JKT, O’Sullivan
CJ, et al
Early versus newer generation devices for transcatheter aortic valve implantation in routine clinical practice: a propensity score matched analysis.
Open Heart. 2018;5(1):e000695.
PubMed [
PMC free article: PMC5786915] [
PubMed: 29387427]
Appendix 1. Selection of Included Studies
Note that this appendix has been formatted for accessibility but has not been copy-edited.
Appendix 2. Characteristics of Included Publications
Note that this appendix has been formatted for accessibility but has not been copy-edited.
Table 6Characteristics of Included Health Technology Assessments and Systematic Reviews
View in own window
| Study citation, country, funding source | Study designs and numbers of primary studies included | Population characteristics | Intervention and comparator(s) | Economic outcomes |
|---|
| Health technology assessments |
|---|
|
Health Technology Wales (2020)22
Wales
Funding source: Health Technology Wales is funded by the Welsh government.
|
Study design: A systematic review of cost-utility analyses. In addition to the review of cost-effectiveness evidence, the HTA included a de novo economic evaluation, which is described in Appendix 2, . Outside of the scope of the current report, the HTA also included a systematic review of clinical effectiveness and reviews of organizational and patient issues.
Literature search strategy: The systematic review incorporated evidence from 2 previous HTA reports. Additional literature searches were performed in MEDLINE, Embase, the Cochrane Library, and clinical trials registries on September 8, 2020, to identify evidence published since the previous HTAs.
Number of included studies: A total of 10 cost-utility analyses were included in the systematic review of cost-effectiveness (all were relevant to the current report).
| Adults with severe symptomatic aortic stenosis who were assessed as being operable but at intermediate surgical risk. Studies of patients at other levels of surgical risk (i.e., low, high, inoperable) were excluded. |
Intervention: TAVI devices.
Comparator: SAVR.
| Economic outcomes:
|
|
Ontario Health (2020)23
Canada
Funding source: Ontario Health is funded by the Ontario government.
|
Study design: A systematic review of cost-benefit analyses, cost-effectiveness analyses, and cost-utility analyses. The HTA also included a systematic review of clinical evidence, a budget impact analysis, and a review of the experiences, preferences, and values of people with severe aortic valve stenosis at low surgical risk, all of which were outside the scope of the current report.
Literature search strategy: Eligible studies were identified from a 2016 Ontario Health HTA that evaluated TAVI for patients with severe aortic stenosis and through an economic literature search performed on July 12, 2019. The search was conducted in Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and the NHS Economic Evaluation Database to identify studies published since January 1, 2015. Additionally, a targeted grey literature search of HTA agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry was performed.
Number of included studies: 1 cost-utility analysis was included in the review (it was relevant to the current report)
| Adults with severe aortic valve stenosis and low surgical risk, as assessed by multidisciplinary heart teams using STS- PROM scores. |
Intervention: TAVI devices (either self-expanding or balloon-expandable, using any implantation route).
Comparator: SAVR
| Economic outcomes:
|
|
HIQA (2019)24
Ireland
Funding source: HIQA is funded by the Irish government.
|
Study design: A systematic review of economic evaluations (e.g., cost-utility analyses or cost-effectiveness analyses). In addition to the review of economic evaluations, the HTA included a de novo primary economic evaluation, which was relevant to the current report and is described in Appendix 2, . Outside of the scope of the current report, the authors conducted a systematic review of clinical effectiveness and safety and reviews of social, organizational, and ethical issues.
Literature search strategy: Electronic searches were conducted in PubMed, Embase, the Cochrane Library, and the University of York CRD database for economic evaluations published between January 1, 2013 and June 28, 2019. In addition, a grey literature search and a search in Scopus were conducted.
Number of included studies: A total of 7 economic evaluations, including 6 cost-utility analyses and 1 cost-effectiveness analysis, were included in the systematic review of cost-effectiveness (all were relevant to the current report).
| Patients with aortic stenosis at low or intermediate risk of surgical complications |
Intervention: TAVI.
Comparator: SAVR.
| Economic outcomes:
|
| Systematic reviews |
|---|
|
Azraai et al. (2020)25
Australia
Funding source: NR.
|
Study design: Systematic review of cost-effectiveness studies.
Literature search strategy: Electronic searches were performed using Ovid MEDLINE, PubMed, Embase, and the Cochrane Database of Systematic Reviews for articles published between January 2010 and November 2019.
Number of included studies: A total of 8 cost-effectiveness studies were included in the systematic review (all were relevant to the current report).
| Studies of people with severe aortic stenosis considered to be at low or intermediate surgical risk were included. |
Intervention: TAVI.
Comparator: SAVR.
| Economic outcomes:
|
|
Gialama et al. (2018)26
Greece
Funding source: NR
|
Study design: Systematic review of economic evaluations.
Literature search strategy: Studies were identified through electronic searches in PubMed and Cochrane conducted in June 2017. There were no search restrictions on publication dates.
Number of included studies: A total of 27 articles were included in the systematic review (11 were relevant to the current report).
| Studies of people with valvular heart disease (i.e., disease of the mitral, aortic, tricuspid, or pulmonary valves) were included. Only studies of people with severe aortic stenosis were considered relevant to the current report. |
Intervention: Any interventions for the treatment of valvular heart disease. Only primary studies that examined TAVI devices were considered relevant to the current report.
Comparators: Any alternative interventions for the treatment of valvular heart disease were eligible for the systematic review. Only economic evaluations that used SAVR as the comparator were considered relevant to the current report.
| Economic outcomes:
Costs QALYs Life-years gained ICERs
|
CRD = Centre for Reviews and Dissemination; HIQA = Health Information and Quality Authority; HTA = health technology assessment; ICER = incremental cost-effectiveness ratio; NHS = National Health Service; NR = not reported. QALY = quality-adjusted life-year; TAVI = transcatheter aortic valve implantation; SAVR = surgical aortic valve replacement; STS-PROM = Society of Thoracic Surgeons predicted risk of mortality.
Table 7Characteristics of Included Economic Evaluations
View in own window
| Study citation, country, funding source | Type of analysis, time horizon, perspective | Population characteristics | Intervention and comparator | Approach | Source of clinical, cost, and utility data used in analysis | Main assumptions |
|---|
|
Himmels et al. (2021)27
Norway
Funding source: NIPH is funded by the Norwegian government.
|
Analysis: Cost-utility analysis conducted as part of an HTA.
Time horizon: 15 years (scenario analyses considered a 1-year time horizon).
Perspective: The Norwegian health care perspective.
| A hypothetical cohort of patients with severe calcific aortic stenosis considered to be at low surgical risk. Patients entered the model at the age of 73 years (based on the PARTNER 3 trial). |
Intervention: TAVI.
Comparator: SAVR.
| A 3-state Markov model with 1-month cycle lengths was analyzed. The 3 health states included: 1) alive and well, 2) post major complications, and 3) dead. Major complications were stroke, acute kidney injury, and myocardial infarction. |
Transition probabilities were derived using clinical
outcomes at 30-days and 1-year from the PARTNER 3 RCT. Mortality risks for patients following major complications were retrieved from the literature. The costs associated with the valve replacement procedures were obtained from Norwegian activity-based payment system. Utility values were derived from EuroQol 5-dimensional scores measured in the PARTNER 2 trial.
|
Patients who experienced a major complication could not recover to the “alive and well” health state
Patients who experienced a minor complication would recover to the “alive and well” health state after 1 cycle
The risk of mortality in patients treated with TAVI or SAVR were not available beyond 1-year; therefore, mortality rates were extrapolated from the available trial data using various methods.
The long-term medical management costs between those treated with TAVI and those treatment with SAVR were assumed equivalent
Uncertainty surrounding cost parameters were assumed to have a gamma distribution
|
|
Lorenzoni et al. (2021)28
Italy
Funding source: Scuola Superiore Sant’Anna and a research grant from Edwards Lifesciences Italia.
|
Analysis: Cost-utility analysis.
Time horizon: 15 years.
Perspective: The Italian National Health System.
| Patients with aortic stenosis considered to be of various levels of surgical risk, including intermediate, high, or inoperable. |
Intervention: TAVI.
Comparator: SAVR (in high- or intermediate-risk patients) or medical treatment (in inoperable patients). Only the analysis that compared TAVI to SAVR was considered relevant to the current report.
| The analysis used a Markov model with a 1-month cycle length, comprising 9 different health states. The 9 health states included: NYHA I, NYHA I with a history of stroke, NYHA II, NYHA II with a history of stroke, NYHA III, NYHA III with a history of stroke, NYHA IV, NYHA IV with a history of stroke, and death. | Effectiveness inputs, transition probabilities, and utility values for TAVI- and SAVR-treated groups were extrapolated from the PARTNER trials and other key clinical studies. Cost inputs were derived from Italian national tariffs. Data from the literature was used to complement cost information not available from the national tariffs. |
Linear extrapolation was used to extend mortality data to the 15-year time horizon
Complication rates were assumed constant beyond the follow-up period observed in key clinical trials
Cost inputs were assumed to have a normal distribution
Beta distributions were applied for the incidence of adverse events and utilities
|
|
Pinar et al. (2021)29
Spain
Funding source: Edwards
Lifesciences SL.
|
Analysis: Cost-utility analysis.
Time horizon: 15 years.
Perspective: The Spanish National Health System.
| Patients with severe symptomatic aortic stenosis considered to be of various levels of surgical risk, including intermediate, high, or inoperable. |
Intervention: TAVI using the SAPIEN 3 system.
Comparator: SAVR (in high- or intermediate-risk patients) or conservative medical treatment (in inoperable patients). Only the analysis that compared TAVI to SAVR was considered relevant to the current report.
| The analysis used a Markov model with a 1-month cycle length, comprising 9 different health states. The 9 health states included: NYHA I, NYHA I with a history of stroke, NYHA II, NYHA II with a history of stroke, NYHA III, NYHA III with a history of stroke, NYHA IV, NYHA IV with a history of stroke, and death. | Transition parameters and probabilities were derived from key clinical studies, including the PARTNER trials. Costs were estimated using published literature, information provided by the accounts service at Hospital Clínico Universitario Virgen de la Arrixaca, checks of diagnosis-related groups, and expert opinion. The utility values assigned to each health state were extracted from the PARTNER 2 trial, where health-related quality of life was measured using EQ-5D scores. Utilities were adjusted to weight for the Spanish population. |
Mortality rates were extrapolated from 1 year in TAVI-treated patients and 2 years in SAVRtreated patients to the 15-year horizon used in the analysis using various functions
Cost inputs were assumed to have a gamma distribution
Beta distributions were applied for utility values
|
|
Zhou et al. (2021)30
Australia
Funding source: The National Heart Foundation of Australia Fellowship, the Viertel Charitable Foundation Award, a National Health and Medical
Research Council of Australia grant, and the Edwards Fellowship
|
Analysis: Cost-utility analysis.
Time horizon: A lifetime horizon.
Perspective: Australian health care system.
| A hypothetical cohort of patients with severe aortic stenosis considered to be at low surgical risk. Patients entered the model at the age of 73 or 74 years. |
Intervention: Balloon-expandable TAVI or self-expanding TAVI.
Comparator: SAVR.
| A decision-analytic Markov model with 30-day cycles. The model included 4 health states: procedure, alive and well, alive with previous stroke, and dead. | Key clinical data inputs were drawn from the PARTNER 3 trial for balloon-expandable TAVI and the Evolut Low-Risk trial for self-expanding TAVI. Additional clinical inputs were derived from Australian life tables and other sources of clinical literature. Cost inputs were retrieved from the Australian Medicare Benefits Schedule and published costs associated with the provision of TAVI and SAVR. Utility values were estimated using EQ-5D values reported following treatment with TAVI or SAVR in the PARTNER S3i intermediate-risk study. |
Risk of stroke beyond 1 year was assumed to be equal in those treated with TAVI and those treated with SAVR
A hazard ratio for mortality of 1.0 was assumed for those who received TAVI compared to those who received SAVR
Procedural costs for the health care system were assumed to equal to the amount reimbursed by Medicare
When standard errors were unavailable to define probability distributions for costs the standard error was assumed to be 1-third of the mean
|
|
Health Technology Wales (2020)22
Wales
Funding source: Health Technology Wales is funded by the Welsh government.
|
Analysis: Cost-utility analysis conducted as part of an HTA.
Time horizon: A lifetime horizon.
Perspective: The UK National Health Service and personal social services.
| People with severe symptomatic aortic stenosis at intermediate surgical risk.
Patients entered the model at the age of 81.6 years and were 55% male (based on the PARTNER 2 trial). |
Intervention: TAVI
Comparator: SAVR
| The analysis used a Markov model with a 1-month cycle length, comprising 3 main health states and 1 complications health state. The main health states were: 1) alive with no complications, 2) disabling stroke, and 3) dead. The complications health state could be transitioned to for 1 cycle before returning to the alive with no complications state. | Mortality and complication rates were obtained from the PARTNER 2 study. Costs of the valve replacement procedure and associated costs were obtained from National Health Service Reference costs 2018/19. Costs of managing complications were National Health Service Reference costs and clinical expert opinion. Utility values were derived from EQ-5D scores measured in the PARTNER 2 study. |
Risk of complications from both procedures was assumed to be 0 after 2 years
Mortality rates beyond 2 years were calculated from general population mortality multiplied by a hazard ratio of 1.15, regardless of treatment received (i.e., TAVI and SAVR were assumed equivalent)
Quality of life 2 years post-procedure was assumed equivalent between TAVI and SAVR patient groups
|
|
Inoue et al. (2020)31
Japan
Funding source: Edwards Lifesciences Limited.
|
Analysis: Cost-utility analysis.
Time horizon: A lifetime horizon.
Perspective: Public health care payers.
|
High surgical risk and inoperable patients with severe aortic stenosis.
High-risk patients entered the model at the age of 81; inoperable patients entered at the age of 83.
|
Intervention: Transfemoral TAVI using the SAPIEN XT system.
Comparator: SAVR (in high-risk patients) or supportive pharmacotherapy (in inoperable patients). Only the analysis that compared TAVI to SAVR was considered relevant to the current report.
| A 2-phase economic model that used a decision tree model for the initial 2 years of analysis followed by a Markov model with 1-year cycles. The decision tree modelled the incidence of myocardial infarction, stroke, renal failure, new pacemaker implantation, new atrial fibrillation, heart failure hospitalization and death at 6, 12, and 24 months. The Markov model included 2 health states: alive and dead. | Clinical inputs for TAVI and comparators were retrieved using a systematic review of the literature. Costs associated with treatments were calculated using a medical claims database provided by Medical Data Vision Co, Ltd. Utility values after TAVI were extracted from the PARTNER trial, whereas utility values from other health states were taken from a previously published economic evaluation. |
The cost of post-operative follow-up in years 3 and beyond was assumed to be the same as year 2
The probabilistic sensitivity analysis assumed a beta distribution for transition probabilities and utility values and a gamma distribution for costs
|
|
Kuntjoro et al. (2020)32
Singapore
Funding source: The Singapore Ministry of Health (through a National Medical Research
Council research grant)
|
Analysis: Cost-utility analysis.
Time horizon: 8-year horizon (scenario analyses considered a 5-year time horizon)
Perspective: The National University Health System in Singapore.
| A hypothetical cohort of patients with severe aortic stenosis considered to be at intermediate or low surgical risk. Patients entered the model at the age of 82 years. |
Intervention: Transfemoral
TAVI using balloon-expandable SAPIEN systems.
Comparator: SAVR.
| A 2-phase economic model that used a decision tree model for the initial 30 days after the procedure followed by a long-term Markov model with 1-year cycles. | Clinical parameters, such as post-operative mortality rates and risk for complications, were derived from the PARTNER 2A RCT, the PARTNER 2 S3 observational trial, and Singapore life tables. Cost inputs were retrieved from the National University Health System database, data published by the National Kidney Foundation, or from other published sources. Health utility values were taken from the Singapore population norm for EQ-5D using local preference weights. |
The risk for chronic complications was equivalent between patients treated with TAVI and SAVR after 2 years (based on clinical literature that suggested there is no difference within the first 2 years)
Annual mortality rates were derived from 5-year mortality rates and assumed a linear increase in mortality over time
Patients who developed chronic complications (e.g., stroke, acute kidney injury, myocardial infarction) were assumed to stay in those health states until they died
Paravalvular leak was not considered as a potential complication in the long-term model due to data unavailability
|
|
HIQA (2019)24
Ireland
Funding source: HIQA is funded by the Irish government.
|
Analysis: Cost-utility analysis conducted as part of an HTA.
Time horizon: 15 years (scenario analyses considered lifetime and 5-year time horizons).
Perspective: The publicly funded health and social care system in Ireland.
| Patients with severe symptomatic aortic stenosis at low (e.g., STS-PROM < 4%) or intermediate risk (e.g., STS-PROM ≥ 4 and < 8%) of surgical complications. At model entry patients were 76 years of age and 55% were male. |
Intervention: TAVI
Comparator: SAVR.
| A decision-analytic model that comprised a 9 state Markov model that simulated patient outcomes in 1-month cycles. The health states included “alive and well,” “major complications,” “post major complications,” “re-hospitalization,” and “death.” The “major complications” and “post major complications” each included 3 health states (i.e., acute kidney injury, disabling stroke, and myocardial infarction) to reflect the different risk of mortality associated with each complication. | Clinical effectiveness inputs for intermediate-risk patients were derived from the PARTNER 2 trial. This study was chosen following a systematic review of the literature, and the data from these patients was deemed most appropriate to use in the model. Similarly, data from the PARTNER 3 trial was used to model clinical effectiveness in low-risk patients following a systematic review of the literature. All-cause mortality data beyond 2 years in intermediate-risk patients and 1 year in low-risk patients were calculated from national life tables for Ireland from 2015, after applying a higher relative risk from published literature. Cost estimates were derived from relevant Diagnostic Related Group codes in Ireland or from previously published HTAs. Utility estimates were obtained from the PARTNER 2 RCT, where patients’ healthrelated quality of life was measuring using the 3-level EQ-5D questionnaire. |
There was no difference in rates of clinical events in both arms beyond the observed trial data (i.e., 2 years in intermediate-risk patients, 1 year in low-risk patients) due to a lack of evidence
Beta distributions were assumed for all probabilities in the probabilistic analysis
Lognormal distributions were assumed for all relative risks
All cost inputs assumed lognormal distributions in the probabilistic analysis
Health utilities of patients treated with TAVI considered to be at low-risk were assumed to be the same as those at intermediate-risk
|
HIQA = Health Information and Quality Authority; HTA = health technology assessment; NIPH = Norwegian Institute of Public Health; NYHA = New York Heart Association; PARTNER = Placement of AoRTic TraNscathetER Valve; TAVI = transcatheter aortic valve implantation; RCT = randomized controlled trial. SAVR = surgical aortic valve replacement; STS-PROM = Society of Thoracic Surgeons predicted risk of mortality.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has been formatted for accessibility but has not been copy-edited.
Table 8Strengths and Limitations of Systematic Reviews Using AMSTAR 220
View in own window
| Strengths | Limitations |
|---|
| Azraai et al. (2020)25 |
|---|
|
The objectives and inclusion criteria were clearly stated and included components of population, intervention, comparator, and outcomes
The choice of included study designs (i.e., any studies presenting cost-effectiveness data) was explained
Multiple databases were searched (Ovid MEDLINE, PubMed, Embase, and the Cochrane Database of Systematic Reviews). Additionally, reference lists from identified studies were further screened for any other eligible studies
Key search terms and search restrictions were provided (e.g., studies published in English between January 2010 and November 2019 were eligible)
A flow chart of study selection was provided
The review authors described the included studies in adequate detail
|
It was unclear whether the review methods were established before conducting the review (no mention of a protocol)
A grey literature search was not completed
It was unclear if study selection and data extraction were conducted in duplicate
The quality of included studies was not assessed
A list of studies excluded after full-text review was not provided (although the reasons for exclusion were)
Review authors did not report on sources of funding for the included primary studies
Review authors did not state their potential conflicts of interest
The source of funding for the review was not disclosed
|
| Health Technology Wales (2020)22 |
|---|
|
The objectives and inclusion criteria were clearly stated and included components of population, intervention, comparator, and outcomes
The choice of included study designs (i.e., economic evaluations) was explained
Evidence from 2 previously published HTA reports was included in the analysis. Additionally, literature searches were performed in multiple databases (MEDLINE, Embase, the Cochrane Library, and clinical trials registries) to identify evidence published since the HTAs
Key search terms and search restrictions were provided (e.g., studies published in English were eligible)
A flow chart of study selection was provided
The review authors described the included studies in adequate detail
The quality of included primary studies was assessed using a satisfactory technique
Review authors reported on sources of funding for the included primary studies
Sources of funding were disclosed and were unlikely to have had an effect on the findings of the review
|
It was unclear whether the review methods were established before conducting the review (no mention of a protocol)
A grey literature search was not completed
It was unclear if study selection, data extraction, and quality assessment were conducted in duplicate
A list of studies excluded after full-text review was not provided (although the reasons for exclusion were)
Review authors did not state their potential conflicts of interest
|
| Ontario Health (2020)23 |
|---|
|
The objectives and inclusion criteria were clearly stated and included components of population, intervention, comparator, and outcomes
The choice of included study designs (i.e., cost-benefit analyses, cost-effectiveness analyses, or cost-utility analyses) was explained
Evidence from a previously published HTA report was included in the analysis. Additionally, literature searches were performed in multiple databases (Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and the NHS Economic Evaluation Database) to identify evidence published since the HTAs and a targeted grey literature search of HTA agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry was performed
Key search terms and search restrictions were provided (e.g., studies published in English between January 1, 2011, and July 12, 2019 were eligible)
A flow chart of study selection was provided
The review authors described the included studies in adequate detail
The quality of included primary studies was assessed using a satisfactory technique (i.e., a modified quality appraisal checklist for economic evaluations originally developed by NICE)
Sources of funding were disclosed and were unlikely to have had an effect on the findings of the review
|
It was unclear whether the review methods were established before conducting the review (no mention of a protocol)
Study selection was performed by a single reviewer
It was unclear if data extraction and quality assessment were conducted in duplicate
A list of studies excluded after full-text review was not provided (although the reasons for exclusion were)
Review authors did not report on sources of funding for the included primary studies
Review authors did not state their potential conflicts of interest
|
| HIQA (2019)24 |
|---|
|
The objectives and inclusion criteria were clearly stated and included components of population, intervention, comparator, and outcomes
The choice of included study designs (i.e., economic evaluations) was explained
Multiple databases were searched (PubMed, Embase, the Cochrane Library, and the University of York CRD database for economic evaluations). In addition, a grey literature search and a search in Scopus were conducted
Key search terms and search restrictions were provided (e.g., studies published in any language between January 1, 2013 and June 28, 2019 were eligible)
A flow chart of study selection was provided
Full-text screening was conducted by 2 reviewers
Data extraction and quality assessment were conducted by 2 independent reviewers (disagreements were resolved through discussion, or by a third reviewer)
The review authors described the included studies in adequate detail
The quality of included primary studies was assessed using a satisfactory technique (i.e., the Consensus on Health Economic Criteria list)
Review authors reported on sources of funding for the included primary studies
Review authors stated that they had no conflicts of interest related to this review
Sources of funding were disclosed and were unlikely to have had an effect on the findings of the review
|
It was unclear whether the review methods were established before conducting the review (no mention of a protocol)
The first level of screening (i.e., title and abstracts) was performed by a single reviewer
A list of studies excluded after full-text review was not provided (although the reasons for exclusion were)
|
| Gialama et al. (2018)26 |
|---|
|
The objectives and inclusion criteria were clearly stated and included components of population, intervention, comparator, and outcomes
The choice of included study designs (i.e., economic evaluations) was explained
Multiple databases were searched (PubMed and Cochrane databases). Additionally, the search was supplemented using internet search engines and reference lists from identified studies were further screened for any other eligible studies
Key search terms and search restrictions were provided (e.g., studies published before June 2017 in English were eligible)
A flow chart of study selection was provided
The review authors described the included studies in adequate detail
The quality of included primary studies was assessed using a satisfactory technique (i.e., 35-item British Medical Journal checklist to economic evaluations)
Review authors reported on sources of funding for the included primary studies
Review authors stated that they had no conflicts of interest related to this review
|
It was unclear whether the review methods were established before conducting the review (no mention of a protocol)
It was unclear if study selection, data extraction, and quality assessment were conducted in duplicate
A list of studies excluded after full-text review was not provided (although the reasons for exclusion were)
The source of funding for the review was not disclosed
|
AMSTAR 2 = A MeaSurement Tool to Assess systematic Reviews 2; CRD = Centre for Reviews and Dissemination; HIQA = Health Information and Quality Authority; HTA = health technology assessment; NICE = National Institute for Health and Care Excellence.
Table 9Strengths and Limitations of Economic Evaluations Using the Drummond Checklist21
View in own window
| Strengths | Limitations |
|---|
| Himmels et al. (2021)27 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The designs and results of effectiveness studies from which assumptions were drawn were provided The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Details of the subjects from whom valuations were obtained were given Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2020 Norwegian kroner) The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (15-year horizon) The discount rate for costs and benefits was stated (4% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
|
Model inputs were taken from single trials, rather than a synthesis or meta-analysis of estimates from multiple sources
No description of currency price adjustments for inflation was provided
No justification for the selected discount rate was provided
The findings of this Norway-based study may not be generalizable to the Canadian health system
|
| Lorenzoni et al. (2021)28 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The designs and results of effectiveness studies from which assumptions were drawn were provided The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2019 euros) The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (15-year horizon) The discount rate for costs and benefits was stated (3% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
|
Limited information was provided on the characteristics of patient populations from whom model inputs were obtained
Model inputs were taken from single trials, rather than a synthesis or meta-analysis of estimates from multiple sources
No description of currency price adjustments for inflation was provided
No justification for the selected discount rate was provided
This work was funded by industry
The findings of this Italy-based study may not be generalizable to the Canadian health system
|
| Pinar et al. (2021)29 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The results of effectiveness studies from which assumptions were drawn were provided The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2019 euros) The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (15-year horizon) The discount rate for costs and benefits was stated (3% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
|
The designs of effectiveness studies from which assumptions were drawn were not provided
Limited information was provided on the characteristics of patient populations from whom model inputs were obtained
Model inputs were taken from single trials, rather than a synthesis or meta-analysis of estimates from multiple sources
No description of currency price adjustments for inflation was provided
No justification for the selected discount rate was provided
This work was funded by industry
The findings of this Spain-based study may not be generalizable to the Canadian health system
|
| Zhou et al. (2021)30 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The designs and results of effectiveness studies from which assumptions were drawn were provided The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Details of the subjects from whom valuations were obtained were given Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2019 Australian dollars) The details of currency price adjustments for inflation were given The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (lifetime horizon) The discount rate for costs and benefits was stated and justified (5% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
Sources of funding were disclosed and were unlikely to have had an effect on the findings of the analysis Study authors stated their potential conflicts of interest (2 authors stated they had no conflicts of interest to declare; 4 authors declared various ties to industry)
|
Model inputs were taken from single trials, rather than a synthesis or meta-analysis of estimates from multiple sources
The findings of this Australia-based study may not be generalizable to the Canadian health system
|
| Health Technology Wales (2020)22 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The designs and results of effectiveness studies from which assumptions were drawn were provided The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Details of the subjects from whom valuations were obtained were given Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2019 pound sterling) The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (lifetime horizon) The discount rate for costs and benefits was stated and justified (3.5% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
|
Model inputs were taken from single trials, rather than a synthesis or meta-analysis of estimates from multiple sources
No description of currency price adjustments for inflation was provided
Study authors did not state their potential conflicts of interest
The findings of this Wales-based study may not be generalizable to the Canadian health system
|
| Inoue et al. (2020)31 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The designs and results of effectiveness studies from which assumptions were drawn were provided The clinical inputs for the model were derived from a systematic review of the literature The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Details of the subjects from whom valuations were obtained were given Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2016 Japanese yen) The details of currency price adjustments for inflation were given The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (lifetime horizon) The discount rate for costs and benefits was stated (2% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
|
No justification for the selected discount rate was provided
This work was funded by industryThe findings of this Japan-based study may not be generalizable to the Canadian health system
|
| Kuntjoro et al. (2020)32 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The designs and results of effectiveness studies from which assumptions were drawn were provided The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Details of the subjects from whom valuations were obtained were given Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2017 Singapore dollars) The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (8-year horizon) The discount rate for costs and benefits was stated (3% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
|
Model inputs were taken from single trials, rather than a synthesis or meta-analysis of estimates from multiple sources
No description of currency price adjustments for inflation was provided
No justification for the selected discount rate was providedStudy authors did not state their potential conflicts of interest
The findings of this Singapore-based study may not be generalizable to the Canadian health system
|
| HIQA (2019)24 |
|---|
Study design
The research question, economic importance of the research question, and rationale for choosing alternative interventions compared were clearly stated The treatment strategies being compared were clearly described The form of economic evaluation used was stated The viewpoint/perspective of the analysis was clearly stated and justified The choice of form of economic evaluation was justified in relation to the questions addressed
Data collection
The sources of effectiveness estimates and treatment costs were provided The designs and results of effectiveness studies from which assumptions were drawn were provided The clinical inputs for the model were derived from a systematic review of the literature The primary outcome measures for the economic evaluation were clearly stated Methods to value benefits were stated Details of the subjects from whom valuations were obtained were given Methods for the estimation of quantities and unit costs were described Currency and price data were recorded (2019 euros) The structure of the model was clearly described using figures
Analysis and interpretation of results
Time horizon of costs and benefits was stated (15-year horizon) The discount rate for costs and benefits was stated and justified (4% per year) The approach to sensitivity analysis was given The choice of variables for the sensitivity analysis was justified Incremental analyses were reported The answer to the study question was given Conclusions followed from the data reported Conclusions were accompanied by appropriate caveats
Miscellaneous
|
No description of currency price adjustments for inflation was provided
Study authors did not state their potential conflicts of interest
The findings of this Ireland-based study may not be generalizable to the Canadian health system
|
Appendix 4. Main Study Findings and Authors’ Conclusions
Note that this appendix has been formatted for accessibility but has not been copy-edited.
Summary of Findings Included Systematic Reviews
Azraai et al. (2020)25
Main study findings
Systematic review that evaluated the cost-effectiveness of TAVI for patients with aortic stenosis at low to intermediate surgical risk.
Relevant primary studies: A total of 8 cost-effectiveness studies were included in the systematic review, all of which were relevant to the current report. Relevant results are summarized individually by primary study.
Intermediate-risk patients
Baron et al. (2019)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Goodall et al. (2019)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Osnabrugge et al. (2012)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Tam et al. (2018a)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Tam et al. (2018b)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Zhou et al. (2019a)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Low-risk patients
Geisler et al. (2019)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
At a willingness-to-pay threshold of DKK 1,130,000 per QALY, TAVI had a 78% probability of being cost-effective
Zhou et al. (2019b)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Authors’ conclusion
“The present systematic review showed that TAVI is potentially cost-effective alternative to SAVR in patients with intermediate surgical risk, possibly extending to those with low surgical risk. Although the raw costs of TAVI is greater, the procedure has been associated with a significant gain in QALY compared to SAVR (p. 1167).”25
Health Technology Wales (2020)22
Main study findings
Systematic review (conducted as part of an HTA) that summarized the evidence regarding the cost-effectiveness of TAVI for severe symptomatic aortic stenosis in adults at intermediate surgical risk.
Relevant primary studies: A total of 10 cost-utility analyses were included in the systematic review, all of which were relevant to the current report. Relevant results are summarized individually by primary study.
Baron et al. (2019)
Total costs
SAVR: US$235,312; SAPIEN XT TAVI: US$227,363
SAVR: US$240,871; SAPIEN 3 TAVI: US$231,179
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
SAPIEN XT TAVI: 0.15
SAPIEN 3 TAVI: 0.27
ICER (incremental costs/incremental QALYs; versus SAVR)
SAPIEN XT TAVI: Dominant (i.e., treatment resulted in more QALYs and less costs)
SAPIEN 3 TAVI: Dominant (i.e., treatment resulted in more QALYs and less costs)
Probability of cost-effectiveness
SAPIEN XT TAVI: NR
SAPIEN 3 TAVI: NR
Goodall et al. (2019)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Health Information and Quality Authority (2019)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Kodera et al. (2018)
Total costs
SAVR: ¥6,316,178; TAVI: ¥8,039,694
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
At a willingness-to-pay threshold of ¥5,000,000 per QALY, TAVI had a 46% probability of being cost-effective
Norwegian Institute of Public Health (2019)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Scottish Health Technologies Group (2019)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Tam et al. (2018a)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Tam et al. (2018b)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Tarride et al. (2019)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Zhou et al. (2019a)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Authors’ conclusion
“The results of the economic analysis suggested that TAVI is more effective and more costly than SAVR but not cost-effective as the ICER exceeds the threshold of £20,000 per QALY. This result was consistent with the analysis by SHTG and other analyses in this area. However, some of the analyses in other settings have found contrasting results, with TAVI found to be less costly and more effective than SAVR (i.e. dominant). The contrasting results primarily reflect differences in the procedure costs for TAVI and SAVR (p. 35).”22
Ontario Health (2020)23
Main study findings
Systematic review (conducted as part of an HTA) that evaluated the cost-effectiveness of TAVI for adults with severe aortic valve stenosis who are at low surgical risk.
Relevant primary studies: A total of 1 cost-utility analysis was included in the systematic review. This analysis was relevant to the current report.
Tam et al. (2020)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
At a willingness-to-pay thresholds of CA$50,000 and CA$100,000 per QALY, balloon-expandable TAVI had 53% and 59% probabilities of being cost-effective, respectively
At a willingness-to-pay thresholds of CA$50,000 and CA$100,000 per QALY, self-expanding TAVI had < 5% and < 10% probabilities of being cost-effective, respectively
Authors’ conclusion
“Our review of the literature identified one published cost-effectiveness analysis that compared TAVI with SAVR in adults with severe aortic valve stenosis who were at low surgical risk. The study was directly applicable and conducted from the perspective of the Ontario Ministry of Health. The TAVI procedure might be cost-effective for patients at low surgical risk; however, there is some uncertainty in this result (p. 56).”23
HIQA (2019)24
Main study findings
Systematic review (conducted as part of an HTA) that investigated the cost-effectiveness of TAVI compared to SAVR in patients with severe symptomatic aortic stenosis at low or intermediate risk of surgical complications.
Relevant primary studies: A total of 7 economic evaluations were included in the systematic review, all of which were relevant to the current report. Relevant results are summarized individually by primary study.
Baron et al. (2019)
Total costs
SAVR: US$235,312; SAPIEN XT TAVI: US$227,363
SAVR: US$240,871; SAPIEN 3 TAVI: US$231,179
ICER (incremental costs/incremental QALYs; versus SAVR)
SAPIEN XT TAVI: Dominant (i.e., treatment resulted in more QALYs and less costs)
SAPIEN 3 TAVI: Dominant (i.e., treatment resulted in more QALYs and less costs)
Goodall et al. (2019)
ICER (incremental costs/incremental QALYs; versus SAVR)
Kaier et al. (2019)
Risk-adjusted in-hospital mortality
Kodera et al. (2018)
Total costs
SAVR: ¥6,316,178; TAVI: ¥8,039,694
ICER (incremental costs/incremental QALYs; versus SAVR)
Tam et al. (2018a)
ICER (incremental costs/incremental QALYs; versus SAVR)
Tam et al. (2018b)
ICER (incremental costs/incremental QALYs; versus SAVR)
Zhou et al. (2019a)
ICER (incremental costs/incremental QALYs; versus SAVR)
Authors’ conclusion
“This systematic review identified seven studies to date that evaluated the cost-effectiveness of TAVI versus SAVR in intermediate risk patients. The cost-effectiveness of the device was generally supported in these studies; however, a number of concerns regarding the quality and credibility of the economic evaluations were identified. These largely related to model structure (for example, many studies modelled implausible health state transitions) and choice of input parameters (for example, few studies comprehensively evaluated postoperative complications). The systematic review found no studies that considered the cost-effectiveness of the device in patients at low surgical risk (p. 82–83).”
Gialama et al. (2018)26
Main study findings
Systematic review that assessed the cost-effectiveness of interventions for valvular heart disease.
Relevant primary studies: The systematic review included 24 studies that investigated various transcatheter interventions for valvular heart disease; however, only primary studies that compared TAVI versus SAVR for the treatment of severe aortic stenosis were relevant to the current report (11 studies). Relevant results are summarized individually by primary study.
Doble et al. (2012)
Incremental QALYs (versus SAVR)
Incremental life-years gained (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Fairbairn et al. (2013)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
£20,000 to £30,000 per QALY were used as the willingness-to-pay thresholds; however, the probability that TAVI was cost-effective at these thresholds was NR
Gada et al. (2012a)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Gada et al. (2012b)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Neyt et al. (2012)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Orlando et al. (2013)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
£20,000 to £30,000 per QALY were used as the willingness-to-pay thresholds; however, the probability that TAVI was cost-effective at these thresholds was NR
Osnabrugge et al. (2012)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
Reynolds et al. (2012)
Total costs
SAVR: US$98,434; transfemoral-transapical TAVI: US$100,504
SAVR: US$97,992; transfemoral TAVI: US$96,743
SAVR: US$99,499; transapical TAVI: US$109,405
QALYs
SAVR: 0.606; transfemoral-transapical TAVI: 0.633
SAVR: 0.591; transfemoral TAVI: 0.659
SAVR: 0.641; transapical TAVI: 0.570
Life-years gained
SAVR: 0.817; transfemoral-transapical TAVI: 0.858
SAVR: 0.813; transfemoral TAVI: 0878
SAVR: 0.826; transapical TAVI: 0.811
ICER (incremental costs/incremental QALYs; versus SAVR)
Transfemoral-transapical TAVI: US$76,877/QALY
Transfemoral TAVI: Dominant (i.e., treatment resulted in more QALYs and less costs)
Transapical TAVI: Dominated (i.e., treatment resulted in less QALYs and more costs)
Probability of cost-effectiveness
Reynolds et al. (2016)
ICER (incremental costs/incremental QALYs; versus SAVR)
ICER (incremental costs/incremental life-year gained; versus SAVR)
Probability of cost-effectiveness
US$50,000 and US$150,000 per QALY were used as the willingness-to-pay thresholds; however, the probability that TAVI was cost-effective at these thresholds was NR
Ribera et al. (2015)
Total costs
SAVR: €23,288; Edwards SAPIEN self-expandable TAVI: €32,087; Medtronic CoreValve balloon-expandable TAVI: €32,111
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
SHTG (2010)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
£20,000 to £30,000 per QALY were used as the willingness-to-pay thresholds; however, the probability that TAVI was cost-effective at these thresholds was NR
Authors’ conclusion
“…the cost-effectiveness of TAVI compared with SAVR in high-risk operable patients, based on the current data, is uncertain. Some studies show it to dominate, others to be dominated, others to be cost-effective and other not. Hence, is not possible to reach any safe conclusions. The limited evidence available, supports that whether TAVI compared with SAVR is cost-effective may depend on the access route (transfemoral or transapical), the procedural cost, and the patients selected, since in general terms TAVI and SAVR have demonstrated similar survival and only small differences in benefit in terms of quality of life. More research is needed in this area (p. 89).”26
Summary of Findings of Included Economic Evaluations
Himmels et al. (2021)27
Main study findings
Cost-utility analysis (conducted as part of an HTA) that examined the cost-effectiveness of TAVI versus SAVR in patients with severe calcific aortic stenosis considered to be at low surgical risk from the Norwegian health care perspective.
Summary of findings
Base-case results
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probabilities of cost-effectiveness
Scenario analysis (minimal cost of SAVR procedures)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probability of cost-effectiveness
TAVI was cost-effective at a willingness-to-pay threshold over NOK 436,400
SAVR was cost-effective at a willingness-to-pay threshold under NOK 436,400
Scenario analysis (maximal cost of SAVR procedures)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probabilities of cost-effectiveness
Scenario analysis (time horizon 1 year)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probabilities of cost-effectiveness
Authors’ conclusion
“The cost-utility analysis indicated that TAVI for patients at low surgical risk was marginally more effective (incremental effectiveness: 0.05 QALYs) and less costly (saving of NOK 35000) than SAVR. The analysis is based on 1-year follow-up data from the PARTNER 3 study and long-term mortality and adverse events for TAVI and SAVR beyond this period remain unclear. The results are sensitive to variations in procedure costs (p. 66).”27
Lorenzoni et al. (2021)28
Main study findings
Cost-utility analysis that modelled the cost-effectiveness of TAVI versus SAVR (in high- or intermediate-risk patients) or medical treatment (in inoperable patients) in patients with severe symptomatic aortic stenosis. Findings from the analysis in inoperable patients were not summarized as they were not relevant to the current report.
Summary of findings
Base-case results in intermediate-risk patients
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
Incremental life-years gained (versus SAVR)
ICER (incremental costs/incremental QALYs)
ICER (incremental costs/incremental life-year gained)
Base-case results in high-risk patients
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
Incremental life-years gained (versus SAVR)
ICER (incremental costs/incremental QALYs)
ICER (incremental costs/incremental life-years gained)
Probabilities of cost-effectiveness
“Cost-effectiveness acceptability curves, shown in Fig. 5, suggest that considering conventional thresholds defined at national level (typically comprised between €25,000–40,000/QALY), TAVI showed high probability (in the range of about 90–100%) of being cost-effective in all risk groups, both when considering the upper and lower limits of the range of value generally considered in Italy (p. 8).”
28
Sensitivity analyses
Sensitivity analyses suggested that mortality along the time horizon, major incidence of stroke in the short-term, and repeated hospitalizations for aortic stenosis were among the main drivers of base-case results.
Authors’ conclusion
“The results of the cost-effectiveness analysis performed show that, considering the Italian National Health System perspective, TAVI would be considered highly cost-effective at frequently cited willingness to pay thresholds. Similar conclusions emerged over a range of analyses performed and also modelling a scenario considering micro-costing data. Indeed, the diverse of analyses performed offer the possible range defining the value for money of TAVI and offer important messages to clinicians and decision maker on both the overall value of TAVI, but also on the feasibility of considering the procedure over diverse risk groups, some of which were rarely considered as candidate for TAVI procedure both in view of the limited evidence related to both clinical and economic implications (p. 12).”28
Pinar et al. (2021)29
Main study findings
A cost-utility analysis that investigated the cost-effectiveness of TAVI using the SAPIEN 3 system versus SAVR (in high- or intermediate-risk patients) or conservative medical treatment (in inoperable patients) from the perspective of the Spanish National Health System. Only the analysis that compared TAVI to SAVR was considered relevant to the current report.
Summary of findings
Base-case results in intermediate-risk patients
ICER (incremental costs/incremental QALYs)
ICER (incremental costs/incremental life-years gained)
Base-case results in high-risk patients
ICER (incremental costs/incremental QALYs)
ICER (incremental costs/incremental life-years gained)
Probabilistic sensitivity analyses
TAVI was the cost-effective option in 74.9% of simulations and dominant in 24.3% of simulations in intermediate-risk patients, based on a willingness-to-pay threshold of €30,000 per QALY gained
TAVI was cost-effective in 87.8% of simulations and dominant in 12.2% of simulations in high-risk patients, based on a willingness-to-pay threshold of €30,000 per QALY gained
Authors’ conclusion
“Treatment of aortic stenosis by S3 TAVI is an effective option for inoperable patients, patients at high surgical risk, and those at intermediate risk and is, moreover, highly likely to be the most cost-effective strategy. With the increasing health care demands linked to population aging, there is a growing need for technological innovations that permit less invasive interventions to simplify procedures while maintaining clinical standards and optimizing resource use (p. 7).”29
Zhou et al. (2021)30
Main study findings
Cost-utility analysis that examined the cost-effectiveness of balloon-expandable or self-expanding TAVI versus SAVR in patients with severe aortic stenosis considered to be at low surgical risk, from the perspective of the Australian health care system.
Summary of findings
Base-case results for balloon-expandable TAVI
Incremental costs (versus SAVR)
Incremental life-years gained (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental life-years gained)
ICER (incremental costs/incremental QALYs)
Base-case results for self-expanding TAVI
Incremental costs (versus SAVR)
Incremental life-years gained (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental life-years gained)
ICER (incremental costs/incremental QALYs)
Probabilities of cost-effectiveness
Probabilistic sensitivity analyses suggested that balloon-expandable TAVI was cost-effective in 78% of Monte Carlo iterations at a willingness-to-pay threshold of AU$50,000 per QALY gained. At a willingness-to-pay threshold of AU$100,000 per QALY gained balloon-expandable TAVI was cost-effective in 88% of iterations.
Self-expanding TAVI was considered cost-effective in 70% and 80% of iterations when willingness-to-pay thresholds of AU$50,000 and AU$100,000 were applied, respectively
Sensitivity analyses
“Balloon-expandable TAVI remained cost-effective compared to SAVR when most input parameters were varied across their 95% confidence intervals. The ICER only exceeded the willingness-to-pay threshold of AU$50,000 per QALY gained if the cost of the TAVI valve exceeded A$35,511 (versus A$26,250 in the base case) (p. 550).”
“For self-expanding TAVI, findings were also robust to changes in most model inputs over plausible ranges. However, compared with the balloon-expandable TAVI analysis, results were more sensitive to key cost drivers such as the price of the transcatheter valve and length of intensive care unit stay. As shown in Figure 2B, self-expanding TAVI was no longer cost-effective if the price of the TAVI valve exceeded AU$27,286 or if TAVI intensive care unit length of stay exceeded the base-case estimate of 2 days (p. 550).”
Authors’ conclusion
“Among low-risk patients with severe symptomatic AS, both balloon-expandable and self-expanding TAVI are likely to be cost-effective compared with SAVR from the perspective of the Australian health care system (p. 553).”30
Health Technology Wales (2020)22
Main study findings
A cost-utility analysis (conducted as part of an HTA) that estimated the cost-effectiveness of TAVI compared to SAVR for people with severe symptomatic aortic stenosis at intermediate surgical risk. The analysis was conducted from the perspective of the UK National Health Service and personal social services.
Summary of findings
Base-case results
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probabilities of cost-effectiveness
Sensitivity analyses
Baseline age = 70 years
ICER (incremental costs/incremental QALYs; versus SAVR)
Baseline age = 75 years
ICER (incremental costs/incremental QALYs; versus SAVR)
Baseline age = 80 years
ICER (incremental costs/incremental QALYs; versus SAVR)
Baseline age = 85 years
ICER (incremental costs/incremental QALYs; versus SAVR)
Only statistically significant differences in complications included
ICER (incremental costs/incremental QALYs; versus SAVR)
TAVI and SAVR costs based on HIQA estimates
ICER (incremental costs/incremental QALYs; versus SAVR)
Additional sensitivity analyses are available in the publication.22
Authors’ conclusion
“The results of the analysis show that TAVI was marginally more effective than SAVR but substantially more costly. The resulting ICER of £94,512 per QALY is substantially higher than the £20,000 per QALY threshold indicating that TAVI is not cost-effective (p. 72).”22
Inoue et al. (2020)31
Main study findings
Cost-utility analysis that investigated the cost-effectiveness of transfemoral TAVI using the SAPIEN XT system versus SAVR (in high surgical risk patients) or supportive pharmacotherapy (in inoperable patients) from the public health care perspective in Japan. Only findings from the analysis that compared TAVI to SAVR were considered relevant to the current report.
Summary of findings
Base-case results
Total costs
SAVR: ¥6,169,068; TAVI: ¥7,725,818
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probabilities of cost-effectiveness
Using a willingness-to-pay threshold of ¥5,000,000/QALY, the probability of TAVI being cost-effective compared with SAVR was 99.9%
Scenario analysis (based on domestic studies)
Total costs
SAVR: ¥6,116,945; TAVI: ¥7,098,560
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Sensitivity analyses
Authors’ conclusion
“This is the first study evaluating the cost-effectiveness of TAVI in which a systematic literature review was conducted and Japanese claims data were used. The results demonstrated that transfemoral TAVI was cost-effective compared with SAVR for high-risk patients and with standard of care for inoperable patients. Assuming a cost-effectiveness threshold of ¥5 million/QALY, the probabilities of transfemoral TAVI being cost-effective compared with SAVR and standard of care were 99.9% and 93.9%, respectively, validating the robustness of these findings (p. 89).”31
Kuntjoro et al. (2020)32
Main study findings
A cost-utility analysis that examined the cost-effectiveness of transfemoral TAVI using balloon-expandable SAPIEN systems compared to SAVR in patients with severe aortic stenosis considered to be at intermediate or low surgical risk from the perspective of the National University Health System in Singapore.
Summary of findings
Base-case results
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs; versus SAVR)
Probabilities of cost-effectiveness
Deterministic sensitivity analyses
“The model was the most sensitive to changes of operational costs for transfemoral TAVI and SAVR. However, the models were robust as ICERs remain similar when applying significant changes on parameters (p. 428).”
32
Scenario analyses
“Shortening the time horizon while holding all other base-case assumptions constantly resulted in larger ICERs for transfemoral TAVI. Using the events rates based on the newer TAVI systems generated more favourable results for transfemoral TAVI. Assuming no new cases for long-term complications or the 2 treatments had same probabilities of stroke, myocardial infarction, and acute kidney injury beyond 2 years also led to higher ICERs (p. 428).”
32
Authors’ conclusion
“From the perspective of the Singapore healthcare system, transfemoral TAVI is highly likely to be a cost-effective treatment option for intermediate and low risk patients with severe symptomatic aortic stenosis, in comparison with SAVR. Our study would provide the first evidence for the potential of TAVI to be an alternative treatment to the conventional SAVR in Singapore. The positive result might contribute to the future formulation of local clinical practice guidelines or health policies (p. 432).”32
HIQA (2019)24
Main study findings
Cost-utility analysis (conducted as part of an HTA) that assessed the cost-effectiveness of TAVI versus SAVR in patients with severe symptomatic aortic stenosis at low (e.g., Society of Thoracic Surgeons predicted risk of mortality [STS-PROM] < 4%) or intermediate risk (e.g., STS-PROM ≥ 4 and < 8%) of surgical complications from the perspective of the publicly funded health and social care system in Ireland.
Summary of findings
Base-case results in intermediate-risk patients
Total costs
SAVR: €42,879 (95% confidence interval (CI) = €36,493 to €49,946); TAVI: €42,681 (95% CI = €36,584 to €49,475)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs)
Incremental net monetary benefit
Base-case results in low-risk patients
Total costs
SAVR: €38,643 (95% CI = €31,071 to €48,328); TAVI: €38,256 (95% CI = €31,064 to €47,690)
Incremental costs (versus SAVR)
Incremental QALYs (versus SAVR)
ICER (incremental costs/incremental QALYs)
Incremental net monetary benefit
Probabilities of cost-effectiveness
Sensitivity and scenario analyses
“The cost-utility findings were robust to a wide range of sensitivity and scenario analyses (p. vi).”
24
Authors’ conclusion
“In both the intermediate and low surgical risk populations, TAVI was less costly and delivered more QALYs than SAVR (due to the short-term improvement in patients’ health-related quality of life). The probability that TAVI was cost-effective at the €20,000 per QALY gained threshold was 61.8% in intermediate risk patients and 57.1% in low risk patients (p. vi).”24
Appendix 5. Overlap Between Included Systematic Reviews
Note that this appendix has been formatted for accessibility but has not been copy-edited.
Table 10Overlap in Relevant Primary Studies Between Included Systematic Reviews
View in own window
| Primary study citation | Azraai et al. (2020)25 | Health Technology Wales (2020)22 | Ontario Health (2020)23 | HIQA (2019)24 | Gialama et al. (2018)26 |
|---|
|
Baron
SJ, et al
Circulation. 2019;139(7):877–888. [PubMed: 30586747]
| Yes | Yes | NI | Yes | NI |
|
Doble
B, et al
J Thorac Cardiovasc Surg. 2013;146(1):52–60.e3. [PubMed: 22795437]
| NI | NI | NI | NI | Yes |
|
Fagerlund
BC, et al
Oslo (NO): Norwegian Institute of Public Health; 2019.
| NI | Yes | NI | NI | NI |
|
Fairbairn
TA, et al
Heart. 2013;99(13):914–920. [PubMed: 23696198]
| NI | NI | NI | NI | Yes |
|
Gada
H, et al
Am J Cardiol. 2012;109(9):1326–1333. [PubMed: 22335853]
| NI | NI | NI | NI | Yes |
|
Gada
H, et al
Ann Cardiothorac Surg. 2012;1(2):145–155. [PMC free article: PMC3741749] [PubMed: 23977485]
| NI | NI | NI | NI | Yes |
|
Geisler
BP, et al
EuroIntervention. 2019;15(11):e959–e967. [PubMed: 31422922]
| Yes | NI | NI | NI | NI |
|
Goodall
G, et al
J Med Econ. 2019;22(4):289–296. [PubMed: 30547704]
| Yes | Yes | NI | Yes | NI |
|
Kaier
K, et al
Eur J Health Econ. 2019;20(4):625–632. [PubMed: 30600467]
| NI | NI | NI | Yes | NI |
|
Kodera
S, et al
J Cardiol. 2017;71(3):223–229. [PubMed: 29153740]
| Yes | Yes | NI | NI | NI |
|
Neyt
M, et al
BMJ Open. 2012;2(3):e001032
| NI | NI | NI | NI | Yes |
|
Orlando
R, et al
Health Technol Assess. 2013;17(33):1–86. [PMC free article: PMC4781377] [PubMed: 23948359]
| NI | NI | NI | NI | Yes |
|
Osnabrugge
RLJ, et al
Ann Thorac Surg. 2012;94(6):1954–1960. [PubMed: 22959568]
| Yes | NI | NI | NI | Yes |
|
Reynolds
MR, et al
J Am Coll Cardiol. 2016;67(1):29–38. [PMC free article: PMC4959424] [PubMed: 26764063]
| NI | NI | NI | NI | Yes |
|
Reynolds
MR, et al
J Am Coll Cardiol. 2012;60(25):2683–2692. [PubMed: 23122802]
| NI | NI | NI | NI | Yes |
|
Ribera
A, et al
Int J Cardiol. 2015;182:321–328 [PubMed: 25585368]
| NI | NI | NI | NI | Yes |
|
Evidence Note 91. Edinburgh (UK): Scottish Health Technologies Group; 2019.
| NI | Yes | NI | NI | NI |
|
Evidence Development Pilot Project. Edinburgh (UK): Scottish Health Technologies Group; 2010.
| NI | NI | NI | NI | Yes |
|
Tam
DY, et al
Ann Thorac Surg. 2018;106(3):676–684 [PubMed: 29730344]
| NI | NI | Yes | NI | NI |
|
Tam
DY, et al
Eur Heart J Qual Care Clin Outcomes. 2020;0:1–8.
| Yes | Yes | NI | Yes | NI |
|
Tam
DY, et al
J Thorac Cardiovasc Surg. 2018;155(5):1978–1988.e1. [PubMed: 29454487]
| Yes | Yes | NI | Yes | NI |
|
Tarride
JE, et al
Clinicoecon Outcomes Res. 2019;11:477–486. [PMC free article: PMC6677373] [PubMed: 31551658]
| NI | Yes | NI | NI | NI |
|
Zhou
J, et al
Circulation. 2019;140:A14484–A.
| Yes | NI | NI | NI | NI |
|
Zhou
J, et al
Int J Cardiol. 2019;294:17–22. [PubMed: 31255453]
| Yes | Yes | NI | Yes | NI |
NI = the primary study was not included in the systematic review; HIQA = Health Information and Quality Authority; SHTG = Scottish Health Technologies Group; Yes = the primary study was included in the systematic review.
Appendix 6. References of Potential Interest
Note that this appendix has been formatted for accessibility but has not been copy-edited.
Health Technology Assessments — Published Prior to 2018
- 1.
Overviews of Systematic Reviews — Published Prior to 2018
- 2.
Kularatna
S, Byrnes
J, Mervin
MC, Scuffham
PA. Health Technology Assessments Reporting Cost-Effectiveness of Transcatheter aortic valve Implantation.
Int J Technol Assess Health Care. 2016
Jan;32(3):89–96.
PubMed [
PubMed: 27491522]
Review Articles
- 3.
Adams
HSL, Ashokkumar
S, Newcomb
A, MacIsaac
AI, Whitbourn
RJ, Palmer
S. Contemporary review of severe aortic stenosis.
Intern Med J. 2019
Mar;49(3):297–305.
PubMed [
PubMed: 30091235]
- 4.
- 5.
Witberg
G, Patterson
T, Redwood
S, Prendergast
B. Future Directions. Transcatheter aortic valve Implantation for Low-risk Patients: Inevitable Evolution or a Step Too Far?
Rev Esp Cardiol (Engl). 2019
Aug;72(8):664–671.
PubMed [
PubMed: 30930254]
- 6.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Questions or requests for information about this report can be directed to ac.HTDAC@stseuqeR